Background: Mmp1a is the postulated mouse genetic homologue of MMP1, but its functions in cancer are unknown.
Results: Endogenous Mmp1a promotes invasion, tumorigenesis, and metastasis of lung cancer and melanoma.
Conclusion: Mmp1a is a functional MMP1 homologue in mouse models of tumorigenesis.
Significance: This is the first report that directly characterizes a function for Mmp1a in mouse (patho)physiology.
Keywords: 7-Helix Receptor, Cancer Biology, Invasion, Matrix Metalloproteinase (MMP), Metastasis, Mouse
Abstract
Matrix metalloprotease-1 (MMP1), a collagenase and activator of the G protein-coupled protease activated receptor-1 (PAR1), is an emerging new target implicated in oncogenesis and metastasis in diverse cancers. However, the functional mouse homologue of MMP1 in cancer models has not yet been clearly defined. We report here that Mmp1a is a functional MMP1 homologue that promotes invasion and metastatic progression of mouse lung cancer and melanoma. LLC1 (Lewis lung carcinoma) and primary mouse melanoma cells harboring active BRAF express high levels of endogenous Mmp1a, which is required for invasion through collagen. Silencing of either Mmp1a or PAR1 suppressed invasive stellate growth of lung cancer cells in three-dimensional matrices. Conversely, ectopic expression of Mmp1a conferred an invasive phenotype in epithelial cells that do not express endogenous Mmp1a. Consistent with Mmp1a acting as a PAR1 agonist in an autocrine loop, inhibition or silencing of PAR1 resulted in a loss of the Mmp1a-driven invasive phenotype. Knockdown of Mmp1a on tumor cells resulted in significantly decreased tumorigenesis, invasion, and metastasis in xenograft models. Together, these data demonstrate that cancer cell-derived Mmp1a acts as a robust functional homologue of MMP1 by conferring protumorigenic and metastatic behavior to cells.
Introduction
Matrix metalloproteases (MMPs)2 are a family of 25 zinc-dependent endopeptidases that allow cells to both sense and remodel their environment through cleavage of extracellular factors and matrix proteins such as collagen (1–3). There are three secreted collagenases with different specificities identified in humans, namely MMP1, MMP8, and MMP13 (4). In particular, MMP1 has been implicated in a wide range of pathophysiologic processes, including arthritis, atherosclerosis, thrombosis, tumorigenesis, and metastasis (5–11). MMP1 overexpression is associated with many cancer types, including lung, breast, and melanoma, and often correlates with a poor clinical prognosis (8, 12–18). An insertional polymorphism in the human MMP1 promoter that leads to elevated MMP1 transcription has also been associated with increased risk of development and metastasis of non-small cell lung cancer and with increased invasiveness in cutaneous melanoma (19–21).
Although MMP1 cleaves many secreted factors and matrix proteins important for tumor progression and invasion, a newly identified mechanism of tumor promotion is through non-canonical activation of protease-activated receptor-1 (PAR1) (22–24). PAR1 is a G protein-coupled receptor that is activated by cleavage of its extracellular N-terminal domain (25). Cleavage reveals a tethered ligand that activates the receptor in an unusual intramolecular binding mode (26), which triggers transmembrane signaling to intracellular G proteins (27). PAR1 signaling activates oncogenic transformation (28), including mitogenesis, survival, gene transcription, and migration/invasion pathways (22, 29–32).
Similar to MMP1, PAR1 is overexpressed frequently by variety of cancer types, including melanoma, lung, breast, and ovarian cancers (33–38). Tumorigenesis, angiogenesis, and experimental metastasis of several cancers can be inhibited effectively by pharmacologic blockade of PAR1 or knockdown of PAR1 gene expression (37, 39–41). Recent work indicates that dual expression of MMP1 and PAR1 on cancer cells is significantly associated with increased tumor recurrence and stage in hepatocellular carcinoma patients and invasion and lymph node metastasis in primary gall bladder carcinoma (42, 43).
Given the emerging importance of MMP1 and PAR1 in human cancer pathogenesis, it is useful to develop relevant mouse models to understand the complex pathobiology and potential therapeutic relevance of the MMP1-PAR1 axis in cancer. However, the functional mouse homologue of MMP1 in murine cancers has not yet been defined clearly. Mapping of the Mmp gene locus revealed a rodent-specific duplication of MMP1, resulting in Mmp1a (Mcol-A) and Mmp1b (Mcol-B) genes (44). Mmp1a and Mmp1b are 74% identical to human MMP1 and 82% identical to each other. When expressed in bacteria, Mmp1a but not Mmp1b exhibited collagenolytic activity in vitro (44). Mmp1a and Mmp1b contain a RGD motif in their catalytic domains, which is characteristic of MMP1. The location of the Mmp1a gene between Mmp10 and Mmp3 in the mouse MMP cluster on chromosome 9 is identical to human MMP1, whereas the Mmp1b gene is inverted and located 73 kb further away, between the Mmp3 and Mmp12 genes.
The tissue expression of Mmp1a appears to be limited in healthy adult mouse tissue as occurs for MMP1 in humans; however, elevated Mmp1a expression levels have been documented in multiple different disease states, including sepsis, wound healing, lung injury, and arthritis (45–49). Moreover, Mmp1a mRNA was found to be significantly up-regulated in the stroma of breast cancer xenografts driven by ectopic expression of the PAR1 oncogene (22).
To determine whether Mmp1a-PAR1 signaling is relevant in mouse cancer biology, we studied a mouse-derived lung cancer cell line, LLC1, and two primary cell lines isolated from spontaneous BRAF V600E/p19ARF−/− mouse melanomas. LLC1 is a clonal cell line derived from the Lewis lung carcinoma tumor widely used in C57BL/6 tumor allografts (50). BRAF is a serine-threonine kinase that is activated by RAS and is frequently mutated in human melanomas, with the constitutively active V600E form occurring in >50% of patient melanomas (51).
Here, we demonstrate that LLC1 cells express high levels of endogenous Mmp1a and PAR1 and autocrine Mmp1a-PAR1 signaling is required for LLC1 invasion. Silencing of Mmp1a expression results in significantly decreased invasion and decreased tumor growth in mice. Furthermore, suppression of Mmp1a expression inhibits experimental metastasis of LLC1 cells to the lungs. This signaling pathway is also conserved in mouse melanoma. BRAF V600E/p19ARF−/− melanoma cells express Mmp1a and PAR1, and Mmp1a-PAR1 activity is required for melanoma cell invasion. Together, these results demonstrate that the newly described Mmp1a matrix metalloprotease has oncogenic functions in mice by enhancing both tumorigenesis and metastasis.
EXPERIMENTAL PROCEDURES
Reagents
The rabbit anti-Mmp1a antibody was generated as described previously (49). Rabbit anti-Myc antibody was from Cell Signaling. Goat anti-mPAR1 (S19) antibody was from Santa Cruz Biotechnology. The N-palmitoylated PAR1 pepducin P1pal-7 (PZ-128) was synthesized by the Tufts University Core Facility as described previously (52). The MMP inhibitors MMP Inh I (IC50 = 1 μm (MMP1), 30 μm (MMP3), 1 μm (MMP8), and 150 μm (MMP9), MMP Inh II (IC50 = 24 nm (MMP1), 18.4 nm (MMP3), 30 nm (MMP7) and 2.7 nm (MMP9)), and MMP Inh V (IC50 = 0.73 nm (MMP2), 42 nm (MMP3), 1.1 nm (MMP8), 2.1 nm (MMP9), 0.45 nm (MMP12), 1.1 nm (MMP13)) were from EMD Biosciences. An inactive MMP Inh I analogue (X-MInhI) lacking the C-terminal hydroxamate (4-Abz-Gly-Pro-d-Leu-d-Ala-OH) was synthesized by the Tufts University Core Facility.
Cell Lines and Culture
LLC1 and HEK293T cells (ATCC) were maintained in DMEM supplemented with 10% FBS, 1% penicillin-streptomycin. C57MG cells were a kind gift from Dr. Lynn Matrisian (Vanderbilt University, Nashville, TN) and were maintained as described previously (53). The 4228 and 4246 melanoma cell lines were isolated from spontaneous cutaneous melanomas arising in transgenic mice expressing BRAF V600E under the mouse tyrosinase promoter (line 476) that were crossed to C57BL/6 p19ARF exon 1b knock-out animals (54, 55). 4228 and 4246 cells were isolated by collagenase/hyaluronidase digestion of tumor fragments for 30 min and were cultured in RPMI supplemented with 10% FBS.
