Background: UDP-glucuronosyltransferase 2B15 (UGT2B15) is the only 5α-dihydrotestosterone (DHT)-metabolizing enzyme in prostate luminal cells.
Results: UGT2B15 requires regulated phosphorylation to metabolize DHT.
Conclusion: PKCα and Src support phosphorylation of UGT2B15 at five sites involving two signaling pathways and cross-talk.
Significance: Strategically controlled DHT synthesis and complex UGT2B15 phosphorylation impose low DHT turnover and presumably homeostatic levels of DHT-occupied androgen receptor for prostate-specific functions.
Keywords: Drug Metabolism, Phosphorylation, Prostate, Src, UDP-glucuronosyltransferase, Basal Cells, Luminal Cells, Dihydrotestosterone (DHT) Metabolism, PKCα, Src Kinase, UGT2B15
Abstract
Because human prostate-distributed UDP-glucuronosyltransferase (UGT) 2B15 metabolizes 5α-dihydrotestosterone (DHT) and 3α-androstane-5α,17β-diol metabolite, we sought to determine whether 2B15 requires regulated phosphorylation similar to UGTs already analyzed. Reversible down-regulation of 2B15-transfected COS-1 cells following curcumin treatment and irreversible inhibition by calphostin C, bisindolylmaleimide, or röttlerin treatment versus activation by phorbol 12-myristate 13-acetate indicated that 2B15 undergoes PKC phosphorylation. Mutation of three predicted PKC and two tyrosine kinase sites in 2B15 caused 70–100 and 80–90% inactivation, respectively. Anti-UGT-1168 antibody trapped 2B15-His-containing co-immunoprecipitates of PKCα in 130–140- and >150-kDa complexes by gradient SDS-PAGE analysis. Complexes bound to WT 2B15-His remained intact during electrophoresis, whereas 2B15-His mutants at phosphorylation sites differentially dissociated. PKCα siRNA treatment inactivated >50% of COS-1 cell-expressed 2B15. In contrast, treatment of 2B15-transfected COS-1 cells with the Src-specific activator 1,25-dihydroxyvitamin D3 enhanced activity; treatment with the Src-specific PP2 inhibitor or Src siRNA inhibited >50% of the activity. Solubilized 2B15-His-transfected Src-free fibroblasts subjected to in vitro [γ-33P]ATP-dependent phosphorylation by PKCα and/or Src, affinity purification, and SDS gel analysis revealed 2-fold more radiolabeling of 55–58-kDa 2B15-His by PKCα than by Src; labeling was additive for combined kinases. Collectively, the evidence indicates that 2B15 requires regulated phosphorylation by both PKCα and Src, which is consistent with the complexity of synthesis and metabolism of its major substrate, DHT. Whether basal cells import or synthesize testosterone for transport to luminal cells for reduction to DHT by 5α-steroid reductase 2, comparatively low-activity luminal cell 2B15 undergoes a complex pattern of regulated phosphorylation necessary to maintain homeostatic DHT levels to support occupation of the androgen receptor for prostate-specific functions.
Introduction
Several factors demonstrate that importance of 5α-dihydrotestosterone (DHT)4 homeostasis to prostate health (1). Findings indicate adequate levels of DHT are essential for prostate growth, development, and differentiation, and elevated levels are associated with prostate diseases. Insufficient DHT levels cause developmental defects in the prostate, and conditions that lead to abnormally high DHT levels cause serious virilization syndromes, indicating that regulation of DHT levels is essential. To initiate prostate organogenesis, the urogenital sinus mesenchyme and urogenital sinus epithelium interact upon receiving signals from DHT-occupied androgen receptors in urogenital sinus mesenchyme/stromal cells, giving rise to basal and luminal epithelia (2). In preparation for DHT to reach prostate luminal cells (3) to support androgen-dependent functions, gonad-synthesized testosterone (4) or adrenal-synthesized dehydroepiandrosterone (DHEA) steroid precursor (4) is transported to prostate basal cells via blood (see Fig. 1A) (5). The fact that human basal cells contain appropriate steroidogenic enzymes detected by in situ hybridization analysis (6) suggests that the cells convert DHEA to testosterone. The one-to-one stratification of basal and luminal cells with intercellular gap-junction connections/structures seen in electron micrographs by El-Alfy et al. (6) (see Fig. 1A) provides indirect evidence that small-molecule exchanges occur between the two cell types and not between blood and luminal cells (6). Moreover, the presence of 5α-steroid reductase 2 (5α-SR-2) primarily in luminal cells (7) suggests that testosterone is largely converted to DHT in luminal cells evidently following its movement from basal epithelia (4, 8) and that in situ synthesized DHT is the primary androgen source for occupation of the receptor. The DHT-occupied androgen receptor in luminal cells mediates all prostate-specific functions. The importance of testosterone reduction to DHT by 5α-SR-2 has been confirmed by malformation of prostate external genitalia during development due to type 2 enzyme deficiencies or in animals treated with 5α-SR-2 inhibitors (1) that lead to insufficient DHT levels.
