Background: Cellular uptake of retinol bound to its serum binding protein depends on a cell surface receptor.
Results: Functional coupling of this receptor with lecithin:retinol acyltransferase is required for the regulated cellular uptake of retinol.
Conclusion: The lecithin: retinol acyltransferase is critical for retinol uptake and homeostasis.
Significance: Blood retinol homeostasis is associated with blinding retinopathies and diabetes.
Keywords: Membrane, Retinoid, Retinoid-binding Protein (RBP), Transport, Vitamin A, Fenretinide, LRAT, STRA6, Cellular Retinol Uptake
Abstract
Vitamin A (all-trans-retinol) must be adequately distributed within the mammalian body to produce visual chromophore in the eyes and all-trans-retinoic acid in other tissues. Vitamin A is transported in the blood bound to retinol-binding protein (holo-RBP), and its target cells express an RBP receptor encoded by the Stra6 (stimulated by retinoic acid 6) gene. Here we show in mice that cellular uptake of vitamin A from holo-RBP depends on functional coupling of STRA6 with intracellular lecithin:retinol acyltransferase (LRAT). Thus, vitamin A uptake from recombinant holo-RBP exhibited by wild type mice was impaired in Lrat−/− mice. We further provide evidence that vitamin A uptake is regulated by all-trans-retinoic acid in non-ocular tissues of mice. When in excess, vitamin A was rapidly taken up and converted to its inert ester form in peripheral tissues, such as lung, whereas in vitamin A deficiency, ocular retinoid uptake was favored. Finally, we show that the drug fenretinide, used clinically to presumably lower blood RBP levels and thus decrease circulating retinol, targets the functional coupling of STRA6 and LRAT to increase cellular vitamin A uptake in peripheral tissues. These studies provide mechanistic insights into how vitamin A is distributed to peripheral tissues in a regulated manner and identify LRAT as a critical component of this process.
Introduction
Vitamin A (all-trans-retinol (ROL)2) plays a pivotal role in vision, embryonic development, immunity, and metabolic control. ROL is the precursor for at least two critical metabolites, 11-cis-retinal, the chromophore of G protein-coupled receptors that mediate phototransduction in the eyes (1), and all-trans-retinoic acid (ATRA), the ligand of retinoic acid receptors that, along with retinoid X receptors, regulate the expression of numerous target genes (2). To support retinoid-dependent processes in tissues, mammals have evolved two major transport systems to distribute and store vitamin A (3). Absorbed dietary ROL is converted to retinyl esters (REs) by enterocytes of the intestine (4) and packaged into chylomicrons that are secreted into the lymph (5). A portion of postprandial circulating REs is taken up by peripheral tissues in a process involving extracellular hydrolysis by lipoprotein lipase (6). But the large remainder (about 70%) in chylomicron remnants is cleared by hepatocytes and hydrolyzed back to ROL (7), transferred to hepatic stellate cells, and esterified for storage (8). Mobilization of vitamin A from liver stores is critically dependent on the ROL-binding protein (RBP) (9). This 21-kDa protein is produced in hepatocytes and secreted into the circulation in a ROL-dependent manner. In the blood, holo-RBP forms a protein·protein complex with the 55-kDa transthyretin (TTR) homotetramer at a 1:1 molar ratio (10). TTR·RBP complex formation is required for normal blood ROL homeostasis and prevents filtration of the relatively small holo-RBP molecule through kidney glomeruli (10). Despite the membrane permeability of ROL, a RBP receptor was proposed long ago (11), and this was recently identified to be encoded by the Stra6 (stimulated by retinoic acid 6) gene (12). Stra6 is widely expressed in mammalian embryos and in several retinoid-metabolizing peripheral tissues of adults, including the eyes, brain, and lung. The liver, as the major organ for retinoid storage and RBP secretion, does not express this protein (12, 13). Recent studies in a Stra6 knock-out mouse model confirmed the importance of STRA6 for ocular retinoid uptake (14).
Cell culture studies indicate that the flux of ROL between RBP and STRA6 is bidirectional and that cellular accumulation of ROL depends on the cellular vitamin A-binding protein 1 (CRBP1) and lecithin:ROL acyltransferase (LRAT) (15, 16). LRAT is a microsomal membrane-anchored enzyme that converts ROL into RE by transferring palmitate from the sn-2 position of lecithin (17). Previously, LRAT function has mainly been investigated in the context of vision and storage of dietary vitamin A in tissues. Lrat−/− mice lack vitamin A stores in the liver and are highly susceptible to dietary vitamin A deficiency (8, 18); these animals also are blind due to the critical role of Lrat in processing visual chromophore (19). Co-expression of Stra6 and Lrat in several peripheral tissues implicates LRAT in vitamin A uptake as well. Finally, both Stra6 and Lrat are ATRA-regulated target genes (13, 20). These observations prompted us to analyze the role of LRAT in vitamin A homeostasis with mouse models to elucidate the putative interdependence of STRA6 and LRAT in cellular vitamin A uptake and its regulation.
EXPERIMENTAL PROCEDURES
Animals
Twelve-week-old female Lrat−/− (19) and wild type control mice with a C57/BL6;129Sv mixed genetic background were used for most experiments described. Mice provided regular chow with ad libitum access to food and water were maintained at 24 °C in a 12-h/12-h light/dark cycle. For dietary vitamin A experiments, 8-week-old wild mice were provided either a vitamin A-sufficient (4,000 IU of vitamin A/kg) or a vitamin A-free diet for 20 weeks with diets prepared by Research Diets, Inc. (New Brunswick, NJ). All animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committee and conformed to recommendations of both the American Veterinary Medical Association Panel on Euthanasia and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Expression and Purification of Human Serum RBP
RBP expression and purification from Escherichia coli was accomplished essentially as described previously (21). Briefly, human RBP (hRBP) cDNA was cloned into a pET3a expression vector and expressed in BL-21 DE3 cells according to a standard protocol. Bacterial cells were harvested and lysed by osmotic shock. Insoluble material was pelleted by centrifugation, washed, and solubilized in 7 m guanidine hydrochloride and 10 mm dithiothreitol. After overnight incubation, insoluble material was removed by ultracentrifugation, and the supernatant was used for the hRBP refolding procedure. hRBP was refolded by the dropwise addition of solubilized material into a mixture containing 150 μCi of [11,12-3H]ROL ([3H]ROL) (PerkinElmer Life Sciences) and non-radiolabeled ROL (Sigma) at a final concentration of 1 mm. Refolded holo-hRBP was dialyzed against 10 mm Tris/HCl buffer, pH 8.0, and loaded onto a DE53 anion exchange chromatography column (Whatman, Piscataway, NJ). Holo-hRBP was eluted with linear gradient of NaCl (0–1 m) in 10 mm Tris/HCl buffer, pH 8.0. Collected fractions were examined by SDS-PAGE and UV-visible spectroscopy to ensure a proper protein/retinoid ratio. Fractions containing at least 90% holo-hRBP were pooled together and concentrated in a Centricon centrifugal filter device (cut-off 10,000 Da) (Millipore, Billerica, MA) to 5 mg/ml. [3H]ROL was quantified in a scintillation counter (Beckman Coulter, Indianapolis, IN). Holo-hRBP aliquots were stored at −80 °C until used.
Intraperitoneal Injection of Holo-RBP and Measurement of [3H]ROL Accumulation in Mouse Tissues
Holo-hRBP (100 μl containing 0.4 μCi of [3H]ROL and 5 nmol of non-radiolabeled ROL) was intraperitoneally injected into wild type and Lrat−/− mice. After injection, mice were sacrificed at different time points (1, 2, 3, and 6 h), and tissue and blood were harvested. Serum (100 μl) and either one eye or tissue (10 mg) from lung, kidney, or adipose tissue homogenized in 100 μl of PBS (137 mm NaCl, 2.7 mm KCl, 4.3 mm Na2HPO4, 1.4 mm KH2PO4, pH 7.3) was mixed with 5 ml of scintilliation mixture (Opti-Fluor, PerkinElmer Life Sciences), and [3H]ROL content was quantified in a Beckman LS 6500 scintillation counter (Beckman Coulter).