Preparation of Conditioned Media
To harvest conditioned media from all cell lines, 4 × 106 cells were plated in complete media in a 10 cm dish. After 6 h, the medium was then changed to 0.1% FBS. At 48 h, medium was harvested and centrifuged to remove cellular debris.
Plasmid Constructs
Mmp1a and Mmp1b cDNAs were purchased from Open Biosystems and cloned via SgfI and MluI sites into pCMV6-Entry (Origene) with a C-terminal His-Myc tag. Human MMP1-pCMV6-Entry was from Origene. E219A-MMP1 and E216A-Mmp1a mutants were generated by QuikChange site-directed mutagenesis (Agilent). Mmp1a and E216A-Mmp1a were also cloned into the EcoRI and SalI sites of pBabe-Puro (Addgene) for retrovirus generation.
RNA Isolation and Quantitative Real-time PCR
Total RNA was extracted from cell lines or flash-frozen whole tumor homogenates, using the RNeasy mini kit and treated with on-column DNase digestion (Qiagen). RNA was reverse-transcribed using a standard reaction. Real-time PCR was conducted using a SYBR Green master mix (Qiagen) and a 40-cycle thermocycling protocol. Mmp1a primers for real-time PCR were as described previously (45). Mouse PAR1 (F2R) and GAPDH real-time primers (5′ to 3′) were CTCCTCAAGGAGCAGACCAC (PAR1-F), CAAGAAAGAAGATGGCGGAG (PAR1-R), AGAACATCATCCCTGCATCC (GAPDH-F), and CACATTGGGGGTAGGAACAC (GAPDH-R).
Knockdown of Mmp1a
Mmp1a-targeted, PAR1-targeted, and control luciferase-targeted shRNAs in pLKO.1-Puro were purchased from Sigma. The sense strand of the shRNA target sequences were as follows: CGCTGAGTACTTCGAAATGTC (shLuc), CCTGGAATTGATGATAAAGTT (shMmp1a-1), CCGTGATTCTAGTACATGGTT (shMmp1a-2), and AGGGCAGTCTACTTAAATATA (shPAR1). Lentiviral particles were generated by triple transfection of pLKO.1-Puro, pMD.G, and pCMV-dR8.9 in HEK293T using the calcium phosphate method (56). Retroviral particles for C57MG infection were generated by calcium phosphate transfection of pBabePuro constructs in Phoenix-Ampho cells. Both lentiviral and retroviral supernatants were harvested 24 h after transfection. All cells were transduced overnight with viral supernatant diluted 1:1000 (lentivirus) or 1:10 (retrovirus) in the presence of 8 μg/ml polybrene. Cells were selected with 3 μg/ml puromycin for 5 days beginning 48 h after transduction.
Boyden Chamber Invasion
All invasion assays were performed using Boyden chambers with 8-μm pore size (Costar). For Matrigel invasion, 50 μg Matrigel (BD Biosciences) was diluted in serum-free DMEM and layered on a Boyden chamber membrane. For collagen invasion, 50 μg of rat tail type I collagen (BD Biosciences) was cross-linked on a Boyden membrane according to the manufacturer's protocol. For all invasion assays, 10% FBS/DMEM was used as a chemoattractant in the lower chamber. The upper chamber contained 25,000 (Matrigel invasion) or 20,000 (collagen invasion) LLC1 cells in 1% FBS DMEM. After 48 h, non-invasive cells were removed, and membranes were stained with Hema-3 stain system (Thermo Fisher). Invasion was quantified by counting number of cells per nine fields (membrane diameter) as described previously (57).
Three-dimensional Growth/Invasion Assays
For three-dimensional growth assays, 5,000 shLuc-, shMmp1a-2- or shPar1-transduced LLC1 cells in 100 μl of Matrigel were layered on top of a 24-well plate that had been precoated with Matrigel. Once gels had polymerized, 100 μl of complete medium was added to coat the gel. Three-dimensional cultures were maintained at 37 °C/5% CO2/humid air for 7 days. The degree of invasiveness was measured for each colony as follows: Grade 0 colony with no invasive protrusions; Grade 1 colony with invasive protrusions that are no longer than the central radius of the colony; Grade 2 colony with invasive protrusions that are longer than the central colony radius. The colonies in at least 10 representative fields per culture were scored to determine the percentage of each colony type.
Three-dimensional Media Release Assay
HEK293T cells were transfected transiently using the calcium phosphate method with vector, Mmp1a, Mmp1b, MMP1, E219A-MMP1, or E216A-Mmp1a with C-terminal His-Myc tags. Transfected cells (2 × 105) were embedded in 1 ml of type I rat tail collagen supplemented with trypsin (10 μg/ml) to activate latent pro-MMPs at a final concentration of 1 mg/ml of collagen and overlaid with 1 ml of serum-free medium as reported previously (58). After 24 h incubation at 37 °C/5% CO2/humid air, the liquid medium was removed gently and weighed to determine the amount of collagen degradation.
Mice and Xenograft Models
Six-week-old female C57BL/6 mice were obtained from Charles River Laboratories. For subcutaneous xenograft models, 2 × 105 shLuc- or shMmp1a-2-transduced LLC1 cells in 100 μl of sterile PBS were injected into the abdominal fat pad (1–2 inoculations per mouse). Twelve days after implantation, palpable tumor growth was measured every other day, and tumor volume was calculated based on the equation (length × width2)/2. After 26 days, tumors were flash frozen for mRNA analysis or formalin-fixed for histology.
For experimental metastasis, 1 × 106 shLuc- or shMmp1a-2-transduced LLC1 cells in 200 μl of sterile PBS were injected into the tail vein. After 28 days, lungs were harvested and formalin-fixed for histology. The number of metastases per animal was determined in a blinded fashion by counting three H&E-stained coronal lung sections per mouse.
Statistical Analysis
All data are presented as the mean and the S.E. Student's t test (unpaired) was used to determine statistical significance, defined as p < 0.05.
RESULTS
Mmp1a and PAR1 Signaling Promotes Cancer Cell Invasion
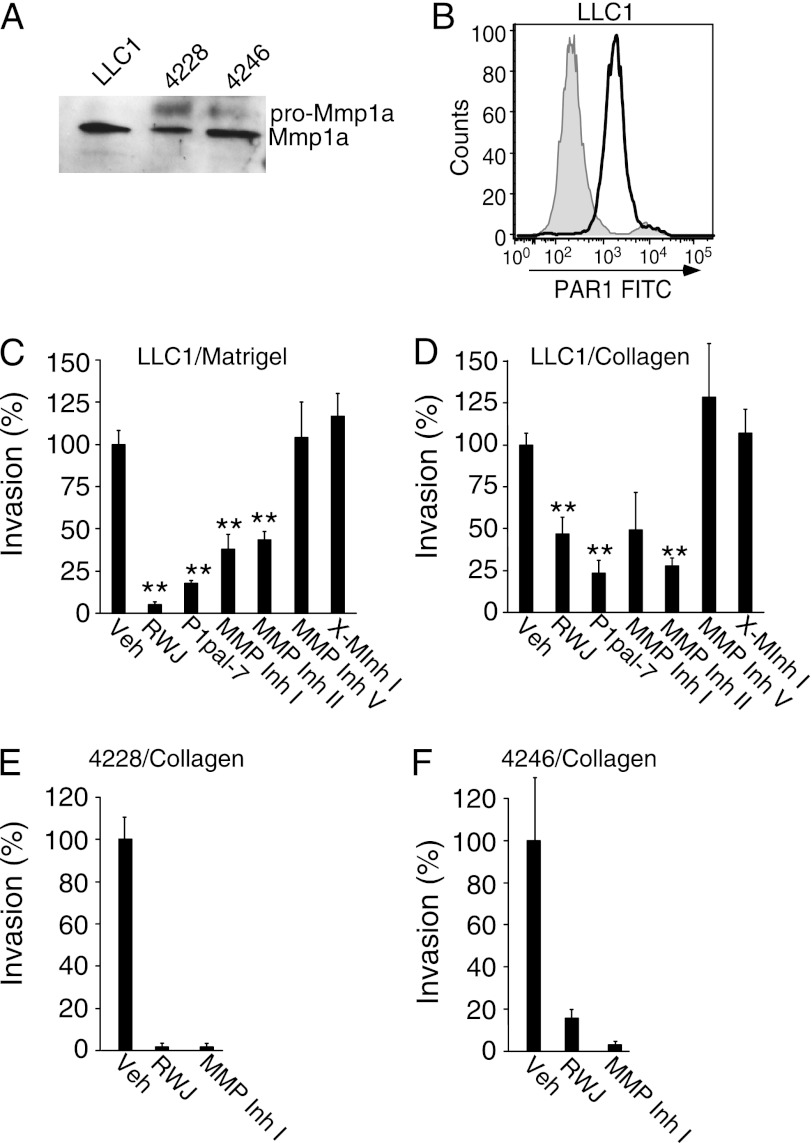
MMP1 and PAR1 are frequently overexpressed in human lung cancers and melanoma (12, 17, 24, 38, 40); therefore, we examined whether PAR1 and Mmp1a were co-expressed using Western blot and FACS analysis in the mouse lung cancer cell line, LLC1, and the primary melanoma cell lines, 4228 and 4246, isolated from spontaneous, BRAF V600E/p19ARF−/− mouse melanomas. A strong protein band at 46 kDa, corresponding to active Mmp1a, was secreted from LLC1 lung carcinoma, and 4228 and 4246 melanoma cells (Fig. 1A). A weaker band at 56 kDa, corresponding in size to pro-MMP1, was detected in 4228 and 4246 melanoma cells (Fig. 1A). All three cell lines had strong surface expression of PAR1 as determined by flow cytometry (Fig. 1B and supplemental Fig. S1).
