Background: Fcabs are small antibody-like molecules that medicate effector functions.
Results: Single N-glycan residues on Fcabs significantly contribute to therapeutically relevant effector functions.
Conclusion: Therapeutic proteins with optimized efficacy can be generated by glycoengineering.
Significance: Enhanced understanding of N-glycan impacts on the biological activity of a protein.
Keywords: Antibodies, Biotechnology, Drug Design, FCgamma Receptors, Glycosylation, Immunology, IgG-Fc Fragments, Effector Function, Glycoengineering, Plants
Abstract
Recent studies have demonstrated that IgG-Fc fragments (Fcabs) can be engineered to form antigen-binding sites with antibody properties. Thus they may serve as an attractive alternative to conventional antibodies in therapeutic applications. The critical influence of Fc glycosylation on effector functions of IgGs is well documented; however, whether this applies to Fcabs is not known. Here we used human cells, wild type, and glycoengineered plants to generate four different glycoforms of H10-03-6, an Fcab with engineered HER2/neu-binding sites. Plant-derived H10-03-6 differed in the presence/absence of single oligosaccharide residues, i.e., core fucose and xylose, and terminal galactose. All of the glycoforms had similar binding to HER2/neu expressed on human tumor cells. By contrast, glycoforms that lacked core oligosaccharide modifications (i.e., core α1,3-fucose and β1,2-xylose) showed significantly enhanced binding to the Fcγ receptor IIIa, irrespective of whether plant or human expression systems were used. Consistent with this finding, plant-derived H10-03-6 glycoforms lacking core N-glycan residues mediated higher antibody-dependent cellular cytotoxicity against human tumor cells. No alteration in γ-receptor binding and antibody-dependent cellular cytotoxicity activity was observed upon decoration of N-glycans by terminal galactose. The results point to a significant impact of distinct N-glycan residues on effector functions of Fcabs. Moreover, the outcomes imply that the effector functions mediated by H10-03-6 can be optimized by altering the N-glycosylation profile. Biasing vaccine-induced immune responses toward optimal Fc glycosylation patterns could result in improved vaccine efficacy.
Introduction
Recombinant mAbs, mainly of the IgG class, have become some of the most promising therapeutics produced by the biopharmaceutical industry (1). Trastuzumab (Herceptin®), used to treat certain breast cancers, is among the most successful therapeutic mAbs (2). It binds to human epidermal growth factor receptor 2 (HER2),2 which when overexpressed on certain cells has been shown to play an important role in the pathogenesis and progression of some types of breast cancer. In addition to full-length IgGs, a variety of antibody fragments have been developed that can theoretically be engineered to bind specifically to any target molecule (3). The small size of such fragments offers the advantage of potentially improved tissue penetration, as well as production benefits. However, alternative binders that are mainly based on antigen-binding Fab domains of IgGs generally have shortcomings for therapeutic application, like their inability to elicit effector functions and a limited in vivo half-life (4). IgG-Fc-based molecules are an alternative that potentially overcomes these hurdles because this domain mediates important interactions with host cells and induces effector functions (like ADCC and complement-dependent cytotoxicity). Recently, antigen-binding Fc fragments (Fcab) that carry engineered HER2/neu-binding sites in structural loops of Fc fragments were described (5). One of these Fcabs (H10-03-6) showed features of full-length IgG antibodies such as antigen binding, effector functions, and a long half-life (5), suggesting that the Fc-based antigen-binding molecules could serve as an attractive alternative small-sized antibody format.
A series of studies have demonstrated the critical role of the oligosaccharides attached at a single conserved site of the Fc domain for the effector functions of the antibody (6). Thus glycosylation has been a focus of interest for the biopharmaceutical industry for several years, and cell lines have been engineered in efforts to optimize antibody products by alteration of the N-glycosylation profile (6). However, because of the large endogeneous glycosylation repertoire of mammalian cells, the system commonly used to produce mAbs, it is currently difficult to generate mAbs with homogeneous profiles, which hampers investigation of the possible biological impacts of specific N-glycan residues. Despite these shortcomings, clinical trials using therapeutic glycoengineered mAbs are underway, and their remarkable physiological activities in vivo have attracted attention as next generation therapeutic antibody approaches with improved efficacy (7, 8). Notably, an afucosylated version of trastuzumab has shown superior efficacy in treating in vivo models of HER2-amplified breast cancer (7). Nevertheless, because of an overall alteration of the glycosylation profile during the production process of this glycovariant compared with the commercially available Herceptin, it cannot be excluded that these differences may contribute at least to some extent to the altered mAb activity. Whether the glycosylation status also translates to Fc-based fragments like H10-03-6 is entirely unknown.
The capacity of plants to produce large amounts of recombinant protein is well established (9). Nicotiana-based manufacturing of antibody products in particular has been driven forward by two major technological advances: (a) viral-based transient expression allowing high accumulation of antibody within days (10) and (b) glycoengineered plants enabling the production of antibodies with a targeted human-like glycosylation pattern.3
In this study, we set out to explore the impact of N-linked oligosaccharides on the functionalities of H10-03-6 (5). To this end, we used a plant-based expression system to generate H10-03-6 variants that differed in their glycosylation pattern and compared their functional activities with those of a human cell-produced orthologue. The data showed similar binding to the relevant antigen HER2/neu of all variants, irrespective of their glycosylation pattern. By contrast, binding to the Fcγ receptor IIIa (FcγRIIIa) was significantly increased in N-glycan variants lacking core α1,3-fucose and β1,2-xylose linkages. Enhanced FcγRIIIa binding correlated with improved ADCC activity. Elongation of complex N-glycans by terminal galactose influenced neither receptor binding nor ADCC activity. These results highlight the importance of N-glycan composition present on Fcabs.