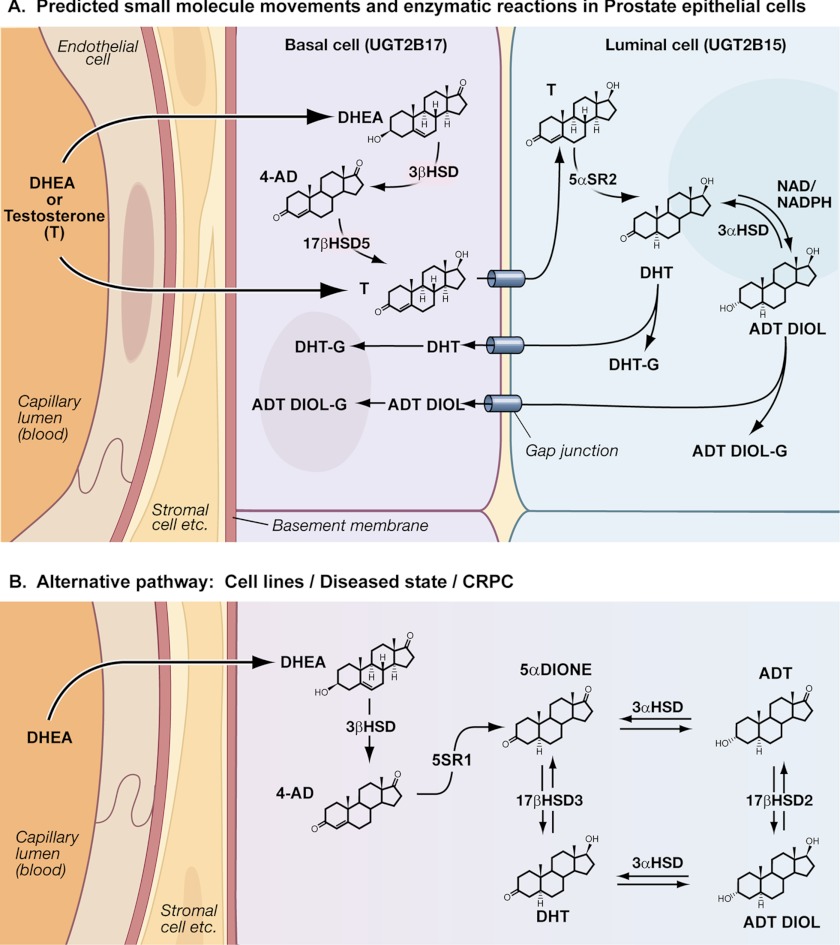
FIGURE 1.
A, distribution of normal prostate steroidogenic and UGT isozymes according to the publications cited. Based on the studies cited, prostate DHT synthesis and its metabolism are summarized in a schematic. Electron micrographs show 1:1 stratification of human prostate basal/luminal cells with intervening gap junction structures that likely allow movement of small molecules between the two cells (6). Structural integrities allow the transport of the steroid precursor DHEA (5) from blood to basal cells, and not to luminal cells. Appropriate steroidogenic enzymes for DHEA conversion to testosterone are in basal cells (5, 6). The fact that 5α-SR-2 is detected primarily in luminal cells (7) suggests that testosterone is transported to luminal cells via gap junctions to undergo reduction to DHT to support DHT occupation of the androgen receptor to carry out prostate-specific functions. Whereas 2B15 is distributed in luminal cells (16) and it metabolizes the active DHT (14), it is likely that 2B15 generates both DHT- and 5α-androstane-3α, 17β-diol-glucuronides (ADT DIOL-G), which exit via gap junctions to basal cells for excretion into blood for facilitated excretion. Moreover, the entry of luminal cell-reduced testosterone (DHT) to basal cells allows 2B17 (15, 16) to participate in DHT metabolism. B, a recently described testosterone bypass pathway in cell lines (9) and intratumoral tissue (10) shows that 4-androstenedione (4-AD) is reduced consecutively by 5α-SR-1 and 17β-hydroxysteroid dehydrogenase (17βHSD) to DHT. Both 5α-androstanedione and DHT are interconvertible by 3α-hydroxysteroid dehydrogenases (3αHSD) to androsterone (ADT) and 5α-androstane-3α,17β-diol (ADT DIOL), respectively. 5α-Androstanedione and DHT, as well as androsterone and 5α-androstane-3α,17β-diol, are also interconvertible by 17β-hydroxysteroid dehydrogenases 2 and 3, respectively. CRPC, castration-resistant prostate cancer.