FIGURE 1.
Endogenous Mmp1a and PAR1 regulate invasion of mouse lung cancer and melanoma cells. A, Western blot analysis of secreted Mmp1a (pro-Mmp1a ∼ 56 kDa, Mmp1a ∼ 46 kDa) in media from LLC1 lung cancer and 4228 or 4246 melanoma cells. B, flow cytometry analysis of PAR1 surface expression on LLC1 cells using S19 FITC-PAR1 Ab versus isotype control (gray). C and D, LLC1 invasion through Matrigel (C) or type I collagen (D) in the absence or presence of PAR1 inhibitors (P1pal-7 (5 μm) or RWJ-58259 (3 μm), or MMP inhibitor (MMP Inh I) (3 μm) and MMP Inh II (5 μm), MMP Inh V (1 μm) and X-MInhI (3 μm)). All data represent mean ± S.E. of three experiments. **, p < 0.005. Veh, vehicle.
We next examined whether Mmp1a and PAR1 impacted invasion of LLC1 cells through the extracellular matrix (22, 24). LLC1 cells readily invaded through Matrigel (Fig. 1C) and collagen (Fig. 1D). Pharmacological inhibition of PAR1 using a small molecule antagonist, RWJ-58259 (59) or a cell-penetrating pepducin antagonist of PAR1, P1pal-7 (60), decreased LLC1 invasion by up to 95 and 75% for Matrigel and collagen, respectively. Furthermore, MMP Inh I and MMP Inh II, which both preferentially target MMP1, reduced invasion of LLC1 cells through Matrigel and collagen invasion by 60–70% (Fig. 1, C and D). In contrast, MMP Inh V, which blocks a variety of MMPs, including MMP-2, -3, -8, -9, -12, -13 but not MMP-1, did not impact LLC1 invasion. Additionally, an inactive control MMP Inh I (X-MInh I) lacking the C-terminal hydroxamate had no effect on LLC1 invasion. These results indicate that both Mmp1a and PAR1 are required for invasion of LLC1 cells through collagen.
We determined whether Mmp1a and PAR1 were also required for melanoma cell invasion. Collagen invasion assays were performed on the primary 4228 and 4246 melanoma cells. Inhibition of PAR1 with RWJ-58259 or Mmp1a with MMP Inh I in 4228 and 4246 melanoma cells reduced collagen invasion by 85–97% (Fig. 1, E and F). Together, these data suggest that lung and melanoma cells expressing Mmp1a and PAR1 are highly invasive and that Mmp1a-PAR1 signaling may lead to a highly malignant cellular phenotype.
Enzymatic Activity of Mmp1a Confers Invasive Potential through PAR1
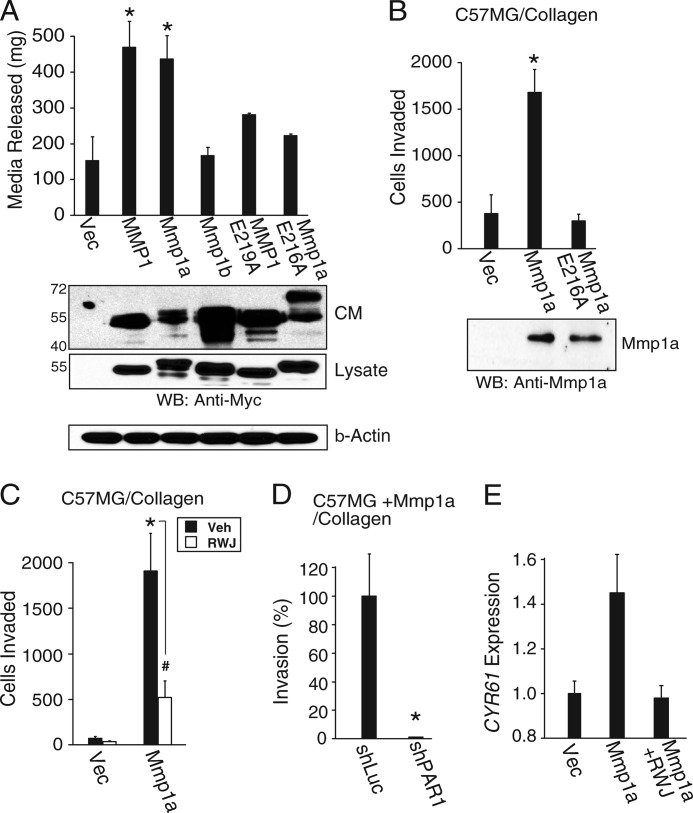
To directly show that Mmp1a has collagenase activity in mammalian expression systems, full-length human MMP1, Mmp1a, and Mmp1b were expressed in HEK293T cells. All MMP constructs contained a C-terminal Myc tag and protein expression levels in the conditioned media and cell lysates were found to be comparable (Fig. 2A, lower panel). The MMP-expressing cells were embedded into three-dimensional collagen gels supplemented with trypsin to activate latent MMPs and collagenolysis assessed by the release of trapped liquid from the gels (58). After 24 h, gels containing mouse Mmp1a-expressing cells had significantly degraded comparable amounts of type I collagen as human MMP1-expressing cells (Fig. 2A). Conversely, Mmp1b did not confer any additional collagenase activity over basal levels.
FIGURE 2.
Mmp1a confers collagenase activity and invasive behavior through PAR1. A, collagenase activity of MMP-Myc transfected HEK293T cells plated in type I collagen gels as measured by the conversion of collagen gel to liquid. Corresponding MMP protein expression in the conditioned media (CM; 40 μl) and lysates (40 μg) was determined by Western blot (lower panel). B, invasion of Mmp1a-null C57MG cells ectopically expressing Mmp1a, inactive E216A Mmp1a, or vector control, through type I collagen toward a gradient of 10% FBS. Corresponding MMP protein expression in the conditioned media was determined by Western blot (WB; lower panel). C and D, Mmp1a-driven invasion of C57MG cells requires PAR1 activity. C, C57MG cells ectopically expressing Mmp1a or vector control, were allowed to invade through type I collagen toward a gradient of 10% FBS, in the presence or absence of the PAR1 inhibitor RWJ-58259 (5 μm). D, Mmp1a-driven invasion of C57MG cells ectopically expressing Mmp1a following stable transduction with a PAR1-targeted shRNA (shPAR1) or control (shLuc). Cells were allowed to invade through type I collagen. E, induction of a PAR1-target gene, CYR61, in Mmp1a-null C57MG cells in response to conditioned media from Mmp1a-null C57MG (vec) or C57MG cells ectopically expressing Mmp1a in the presence or absence of RWJ-58259 (5 μm). Data represent means ± S.E. of three experiments. *, p < 0.05; #, p < 0.10. Veh, vehicle.
To confirm that Mmp1a catalytic activity was directly responsible for the collagenase activity observed, the critical active site glutamate of MMP1 (Glu-219) and Mmp1a (Glu-216) were mutated to alanine (61). The E219A-MMP1 and E216A-Mmp1a mutants had no significant collagenase activity, consistent with the requirement of the conserved active site glutamate for function of both the human and mouse homologues.
We then examined whether gain-of-exogenous expression of Mmp1a bestows an invasive phenotype on the PAR1-expressing cell line C57MG. C57MG is a mouse mammary epithelial-derived cell line that does not express Mmp1a (Fig. 2B, lower panel) (62). C57MG cells were stably transduced with vector, Mmp1a, or E216A-Mmp1a and tested for invasive activity. Expression of Mmp1a in C57MG cells caused a significant 4-fold increase in collagen invasion above vector control, an effect that was lost with the active site defective Mmp1a mutant, E216A (Fig. 2B). Blockade of PAR1 with RWJ-58259 caused a 75% loss of invasion in the Mmp1a-transduced cells, and the PAR1-expressing C57MG cells did not invade in the absence of Mmp1a (vector control) (Fig. 2C). Additionally, treatment of C57MG cells ectopically expressing Mmp1a (C57MG + Mmp1a) with a PAR-targeted shRNA reduced mPAR1 surface expression by 80% and completely abolished collagen invasion relative to shLuc control (Fig. 2D).