Treatment with RBP-lowering Agents; Blood and Tissue Collection
At 8:00 a.m., a small blood sample was collected from the tail vein of each animal to determine the RBP level at the time point (t = 0) and kept on ice until serum separation. Immediately thereafter, mice were gavaged with 30 mg/kg body weight of either fenretinide (Toronto Research Chemicals), A1120 (Sigma), or ATRA (Sigma) dissolved in 200 μl of canola oil with the same volume of canola oil used as a vehicle control. Tail vein blood samples were collected at 4, 8, and 24 h after treatment. Mice then were anesthetized by intraperitoneal injection of a mixture containing ketamine (80 mg/kg body weight) and xylazine (20 mg/kg body weight) in 10 mm sodium phosphate, pH 7.2, with 100 mm NaCl, and blood was drawn directly from the heart after snipping the right atrium. Then mice were perfused with 10 ml of PBS and killed by cervical dislocation. Liver, kidney, lung, eyes, and gonadal adipose tissue were dissected out, weighed, and immediately snap-frozen in liquid nitrogen before storage at −80 °C until further use.
Analysis of RBP Secretion by HepG2 Cells
Human hepatoma HepG2 cells were maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin sulfate and cultured at 37 °C in 5% CO2. When cells reached confluence, the medium was removed, and cells were washed twice with PBS. Then cells were preincubated for 30 min in DMEM containing 10 mg/ml bovine serum albumin (Sigma) and 20 μg/ml cyclohexamide (Sigma). ATRA (Sigma) dissolved in ethanol was added to the medium at a final concentration of 2 μm, and cells were incubated for 2 h at 37 °C. Then ROL was added at a final concentration of 2 μm, and cells were incubated for an additional 1 h. Ethanol only (0.1%, v/v) was used as the vehicle controls. Cells and medium were harvested and frozen separately at −80 °C until further analysis.
Immunoblotting
All tissue samples were homogenized in M-PER mammalian protein extraction reagent (Thermo Scientific, Marietta, OH) following the manufacturer's instructions. For RBP determinations in liver, 20 μg of total protein was used. To determine RBP serum levels, 2 μl of serum was diluted with 40 μl of PBS containing Complete Mini EDTA-free protease inhibitor (Roche Applied Science), and 4 μl of this solution was used for immunoblot analysis. To quantify RBP protein levels in HepG2 cells, 10–20 μg of total protein cell lysate and 50 μl of cell-free medium were subjected to immunoblot analyses. Samples were subjected to SDS-PAGE and then electroblotted onto PVDF membranes (Bio-Rad). For LRAT and CRBP1 determinations in liver, lung, and adipose tissue, 10–30 μg of total protein was fractionated by SDS-PAGE and immunoblotted as described above. Membranes then were blocked with fat-free milk powder (5%, w/v) dissolved in Tris-buffered saline (15 mm NaCl and 10 mm Tris/HCl, pH 7.5) containing 0.01% Tween 100 (TBS-T), washed, and incubated overnight at 4 °C with the appropriate primary antibody. For RBP detection, a rabbit anti-human RBP serum (DakoCytomation, Denmark) was used at a 1:1000 dilution. For CRBP1 detection, a polyclonal rabbit antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was employed at a 1:2000 dilution. For LRAT detection, a non-commercial anti-LRAT monoclonal antibody was used at a dilution of 1:2000 (19). Either β-actin antiserum (Cell Signaling, Boston, MA) at a dilution of 1:1000 or Ponceau S staining solution (Boston BioProducts, Ashland, MA) served as a loading control for total protein visualization. Secondary antibodies employed were either horseradish peroxidase-conjugated anti-rabbit IgG (Promega, Madison, WI) or anti-mouse IgG (Promega) used at a dilution of 1:5000. Immunoblots were developed with the ECL system (GE Healthcare). Quantification of scanned immunoblot single bands was performed with ImageJ software.
mRNA Isolation and Quantitative PCR Analysis
Total mRNA isolation was carried out with the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA concentration and purity was measured with a Nano-drop spectrophotometer (ND-1000, Thermo Scientific). The Applied BioSystems retrotranscription kit (Applied BioSystems, Carlsbad, CA) was employed to reverse transcribe 1 μg of total RNA to cDNA. qRT-PCR was carried out with TaqMan probes (Applied BioSystems) for Cyp26a1 (Mm00514486_m1), Stra6 (Mm00486457_m1), Lrat (Mm00469972_m1), and RBP (Mn00803266_m1). β-Actin (Mm01205647_g1) and 18S rRNA (4319413E) probe sets were used as endogenous controls. All real-time experiments were done with an ABI Step-One Plus qRT-PCR instrument (Applied BioSystems).
HPLC Analysis of Retinoids
Non-polar retinoids were extracted from either 70–100 μl of serum or 30 mg of tissue under a dim red safety light as described previously (22). The extraction solution was composed of 200 μl of methanol, 400 μl of acetone, and 500 μl of hexanes. The organic phase was removed, and the extraction was repeated with 500 μl of hexanes. Then the collected organic phases were pooled and dried in a SpeedVac (Eppendorf, Hamburg, Germany). The residue was dissolved in 200 μl of HPLC solvent (hexane/ethyl acetate, 90:10, v/v). HPLC was performed with a normal phase Zobax Sil (5 μm, 4.6 × 150 mm) column (Agilent, Santa Clara, CA). Isocratic chromatographic separation was achieved with 10% ethyl acetate/hexane at a flow rate of 1.4 ml/min. For quantification of retinoids, the HPLC was scaled previously with the pattern compound ROL and RE (Sigma).
Statistical Analyses
Values are expressed as means ± S.E. Statistical significance of differences derived from either the two-way ANOVA or two-tailed Student's t test were analyzed by SPSS 14.0 for windows (SPSS, Chicago, IL), with the threshold of significance set at p < 0.05.
RESULTS
ROL Uptake from Holo-RBP in Vivo Depends on LRAT Function
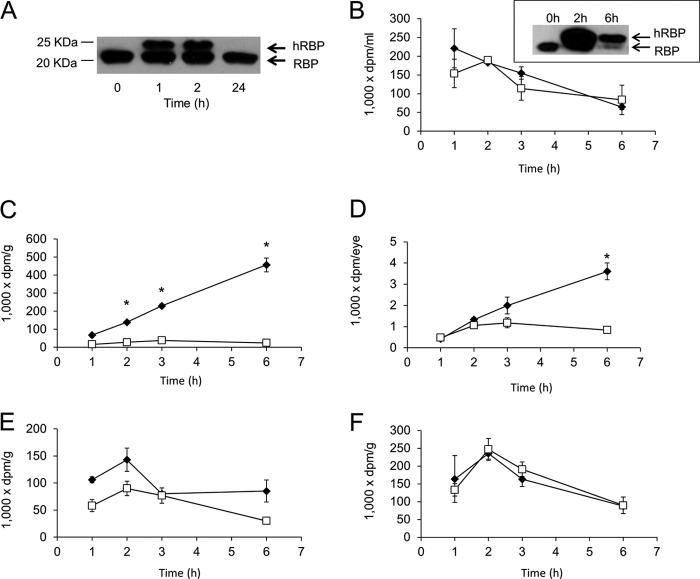
Previous cell culture studies implicated LRAT in the uptake of vitamin A from holo-RBP (12, 15, 16). To analyze the role of LRAT for this process in vivo, we devised a method to measure cellular vitamin A uptake from holo-RBP in mice. It had been shown that human RBP can substitute for blood ROL transport in Rbp−/− mice (9, 23). Thus, we produced recombinant human holo-RBP and injected it intraperitoneally into mice. Endogenous mouse and recombinant human RBPs showed slightly different eletrophoretic mobility when serum samples were fractionated in 15% SDS-polyacrylamide gels (Fig. 1A). 1 h after administration, hRBP was detectable in the circulation, demonstrating that it was absorbed and transported in the blood stream, but after 24 h, TTR-unbound hRBP was completely cleared from circulation by glomerular filtration (Fig. 1A).
FIGURE 1.
Cellular uptake of retinol from holo-RBP is LRAT-dependent. A, immunoblot analysis of recombinant hRBP and endogenous RBP protein levels in mouse serum after hRBP intraperitoneal injection. B–F, hRBP was expressed in E. coli, purified, and refolded in the presence of a mixture of non-labeled and tritiated [3H]retinol. Then 100 μl of radiolabeled hRBP (corresponding to 0.4 μCi) was injected intraperitoneally into wild type (filled diamonds) and Lrat−/− (open squares) mice. B, levels of [3H]retinol in the serum of these mice. The inset shows an immunoblot analysis of RBP in their serum. Levels of [3H]retinol in the lungs (C), eyes (D), adipose tissue (E), and kidneys (F) of these mice are shown as a function of time. Values indicate means ± S.E. from at least three animals per tissue and genotype ± S.E. (error bars). *, p < 0.05 in Student's t test comparing both genotypes at each time point.