EXPERIMENTAL PROCEDURES
Recombinant Expression and Purification of H10-03-6
To express H10-03-6 in plants, the respective cDNA, which originates from a human IgG1-Fc template (5), was transferred into tobacco mosaic virus-based magnICON® 3′ module vector (12). Agrobacteria transformed with the vector modules necessary to assemble the modified virus for the expression of the H10-03-6 (pICH 11599:H10-03-6, pICH 20111, and pICH 14011) were grown at 29 °C for 24 h. After harvesting by gentle centrifugation (5 min at 3000 × g), the bacteria were resuspended in buffer (10 mm MES, pH 5.6, 10 mm MgSO4), mixed, and diluted to a final A600 of ∼0.1–0.2 for subsequent co-infiltration as described recently (13). Nicotiana benthamiana WT and ΔXTFT (N. benthamiana glycosylation mutant lacking core β1,2-xylose and core α1,3-fucose) plants were grown in a growth chamber at 22 °C with a 16-h light/8-h dark photoperiod. In experiments aimed to modulate plant glycosylation toward human-like galactosylation, agrobacteria transformed with a binary vector containing a modified version of the β1,4-galacsyltransferases (14) were prepared the same way and added to the infiltration mixture. Leaves from 4–5-week-old plants were used for agroinfiltration experiments. Five or six days post-infiltration H10-03-6 was harvested and purified by protein A affinity chromatography as described previously (15). As a final step, purified H10-03-6 was dialyzed at 4 °C overnight against PBS.
Expression of H10-03-6 in HEK293 freestyle cells was done as described earlier (5). The extracellular domain of human CD16a (Gly-17 to Gln-208) was cloned in pTT5 (16) together with a C-terminal histidine tag and expressed in HEK293 cells stably expressing the Epstein-Barr virus EBNA1 gene (16). Recombinant CD16a was purified from cell supernatants using a Ni2+ ion chromatography column and elution with imidazole.
Size Exclusion HPLC
Size exclusion HPLC was performed with a HP1100 HPLC system (Agilent) using a TSKGel3000SW XL column (Tosoh Biosciences). The samples were analyzed at a concentration of 100 μg/ml (100 μl of injection volume) using 1× PBS without Ca2+ and Mg2+ (PAA Laboratories) as running buffer at a flow rate of 1 ml/min. Signals were detected using a Multi Wavelength Detector at 280 nm.
N-Glycosylation Analyses
N-Glycan analyses of purified H10-03-6 were carried out by liquid chromatography electrospray ionization-mass spectrometry (LC-ESI-MS) of tryptic glycopeptides as recently described (17). Briefly, the purified samples of H10-03-6 were submitted to reducing SDS-PAGE, and the 25-kDa band corresponding to the Fc was cut from the gel, S-alkylated, digested with trypsin, eluted from the gel fragment with 50% acetonitrile, and separated on a Biobasic C18 column (150 × 0.32 mm; Thermo Electron) with a gradient of 1–80% acetonitrile containing 65 mm ammonium formate, pH 3.0. Positive ions were detected with a quadrupole TOF Ultima Global mass spectrometer (Waters, Milford, MA). Summed and deconvoluted spectra of the glycopeptides elution range were used for identification of glycoforms. This method generates two glycopeptides that differ by 482 Da (glycopeptide 1, EEQYNSTYR; glycopeptide 2 TKPREEQYNSTYR).
Surface Plasmon Resonance Experiments
The binding of H10-03-6 glycoforms to recombinant CD16a was determined by surface plasmon resonance using a BIAcore 3000 instrument (GE Healthcare). The running buffer was 0.01 m HEPES, pH 7.4, 150 mm NaCl, 3 mm EDTA, and 0.005% surfactant P20 (HBS-EP buffer supplied by GE). Protein A was immobilized on a research grade CM5 sensor chip (Biacore Inc.) by amine coupling (kit supplied by the manufacturer). Purified H10-03-6 variants were diluted with running buffer to a final concentration of 20 μg/ml and applied onto the CM5 chip at a flow rate of 20 μl/min. The CM5 sensor chip was washed with running buffer, and recombinant CD16a was applied at a concentration of 25 μg/ml and a flow rate of 20 μl/min. As a control, each sample was passed over a reference flow cell containing no ligand (protein A). All of the binding experiments were performed at room temperature. Binding of H10-03-6 variants to Her-2 was determined following the method described recently by Wozniak-Knopp et al. (5).
FACS Staining of Human Breast Cancer Cell Lines
SKBR3 (a human breast adenocarcinoma cell line) cells were obtained from the American Type Culture Collection (HTB-30). The cells were cultured in RPMI 1640 containing 10% fetal calf serum and 8 mm glutamine. The cells were harvested by trypsinization. A total of 1 × 105 cells in PBS containing 0.1% BSA were incubated with H10-03-6 dilutions starting from 5 μg/ml and incubated on ice for 60 min. After removal of excess H10-03-6, the cells were incubated for 60 min with phycoerythrin R-coupled polyclonal anti-human Fc antibody (Sigma). Measurements were performed on a Becton Dickinson FACSCalibur cytometer.
Antibody-dependent Cellular Cytotoxicity
Fresh primary human NK cells were isolated from peripheral blood mononuclear cells by negative selection using magnetic beads (NK isolation kit; Miltenyi Biotec) in an AutoMACS instrument.