We also examined the ability of Mmp1a to directly activate PAR1-dependent gene transcription. CYR61 is a proangiogenic factor that is strongly induced by PAR1 stimulation (30). Treatment of Mmp1a-null C57MG cells with conditioned media from Mmp1a-expressing C57MG cells resulted in a 45% induction of CYR61 mRNA, as compared with conditioned media from vector control C57MG cells (Fig. 2E). Inhibition of PAR1 with RWJ-58259 reduced CYR61 expression to basal levels. Together, these data indicate that Mmp1a requires the activity of PAR1 to confer a proinvasive/angiogenic phenotype to mouse epithelial-derived cells.
Knockdown of Mmp1a and PAR1 Suppresses Invasive Phenotype of LLC1 Lung Cancer Cells
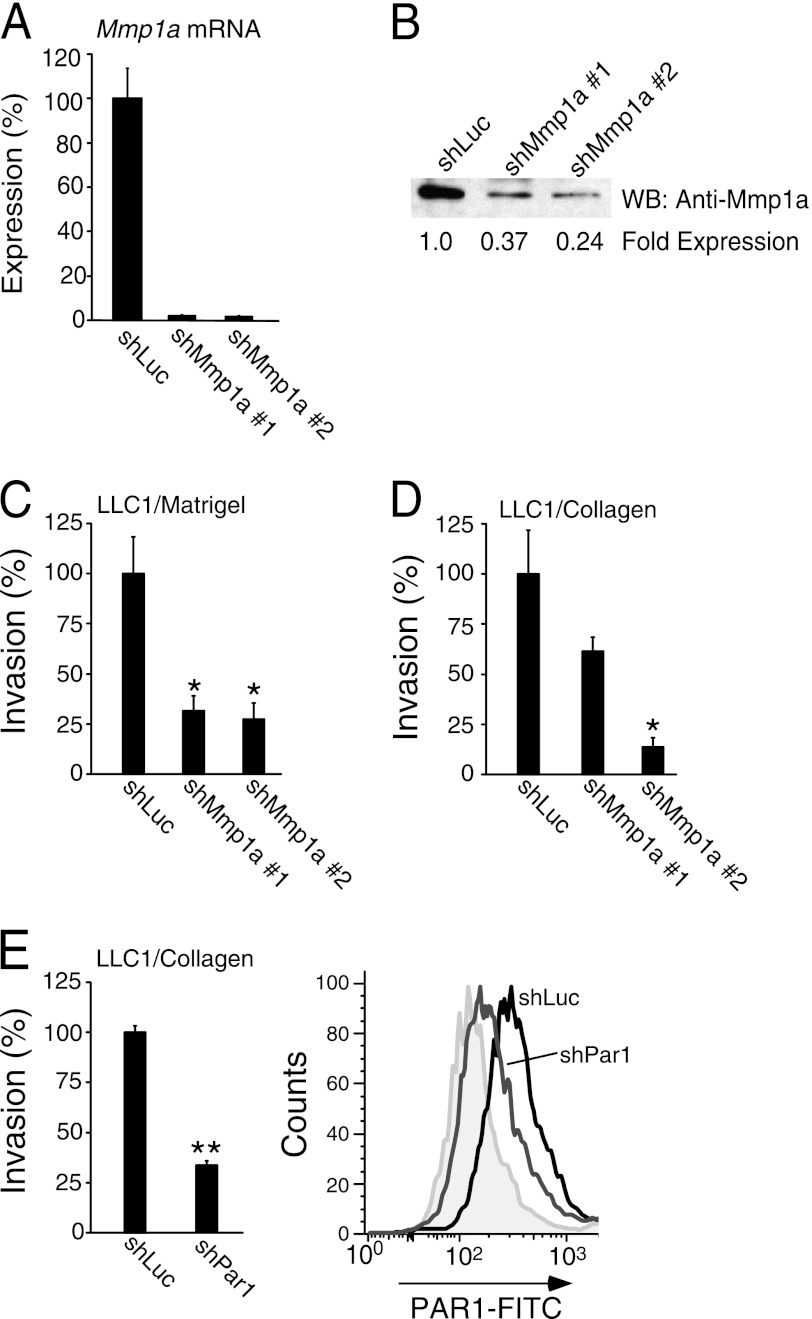
A screen of a panel of Mmp1a-targeted shRNAs identified two constructs (shMmp1a-1 and -2) which caused a >95% reduction in Mmp1a mRNA in LLC1 cells, as measured by quantitative RT-PCR (Fig. 3A). These shRNAs were specific and did not affect expression of the most related mRNA transcripts from Mmp1b and Mmp13 (data not shown). As shown in Fig. 3B, a strong band at 46 kDa corresponding to Mmp1a was detected in shLuc-LLC1, which was reduced by 63 and 76% upon transduction with shMmp1a-1 and -2, respectively. Consistent with the previously observed effects of pharmacologic inhibition of Mmp1a, silencing of Mmp1a expression with shMmp1a-2 significantly reduced LLC1 invasion through Matrigel and type I collagen by 70–85% (Fig. 3, C and D). Highly similar results were observed following silencing of Mmp1a in the 4228 and 4246 melanoma cell lines, with 80–90% reduction in Mmp1a mRNA expression (supplemental Fig. S2A) and suppression of collagen invasion by 70–75% (supplemental Fig. S2, B and C). Likewise, 75% knockdown of PAR1 surface expression with shPAR1 led to 70% reduction in LLC1 invasion (Fig. 3E), consistent with the effects of Mmp1a invasion being mediated through PAR1.
FIGURE 3.
Knockdown of Mmp1a-PAR1 decreases invasion of LLC1 lung cancer cells. A and B, Mmp1a mRNA (A) and Mmp1a protein expression (B) following stable, lentiviral knockdown in LLC1 cells with Mmp1a-targeted shRNA (shMmp1a-1 or -2) versus shLuc (luciferase) control. C and D, Matrigel (C) and collagen (D) invasion of Mmp1a knockdown LLC1 cells. E, LLC1 cell invasion through collagen following stable transduction with a PAR1-targeted shRNA (shPar1) versus shLuc control (left panel), with FACS analysis of PAR1 surface expression (right panel) using S19 FITC-PAR1 Ab versus isotype control (gray). All data represent means ± S.E. of three experiments. *, p < 0.05; **, p < 0.005. WB, Western blot.