To follow the metabolic fate of ROL bound to RBP, we loaded recombinant human RBP with [3H]ROL. Both Lrat−/− and wild type mice were injected intraperitoneally with a [3H]ROL/hRBP solution corresponding to 0.4 μCi. We then measured levels of [3H]ROL in the serum and tissues of mice at different time points after injection. Both Lrat−/− and wild type mice showed a transient increase of [3H]ROL in the serum that decreased with time (Fig. 1B). Although analysis of the lung and eyes of wild type mice demonstrated a significant uptake of [3H]ROL over time (Fig. 1, C and D), the lungs and eyes of Lrat−/− mice showed no such uptake (Fig. 1, C and D). We also measured [3H]ROL uptake in white adipose tissue that lacks Lrat expression (8). Our analysis revealed that [3H]ROL was detectable in adipose tissue of both genotypes, reaching a maximum after 2 h; by 6 h, [3H]ROL had decreased in the fat of both genotypes (Fig. 1E). We also determined [3H]ROL in the kidneys of these animals. Again, [3H]ROL levels increased, reached a maximum at about 2 h, and then decreased with time in both genotypes (Fig. 1F).
To determine the chemical form of [3H]ROL that accumulated in tissues, we extracted non-polar retinoids from the lungs and kidneys of both Lrat−/− and wild type mice. Retinoids were separated by HPLC, and the flow-through was collected every 2 min (Fig. 2A). Collected samples were dried and analyzed in a scintillation counter (Fig. 2, B and C). About 90% of [3H]ROL detected in the lung of wild type mice eluted in the RE fraction (Fig. 2B), indicating that most of the [3H]ROL absorbed by this tissue was esterified. No such accumulation of [3H]ROL in the RE fraction was noted in Lrat−/− mice. In wild type mice, some [3H]ROL was detected as RE in the kidneys as well (Fig. 2C), but most of the injected [3H]ROL (75% in wild type and 95% in Lrat−/− mice) remained unchanged as free non-esterified ROL, probably reflecting the clearance of TTR-unbound hRBP from the circulation by glomerular filtration.
FIGURE 2.
Retinol accumulates as retinyl esters in wild type mice. A, HPLC chromatogram of non-polar retinoids extracted from a lung of a wild type mouse. RE eluted at 2.5 min, and ROL eluted at 9.2 min. The inset shows the spectrum of the retinyl ester peak. B and C, lipophilic extracts from the lungs (B) and kidneys (C) of animals injected with hRBP loaded with [3H]retinol were separated by HPLC. The flow-through was collected every 2 min (x axis), and [3H]retinol levels (y axis) were quantified with a scintillation counter. Numbers shown on the ordinate are expressed in dpm/g of tissue. Values present means ± S.E. (error bars) of data derived from at least three animals per tissue and genotype. D, serum ROL levels in WT and Lrat−/− mice. E, qRT-PCR analyses for Stra6 mRNA expression in WT and Lrat−/− mice. Values in D and E present means ± S.E. of data from at least five animals per tissue and genotype. F, immunoblot analysis for CRBP1 in the liver, lung, and white adipose tissue (WAT) of wild type and Lrat−/− mice. Representative immunoblots from two animals per genotype are shown.
CRBP1 Does Not Contribute Significantly to Cellular ROL Uptake
We found that wild type but not Lrat−/− mice accumulated [3H]ROL from hRBP in the lung and eyes. To exclude the possibility that these differences were caused by alterations in ROL serum levels and/or Stra6 mRNA levels, we compared age- and sex-matched mice. No significant changes in ROL blood levels were observed between these two genotypes (Fig. 2D). Moreover, Stra6 mRNA levels in the lung and the eyes were comparable (Fig. 2E). Interestingly, Lrat−/− mice displayed significantly elevated Stra6 mRNA levels in white adipose tissue (Fig. 2E).
In cell culture studies, CRBP1 has been implicated in STRA6-mediated vitamin A uptake (16). Interestingly, Lrat−/− mice showed elevated CRBP1 protein in the liver, lung, and adipose tissue as compared with wild type control animals (Fig. 2F). Because [3H]ROL did not accumulate in the eyes and lungs of Lrat−/− mice and Stra6 was expressed in these tissues, we conclude that CRBP1 alone cannot significantly enhance STRA6-mediated ROL uptake in the absence of LRAT.
Retinoic Acid Induces Lrat-dependent Vitamin A Uptake and Storage
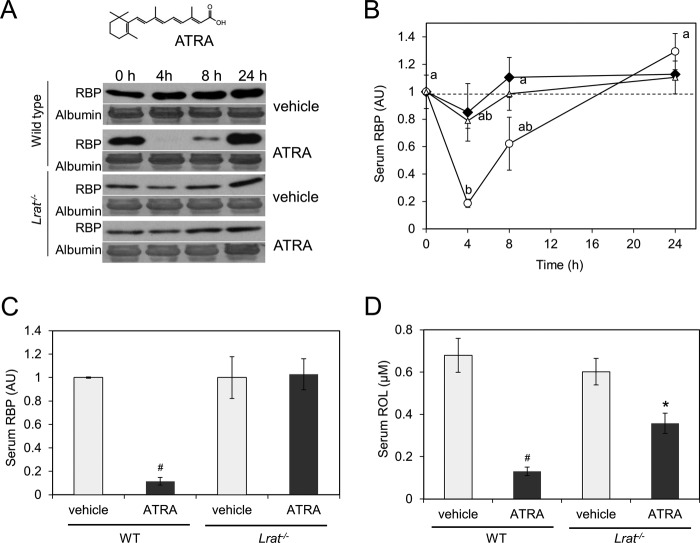
Our analyses revealed that LRAT is critical for cellular ROL uptake from circulating holo-RBP by the lungs and eyes of mice. However, the coupling of STRA6 with LRAT may also contribute to the regulation of blood vitamin A homeostasis because both proteins are encoded by ATRA-regulated genes (13, 20). Thus, we tested whether an acute systemic ATRA treatment would increase ROL uptake from circulating holo-RBP and if LRAT is required for this process. We first recorded dose-response curves in wild type mice treated with ATRA and vehicle alone and collected blood from the tail vein at different time points. Treatment with 30 and 10 mg/kg ATRA significantly decreased serum RBP levels after 4 h, whereas this effect of ATRA was not observed in mice treated with 5 mg/kg (Fig. 3, A and B). We next gavaged both wild type and Lrat−/− mice with ATRA (30 mg/kg) or vehicle alone. Again, 4 h after ATRA treatment, serum RBP levels were dramatically reduced in wild type mice. RBP serum levels then increased by 8 h and reached initial values by 24 h after ATRA treatment (Fig. 4, A and B). In contrast to wild type mice, no changes of RBP serum levels were observed when Lrat−/− mice were treated with the highest dose (30 mg/kg) of ATRA (Fig. 4, A and B).
FIGURE 3.

Retinoic acid dose-dependently decreases serum holo-RBP levels in wild type mice. A, serum RBP levels in wild type mice after a single oral gavage with different doses of ATRA. A small blood sample from the tail vein was collected from each animal (n = 3 per genotype and condition) at different time points (0 h (just before treatment) and 4, 8, and 24 h after treatment). Representative immunoblot analyses for RBP in serum are shown. Albumin stained with Ponceau S was used as the loading control. B, quantification of serum RBP levels after various doses of ATRA is shown. One-way ANOVA, followed by LSD post hoc, was performed (p < 0.05), a ≠ b. Error bars, S.E.
FIGURE 4.
Retinoic acid decreases serum holo-RBP levels in wild type but not in Lrat−/− mice. A and B, serum RBP levels in WT and Lrat−/− mice after a single oral gavage with 30 mg/kg ATRA. A small blood sample from the tail vein was collected from each animal (n = 3 per genotype and condition) at different time points (0 h (just before treatment) and 4, 8, and 24 h after treatment). A, representative immunoblot analyses for RBP in serum. Ponceau S staining for albumin was used as loading control. B, quantification of serum RBP levels in different groups. Black diamonds, vehicle-treated mice; open triangles, Lrat−/− mice treated with ATRA; open circles, WT mice treated with ATRA. One-way ANOVA, followed by LSD post hoc, was performed (p < 0.05), a ≠ b. C and D, serum RBP and ROL levels 4 h after a single gavage with 30 mg/kg ATRA and vehicle only. Serum RBP levels were quantified by immunoblot analysis (C), and ROL levels were quantified by HPLC analysis (D). As a loading control, PVDF membranes were stained with the nonspecific protein dye Ponceau S. The band selected in Fig. 3A corresponds with the molecular mass of the albumin (66 kDa). #, p < 0.0001; *, p < 0.05 for Student's t test comparing each genotype with the vehicle control. One-way ANOVA, followed by LSD post hoc, was performed (p < 0.05), a ≠ b. Error bars, S.E.