For the first time, studies have described an alternative DHT biosynthetic pathway that bypasses testosterone in prostate cell lines (9) and intratumoral prostate tissues (10). Because 4-androstenedione derived from DHEA can be reduced at the 17-keto position to testosterone by a 17β-hydroxysteroid dehydrogenase or at the 5α-position to 5α-androstanedione by a 5α-SR, two consecutive reductive steps starting with 5α-androstanedione reduction by 5α-SR-1 over 5α-SR-2 and 17β-hydroxysteroid dehydrogenase generate DHT (see Fig. 1B). Analyses of intratumoral prostate tissues that ranged from hormone-naïve prostate cancer to castration-resistant prostate cancer revealed that the level of 5α-SR-1 increased as 5α-SR-2 decreased, indicating that 5α-SR-1 becomes the dominant enzyme that controls the development of the alternative pathway (10). This finding strongly indicates that the emergence of this alternative pathway is causative in the development of castration-resistant prostate cancer (9, 10).
Concerning DHT dysregulation, any negative role DHT plays in prostate diseases was posed in recent studies to inquire whether prostate cancer and/or benign prostate hyperplasia (BPH) is reduced by preventive treatment with 5α-SR-2 inhibitors (11–13). Treatment with the specific inhibitor dutasteride led to a 23% reduction in DHT levels over 4 years (11) that was accompanied by improvement in the incidence of BPH with significant reductions in confirmed prostate cancer upon biopsy. These results indicate that reduced DHT levels in males, on average, positively affect both diseases. In addition, reduced androgen receptor levels (11) are consistent with the preventive efficacy of the 5α-SR-2 inhibitor. Combined, the studies have provided significant evidence that dysregulation of DHT levels is associated with BPH and prostate cancer (11–13).
The fact that prostate basal cells and other peripheral androgen target tissues (5) convert DHEA to DHT provides indirect evidence that enzymes dedicated to its synthesis are distributed locally. Moreover, studies have shown the UDP-glucuronosyltransferase (UGT) isozymes (14–16) that convert DHT and its 5α-androstane-3α,17β-diol metabolite to water-soluble derivatives are also locally distributed. UGT attaches either DHT or 5α-androstane-3α,17β-diol to glucuronic acid to produce glucuronides that undergo facilitated excretion. Cloning studies identified two human prostate-distributed UGTs, UGT2B15 (14) and UGT2B17 (15), that carry out this function. Immunohistochemical studies demonstrated that the 2B15 and 2B17 isozymes are distributed in prostate luminal and basal cells, respectively (16). COS-1 cells transiently expressing 2B15 (14) versus HEK293 cells stably expressing 2B17 (15) metabolize DHT and 5α-androstane-3α,17β-diol but at significantly different rates. A more recent quantitative PCR study showed that 2B15 is also widely distributed in other tissues, such as liver, bladder, breast, ovary, testis, and most gastrointestinal organs, compared with 2B17, which is more narrowly distributed in a few other tissues (stomach, colon, and testis) (17). The previously described 2B7 (18), not detected in prostate (19), was also shown to glucuronidate these same androgens (20). Distribution of low-activity DHT-metabolizing 2B15 in luminal cells, which require DHT-occupied androgen receptor to carry out prostate-specific functions (1–3), and distribution of robust DHT-metabolizing 2B17 activity in basal cells, without clearly defined function(s), necessarily prompt speculation that 2B15 has evolved such activity to maintain DHT-occupied androgen receptor complexes for this vital action. Moreover, electron micrographs that show a one-to-one stratified arrangement of basal/luminal cells attached to basement membrane in human prostate (Fig. 1) (6) support a model of exclusive movement of small molecules from blood to basal cells and, in turn, to luminal cells. These considerations indicate that prostate DHT regulation is necessarily complex and is interdependent upon the two epithelial cell types.