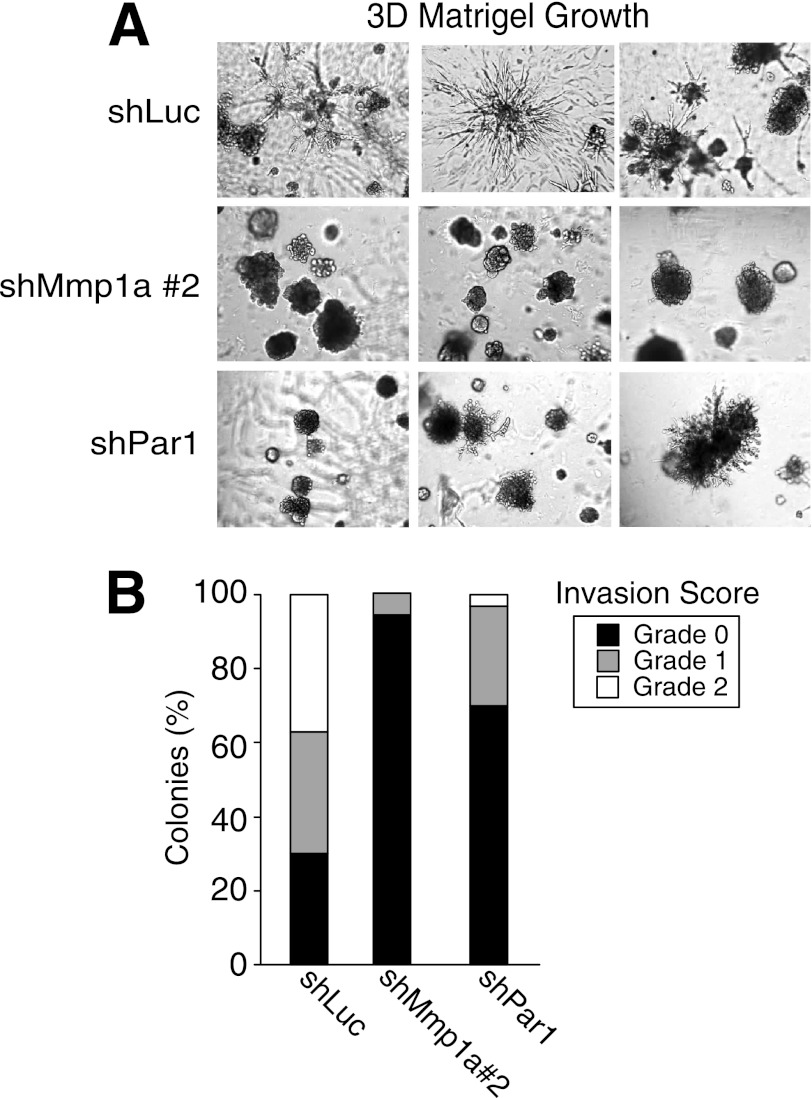
To more closely examine the effects of Mmp1a on the invasive behavior of the LLC1 lung cancer cells, we employed a three-dimensional Matrigel invasion assay. LLC1 cells transduced with negative control shLuc produced numerous grade 1 and 2 stellate colonies with multiple, long projections invading deeply into the three-dimensional Matrigel culture (Fig. 4, A and B). Silencing of Mmp1a with shMmp1a-2 caused a striking loss of invasive stellate colony growth with complete absence of grade 2 colonies (Fig. 4, A and B). PAR1 knockdown resulted in a similar phenotype with the appearance of predominantly non-invasive colonies with the remaining invasive colonies exhibiting truncated stellate projections (Fig. 4, A and B). The suppression of the invasive phenotype following silencing of Mmp1a or PAR1 in the LLC1 lung cancer cells in three-dimensional matrix is highly consistent with the results from the transwell collagen invasion assays, where shMmp1a-2 reduced invasion by 90% and shPAR1 resulted in a 68% reduction in invasion. However, silencing of PAR1 did not completely mimic the effects of Mmp1a silencing, suggesting both PAR1-dependent and PAR1-independent functions for Mmp1a such as direct lysis of collagen.
FIGURE 4.
Mmp1a and PAR1 are required for invasive stellate colony formation of lung cancer cells in three-dimensional matrices. A, growth and invasion of LLC1 cells transduced with control (shLuc), Mmp1a knockdown (shMmp1a-2), or PAR1 knockdown (shPar1) after 7 days in three-dimensional (3D) Matrigel cultures. Digital images were acquired at 60× magnification, n = 3). B, the grade of invasiveness was measured for each colony in three 6× fields and scored (grade 0, 1, 2) as described under “Experimental Procedures.”
Mmp1a Promotes Tumorigenesis and Metastasis of LLC1
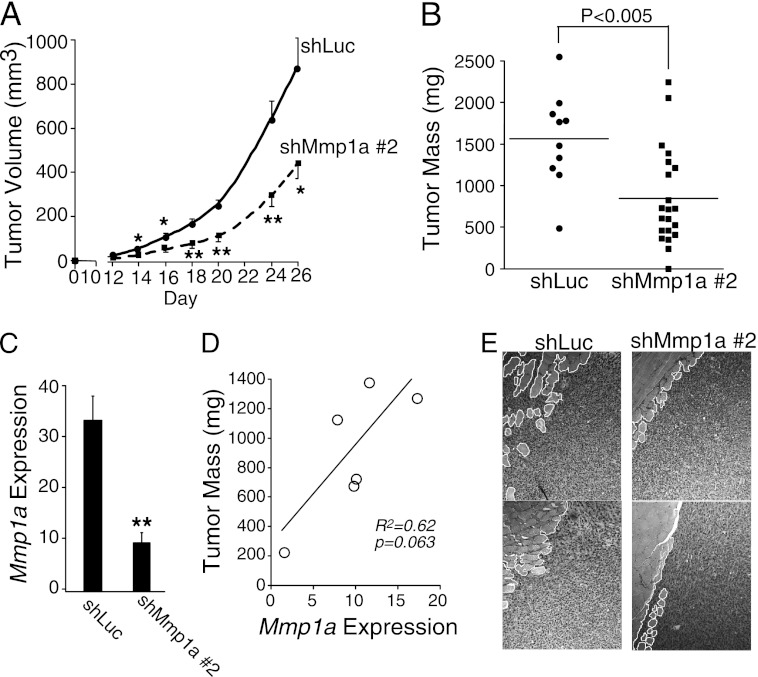
To determine whether Mmp1a plays a role in tumorigenesis and invasion of lung cancer in vivo, tumor xenograft experiments were performed with LLC1 cells. LLC1 cells were injected into the abdominal fat pads of C57BL/6 mice and tumor growth monitored over 26 days. shMmp1a-2 knockdown tumors grew significantly slower than control shLuc LLC1 tumors at all time points (Fig. 5A). At the day 26 end point, shMmp1a-2 tumors weighed significantly less than shLuc tumors (Fig. 5B). Analysis of Mmp1a mRNA levels by real-time PCR in whole tumor homogenates surprisingly showed a 33-fold up-regulation of Mmp1a mRNA as compared with parental shLuc LLC1 cells grown in culture. However, Mmp1a mRNA was reduced on average by 75% in shMmp1a-2 knockdown tumors as compared with shLuc control tumors (Fig. 5C), indicating that the shRNA maintained knockdown of Mmp1a throughout the experiment. Residual elevated Mmp1a mRNA in the tumors may be due to stromal sources. There was a correlation between Mmp1a mRNA levels and tumor size (Fig. 5D), consistent with the notion that residual and/or stromal Mmp1a expression levels may be growth limiting in the shMmp1a-2 tumors. Histologic examination revealed that shMmp1a-2 tumors exhibited decreased tumor invasion into the underlying abdominal musculature (Fig. 5E).
FIGURE 5.
Silencing of Mmp1a suppresses tumor growth and invasion of LLC1 lung cancer cells in mice. A, tumor growth following subcutaneous implantation of 200,000 shLuc (n = 10) or shMmp1a-2 (n = 21) transduced LLC1 cells in the abdominal fat pads of C57BL/6 female mice. B, mass of excised LLC1 tumors at the day 26 end point. C, Mmp1a mRNA expression in whole tumor homogenates (n = 6 per cohort) as determined by real-time PCR and expressed relative to Mmp1a mRNA levels in cultured shLuc LLC1 cells (1-fold). D, correlation between tumor size and Mmp1a mRNA expression of excised shMmp1a-2 tumors. E, invasion of shLuc or shMmp1a LLC1 transduced tumors into the abdominal musculature of mice as assessed by H&E-stained sections from subcutaneous tumors (10× magnification). Tumor/muscle interface is outlined in white. Values shown are mean ± S.E. *, p < 0.05; **, p < 0.01.
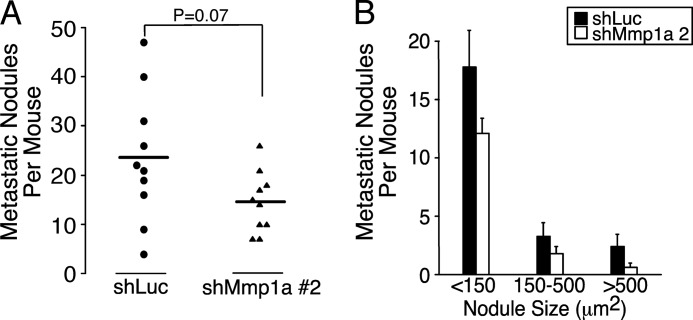
To quantify the effect of Mmp1a knockdown on tumor cell experimental metastasis in vivo, shLuc or shMmp1a-2-silenced LLC1 cells were inoculated into the venous circulation by tail vein injection. At the 28 day end point, lungs were harvested, and metastatic nodules were quantified. Consistent with the hypothesis that Mmp1a promotes a malignant phenotype in epithelial cells, mice were inoculated with Mmp1a knockdown LLC1 cells which had a 40% reduction in metastatic nodules (Fig. 6, A and B). This provides evidence that Mmp1a plays a role in the late events of invasion and metastasis in mouse lung cancer models.
FIGURE 6.
Silencing of Mmp1a reduces experimental metastasis of lung cancer cells in mice. A and B, number (A) and size (B) of metastatic lung nodules per mouse as determined by sum of three coronal sections per animal. LLC1 cells transduced with shMmp1a-2 or shLuc (1 × 106) were injected into the tail vein of C57BL/6 mice, and lungs were harvested 28 days later and analyzed for metastases by histology. n = 10 per cohort.