We then treated wild type and Lrat−/− mice with ATRA (30 mg/kg) or vehicle only and sacrificed animals after 4 h to collect serum and tissues. Measurement of serum RBP levels again revealed a significant decrease in ATRA-treated wild type animals as compared with their vehicle only-treated siblings, but no such decrease was found in Lrat−/− mice (Fig. 4C). We then measured serum ROL levels by HPLC analysis. In wild type mice, serum levels of ROL were significantly lower, mirroring the decrease of RBP (Fig. 4D). Lrat−/− mice also showed a slight reduction of serum ROL levels upon ATRA treatment (Fig. 4D). However, this decrease was significantly lower than in wild type mice.
The rapid decrease of holo-RBP upon ATRA treatment could be caused by increased STRA6 and LRAT activity in tissues and the elimination of ROL-free RBP via glomerular filtration. Therefore, we next measured Stra6 and Lrat mRNA expression levels by qRT-PCR analysis in ATRA-treated animals and compared them with vehicle-treated control animals (Fig. 5). ATRA treatment significantly increased Stra6 and Lrat mRNA expression in the lungs of wild type mice (Fig. 5, A and B). We also confirmed induction of LRAT expression at the protein level (Fig. 5C). Surprisingly, ATRA had no significant effect on ocular Lrat expression, and only a modest increase of Stra6 expression was detected (Fig. 5, D and E). To exclude the possibility that this negative finding was caused by a lack of ATRA in ocular tissues, we determined ocular expression levels of Cyp26A1. qRT-PCR revealed an 18-fold induction of mRNA expression for this ATRA-inducible marker gene (Fig. 5F) (24).
FIGURE 5.
ATRA treatment induces Lrat and Stra6 expression in extraocular tissues of mice. WT and Lrat−/− mice (n = 4/group) were treated with 30 mg of ATRA/kg body weight or with vehicle only. After 4 h, Stra6 and Lrat mRNA expression was measured by qRT-PCR analysis, and LRAT protein levels were determined from immunoblots. A, Stra6 mRNA levels in lungs of WT mice. B, Lrat mRNA expression in lungs of WT mice. C, LRAT protein levels in lungs of WT mice. A representative immunoblot is shown on the left. D, Stra6 mRNA expression in the eyes of WT mice. E, Lrat mRNA expression in the eyes of WT mice. F, Cyp26a1 mRNA expression in the eyes of WT mice. G, Stra6 mRNA levels in adipose tissue of WT mice. H, Stra6 mRNA levels in the lungs of Lrat−/− mice. I, Stra6 mRNA levels in the eyes of Lrat−/− mice. J, Cyp26a1 mRNA expression in the eyes of Lrat−/− mice. K, Stra6 mRNA levels in adipose tissue of Lrat−/− mice. 18S was used as internal control for mRNA expression in the lung, liver, and adipose tissue, and β-actin was used in the eye. Data represent the mean ± S.E. (error bars) *, p < 0.05; Student's t test.
Stra6 mRNA expression also was significantly increased in the lungs of ATRA-treated Lrat−/− mice (Fig. 5H) with only a slight increase found in their eyes (Fig. 5I), despite a 16-fold increase in ocular Cyp26a1 mRNA expression (Fig. 5J). Stra6 expression also was highly increased in white adipose tissue of both treated genotypes (Fig. 5, G and K). Thus, we conclude that concomitant induction of Stra6 and Lrat expression in extraocular tissues, such as the lung, contributes to rapid uptake of vitamin A from holo-RBP in wild type mice and that induction of STRA6 alone is not sufficient to increase such uptake in Lrat−/− mice.
ATRA Retains RBP in Hepatocytes in an LRAT-independent Manner
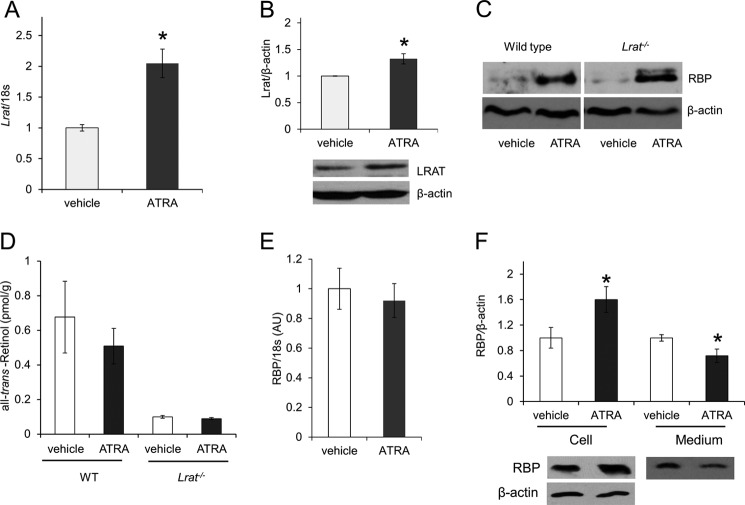
The liver secretes holo-RBP and constitutes a major contributor to blood vitamin A homeostasis. In vitamin A deficiency, lack of hepatic ROL prevents the secretion of holo-RBP into the circulation. Thus, we examined whether an ATRA-dependent induction of Lrat expression in liver could impede holo-RBP release by converting hepatic ROL into its ester form. As expected, qRT-PCR and immunoblot analysis demonstrated an induction of LRAT in ATRA-treated wild type mice as compared with vehicle-treated controls (Fig. 6, A and B). To analyze whether such increased hepatic LRAT expression could lead to hepatic retention of RBP, we measured both RBP and ROL levels in livers of Lrat−/− and wild type mice. Surprisingly, liver RBP levels were significantly increased upon ATRA treatment independent of the genotype as compared with vehicle-treated controls (Fig. 6C). HPLC analysis revealed no changes of liver ROL levels in both genotypes, but Lrat−/− mice showed lower ROL levels relative to wild type mice (Fig. 6D). RBP mRNA levels remained unchanged after treatment with ATRA (Fig. 6E), showing that the observed elevated hepatic RBP did not result from de novo biosynthesis. Thus, we speculate that the LRAT- and ROL-independent increase of hepatic RBP could be explained by the binding of ATRA to RBP in hepatocytes. Previous studies have reported that RBP has similar affinities for ATRA and ROL in vitro (25) and that RBP upon ATRA binding is retained in hepatocytes (26). To confirm this effect of ATRA on RBP release, we treated human hepatoma HepG2 cells with ATRA or vehicle for 2 h and then added ROL. (Cells were preincubated with cyclohexamide to avoid any ATRA-induced changes in gene expression). HepG2 cells incubated with ATRA showed higher levels of RBP as compared with vehicle-treated cells. (Fig. 6F). The opposite was true when we measured RBP levels in the medium (Fig. 6F), indicating that ATRA reduced RBP release from cells. These observations could explain the LRAT-independent increase of RBP in the liver of mice after ATRA treatment. However, accumulation of hepatic RBP in both genotypes and the lack of ATRA effects on circulating holo-RBP in Lrat−/− mice exclude the possibility that this mechanism significantly contributes to the rapid decrease in serum RBP levels observed only in wild type animals.
FIGURE 6.
ATRA inhibits RBP release from hepatocytes. WT and Lrat−/− mice (n = 4/5 group) were treated with 30 mg of ATRA/kg body weight or the same volume of vehicle. A and B, hepatic Lrat mRNA (A) and protein levels (B) in WT mice. C, representative immunoblot analysis of hepatic RBP levels in WT and Lrat−/− mice upon vehicle and ATRA treatments. β-Actin was used as loading control. D, ROL levels in livers of WT and Lrat−/− mice. E, hepatic Rbp mRNA levels. F, HepG2 cells were preincubated with cyclohexamide (20 μg/ml) for 30 min before adding ATRA (2 μm final concentration) or vehicle only. 2 h later, ROL (2 μm final concentration) was added, and cells were incubated for an additional 2 h. Cells and medium were harvested, and RBP protein levels were determined by immunoblot analysis. Data represent means ± S.E. (error bars) from three independent experiments. *, p < 0.05; Student's t test.