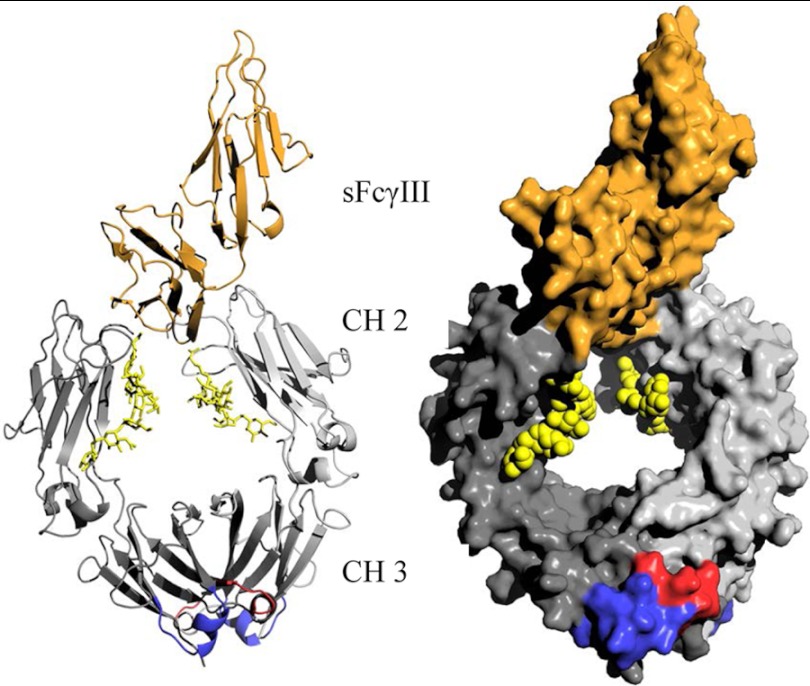
A total of 5 × 105 NK cells were mixed with 1 × 105 SKBR3 cells that had been preincubated with graded concentrations of H10-03-6 for 20 min at 37 °C. After 4 h at 37 °C, 7-aminoactinomycin (Sigma) was added, and the percentage of 7-aminoactinomycin-positive cells was determined by flow cytometry. The image for Fig. 6 was made on the basis of Protein Data Bank entry 1T83 (18).
FIGURE 6.
Cartoon and surface presentation of human Fc from IgG1 (gray) in complex with soluble CD16 (sFcγIII receptor, orange). The location of the CD16 binding site is on the N-terminal side of the CH2 domain of Fc, whereas engineered antigen-binding sites in Fcabs such as the HER2/neu in H10-03-6 are located at the C-terminal part of the CH3 domain (bottom). Each of the two antigen-binding sites in the Fc homodimer (dark and bright gray indicate the two chains) is composed of residues in the AB (red) and in the EF (blue) loop (5). Sugar residues in the N-linked glycosylation sites are indicated in yellow.
RESULTS
Expression of H10-03-6 in N. benthamiana WT and ΔXTFT
In this study we used the viral-based magnICON® pro-vector system (12) for efficient transient expression of the Fc-based antibody-like molecule with HER2/neu-binding sites H10-03-6 (5) in N. benthamiana, a tobacco-related plant species commonly used for recombinant protein expression. The magnICON® 3′ module vector (pICH 11599) carrying the cDNA from H10-03-6 (Fig. 1A) was co-delivered with the 5′ module (pICH 20111) and the integrase module (pICH 14011) (12) into leaves of N. benthamiana WT and the glycosylation mutant lacking core β1,2-xylose and core α1,3-fucose linkages (ΔXTFT) (19). The leaves were harvested 5–6 days post-infiltration, the time point with maximum expression levels. Expression levels were ∼1.5 mg of H10-03-6/g leaf biomass, which corresponds to ∼25% of total soluble proteins. Infiltrated leaves were homogenized, and extracts were subjected to protein A affinity-based purification. Nonreducing SDS-PAGE analysis of purified H10-03-6 exhibited one band at the expected size of 55 kDa in line with the homodimeric nature of human Fc and H10-03-6 produced in HEK293 cells (5). Note, each H10-03-6 molecule contains one HER2/neu-binding site, and consequently the homodimer carries two (identical to full-length mAbs). Marginal or no degradation products were detected (Fig. 1B). In addition, proteins were analyzed by size exclusion HPLC (Fig. 1C). All of the H10-03-6 variants eluted as single peaks at the expected molecular weight, confirming the purity of H10-03-6 preparations and the absence of aggregated H10-03-6 species or contaminants. Subsequently N-glycosylation analysis of H10-03-6 was performed using LC-ESI-MS. The N-glycan profile of H10-03-6 derived from N. benthamiana WT (H10-03-6WT) exhibited a largely homogeneous GnGnXF3 pattern with plant-typical β1,2-xylose and core α1,3-fucose residues (Fig. 2). In addition, some minor glycoforms representing GnMXF3 and Man5 were present. H10-03-6 derived from ΔXTFT (H10-03-6ΔXTFT) carried a single dominant N-glycan species, i.e., GnGn structures (Fig. 2). This glycoform represents human-type glycosylation without core modifications. Additionally, incompletely processed MGn structures were detected. Upon co-expression of a modified version of the human β1,4 galactosyltransferase (14) with H10-03-6 in ΔXTFT plants, highly galactosylated structures were generated (H10-03-6GalT, Fig. 2). Plant-derived H10-03-6 exhibited only minor nonglycosylated fractions (5–10%). The results are in accordance with those obtained by expressing mAbs at lower levels in the same plants (13, 19, 20), demonstrating that high level expression of recombinant mammalian-derived proteins does not alter the quality of the products in terms of proteolytic degradation and glycosylation. LC-ESI-MS analysis of HEK293 cell-derived H10-03-6 (H10-03-6HEK293) revealed the presence of three main glycan species, all of them decorated with core α1,6 fucose: GnGnF6, AGnF6, and AAF6.
FIGURE 1.