DISCUSSION
Emerging evidence suggests that the matrix metalloprotease MMP1 plays a pivotal role in the pathogenesis of multiple human diseases; however, a function for the putative mouse homologue, Mmp1a, has not yet been identified clearly. Here, we report that Mmp1a is highly expressed in mouse lung cancer and is critical for in vivo tumor growth, invasion, and metastasis. Primary melanomas isolated from BRAF V600E p19ARF−/− mice also endogenously express Mmp1a that is essential for invasion, thus providing further support for a pathophysiologic role for Mmp1a in mouse tumor biology.
Gain-of-function migratory and invasive activity of Mmp1a in mouse epithelial cells required the G protein-coupled PAR1 receptor, which had been shown to be an oncogene in human cancers (63). Previous work had also shown the importance of stromal MMP1 activity as being required for PAR1-driven cancer cell growth, tumorigenesis, and invasion of human breast cancer xenografts that lack endogenous MMP1 (22). However, the present study describes for the first time, an autocrine Mmp1a-PAR1 system that promotes lung cancer pathogenesis. A similar autocrine system in human melanomas has recently been reported to promote growth and invasion (24). Blackburn et al. (24) provided evidence that activation of human PAR1 by MMP1 in less advanced melanomas leads to increased transcription of MMP1. Although this would promote increased cancer cell signaling, it has not yet been determined whether the action of cancer cell-derived MMP1 on the stromal component also leads to increased stromal MMP1(a) production in vivo. Stromal MMP1 has been shown to be induced by a PAR1-Cyr61-MMP1 pathway, whereby secreted Cyr61 from human breast cancer cells induces MMP1 expression in human mammary and other stromal fibroblasts in co-culture experiments (30). Media from human breast cancer cells can also induce MMP1 expression in human mammary fibroblasts possibly through a CXCR4-regulated mechanism (64).
Gain-of-MMP1 expression by breast cancer cells has been proposed to be a key component of the secreted protein toolbox necessary for metastasis to the lung and bone (65, 66). Recently, it was shown that expression of MMP1 by stromal cells is correlated with breast cancer subtype and risk of distant metastasis in patients, suggesting that stromal MMP1 expression may also modulate tumor phenotype (67, 68). This suggests the likelihood that there are multiple sources of Mmp1a in the tumor microenvironment, including the stroma (22). Additional studies are required to understand the interplay between autocrine and paracrine MMP1 activity on various tumor types and correlate these with clinical outcomes. Given the conservation of Mmp1a-PAR1 signaling in the murine tumor cells used here, we propose that mouse models may be an appropriate tool for understanding the relative contribution of stromal versus tumor-derived MMP1 in tumorigenesis.
In addition to the Mmp1a collagenase, there are two additional soluble collagenases identified in mice, namely Mmp8 and Mmp13, and a rodent-specific Mmp1a-like duplication, Mmp1b. Consistent with an early report (44), we found that Mmp1b did not have collagenase activity in our systems. Mmp1b contains all the structural and catalytic residues required for a secreted collagenase, yet there are no obvious defects to explain the apparent lack of collagenase activity. Recombinant Mmp1b has been shown to weakly autocatalyze its prodomain over 24 h, and Mmp1b mRNA expression patterns appear to be similar to those of Mmp1a (44, 45). Mmp1b protein previously has been shown to be present in mouse plasma (49); however, it is not yet known what role this secreted protein plays in rodent physiology or disease.
Mmp8 mRNA was not present in LLC1 cells, whereas Mmp13 mRNA was detected. Although we did not directly address a potential role for Mmp13 in these cells, Mmp1a activity was required for the invasive, tumorigenic, and metastatic phenotype of the LLC1 lung cancer cells. Thus, collagen invasion was decreased with inhibitors that targeted MMP1 (MMP Inh I and MMP Inh II), whereas a potent MMP13 inhibitor (MMP Inh V) had no effect. Additionally, there were no changes in Mmp13 mRNA levels with the Mmp1a-targeting shRNA (data not shown) that significantly suppressed invasion, stellate colony growth, tumorigenesis, and metastasis. Similarly, Mmp13 mRNA was also detected in the 4228 and 4246 primary melanoma cell lines but was unaffected by the Mmp1a-targeting shRNA that also suppressed invasion. Together, these data point to Mmp1a as mediating the invasive phenotype in the LLC1 and melanoma cells, with little or no compensatory role for Mmp13.
Moreover, there is increasing evidence that MMP1 and MMP13 are protumorigenic, whereas MMP8 collagenase may have tumor-suppressive activities. Mmp8-deficient mice exhibit increased skin tumorigenesis, and MMP8-inactivating somatic mutations have been identified in patient melanoma samples (69, 70), in marked contrast to the frequently observed overexpression of MMP1 in melanomas (10, 18, 71). This suggests that despite the apparent commonality of collagenase activity, the three secreted collagenases, MMP1, MMP8, and MMP13, have distinct functions in cancer biology and that MMP1/Mmp1a may play a specific role in the invasive and metastatic progression of melanoma and lung cancer.
Supplementary Material
This work was supported in part by National Institutes of Health grants F30-HL104835 (to C. J. F.), CA122992 and HL64701 (to A. K.), CA104406 (to L. C.), CA095798, and CA104322 and a Sackler Family Cancer Award (to P. W. H. and C. L.).

This article contains supplemental Figs. S1 and S2.
- MMP
- matrix metalloprotease.
REFERENCES
- 1. Ugalde A. P., Ordóñez G. R., Quirós P. M., Puente X. S., López-Otín C. (2010) Metalloproteases and the degradome. Methods Mol. Biol. 622, 3–29 [DOI] [PubMed] [Google Scholar]
- 2. Rodríguez D., Morrison C. J., Overall C. M. (2010) Matrix metalloproteinases: What do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim. Biophys. Acta 1803, 39–54 [DOI] [PubMed] [Google Scholar]
- 3. Fanjul-Fernández M., Folgueras A. R., Cabrera S., López-Otín C. (2010) Matrix metalloproteinases: Evolution, gene regulation, and functional analysis in mouse models. Biochim. Biophys. Acta 1803, 3–19 [DOI] [PubMed] [Google Scholar]
- 4. Minond D., Lauer-Fields J. L., Cudic M., Overall C. M., Pei D., Brew K., Visse R., Nagase H., Fields G. B. (2006) The roles of substrate thermal stability and P2 and P1′ subsite identity on matrix metalloproteinase triple-helical peptidase activity and collagen specificity. J. Biol. Chem. 281, 38302–38313 [DOI] [PubMed] [Google Scholar]
- 5. Brinckerhoff C. E., Rutter J. L., Benbow U. (2000) Interstitial collagenases as markers of tumor progression. Clin. Cancer Res. 6, 4823–4830 [PubMed] [Google Scholar]
- 6. Murray G. I., Duncan M. E., O'Neil P., McKay J. A., Melvin W. T., Fothergill J. E. (1998) Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J. Pathol. 185, 256–261 [DOI] [PubMed] [Google Scholar]
- 7. Sukhova G. K., Schönbeck U., Rabkin E., Schoen F. J., Poole A. R., Billinghurst R. C., Libby P. (1999) Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation 99, 2503–2509 [DOI] [PubMed] [Google Scholar]
- 8. Murray G. I., Duncan M. E., O'Neil P., Melvin W. T., Fothergill J. E. (1996) Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat. Med. 2, 461–462 [DOI] [PubMed] [Google Scholar]
- 9. Visse R., Nagase H. (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92, 827–839 [DOI] [PubMed] [Google Scholar]
- 10. Blackburn J. S., Rhodes C. H., Coon C. I., Brinckerhoff C. E. (2007) RNA interference inhibition of matrix metalloproteinase-1 prevents melanoma metastasis by reducing tumor collagenase activity and angiogenesis. Cancer Res. 67, 10849–10858 [DOI] [PubMed] [Google Scholar]