Because ongoing in vitro studies (21–25), as well as evidence from an in vivo UGT model (26), have demonstrated each UGT requires regulated phosphorylation carried out by a different kinase, we sought to determine whether UGT2B15 is also dependent upon phosphorylation given that it has five phosphorylation sites: three PKC and two tyrosine kinase (TK) sites. Studies concerning the regulation of prostate-distributed UGTs that hasten DHT excretion from the body take on great importance because these preventive studies have indicated that elevated DHT is associated with prostate cancer and BPH development (11–13). Hence, questions arise as to whether phosphorylation of 2B15 likely impacts DHT clearance.
EXPERIMENTAL PROCEDURES
Materials and Cell Lines
The UGT2B15 cDNA was cloned and inserted into the pSVL vector (14). The His tag affinity ligand from the pcDNA3.1 vector (Invitrogen) was fused in-frame at the 3′-end of pSVL-UGT2B15 cDNA (21, 22–25). Clones were expressed in COS-1 and Src/Yes/Fyn (SYF−/−) cells (American Type Culture Collection, Manassas, VA). Sources for all reagents used in tissue culture studies and the BCA protein assay kit were obtained as described (25). UGT substrates, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide kit, phorbol 12-myristate 13-acetate (PMA), anti-β-actin antibody, 1,25-dihydroxyvitamin D3, PP2, and kinase inhibitors (curcumin, calphostin C, bisindolylmaleimide, Gö 6976, and röttlerin) were from either Sigma or Calbiochem. PKCα and Src siRNAs were from Dharmacon. Anti-His antibody was from BIOSOURCE (Camarillo, CA). The development of the highly specific anti-UGT-1168 antibody has been described (21, 25). UDP-[14C]glucuronic acid (PerkinElmer Life Sciences) generated radiolabeled products in the glucuronidation assay, which were quantitated using the Cyclone storage phosphor imager (PerkinElmer Life Sciences) (21).
Growth, Transfection, and Treatment of Cells
COS-1 monkey kidney epithelia and SYF−/− mouse fibroblast cells were grown in DMEM with 4 and 10% fetal bovine serum, respectively. Transfection of cells was carried out with Lipofectamine 2000 at 16 h after plating as described (21). Reagents used in cell culture were solubilized in fresh dimethyl sulfoxide to allow a 0.5% final concentration. COS-1 cells were treated with curcumin, PKC inhibitors or activators, PP2, or Src siRNA at 70 or 24 h, respectively, after transfection, which continued as described in the figure legends. The viability of cells treated with curcumin, PKC, or Src effector agents was monitored using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay kit.
Glucuronidation Assay
Cellular extracts were analyzed for glucuronidation as described (25). The common donor substrate UDP-[14C]glucuronic acid (1.4 mm, 1.4 μCi/μmol) for all UGT isozymes was used in all reactions in conjunction with unlabeled dihydrotestosterone or its metabolite, 5α-androstane-3α,17β-diol, as the acceptor substrate. Incubation conditions are described in the figure legends or in the supplemental figure legends. Because of the low activity for 2B15, reactions were continued for 6 h at 37 °C.
Site-directed Mutagenesis of PKC and TK Phosphorylation Sites in UGT2B15
2B15(S124A) mutations were inserted by DNA amplification using PCR and appropriate primers as follows: primers XhoI(−7) S (5′-CTC GAG TCT AGA GAC CAG GAT GTC-3′) and 2B15 383AS (5′-CAG AGC TTG TTA GCG TAG TC-3′) for fragment 1 and primers 2B15 364S (5′-GAC TAC GCT AAC AAG CTC TG-3′) and 2B15(SacI) 928AS (5′-CCA TTT TCT CCA GAG CTC TGC ACA AAC T-3′) for fragment 2. The two fragments were joined in a third PCR using outer primers XhoI(−7) S and 2B15(SacI) 928AS, which produced an ∼940-bp fragment with mutation S124A. A fourth PCR was simultaneously carried out to generate the second part of the full-length cDNA (∼730 bp) using primers 2B15(SacI) 901S (5′-AGT TTG TGC AGA GCT CTG GAG AAA ATG G-3′) and 2B15(SmaI) 1624AS (5′-CCC CGG GGG CTT TTG ATA TAA CTA ATC TC-3′). The 940- and 730-bp fragments were joined by combination in a fifth PCR with the XhoI(−7) S and 2B15(SmaI) 1624AS primers, which generated the 1670-bp full-length cDNA with mutation S124A. This 1670-bp full-length cDNA was digested with XhoI and SmaI and inserted into the XhoI- and SmaI-digested pSVL vector.