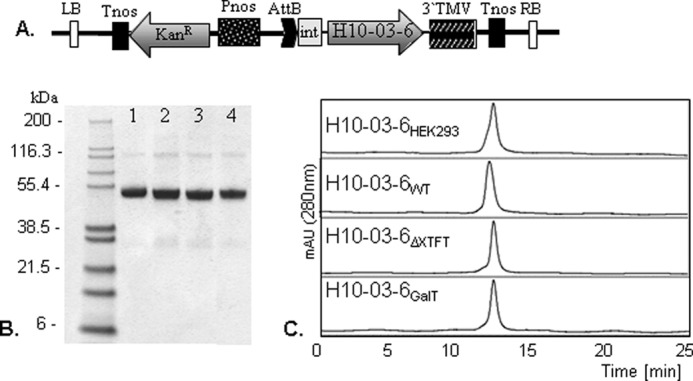
A, schematic representation of the tobacco mosaic virus-based magnICON 3′ provector pICH11599 used in conjugation with the 5′ provector pICH20111 and the binary vector pICH14011 for the transient expression of the H10-03-6 Fc in N. benthamiana. Pnos, nopaline synthase gene promoter; Tnos, nopaline synthase gene terminator; KanR, neomycin phosphotransferase II gene; 3′TMV, 3′-untranslated region (tobacco mosaic virus); AttB, recombination site; int, intron; LB, left border; RB, right border. B, nonreducing SDS-PAGE of H10-03-6 variants produced in HEK293 cells (H10-03-6HEK293, lane 1), N. benthamiana wild type (H10-03-6–6WT, lane 2), N. benthamiana mutant ΔXTFT (H10-03-6ΔXTFT, lane 3), and ΔXTFT co-infiltrated with human β1,4-galactosylatransferase (H10-03-6GalT, lane 4). Left lane, molecular markers in kilodaltons. C, size exclusion HPLC analysis of purified H10-03-6 glycoforms.
FIGURE 2.
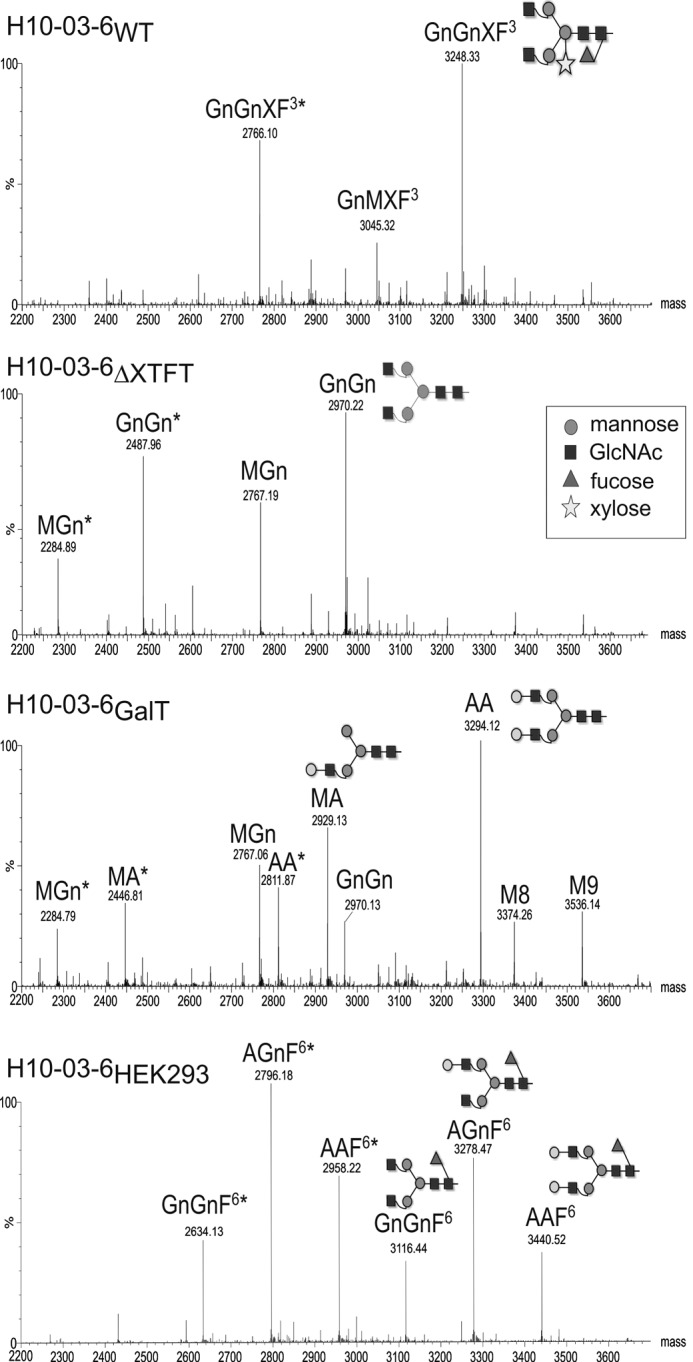
N-Glycan profiles of H10-03-6 expressed in different hosts. N-Glycan analysis was carried out by LC-ESI-MS of tryptic glycopeptides as described (17, 19). Note that during this procedure two glycopeptides are generated that differ by 482 Da. Glycopeptide 1 is indicated with asterisks. See ProGlycAn website for N-glycan abbreviations.
Specific Binding of H10-03-6 Glycoforms to HER2/neu and Fcγ Receptors
To estimate whether plant-derived H10-03-6 glycoforms recognize their cognate antigen, the HER2/neu-positive cell line SKBR3 was used. All of the H10-03-6 glycoforms reacted with SKBR3 cells in a dose-dependent fashion in a way similar to H10-03-6HEK293, resulting in almost identical apparent EC50 values (Fig. 3A). Equivalent binding of plant-derived H10-03-6 versus H10-03-6HEK293 was confirmed by surface plasmon resonance experiments with HER2/neu immobilized on a BIACore chip (Fig. 3B). Together, these data showed that the different glycan structures did not influence antigen binding.
FIGURE 3.