- 11. Trivedi V., Boire A., Tchernychev B., Kaneider N. C., Leger A. J., O'Callaghan K., Covic L., Kuliopulos A. (2009) Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell 137, 332–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Airola K., Karonen T., Vaalamo M., Lehti K., Lohi J., Kariniemi A. L., Keski-Oja J., Saarialho-Kere U. K. (1999) Expression of collagenases-1 and -3 and their inhibitors TIMP-1 and -3 correlates with the level of invasion in malignant melanomas. Br J. Cancer 80, 733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakopoulou L., Giannopoulou I., Gakiopoulou H., Liapis H., Tzonou A., Davaris P. S. (1999) Matrix metalloproteinase-1 and -3 in breast cancer: Correlation with progesterone receptors and other clinicopathologic features. Hum. Pathol. 30, 436–442 [DOI] [PubMed] [Google Scholar]
- 14. Nikkola J., Vihinen P., Vlaykova T., Hahka-Kemppinen M., Kähäri V. M., Pyrhönen S. (2002) High expression levels of collagenase-1 and stromelysin-1 correlate with shorter disease-free survival in human metastatic melanoma. Int. J. Cancer 97, 432–438 [DOI] [PubMed] [Google Scholar]
- 15. van 't Veer L. J., Dai H., van de Vijver M. J., He Y. D., Hart A. A., Mao M., Peterse H. L., van der Kooy K., Marton M. J., Witteveen A. T., Schreiber G. J., Kerkhoven R. M., Roberts C., Linsley P. S., Bernards R., Friend S. H. (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415, 530–536 [DOI] [PubMed] [Google Scholar]
- 16. Poola I., DeWitty R. L., Marshalleck J. J., Bhatnagar R., Abraham J., Leffall L. D. (2005) Identification of MMP-1 as a putative breast cancer predictive marker by global gene expression analysis. Nat. Med. 11, 481–483 [DOI] [PubMed] [Google Scholar]
- 17. Shah S. A., Spinale F. G., Ikonomidis J. S., Stroud R. E., Chang E. I., Reed C. E. (2010) J. Thorac. Cardiovasc. Surg. 139, 984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giricz O., Lauer J. L., Fields G. B. (2010) Variability in melanoma metalloproteinase expression profiling. J. Biomol. Tech. 21, 194–204 [PMC free article] [PubMed] [Google Scholar]
- 19. Rutter J. L., Mitchell T. I., Butticè G., Meyers J., Gusella J. F., Ozelius L. J., Brinckerhoff C. E. (1998) A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 58, 5321–5325 [PubMed] [Google Scholar]
- 20. Ye S., Dhillon S., Turner S. J., Bateman A. C., Theaker J. M., Pickering R. M., Day I., Howell W. M. (2001) Invasiveness of cutaneous malignant melanoma is influenced by matrix metalloproteinase 1 gene polymorphism. Cancer Res. 61, 1296–1298 [PubMed] [Google Scholar]
- 21. Sun T., Gao Y., Tan W., Ma S., Zhang X., Wang Y., Zhang Q., Guo Y., Zhao D., Zeng C., Lin D. (2006) Haplotypes in matrix metalloproteinase gene cluster on chromosome 11q22 contribute to the risk of lung cancer development and progression. Clin. Cancer Res. 12, 7009–7017 [DOI] [PubMed] [Google Scholar]
- 22. Boire A., Covic L., Agarwal A., Jacques S., Sherifi S., Kuliopulos A. (2005) PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120, 303–313 [DOI] [PubMed] [Google Scholar]
- 23. Goerge T., Barg A., Schnaeker E. M., Poppelmann B., Shpacovitch V., Rattenholl A., Maaser C., Luger T. A., Steinhoff M., Schneider S. W. (2006) Tumor-derived matrix metalloproteinase-1 targets endothelial proteinase-activated receptor 1 promoting endothelial cell activation. Cancer Res. 66, 7766–7774 [DOI] [PubMed] [Google Scholar]
- 24. Blackburn J. S., Liu I., Coon C. I., Brinckerhoff C. E. (2009) A matrix metalloproteinase-1/protease activated receptor-1 signaling axis promotes melanoma invasion and metastasis. Oncogene 28, 4237–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. (1991) Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 64, 1057–1068 [DOI] [PubMed] [Google Scholar]
- 26. Seeley S., Covic L., Jacques S. L., Sudmeier J., Baleja J. D., Kuliopulos A. (2003) Structural basis for thrombin activation of a protease-activated receptor: Inhibition of intramolecular liganding. Chem. Biol. 10, 1033–1041 [DOI] [PubMed] [Google Scholar]
- 27. Swift S., Sheridan P. J., Covic L., Kuliopulos A. (2000) PAR1 thrombin receptor-G protein interactions. Separation of binding and coupling determinants in the gα subunit. J. Biol. Chem. 275, 2627–2635 [DOI] [PubMed] [Google Scholar]
- 28. Whitehead I., Kirk H., Kay R. (1995) Expression cloning of oncogenes by retroviral transfer of cDNA libraries. Mol. Cell. Biol. 15, 704–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ossovskaya V. S., Bunnett N. W. (2004) Protease-activated receptors: contribution to physiology and disease. Physiol. Rev. 84, 579–621 [DOI] [PubMed] [Google Scholar]
- 30. Nguyen N., Kuliopulos A., Graham R. A., Covic L. (2006) Tumor-derived Cyr61(CCN1) promotes stromal matrix metalloproteinase-1 production and protease-activated receptor 1-dependent migration of breast cancer cells. Cancer Res. 66, 2658–2665 [DOI] [PubMed] [Google Scholar]
- 31. Arora P., Ricks T. K., Trejo J. (2007) Protease-activated receptor signaling, endocytic sorting, and dysregulation in cancer. J. Cell Sci. 120, 921–928 [DOI] [PubMed] [Google Scholar]
- 32. Yang E., Boire A., Agarwal A., Nguyen N., O'Callaghan K., Tu P., Kuliopulos A., Covic L. (2009) Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Res. 69, 6223–6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Even-Ram S., Uziely B., Cohen P., Grisaru-Granovsky S., Maoz M., Ginzburg Y., Reich R., Vlodavsky I., Bar-Shavit R. (1998) Thrombin receptor overexpression in malignant and physiological invasion processes. Nat. Med. 4, 909–914 [DOI] [PubMed] [Google Scholar]
- 34. Ghio P., Cappia S., Selvaggi G., Novello S., Lausi P., Zecchina G., Papotti M., Borasio P., Scagliotti G. V. (2006) Prognostic role of protease-activated receptors 1 and 4 in resected stage IB non-small cell lung cancer. Clin. Lung Cancer 7, 395–400 [DOI] [PubMed] [Google Scholar]
- 35. Grisaru-Granovsky S., Salah Z., Maoz M., Pruss D., Beller U., Bar-Shavit R. (2005) Differential expression of protease activated receptor 1 (Par1) and pY397FAK in benign and malignant human ovarian tissue samples. Int. J. Cancer 113, 372–378 [DOI] [PubMed] [Google Scholar]
- 36. Dorsam R. T., Gutkind J. S. (2007) G protein-coupled receptors and cancer. Nat. Rev. Cancer 7, 79–94 [DOI] [PubMed] [Google Scholar]
- 37. Agarwal A., Covic L., Sevigny L. M., Kaneider N. C., Lazarides K., Azabdaftari G., Sharifi S., Kuliopulos A. (2008) Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites, and progression of ovarian cancer. Mol. Cancer Ther. 7, 2746–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Depasquale I., Thompson W. D. (2008) Prognosis in human melanoma: PAR-1 expression is superior to other coagulation components and VEGF. Histopathology 52, 500–509 [DOI] [PubMed] [Google Scholar]
- 39. Arora P., Cuevas B. D., Russo A., Johnson G. L., Trejo J. (2008) Persistent transactivation of EGFR and ErbB2/HER2 by protease-activated receptor-1 promotes breast carcinoma cell invasion. Oncogene 27, 4434–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cisowski J., O'Callaghan K., Kuliopulos A., Yang J., Nguyen N., Deng Q., Yang E., Fogel M., Tressel S., Foley C., Agarwal A., Hunt S. W., 3rd, McMurry T., Brinckerhoff L., Covic L. (2011) Targeting protease-activated receptor-1 with cell-penetrating pepducins in lung cancer. Am. J. Pathol. 179, 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Villares G. J., Zigler M., Wang H., Melnikova V. O., Wu H., Friedman R., Leslie M. C., Vivas-Mejia P. E., Lopez-Berestein G., Sood A. K., Bar-Eli M. (2008) Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 68, 9078–9086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liao M., Tong P., Zhao J., Zhang Y., Li Z., Wang J., Feng X., Hu M., Pan Y. (2012) Prognostic value of matrix metalloproteinase-1/proteinase-activated receptor-1 signaling axis in hepatocellular carcinoma. Pathol. Oncol. Res. 18, 397–403 [DOI] [PubMed] [Google Scholar]