ROL Is Preferentially Transported to the Eyes in Long Term Vitamin A Deficiency
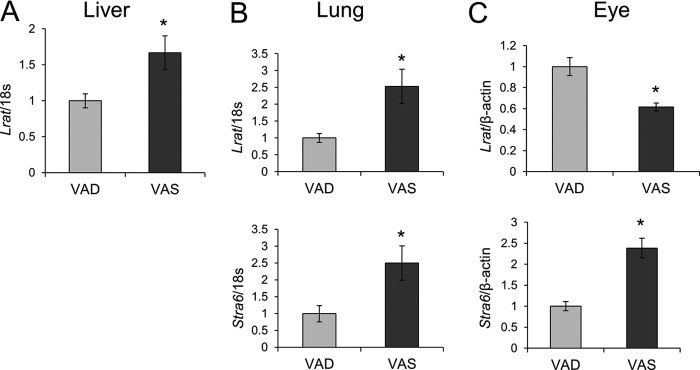
We found that Stra6 and Lrat expression is subject to ATRA regulation in non-ocular peripheral tissues, such as the lung, whereas ATRA did not induce ocular LRAT expression (Fig. 5). This difference suggested that ROL uptake from circulating holo-RBP in the eyes does not depend on the vitamin A status (e.g. it might still occur under conditions of prolonged vitamin A deficiency). Therefore, we analyzed the retinoid content of different tissues in wild type mice subjected to dietary vitamin A restriction for 20 weeks and compared the data with data from vitamin A-sufficient control animals. HPLC analyses showed a profound depletion of vitamin A levels in mice provided a vitamin A-deficient diet (VAD) in the liver and the lung, as compared with siblings given the vitamin A-sufficient (VAS) diet (Fig. 7, A and B). Serum ROL levels also were lower in VAD animals, but this difference did not reach statistical significance (Fig. 7C). In contrast, retinoid content of the eyes was comparable between VAD and VAS animals (Fig. 7D). We also measured Lrat and Stra6 mRNA levels in these tissues (Fig. 8). Lrat mRNA levels were significantly higher in VAS animals as compared with VAD animals in both the liver and the lung (Fig. 8, A and B). Stra6 mRNA levels were also higher in the lungs of the VAS mice (Fig. 8B). In contrast, Lrat mRNA levels in the eye were higher in the VAD compared with the VAS mice, whereas Stra6 mRNA showed the opposite expression pattern (Fig. 8C). This regulation could assure that circulating holo-RBP is mainly utilized by the eyes to support vision under prolonged dietary vitamin A restriction.
FIGURE 7.
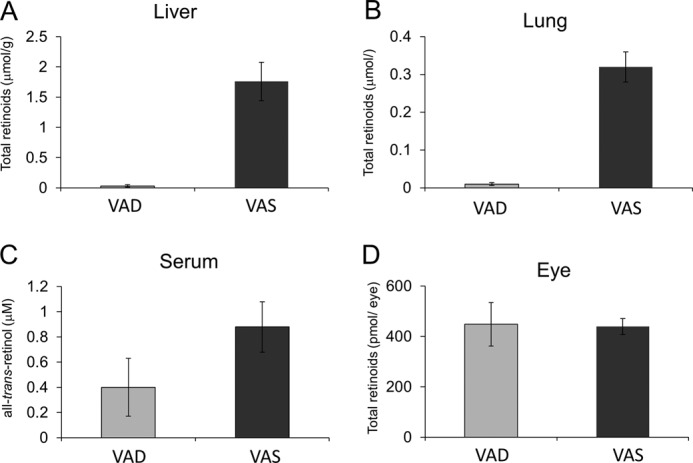
Ocular retinoid homeostasis is maintained in mice subjected to dietary vitamin A restriction. Upon weaning, male wild type mice (n = 5/condition) were maintained on either a vitamin A-deficient or -sufficient (4000 IU of vitamin A/kg) diet. After 20 weeks, animals were sacrificed, and retinoid levels were determined in the serum and tissues. A, total retinoid levels in the liver. B, total retinoid levels in the lungs. C, total retinoid levels in the eyes. D, ROL levels in the serum.
FIGURE 8.
Stra6 and Lrat expression is influenced by the vitamin A status of animals. Upon weaning, male wild type mice (n = 5/condition) were maintained on either a VAD or VAS (4000 IU of vitamin A/kg) diet. After 20 weeks, animals were sacrificed, and total RNA was isolated from the liver, lungs, and eyes. Stra6 and Lrat mRNA expression was measured by qRT-PCR analysis. A, Lrat mRNA levels in the liver. B, Lrat and Stra6 mRNA expression in lungs. C, Lrat and Stra6 mRNA expression in lungs. 18S ribosomal RNA was used as the internal control for mRNA expression in the lungs and liver, and β-actin was used for that in the eye. Data represent the means ± S.E. (error bars). *, p < 0.05; Student's t test.
The RBP-lowering Drug Fenretinide Acts in an LRAT-dependent Manner
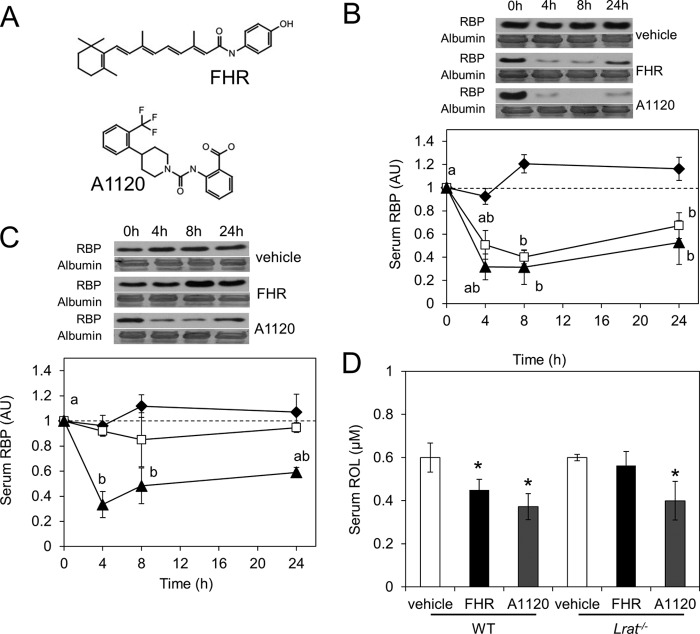
Pharmacological reduction of holo-RBP levels has been proposed as a strategy to combat certain diseases, such as lipofuscin-based retinopathies and type 2 diabetes (27, 28). Treatment of Abca4−/− mice, a model for Stargardt disease, with the synthetic retinoid, fenretinide (FHR) (Fig. 9A), arrested accumulation of A2E and lipofuscin autofluorescence in the RPE (28). Recent research also has associated elevated blood RBP levels with type 2 diabetes (29). Studies in diabetic mouse models have shown that FHR lowers RBP levels and improves glucose tolerance (27, 29). Another RBP-lowering molecule, A1120 (Fig. 9A), has been identified by a high throughput screening approach (30). In vitro studies indicate that both FHR and A1120 disrupt the protein interaction between RBP and TTR (30). RBP freed from TTR is then cleared from the circulation by glomerular filtration. Surprisingly, despite the RBP-lowering effect of both compounds, only FHR improves the glucose tolerance of diabetic mice (29, 30). However, the mechanism responsible for this difference has not been established.
FIGURE 9.
FHR but not A1220 decreases RBP and retinol serum levels in a LRAT-dependent manner. A, molecular structure of the two RBP-lowering agents used, namely the synthetic retinoid FHR and A1120. B and C, serum RBP levels in wild type (B) and Lrat−/− (C) mice after a single gavage with 30 mg of FHR/kg (white squares), 30 mg of A1120/kg (black triangles), or vehicle (black diamonds). Blood samples from the tail vein of each animal were collected (n = 3/genotype and condition) at the time points shown. Representative immunoblots for serum RBP are displayed above the graphs. Ponceau S staining for albumin was used as loading control. D, after 24 h, mice were sacrificed, and serum was collected for HPLC analysis of ROL levels. As a loading control, PVDF membranes were stained with the nonspecific protein dye Ponceau S. The band selected in Fig. 7, B and C, corresponds with the molecular mass of the albumin (66 kDa). Data represent means ± S.E. (error bars) One-way ANOVA, followed by LSD post hoc, was performed (p < 0.05), a ≠ b. *, p < 0.05; Student's t test comparing the control group with each genotype.