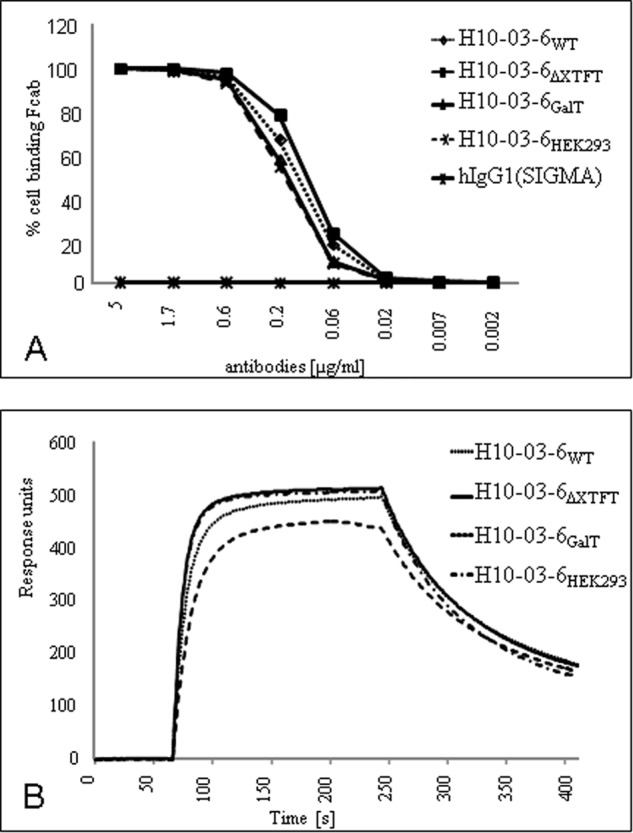
Estimation of H10-03-6 binding capacities. A, binding of H10-03-6 variants to SKBR3 cells using flow cytometry. Irrelevant human IgG1 was used as a staining control. The data are indicated as the percentages of SKBR3 cells binding to H10-03-6 glycovariants. B, binding of H10-03-6 variants to Her-2/neu measured by surface plasmon resonance. A fixed amount of H10-03-6 proteins was injected to chips carrying immobilized recombinant Her-2/neu.
The binding of Fc to the receptors (FcγR), in particular to FcγRIIIa (CD16a), on immune cells and the consequent antibody effector functions are influenced besides other factors by the pattern of Fc glycosylation (6, 21). Several studies have demonstrated that upon elimination of core fucose, mAbs mediate enhanced binding to FcγRIIIa and as a consequence elicit significantly enhanced ADCC (22, 23). In this study binding of H10-03-6 glycoforms to FcγRIIIa (CD16a) was measured by using surface plasmon resonance. First, the protein A binding capacity of H10-03-6 was estimated. Equal affinities were detected for all glycoforms (Fig. 4). Subsequently, soluble CD16a was added to protein A-captured H10-03-6. The data demonstrated that H10-03-6WT and H10-03-6HEK293 exhibit similar binding, whereas glycoforms lacking core modifications (i.e., H10-03-6ΔXTFT and H10-03-6GalT) showed significantly higher affinities to CD16a (Fig. 4). Notably, the elongation of GnGn structures with β1,4-galactose does not seem to alter binding to CD16a, as indicated by comparable binding affinities of H10-03-6ΔXTFT and H10-03-6GalT.
FIGURE 4.

Binding of H10-03-6 variants to protein A and CD16a. Protein A-coated Biacore chips were used to capture H10-03-6 glycovariants. After a washing step, recombinant soluble CD16a was added to the protein A-captured Fcabs followed by an injection of plain buffer. Association and dissociation phases are shown in the sensograms. Signal of an uncoated reference cell was subtracted from all measurements.
Antibody-dependent Cellular Cytotoxicity
ADCC, which relies on the interaction of Fc domains with FcγRs, is considered a major mechanism by which therapeutic antibodies mediate their clinical efficacy. To determine whether increased CD16a binding of H10-03-6ΔXTFT and H10-03-6GalT translates to enhanced immunoeffector functionality, we performed ADCC experiments using the breast cancer cell line SKBR3 as target cells. Notably, all isoforms were able to kill SKBR3 cells in a dose-dependent fashion. H10-03-6WT and H10-03-6HEK293 exhibited similar potencies, whereas glycoforms that lack core modifications (H10-03-6ΔXTFT and H10-03-6GalT) were ∼20–50-fold more potent (Fig. 5 and Table 1). As observed in the FcγRIIIa binding studies, the elongation of GnGn structures with β1,4-galactose does not alter ADCC potency. In the absence of effector NK cells, no killing activity was observed (not shown), indicating that the killing mechanism was due to ADCC and not due to a direct effect of the H10-03-6 on the target cells (Fig. 5).
FIGURE 5.
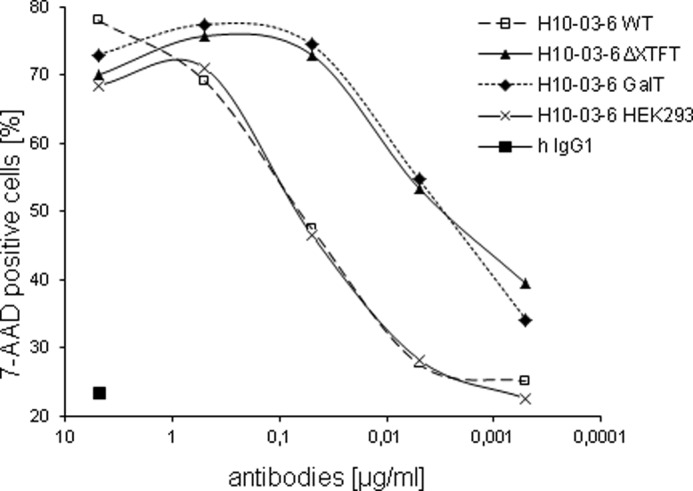
ADCC activity of H10-03-6 variants. SKBR3 target cells were mixed with freshly prepared natural killer cells as effectors at a 1:5 ratio in the presence of increasing concentrations of H10-03-6 variants or human IgG1 as a negative control. Dead cells were enumerated by flow cytometry as a percentage of cells staining positively after addition of 7-aminoactinomycin (7-AAD).