- 43. Du X., Wang S., Lu J., Cao Y., Song N., Yang T., Dong R., Zang L., Yang Y., Wu T., Li J. (2011) Correlation between MMP1-PAR1 axis and clinical outcome of primary gallbladder carcinoma. Jpn. J. Clin. Oncol. 41, 1086–1093 [DOI] [PubMed] [Google Scholar]
- 44. Balbín M., Fueyo A., Knäuper V., López J. M., Alvarez J., Sánchez L. M., Quesada V., Bordallo J., Murphy G., López-Otín C. (2001) Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J. Biol. Chem. 276, 10253–10262 [DOI] [PubMed] [Google Scholar]
- 45. Nuttall R. K., Sampieri C. L., Pennington C. J., Gill S. E., Schultz G. A., Edwards D. R. (2004) Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett. 563, 129–134 [DOI] [PubMed] [Google Scholar]
- 46. Hartenstein B., Dittrich B. T., Stickens D., Heyer B., Vu T. H., Teurich S., Schorpp-Kistner M., Werb Z., Angel P. (2006) Epidermal development and wound healing in matrix metalloproteinase 13-deficient mice. J. Invest. Dermatol. 126, 486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tomita M., Okuyama T., Katsuyama H., Miura Y., Nishimura Y., Hidaka K., Otsuki T., Ishikawa T. (2007) Mouse model of paraquat-poisoned lungs and its gene expression profile. Toxicology 231, 200–209 [DOI] [PubMed] [Google Scholar]
- 48. Pfaffen S., Hemmerle T., Weber M., Neri D. (2010) Isolation and characterization of human monoclonal antibodies specific to MMP-1A, MMP-2, and MMP-3. Exp. Cell Res. 316, 836–847 [DOI] [PubMed] [Google Scholar]
- 49. Tressel S. L., Kaneider N. C., Kasuda S., Foley C., Koukos G., Austin K., Agarwal A., Covic L., Opal S. M., Kuliopulos A. (2011) A matrix metalloprotease-PAR1 system regulates vascular integrity, systemic inflammation, and death in sepsis. EMBO Mol. Med. 3, 370–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bertram J. S., Janik P. (1980) Establishment of a cloned line of Lewis lung carcinoma cells adapted to cell culture. Cancer Lett. 11, 63–73 [DOI] [PubMed] [Google Scholar]
- 51. Davies H., Bignell G. R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M. J., Bottomley W., Davis N., Dicks E., Ewing R., Floyd Y., Gray K., Hall S., Hawes R., Hughes J., Kosmidou V., Menzies A., Mould C., Parker A., Stevens C., Watt S., Hooper S., Wilson R., Jayatilake H., Gusterson B. A., Cooper C., Shipley J., Hargrave D., Pritchard-Jones K., Maitland N., Chenevix-Trench G., Riggins G. J., Bigner D. D., Palmieri G., Cossu A., Flanagan A., Nicholson A., Ho J. W., Leung S. Y., Yuen S. T., Weber B. L., Seigler H. F., Darrow T. L., Paterson H., Marais R., Marshall C. J., Wooster R., Stratton M. R., Futreal P. A. (2002) Mutations of the BRAF gene in human cancer. Nature 417, 949–954 [DOI] [PubMed] [Google Scholar]
- 52. Covic L., Gresser A. L., Talavera J., Swift S., Kuliopulos A. (2002) Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc. Natl. Acad. Sci. U.S.A. 99, 643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vaidya A. B., Lasfargues E. Y., Sheffield J. B., Coutinho W. G. (1978) Murine mammary tumor virus (MuMTV) infection of an epithelial cell line established from C57BL/6 mouse mammary glands. Virology 90, 12–22 [DOI] [PubMed] [Google Scholar]
- 54. Goel V. K., Ibrahim N., Jiang G., Singhal M., Fee S., Flotte T., Westmoreland S., Haluska F. S., Hinds P. W., Haluska F. G. (2009) Melanocytic nevus-like hyperplasia and melanoma in transgenic BRAFV600E mice. Oncogene 28, 2289–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kamijo T., Zindy F., Roussel M. F., Quelle D. E., Downing J. R., Ashmun R. A., Grosveld G., Sherr C. J. (1997) Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659 [DOI] [PubMed] [Google Scholar]
- 56. Graham F. L., van der Eb A. J. (1973) A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52, 456–467 [DOI] [PubMed] [Google Scholar]
- 57. Kamath L., Meydani A., Foss F., Kuliopulos A. (2001) Signaling from protease-activated receptor-1 inhibits migration and invasion of breast cancer cells. Cancer Res. 61, 5933–5940 [PubMed] [Google Scholar]
- 58. Wyatt C. A., Geoghegan J. C., Brinckerhoff C. E. (2005) Short hairpin RNA-mediated inhibition of matrix metalloproteinase-1 in MDA-231 cells: Effects on matrix destruction and tumor growth. Cancer Res. 65, 11101–11108 [DOI] [PubMed] [Google Scholar]
- 59. Andrade-Gordon P., Maryanoff B. E., Derian C. K., Zhang H. C., Addo M. F., Darrow A. L., Eckardt A. J., Hoekstra W. J., McComsey D. F., Oksenberg D., Reynolds E. E., Santulli R. J., Scarborough R. M., Smith C. E., White K. B. (1999) Design, synthesis, and biological characterization of a peptide-mimetic antagonist for a tethered ligand receptor. Proc. Natl. Acad. Sci. U.S.A. 96, 12257–12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kuliopulos A., Covic L. (2003) Blocking receptors on the inside: Pepducin-based intervention of PAR signaling and thrombosis. Life Sci. 74, 255–262 [DOI] [PubMed] [Google Scholar]
- 61. Saffarian S., Collier I. E., Marmer B. L., Elson E. L., Goldberg G. (2004) Interstitial collagenase is a Brownian ratchet driven by proteolysis of collagen. Science 306, 108–111 [DOI] [PubMed] [Google Scholar]
- 62. Lynch C. C., Vargo-Gogola T., Martin M. D., Fingleton B., Crawford H. C., Matrisian L. M. (2007) Matrix metalloproteinase 7 mediates mammary epithelial cell tumorigenesis through the ErbB4 receptor. Cancer Research 67, 6760–6767 [DOI] [PubMed] [Google Scholar]
- 63. Whitehead I. P., Zohn I. E., Der C. J. (2001) Rho GTPase-dependent transformation by G protein-coupled receptors. Oncogene 20, 1547–1555 [DOI] [PubMed] [Google Scholar]
- 64. Eck S. M., Côté A. L., Winkelman W. D., Brinckerhoff C. E. (2009) CXCR4 and matrix metalloproteinase-1 are elevated in breast carcinoma-associated fibroblasts and in normal mammary fibroblasts exposed to factors secreted by breast cancer cells. Mol. Cancer Res. 7, 1033–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Minn A. J., Gupta G. P., Siegel P. M., Bos P. D., Shu W., Giri D. D., Viale A., Olshen A. B., Gerald W. L., Massagué J. (2005) Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kang Y., Siegel P. M., Shu W., Drobnjak M., Kakonen S. M., Cordón-Cardo C., Guise T. A., Massagué J. (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 [DOI] [PubMed] [Google Scholar]
- 67. Boström P., Söderström M., Vahlberg T., Söderström K. O., Roberts P. J., Carpén O., Hirsimäki P. (2011) MMP-1 expression has an independent prognostic value in breast cancer. BMC Cancer 11, 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vizoso F. J., González L. O., Corte M. D., Rodríguez J. C., Vázquez J., Lamelas M. L., Junquera S., Merino A. M., García-Muñiz J. L. (2007) Study of matrix metalloproteinases and their inhibitors in breast cancer. Br J. Cancer 96, 903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Balbín M., Fueyo A., Tester A. M., Pendás A. M., Pitiot A. S., Astudillo A., Overall C. M., Shapiro S. D., López-Otín C. (2003) Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat. Genet. 35, 252–257 [DOI] [PubMed] [Google Scholar]
- 70. Palavalli L. H., Prickett T. D., Wunderlich J. R., Wei X., Burrell A. S., Porter-Gill P., Davis S., Wang C., Cronin J. C., Agrawal N. S., Lin J. C., Westbroek W., Hoogstraten-Miller S., Molinolo A. A., Fetsch P., Filie A. C., O'Connell M. P., Banister C. E., Howard J. D., Buckhaults P., Weeraratna A. T., Brody L. C., Rosenberg S. A., Samuels Y. (2009) Analysis of the matrix metalloproteinase family reveals that MMP8 is often mutated in melanoma. Nat. Genet. 41, 518–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ryu B., Moriarty W. F., Stine M. J., DeLuca A., Kim D. S., Meeker A. K., Grills L. D., Switzer R. A., Eller M. S., Alani R. M. (2011) Global analysis of BRAFV600E target genes in human melanocytes identifies matrix metalloproteinase-1 as a critical mediator of melanoma growth. J. Invest. Dermatol. 131, 1579–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.