Previously, it was shown that, aside from its destabilizing influence on the holo-RBP·TTR complex, FHR also can induce the transcriptional activity of ATRA-dependent regulated genes (31). Thus, we investigated whether the effects of FHR on blood RBP levels are at least in part caused by the induction of Stra6 and Lrat mRNA expression. To confirm that FHR can induce ATRA-regulated target genes, we treated WT mice with a single dose of FHR (30 mg/kg) and then measured Cyp26a1 expression by qRT-PCR in the liver after 24 h. This experiment showed that Cyp26a1 mRNA was 4.5-fold induced after FHR treatment (data not shown). Analysis of hepatic LRAT levels by immunoblot blot also indicated a 3-fold induction (data not shown). To analyze if the reduction of blood RBP levels by FHR is mediated by induction of Stra6 and Lrat mRNA expression in peripheral tissues, we next treated WT and Lrat−/− mice with FHR (30) and included the non-retinoid compound A1120 that does not display this effect as a control. After a single dose of FHR and A1120 (each 30 mg/kg) or vehicle only, we then measured serum RBP levels in tail vein blood by immunoblot analysis. As reported previously in WT mice, treatment with either drug significantly reduced RBP blood levels (Fig. 9B), but in contrast, only A1120 reduced RBP serum levels in Lrat−/− mice (Fig. 9C). At the end of this time course experiment, mice were sacrificed, and blood and tissues were collected. We then measured serum ROL levels by HPLC (Fig. 9D). In mice, serum ROL was significantly decreased after FHR and A1120 treatment as compared with vehicle-treated animals. However, only A1120 decreased ROL levels in Lrat−/− mice as compared with vehicle or FHR-treated Lrat−/− animals. Thus, we conclude that FHR, like ATRA, reduces blood RBP levels in a LRAT-dependent manner, whereas A1220 reduced blood RBP levels independent of LRAT activity.
DISCUSSION
Here we analyzed the role of LRAT in cellular ROL uptake and blood vitamin A homeostasis in vivo (see Fig. 10 for the current model). We show in mice that cellular uptake of ROL from holo-RBP depends on LRAT in peripheral tissues, such as the lungs and eyes. This coupling of ROL transport to an intracellular enzymatic activity traps this small membrane-permeable molecule as REs in target cells. We also provide evidence that this coupling is regulated to accommodate the differential distribution of vitamin A into peripheral tissues under conditions of vitamin A deficiency and excess. Finally, we demonstrated that this regulation is affected by FHR, a drug used clinically to lower holo-RBP levels in certain disease states. The implications of these findings for vitamin A uptake and homeostasis are discussed below.
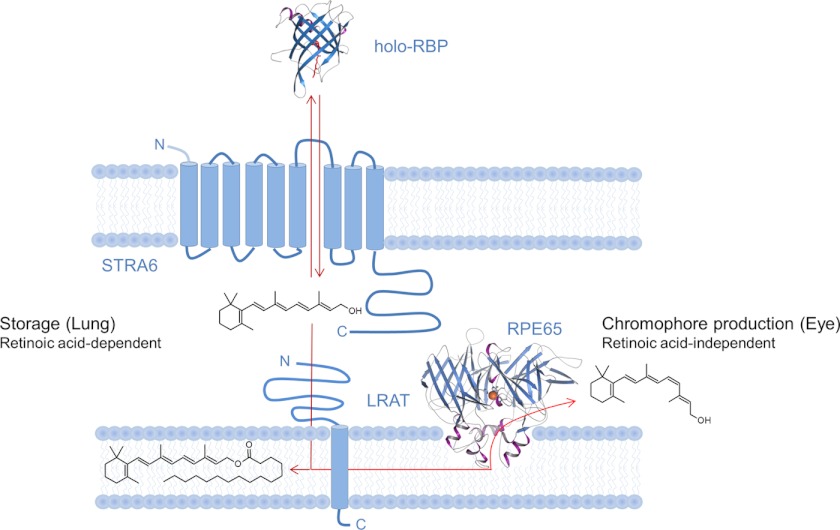
FIGURE 10.
Scheme of the interplay between identified components mediating cellular vitamin A uptake in peripheral tissues. In this process, LRAT enhances the cellular uptake of ROL from holo-RBP via STRA6 by converting absorbed ROL to RE. In lungs, this process is subject to regulation by all-trans-retinoic acid. In the eyes, no such regulation was observed. Moreover, RE can be further converted to 11-cis-retinol by retinal pigment epithelium protein of 65 kDa (RPE65) that displays retinoid isomerase activity.
LRAT Is Critical for Cellular ROL Uptake
ROL is a small lipophilic molecule that can cross biological membranes by passive non-ionic diffusion. This physical property is manifested by both the intestinal uptake of ROL and the cellular uptake of ROL from circulating chylomicrons independent of specific transporters (for a recent review, see Ref. 3). Pharmacological doses of retinoids also can be delivered to target tissues, such as the eyes, without specific transport systems (21). Nevertheless, mammals have evolved a specific ROL transport system wherein RBP mobilizes ROL from RE stores mainly from the liver and distributes it via the circulation to target tissues. Hepatic holo-RBP secretion is strictly dependent on vitamin A, and thus blood holo-RBP levels remain constant as long as stored vitamin A is available from the liver. This regulation guarantees that extrahepatic tissues have access to vitamin A even under conditions when dietary vitamin A is not available. In vitro cell culture studies identified STRA6 as a high affinity RBP receptor that facilitates uptake of ROL by target cells (12), an observation confirmed by in vivo studies in zebrafish and mice (14, 15). Here we identified LRAT as a third critical component of this transport system. This conclusion is based on measurement of [3H]ROL uptake from holo-hRBP in wild type and Lrat−/− mice. Our data showed that serum hRBP levels rapidly increased after intraperitoneal injection and then decreased with time. In the kidney, [3H]ROL levels peaked after 2 h and then declined. Most of the [3H]ROL existed in non-esterified form in this organ, indicating that injected hRBP is eliminated from the circulation by glomerular filtration (10). Nevertheless, the lung and the eyes of wild type mice accumulated increasing amounts of [3H]ROL from injected hRBP over time. HPLC analysis of retinoids in the lung demonstrated that this [3H]ROL existed in esterified form. This accumulation was absent in Lrat−/− mice, demonstrating that the cellular uptake of [3H]ROL from hRBP by this tissue was LRAT-dependent. An LRAT requirement for cellular vitamin A uptake is consistent with our previous finding that STRA6 can also facilitate the efflux of ROL from cells (15). The coupling of STRA6 with LRAT enhances ROL uptake by converting it to its ester form that is readily stored in cellular lipid droplets (32). Such coupling of transport with an energy-consuming intracellular enzymatic reaction is a common characteristic of biological systems (e.g. for trapping of glucose by ATP-dependent phosphorylation). Besides LRAT, CRBP1 was suggested as an intracellular ROL acceptor from in vitro studies (16). Interestingly, we found that Lrat−/− mice displayed relatively high levels of CRBP1 in tissues as compared with control mice used in this study. However, although CRBP1 was present, no significant accumulation of [3H]ROL was observed in peripheral tissues, such as the eyes and lungs of Lrat−/− mice. A minor role for CRBP1 in cellular ROL uptake also is consistent with studies of Crbp1−/− mice that show relatively normal ocular retinoid levels (33).
Like dietary vitamin A from the intestine, stored vitamin A from the liver could be distributed with secreted lipoproteins. Thus, a critical question is why vertebrates have developed a highly specific transport system for the distribution of stored ROL. A common explanation is that such a system helps an animal to endure periods of dietary vitamin A deprivation. Although this argument certainly is valid, a specific transport system as we show here offers additional advantages, such as tissue specificity and regulation, strikingly demonstrated by the selective vitamin A uptake of the retinal pigmented epithelium (RPE). Despite its relatively small surface, this cell layer must acquire large amounts of ROL from the circulation to produce visual chromophore, which then must be delivered to adjacent photoreceptors. The dependence of the RPE on a specific vitamin A uptake system was previously demonstrated in both RBP- and LRAT-deficient mice (9, 19) and more recently in STRA6-deficient mice (14).