TABLE 1.
Determination of EC50 values
ADCC potencies of H10-03-6 glycovariants calculated at half-maximum protein concentrations (EC50) are shown from three independent experiments using three different NK cell donors (d005, d006, and d009). ND, not determined.
| Sample | EC50 |
||
|---|---|---|---|
| d005 | d006 | d009 | |
| pm | |||
| H10-03-6HEK293 | 6700 | 944 | 4052 |
| H10-03-6WT | 9310 | 1387 | 268,805 |
| H10 -03-6ΔXTFT | 150 | 93 | 222 |
| H10-03-6GalT | 680 | 93 | ND |
DISCUSSION
In the future, Fcabs may provide an important alternative to commonly used therapeutic monoclonal antibodies. In contrast to other small sized antibody-like molecules (4), such proteins maintain important Fc-based activities like effector functions and a long half-life. This has been demonstrated recently by the use of the HER2/neu-binding Fc fragment H10-03-6 (5). Because Fcabs are intensively glycosylated, the correct glycosylation status might be a critical determinant when using them in therapeutic applications. Here we show the efficient generation of four different H10-03-6 glycoforms in plants and human cells and the significant impact of individual N-glycan residues on effector functions.
Expression levels of H10-03-6 in plants were up to ∼1.5 mg/g of leaf biomass corresponding to 25% of total soluble proteins. Such high expression levels have been reported for full-length mAbs using similar expression vectors (23, 24). Plant-produced H10-03-6 behaved virtually identically to full-length mAbs with respect to recombinant protein accumulation and biophysical properties (size exclusion HPLC, stability upon purification, protein degradation, and dimer formation). Notably, no degradation of plant-produced H10-03-6 was observed, even after storage for 6 months at 4 °C and 18 months at −20 °C. This is remarkable considering the intensive manipulation of the loop sequences within the CH3 domain (5). Recent studies have shown that targeted modifications of certain amino acids within the CH3 domain of H10-03-6 cause increased thermal stability without affecting antigen binding and important effector functions, further pointing to the great potential for therapeutic application of such newly developed molecules (25).
The glycosylation profile of H10-03-6 produced in N. benthamiana WT and ΔXTFT exhibited the single major N-glycan species, GnGnXF and GnGn, respectively. We found a dominant galactosylated oligosaccharide species (i.e., digalactosylated AA structure) present on H10-03-6GalT, although the profile was not as homogeneous as seen for H10-03-6WT and H10-03-6ΔXTFT. Note that the three plant-derived H10-03-6 glycoforms differed in one or two glycan residues, i.e., presence/absence of the core fucose and xylose and terminal galactose. This allowed a precise investigation of the possible impact of individual oligosaccharide glycan residues on the activity. Such precise investigations are hardly possible using other expression platforms like mammalian, yeast, and insect cells, mainly because of the difficulties in the targeted manipulation of the endogeneous glycosylation pathway. Although the results of intensive glycoengineering in yeast have been remarkable, the efficient generation of complex N-glycans remains a challenging issue (26). Considering the importance of mammalian cells in the production of mAbs, relatively modest results have been achieved in studies permitting targeted glycosylation (6). The system described here may have a broader impact because it allows a straightforward generation of any protein with targeted N-glycan profiles, enabling a more detailed structure-function analysis to better understand so far unknown molecular mechanisms that, for example, regulate the immune system.
Here we show a similar binding profile for all four H10-03-6 glycoforms (plant and human cell-derived) to the antigen HER2 in vitro and in vivo, indicating correct folding of the engineered human protein in plants. The results are in agreement with previous studies that showed no alteration in antigen binding of mAbs despite differences in the glycosylation pattern (19, 20, 27, 28). The ability of H10-03-6-derived human cells to elicit ADCC was demonstrated recently (5). Several studies have shown that this important effector function of mAbs, which mainly relies on the interaction of the Fc-CH2 domain with the Fcγ receptor IIIa (CD16a) expressed on effector cells, is critically influenced by the glycosylation status of the Fc domain. Here we demonstrate that this observation translates to H10-03-6. We show that individual glycoforms bind to CD16a to different degrees, with decreased binding affinities in the order of H10-03-6ΔXTFT > H10-03-6GalT ≫ H10-03-6HEK293 > H10-03-6WT. Notably, the two fucose-free glycovariants, H10-03-6ΔXTFT and H10-03-6GalT, exhibited significantly better binding than the variants produced in N. benthamiana WT and HEK cells, which were extensively decorated with core fucose, either in α1,3 or α1,6 linkage. The differences in binding affinities of H10-03-6 glycoforms are mirrored by their potency to induce ADCC. Fucose-free H10-03-6ΔXTFT and H10-03-6GalT exhibit a 20–50-fold increased ADCC activity compared with the two fucose-carrying variants. These results are in line with those describing enhanced CD16a binding and ADCC potency of fucose-free full-length mAbs (7, 20). It seems that despite intensive sequence manipulation, the mode of downstream action of H10-03-6 matches mAb-Fc-induced effector functions. In fact, the crystal structure of Fc fragment in complex with soluble CD16 (sFcγRIII) corroborates our observation (Fig. 6). The structure shows that the binding site of CD16 on Fc is located at the N-terminal side of the CH2 domain in close physical proximity to the Fc carbohydrates (29). Structural analyses showed that the interaction between Fc and FcγRIII is limited to amino acid residues within the Fc-CH2 domain and is therefore on the opposite end of the Fc fragment relative to the engineered HER2/neu antigen-binding site (Fig. 6). Conformational changes that may have occurred upon introducing a HER2/neu-binding site to Fc CH3 obviously did not have an effect on the CH2 domain. Recent studies using an afucosylated mAb reveal a unique type of interface consisting of carbohydrate-carbohydrate interactions between glycans of the receptor and the afucosylated Fc. In contrast, in the complex structure with fucosylated Fc, these contacts are weakened or nonexistent, explaining the decreased affinity for the receptor (30). Our results indicate that H10-03-6 acts in a similar mode.