2B15(S172A) was also generated by a similar strategy using the two inside primers 2B15(S172A) 502S (5′-CCC TTT CTG TAC GCT CTT CGA-3′) and 2B15(S172A) 522AS (5′-TCG AAG AGC GTA CAG AAA GGG-3′) to introduce the mutation at Ser172, substituting the alanine codon in both fragments 1 and 2 in the first and second reactions, respectively, with the XhoI(−7) S and 2B15(SacI) 928AS outside primers. Fragments 1 and 2 were again combined in the third reaction using the outside primers, and the full-length cDNA was generated in the fourth and fifth reactions as described for 2B15(S124A). Similarly, 2B15(S422A) was generated using primers 2B15(S422A) 1254S (5′-CAG GAC CAT GGG AAG TAG-3′) and 2B15(S422A) 1271AS (5′-CTA CTT CCC ATG GTC CTG-3′) to introduce Ser422 in both fragments 1 and 2 again with the 2B15(SacI) 901S and 2B15(SmaI) 1624AS outside primers. Again, the third PCR completed the synthesis of the 2B15 cDNA with 2B15(S422A) Xho(−7) S and 2B15(SmaI) 1624AS and the wild-type fragment (−7 to 928) as described for 2B15(S124A).
In addition, the 2B15(Y99F) mutation was introduced using primers 2B15(Y99F) 280S (5′-CTC GAT AGA TGG ATA TTC GGT G-3′) and 2B15(Y99F) 301AS (5′-CAC CGA ATA TCC ATC TAT CGA G-3′), surrounding Tyr99, as described for 2B15(S124A). Similarly, 2B15(Y237F) was generated using primers 2B15(Y237F) 698S (5′-GGG ACC AGT TTT TCA GTG AAG-3′) and 2B15(Y237F) 718AS (5′-CTT CAC TGA AAA ACT GGT CCC-3′) as described for 2B15(S124A).
Insertion of His Tag into UGT2B15 cDNA
The 2B15-His construct was made using WT 2B15 and pUGT1A7-His as templates. Initially, a PCR fragment was generated using primers XhoI(−7) S and 2B15(SacI) 928AS. Then, a PCR fragment was synthesized using primers 2B15(SacI) 901S and 2B15-thrombin-His AS (5′-CTC GGA TCC GGA TCC ACG CGG AAC CAG ATC TCT TTT C-3′). Subsequently, a third PCR fragment was generated using pUGT1A7-His as a template, 2B15-thrombin-His S (5′-GAA AAG AGA TCT GGT TCC GCG TGG ATC CGG ATC CGA G-3′), and PmeI-SmaI AS (5′-GGT TTA AAC CCC GGG TCA ATG ATG ATG ATG ATG-3′). The second and third fragments were combined by joint PCR using primers 2B15(SacI) 901S and PmeI-SmaI AS (5′-GGT TTA AAC CCC GGG TCA ATG ATG ATG ATG ATG-3′) with the His tag and a thrombin cleavage site after the 2B15 complete encoded message. This combined product was joined with fragment 1 using primers XhoI(−7) S and PmeI-SmaI AS. This 1730-bp full-length cDNA containing the His-coding sequence was digested with XhoI and SmaI before ligation into the pSVL vector previously digested with XhoI and SmaI to generate pSVL-UGT2B15-His with a His tag. 2B15(S124A)-His, 2B15(S172A)-His, 2B15(S422A)-His, 2B15(Y99F)-His, 2B15(Y237F)-His, and 2B15(S124A/S422A)-His constructs were also made simultaneously.
Western Blot Analysis of UGT2B15-expressing COS-1 Cells
Relative amounts of UGT in transfected COS-1 cells were established by Western blotting as described (21, 23–25). Twenty-five μg of solubilized cell homogenate was added to SDS sample buffer, applied to SDS-polyacrylamide gel, and subjected to electrophoresis. Following electrotransblotting of proteins onto nitrocellulose membrane, blots were processed as described previously (25) for exposure to x-ray films. In order, blots were exposed to antibody specific for PKCα and other PKC isozymes according to the suppliers' instructions, followed by the appropriate HRP-conjugated secondary antibody before visualizing the reactive pattern as described for anti-UGT antibody (21).
Co-immunoprecipitation of UGT2B15, Its Mutants, and PKCα
To carry out co-immunoprecipitation studies, 2B15-His- or mutant 2B15-His-expressing COS-1 cells were harvested and processed for immunoprecipitation using anti-UGT-1168 antibody as described previously (23–25). Immunoprecipitates were resolved in 4–15% gradient SDS-PAGE systems and transblotted as described for Western blot analysis using the antibodies indicated in the figure legends.