ATRA Regulation of Cellular Vitamin A Uptake
ATRA-dependent regulation of ROL uptake activity by peripheral tissues was recently demonstrated in neonatal lungs of rats (34). Supplementation of animals with ATRA and synthetic analogs resulted in higher RE stores in this tissue. In contrast, a decline of serum RBP did not occur in Lrat−/− mice after ATRA treatment, despite their increased Stra6 expression. It was previously reported that Lrat−/− mice on ROL-sufficient diets show increased Cyp26a1 mRNA expression in liver (18), indicating that ATRA may undergo a more pronounced first pass metabolism in this mouse mutant. However, we believe that this characteristic does not conclusively explain differences between wild type and Lrat−/− mice for the following reasons. Pharmacological doses of ATRA induced Stra6 and Cyp26a1 mRNA expression to a similar extent in Lrat−/− and wild type mice. Additionally, wild type mice showed a pronounced decline of RBP when gavaged with a 3-fold lower amount of ATRA than Lrat−/− mice. Furthermore, the synthetic retinoid FHR also reduced RBP levels in wild type but not Lrat−/− mice, but FHR, unlike ATRA, displays a longer half-life and does not show evidence of autoinduced increased metabolism by Cyp26a1 (see Ref. 35 and references therein). Indeed, RBP serum levels were still reduced after 24 h in wild type mice after FHR treatment, whereas Lrat−/− mice showed no such reduction. Thus, our findings best fit a model for coupling ROL transport to intracellular esterification. In wild type mice, a concomitant induction of STR6 and LRAT activities by ATRA increases cellular ROL uptake, whereas the induction of STRA6 alone in Lrat−/− mice does not suffice. In summary, our data suggest that coupling of STRA6 and LRAT function also facilitates control of cellular ROL uptake. When challenged with a pharmacological dose of ATRA, wild type mice exhibited a rapid decline in serum RBP.
Intriguingly, we found that Stra6 and Lrat were not ATRA-responsive in the eyes. This lack of ATRA regulation is explainable by the dual role of LRAT in the eyes for both uptake of ROL and participation in the visual cycle. ATRA responsiveness of ocular LRAT activity probably would affect visual chromophore metabolism. Moreover, ATRA unresponsiveness of Stra6 and Lrat expression in the eyes versus their responsiveness in tissues, such as the lung, provides an additional advantage. In vitamin A deficiency, Lrat expression is greatly reduced in tissues such as the lung because of ATRA-dependence (36). As a consequence, stored ROL is mainly delivered and taken up by the eyes to maintain vision. Indeed, we found that ocular retinoid levels were comparable between VAS and VAD animals, whereas lung and liver stores of VAD animals were largely depleted. This regulation may ensure that there is no competition between the eyes and peripheral tissues, such as the lungs, for ROL remaining during vitamin A depletion.
The RBP Lowering Effect of FHR Versus A1220
Lowering of blood holo-RBP by the synthetic retinoid FHR could be a strategy to fight certain diseases states. We provide evidence that the regulation of STRA6 and LRAT in cellular vitamin A uptake is the target of this drug. In our experiments, FHR reduced holo-RBP levels in wild type but not in Lrat−/− mice. In contrast, the RBP-lowering drug, A1220, acted independently of LRAT by reducing holo-RBP in both wild type and Lrat−/− mice. The latter finding suggests that FHR acts through retinoic acid receptors to induce Stra6 and Lrat expression rather than by disrupting the holo-RBP·TTR complex as previously proposed (37). Indeed, it was previously shown and confirmed here that this synthetic ATRA derivative can induce retinoic acid receptor-regulated target genes when administered to animals (31). This observation also could pertain to the effects of FHR on diabetes. FHR treatment reduced both elevated RBP levels and insulin resistance in mice with type 2 diabetes (29), but treatment with A1220 did not affect insulin resistance despite its pronounced RBP lowering effect (30). Therefore, it was concluded that lowering RBP levels does not suffice to improve insulin resistance in diabetic mice (30). Interestingly, several studies have shown that ATRA reduces obesity and lowers insulin resistance in mice (38–40). Thus, the effects of FHR on insulin resistance could at least in part be explained by its capability to increase retinoid signaling in tissues.
The finding that FHR and A1220 act through different mechanisms could be relevant to the pharmacological treatment of retinal diseases associated with the accumulation of aberrant retinoid cycle products, including Stargardt disease and age-related macular degeneration. Treatment of Abca4−/− mice, a model for such diseases, with FHR arrested accumulation of A2E and lipofuscin autofluorescence in the RPE by reducing ocular retinoid levels (28). An effect of FHR on ocular retinoid metabolism and visual performance also has been reported in patients treated with this drug to fight certain forms of cancer (41, 42). As we show here, FHR acts by inducing Lrat-dependent cellular vitamin A uptake by non-ocular peripheral tissues, such as the lungs. In contrast, A1220 acts LRAT-independently. Thus, FHR promotes redistribution of circulating vitamin A from ocular toward non-ocular tissues, whereas A1220 eliminates holo-RBP from the circulation. Future comparisons of the efficacy and safety of both drugs in the treatment of mouse models with various retinal diseases and diabetes should be of compelling interest.
Acknowledgment
We are grateful to Dr. Leslie Webster for critical comments and important suggestions regarding the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants EY019641 and EY020551.
- ROL
- all-trans-retinol
- ATRA
- all-trans-retinoic acid
- FHR
- fenretinide (4-hydroxy(phenyl)retinamide)
- RBP
- retinol-binding protein
- hRBP
- human retinol-binding protein
- [3H]retinol
- all-trans-[11,12-3H]retinol
- [3H]ROL
- [11,12-3H]ROL
- LRAT
- lecithin:retinol acyltransferase
- RE
- retinyl ester
- TTR
- transthyretin
- ANOVA
- analysis of variance
- qRT-PCR
- quantitative RT-PCR
- VAD
- vitamin A-deficient
- VAS
- vitamin A-sufficient
- LSD
- least squares difference
- LSD
- least significant difference.
REFERENCES
- 1. Palczewski K. (2006) G protein-coupled receptor rhodopsin. Annu. Rev. Biochem. 75, 743–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chambon P. (1996) A decade of molecular biology of retinoic acid receptors. FASEB J. 10, 940–954 [PubMed] [Google Scholar]
- 3. D'Ambrosio D. N., Clugston R. D., Blaner W. S. (2011) Vitamin A metabolism. An update. Nutrients 3, 63–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wongsiriroj N., Piantedosi R., Palczewski K., Goldberg I. J., Johnston T. P., Li E., Blaner W. S. (2008) The molecular basis of retinoid absorption. A genetic dissection. J. Biol. Chem. 283, 13510–13519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paik J., Vogel S., Quadro L., Piantedosi R., Gottesman M., Lai K., Hamberger L., Vieira Mde M., Blaner W. S. (2004) Vitamin A. Overlapping delivery pathways to tissues from the circulation. J. Nutr. 134, 276S–280S [DOI] [PubMed] [Google Scholar]
- 6. van Bennekum A. M., Kako Y., Weinstock P. H., Harrison E. H., Deckelbaum R. J., Goldberg I. J., Blaner W. S. (1999) Lipoprotein lipase expression level influences tissue clearance of chylomicron retinyl ester. J. Lipid Res. 40, 565–574 [PubMed] [Google Scholar]
- 7. Goodman D. W., Huang H. S., Shiratori T. (1965) Tissue Distribution and Metabolism of Newly Absorbed Vitamin A in the Rat. J. Lipid Res. 6, 390–396 [PubMed] [Google Scholar]
- 8. O'Byrne S. M., Wongsiriroj N., Libien J., Vogel S., Goldberg I. J., Baehr W., Palczewski K., Blaner W. S. (2005) Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J. Biol. Chem. 280, 35647–35657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quadro L., Blaner W. S., Salchow D. J., Vogel S., Piantedosi R., Gouras P., Freeman S., Cosma M. P., Colantuoni V., Gottesman M. E. (1999) Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 18, 4633–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Episkopou V., Maeda S., Nishiguchi S., Shimada K., Gaitanaris G. A., Gottesman M. E., Robertson E. J. (1993) Disruption of the transthyretin gene results in mice with depressed levels of plasma retinol and thyroid hormone. Proc. Natl. Acad. Sci. U.S.A. 90, 2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blaner W. S. (2007) STRA6, a cell surface receptor for retinol-binding protein. The plot thickens. Cell Metab. 5, 164–166 [DOI] [PubMed] [Google Scholar]