Note that elongation of GnGn oligosaccharides with terminal galactose does not alter CD16a binding and ADCC activity, as indicted by the similar properties of H10-03-6ΔXTFT and H10-03-6GalT, which differ only in the presence/absence of terminal galactose. To date, conflicting results have been published regarding the role of this N-glycan residue in modulating IgG activity (11). Recently we showed that highly galactosylated mAbs against HIV mediate increased neutralization of the virus in vitro (19). However, it seems that this observation is not directly comparable with in vivo effector functions, like antibody-dependent cell-mediated virus inhibition, equivalent to ADCC activity of anti-cancer antibodies (20). Interestingly, the degree of antibody galactosylation in the serum can vary during physiological stages (e.g., pregnancy and aging), indicating an active role for this N-glycan residue in modulating IgG activity in vivo. However, the exact contribution of this oligosaccharide residue is not known. The expression system described here provides a suitable platform to efficiently generate highly galactosylated mAbs and Fc fragments to permit further structure-function studies.
In summary, our results demonstrate that the N-glycosylation status of Fc-based antibody-like molecules, such as H10-03-6, has a significant impact on their effector function, a feature shared with full-length mAbs. Importantly, modifying the glycosylation pattern of H10-03-6 results in improvements of potentially critical therapeutic activities such as ADCC. Although we did not specifically measure other Fc-Fcγ receptor-mediated activities, such as phagocytosis, it is likely that these functions are also influenced by Fc glycosylation. Our results may have implications for passive immunotherapy and suggest the need for in vivo studies comparing different glycovariants. At present, glycoengineering studies are centered on the development of more potent therapeutic antibodies and other glycoprotein-based drugs. Beyond biotechnological exploitation, it would be of great interest to investigate general glycosylation-associated regulation mechanisms occurring in nature. Given the speed and ease with which certain quantities of different glycovariants of Fcabs were produced, the plant-based expression systems detailed in this study are of great use not only for the research and development of pharmaceutical proteins but also for basic scientific research.
Supplementary Material
Acknowledgments
We thank Pia Gattinger (Department of Applied Genetics and Cell Biology, BOKU-Wien) for excellent technical support and Friedrich Altmann (Department of Chemistry, BOKU-Wien) for supervising MS analyses.
This work was supported by Der Wissenschaftsfonds Grant FWF-TR, Die Österreichische Forschungsförderungs-Gesellschaft Grant L575-B13, and Laura Bassi Centres of Expertise Grant 822757.

This article contains supplemental Fig. S1.
Loos, A., and Steinkellner, H. (2012) Arch. Biochem. Biophys. dx.doi.org/10.1016/j.abb.2012.05.01.
- HER2
- human epidermal growth factor receptor 2
- ADCC
- antibody-dependent cellular cytotoxicity
- LC-ESI-MS
- liquid chromatography-electrospray ionization mass spectrometry
- Fcab
- antigen-binding Fc fragment
- FcγR
- Fcγ receptor.
REFERENCES
- 1. Reichert J. M. (2008) Monoclonal antibodies as innovative therapeutics. Curr. Pharm. Biotechnol. 9, 423–430 [DOI] [PubMed] [Google Scholar]
- 2. Nelson A. L., Dhimolea E., Reichert J. M. (2010) Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug Discov. 9, 767–774 [DOI] [PubMed] [Google Scholar]
- 3. Wurch T., Lowe P., Caussanel V., Bes C., Beck A., Corvaia N. (2008) Development of novel protein scaffolds as alternatives to whole antibodies for imaging and therapy. Status on discovery research and clinical validation. Curr. Pharm. Biotechnol. 9, 502–509 [DOI] [PubMed] [Google Scholar]
- 4. Nelson A. L., Reichert J. M. (2009) Development trends for therapeutic antibody fragments. Nat. Biotechnol. 27, 331–337 [DOI] [PubMed] [Google Scholar]
- 5. Wozniak-Knopp G., Bartl S., Bauer A., Mostageer M., Woisetschläger M., Antes B., Ettl K., Kainer M., Weberhofer G., Wiederkum S., Himmler G., Mudde G. C., Rüker F. (2010) Introducing antigen-binding sites in structural loops of immunoglobulin constant domains: Fc fragments with engineered HER2/neu-binding sites and antibody properties. Protein Eng. Des. Sel. 23, 289–297 [DOI] [PubMed] [Google Scholar]
- 6. Jefferis R. (2009) Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 8, 226–234 [DOI] [PubMed] [Google Scholar]
- 7. Junttila T. T., Parsons K., Olsson C., Lu Y., Xin Y., Theriault J., Crocker L., Pabonan O., Baginski T., Meng G., Totpal K., Kelley R. F., Sliwkowski M. X. (2010) Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res. 70, 4481–4489 [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto K., Utsunomiya A., Tobinai K., Tsukasaki K., Uike N., Uozumi K., Yamaguchi K., Yamada Y., Hanada S., Tamura K., Nakamura S., Inagaki H., Ohshima K., Kiyoi H., Ishida T., Matsushima K., Akinaga S., Ogura M., Tomonaga M., Ueda R. (2010) Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J. Clin. Oncol. 28, 1591–1598 [DOI] [PubMed] [Google Scholar]
- 9. Ma J. K., Barros E., Bock R., Christou P., Dale P. J., Dix P. J., Fischer R., Irwin J., Mahoney R., Pezzotti M., Schillberg S., Sparrow P., Stoger E., Twyman R. M. (2005) Molecular farming for new drugs and vaccines. Current perspectives on the production of pharmaceuticals in transgenic plants. EMBO Rep. 6, 593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whaley K. J., Hiatt A., Zeitlin L. (2011) Emerging antibody products and Nicotiana manufacturing. Human Vaccines 7, 349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lux A., Nimmerjahn F., Pulendran B., Katsikis P. D., Schoenberger S. P. (2011) Impact of differential glycosylation on IgG activity crossroads between innate and adaptive immunity III, in Crossroads between Innate and Adaptive Immunity III, pp. 113–124, Springer, New York [Google Scholar]
- 12. Marillonnet S., Thoeringer C., Kandzia R., Klimyuk V., Gleba Y. (2005) Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat. Biotechnol. 23, 718–723 [DOI] [PubMed] [Google Scholar]
- 13. Castilho A., Bohorova N., Grass J., Bohorov O., Zeitlin L., Whaley K., Altmann F., Steinkellner H. (2011) Rapid high yield production of different glycoforms of Ebola virus monoclonal antibody. PLoS One 6, e26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strasser R., Castilho A., Stadlmann J., Kunert R., Quendler H., Gattinger P., Jez J., Rademacher T., Altmann F., Mach L., Steinkellner H. (2009) Improved virus neutralization by plant-produced anti-HIV antibodies with a homogeneous β1,4-galactosylated N-glycan profile. J. Biol. Chem. 284, 20479–20485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castilho A., Strasser R., Stadlmann J., Grass J., Jez J., Gattinger P., Kunert R., Quendler H., Pabst M., Leonard R., Altmann F., Steinkellner H. (2010) In planta protein sialylation through overexpression of the respective mammalian pathway. J. Biol. Chem. 285, 15923–15930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Durocher Y., Perret S., Kamen A. (2002) High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 30, E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stadlmann J., Pabst M., Kolarich D., Kunert R., Altmann F. (2008) Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics 8, 2858–2871 [DOI] [PubMed] [Google Scholar]
- 18. Radaev S., Motyka S., Fridman W. H., Sautes-Fridman C., Sun P. D. (2001) The structure of a human type III Fcgamma receptor in complex with Fc. J. Biol. Chem. 276, 16469–16477 [DOI] [PubMed] [Google Scholar]
- 19. Strasser R., Stadlmann J., Schähs M., Stiegler G., Quendler H., Mach L., Glössl J., Weterings K., Pabst M., Steinkellner H. (2008) Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol. J. 6, 392–402 [DOI] [PubMed] [Google Scholar]
- 20. Forthal D. N., Gach J. S., Landucci G., Jez J., Strasser R., Kunert R., Steinkellner H. (2010) Fc-glycosylation influences Fcγ receptor binding and cell-mediated anti-HIV activity of monoclonal antibody 2G12. J. Immunol. 185, 6876–6882 [DOI] [PubMed] [Google Scholar]
- 21. Bruhns P., Iannascoli B., England P., Mancardi D. A., Fernandez N., Jorieux S., Daëron M. (2009) Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 113, 3716–3725 [DOI] [PubMed] [Google Scholar]
- 22. Ferrara C., Stuart F., Sondermann P., Brünker P., Umaña P. (2006) The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J. Biol. Chem. 281, 5032–5036 [DOI] [PubMed] [Google Scholar]
- 23. Shields R. L., Lai J., Keck R., O'Connell L. Y., Hong K., Meng Y. G., Weikert S. H., Presta L. G. (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcγ RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 [DOI] [PubMed] [Google Scholar]
- 24. Bendandi M., Marillonnet S., Kandzia R., Thieme F., Nickstadt A., Herz S., Fröde R., Inogés S., Lòpez-Dìaz de Cerio A., Soria E., Villanueva H., Vancanneyt G., McCormick A., Tusé D., Lenz J., Butler-Ransohoff J. E., Klimyuk V., Gleba Y. (2010) Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin's lymphoma. Ann. Oncol. 21, 2420–2427 [DOI] [PubMed] [Google Scholar]
- 25. Wozniak-Knopp G., Stadlmann J., Rüker F. (2012) Stabilisation of the Fc fragment of human IgG1 by engineered intradomain disulfide bonds. PLoS One 7, e30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li H., Sethuraman N., Stadheim T. A., Zha D., Prinz B., Ballew N., Bobrowicz P., Choi B. K., Cook W. J., Cukan M., Houston-Cummings N. R., Davidson R., Gong B., Hamilton S. R., Hoopes J. P., Jiang Y., Kim N., Mansfield R., Nett J. H., Rios S., Strawbridge R., Wildt S., Gerngross T. U. (2006) Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat Biotechnol 24, 210–215 [DOI] [PubMed] [Google Scholar]
- 27. Loos A., Van Droogenbroeck B., Hillmer S., Grass J., Kunert R., Cao J., Robinson D. G., Depicker A., Steinkellner H. (2011) Production of monoclonal antibodies with a controlled N-glycosylation pattern in seeds of Arabidopsis thaliana. Plant Biotechnol. J. 9, 179–192 [DOI] [PubMed] [Google Scholar]
- 28. Loos A., Van Droogenbroeck B., Hillmer S., Grass J., Pabst M., Castilho A., Kunert R., Liang M., Arcalis E., Robinson D. G., Depicker A., Steinkellner H. (2011) Expression of antibody fragments with a controlled N-glycosylation pattern and induction of endoplasmic reticulum-derived vesicles in seeds of Arabidopsis. Plant Physiol. 155, 2036–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sondermann P., Huber R., Oosthuizen V., Jacob U. (2000) The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature 406, 267–273 [DOI] [PubMed] [Google Scholar]
- 30. Ferrara C., Grau S., Jäger C., Sondermann P., Brünker P., Waldhauer I., Hennig M., Ruf A., Rufer A. C., Stihle M., Umaña P., Benz J. (2011) Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. U.S.A. 108, 12669–12674 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.