- 12. Kawaguchi R., Yu J., Honda J., Hu J., Whitelegge J., Ping P., Wiita P., Bok D., Sun H. (2007) A membrane receptor for retinol-binding protein mediates cellular uptake of vitamin A. Science 315, 820–825 [DOI] [PubMed] [Google Scholar]
- 13. Bouillet P., Sapin V., Chazaud C., Messaddeq N., Décimo D., Dollé P., Chambon P. (1997) Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech. Dev. 63, 173–186 [DOI] [PubMed] [Google Scholar]
- 14. Ruiz A., Mark M., Jacobs H., Klopfenstein M., Hu J., Lloyd M., Habib S., Tosha C., Radu R. A., Ghyselinck N. B., Nusinowitz S., Bok D. (2012) Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest. Ophthalmol. Vis. Sci. 53, 3027–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Isken A., Golczak M., Oberhauser V., Hunzelmann S., Driever W., Imanishi Y., Palczewski K., von Lintig J. (2008) RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab. 7, 258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawaguchi R., Yu J., Ter-Stepanian M., Zhong M., Cheng G., Yuan Q., Jin M., Travis G. H., Ong D., Sun H. (2011) Receptor-mediated cellular uptake mechanism that couples to intracellular storage. ACS Chem. Biol. 6, 1041–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruiz A., Winston A., Lim Y. H., Gilbert B. A., Rando R. R., Bok D. (1999) Molecular and biochemical characterization of lecithin retinol acyltransferase. J. Biol. Chem. 274, 3834–3841 [DOI] [PubMed] [Google Scholar]
- 18. Liu L., Gudas L. J. (2005) Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J. Biol. Chem. 280, 40226–40234 [DOI] [PubMed] [Google Scholar]
- 19. Batten M. L., Imanishi Y., Maeda T., Tu D. C., Moise A. R., Bronson D., Possin D., Van Gelder R. N., Baehr W., Palczewski K. (2004) Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J. Biol. Chem. 279, 10422–10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsuura T., Gad M. Z., Harrison E. H., Ross A. C. (1997) Lecithin:retinol acyltransferase and retinyl ester hydrolase activities are differentially regulated by retinoids and have distinct distributions between hepatocyte and nonparenchymal cell fractions of rat liver. J. Nutr. 127, 218–224 [DOI] [PubMed] [Google Scholar]
- 21. Golczak M., Maeda A., Bereta G., Maeda T., Kiser P. D., Hunzelmann S., von Lintig J., Blaner W. S., Palczewski K. (2008) Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J. Biol. Chem. 283, 9543–9554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hessel S., Eichinger A., Isken A., Amengual J., Hunzelmann S., Hoeller U., Elste V., Hunziker W., Goralczyk R., Oberhauser V., von Lintig J., Wyss A. (2007) CMO1 deficiency abolishes vitamin A production from β-carotene and alters lipid metabolism in mice. J. Biol. Chem. 282, 33553–33561 [DOI] [PubMed] [Google Scholar]
- 23. Quadro L., Blaner W. S., Hamberger L., Van Gelder R. N., Vogel S., Piantedosi R., Gouras P., Colantuoni V., Gottesman M. E. (2002) Muscle expression of human retinol-binding protein (RBP). Suppression of the visual defect of RBP knockout mice. J. Biol. Chem. 277, 30191–30197 [DOI] [PubMed] [Google Scholar]
- 24. Abu-Abed S. S., Beckett B. R., Chiba H., Chithalen J. V., Jones G., Metzger D., Chambon P., Petkovich M. (1998) Mouse P450RAI (CYP26) expression and retinoic acid-inducible retinoic acid metabolism in F9 cells are regulated by retinoic acid receptor γ and retinoid X receptor α. J. Biol. Chem. 273, 2409–2415 [DOI] [PubMed] [Google Scholar]
- 25. Cogan U., Kopelman M., Mokady S., Shinitzky M. (1976) Binding affinities of retinol and related compounds to retinol-binding proteins. Eur. J. Biochem. 65, 71–78 [DOI] [PubMed] [Google Scholar]
- 26. Kaji E. H., Lodish H. F. (1993) Unfolding of newly made retinol-binding protein by dithiothreitol. Sensitivity to retinoids. J. Biol. Chem. 268, 22188–22194 [PubMed] [Google Scholar]
- 27. Preitner F., Mody N., Graham T. E., Peroni O. D., Kahn B. B. (2009) Long-term Fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 297, E1420–E1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Radu R. A., Han Y., Bui T. V., Nusinowitz S., Bok D., Lichter J., Widder K., Travis G. H., Mata N. L. (2005) Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores. A potential therapy for treatment of lipofuscin-based retinal diseases. Invest. Ophthalmol. Vis. Sci. 46, 4393–4401 [DOI] [PubMed] [Google Scholar]
- 29. Yang Q., Graham T. E., Mody N., Preitner F., Peroni O. D., Zabolotny J. M., Kotani K., Quadro L., Kahn B. B. (2005) Serum retinol-binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356–362 [DOI] [PubMed] [Google Scholar]
- 30. Motani A., Wang Z., Conn M., Siegler K., Zhang Y., Liu Q., Johnstone S., Xu H., Thibault S., Wang Y., Fan P., Connors R., Le H., Xu G., Walker N., Shan B., Coward P. (2009) Identification and characterization of a non-retinoid ligand for retinol-binding protein 4, which lowers serum retinol-binding protein 4 levels in vivo. J. Biol. Chem. 284, 7673–7680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao Z., Matsuura T., Popoff K., Ross A. C. (1994) Effects of N-(4-hydroxyphenyl)-retinamide on the number and cytotoxicity of natural killer cells in vitamin A-sufficient and -deficient rats. Nat. Immun. 13, 280–288 [PubMed] [Google Scholar]
- 32. Imanishi Y., Gerke V., Palczewski K. (2004) Retinosomes. New insights into intracellular managing of hydrophobic substances in lipid bodies. J. Cell Biol. 166, 447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saari J. C., Nawrot M., Garwin G. G., Kennedy M. J., Hurley J. B., Ghyselinck N. B., Chambon P. (2002) Analysis of the visual cycle in cellular retinol-binding protein type I (CRBPI) knockout mice. Invest. Ophthalmol. Vis. Sci. 43, 1730–1735 [PubMed] [Google Scholar]
- 34. Wu L., Ross A. C. (2010) Acidic retinoids synergize with vitamin A to enhance retinol uptake and STRA6, LRAT, and CYP26B1 expression in neonatal lung. J. Lipid Res. 51, 378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cooper J. P., Hwang K., Singh H., Wang D., Reynolds C. P., Curley R. W., Jr., Williams S. C., Maurer B. J., Kang M. H. (2011) Fenretinide metabolism in humans and mice. Utilizing pharmacological modulation of its metabolic pathway to increase systemic exposure. Br. J. Pharmacol. 163, 1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zolfaghari R., Ross A. C. (2002) Lecithin:retinol acyltransferase expression is regulated by dietary vitamin A and exogenous retinoic acid in the lung of adult rats. J. Nutr. 132, 1160–1164 [DOI] [PubMed] [Google Scholar]
- 37. Berni R., Formelli F. (1992) In vitro interaction of fenretinide with plasma retinol-binding protein and its functional consequences. FEBS Lett. 308, 43–45 [DOI] [PubMed] [Google Scholar]
- 38. Felipe F., Bonet M. L., Ribot J., Palou A. (2004) Modulation of resistin expression by retinoic acid and vitamin A status. Diabetes 53, 882–889 [DOI] [PubMed] [Google Scholar]
- 39. Ribot J., Felipe F., Bonet M. L., Palou A. (2001) Changes of adiposity in response to vitamin A status correlate with changes of PPARγ2 expression. Obes. Res. 9, 500–509 [DOI] [PubMed] [Google Scholar]
- 40. Berry D. C., Noy N. (2009) All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor β/δ and retinoic acid receptor. Mol. Cell Biol. 29, 3286–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaiser-Kupfer M. I., Peck G. L., Caruso R. C., Jaffe M. J., DiGiovanna J. J., Gross E. G. (1986) Abnormal retinal function associated with fenretinide, a synthetic retinoid. Arch. Ophthalmol. 104, 69–70 [DOI] [PubMed] [Google Scholar]
- 42. Decensi A., Torrisi R., Polizzi A., Gesi R., Brezzo V., Rolando M., Rondanina G., Orengo M. A., Formelli F., Costa A. (1994) Effect of the synthetic retinoid fenretinide on dark adaptation and the ocular surface. J. Natl. Cancer Inst. 86, 105–110 [DOI] [PubMed] [Google Scholar]