Treatment of UGT2B15-expressing COS-1 Cells with PKCα siRNA
Exposure of transfected COS-1 cells was carried out as described previously (25).
Treatment of UGT2B15-expressing COS-1 Cells with Src Kinase Effector Agents 1,25-Dihydroxyvitamin D3, PP2-specific Inhibitor, and Src Kinase siRNA
Because studies have shown that COS-1 cells contain the 1,25-dihydroxyvitamin D3 receptor (27, 28), we treated COS-1 cells 70 h after 2B15 transfection with different concentrations of 1,25-dihydroxyvitamin D3. The treated cells were allowed to incubate for different times before harvesting and storing at −80 °C for assay for glucuronidation of androgens as described in the figure legends. At 58 h after 2B15 transfection, which was followed by 12 h of phosphate-free conditions, cells were treated with PP2 for 2 h, or 24 h after transfection, cells were treated with Src kinase siRNA versus non-target siRNA, which was continued for 44 h. Cells were then harvested and stored at −80 °C until the samples were assayed for glucuronidation of DHT and 5α-androstane-3α,17β-diol as described in the figure legends.
In Vitro Phosphorylation of UGT2B15-His Expressed in Src-free Cells
To confirm that PKCα and/or Src phosphorylates 2B15, non-transfected and pSVL-2B15-His-transfected SYF−/− fibroblast cells were cultured for 60 h before harvesting in PBS buffer as described previously (21). Cells were solubilized in 5 mm CHAPS, 100 μm Na3VO4, 5 mm NaF, and protease inhibitor mixture (Sigma) in 50 mm phosphate buffer (pH 7.8) as described previously (21, 25). Equal aliquots of Src-TK alone, PKCα alone, Src-TK and PKCα combined, or the appropriate buffer were distributed in reaction systems from a kit (Millipore) to which had been added either solubilized non-transfected or UGT2B15-transfected cellular protein and [γ-33P]ATP (3000 Ci/mmol; Perkin Elmer) as suggested by the manufacturer. Samples were incubated for 30 min at 30 °C before storing at −20 °C (21). Samples were purified by His affinity chromatography as described (21) and subjected to resolution in a 4–15% gradient SDS-PAGE system. The gel was dried, exposed to x-ray film, and developed for a photograph.
RESULTS
Relative Activity for UGT2B15(Asp85) and UGT2B15(Tyr85)
Considering that two different UGT2B15 alleles exist in the population containing either Asp85 or Tyr85 due to a SNP (29), UGT2B15(Asp85) (14) was analyzed in this study.
Effect of Curcumin Treatment on UGT2B15 Activity
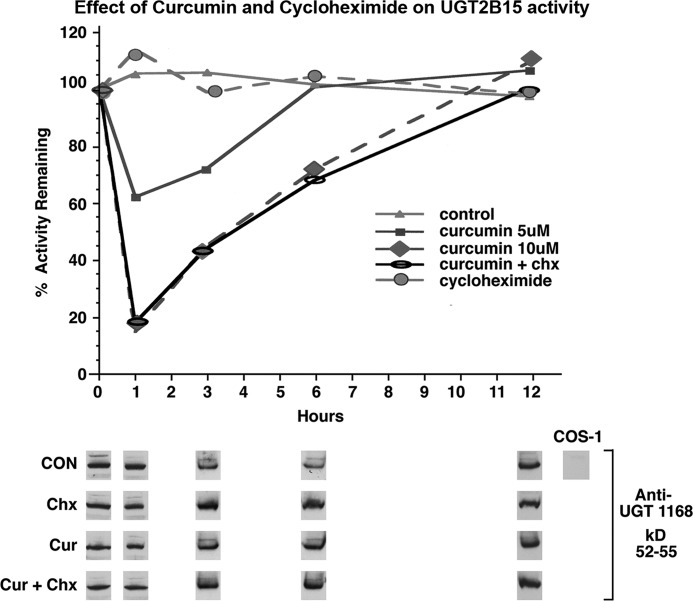
Whereas curcumin treatment of 10 different recombinant UGT isozymes expressed in cell culture led to the observation that each was differentially inhibited (25), further analysis of UGT1A1 (23), UGT1A7 (24, 25), UGT1A10 (24, 25), and UGT2B7 (21) revealed that each required regulated phosphorylation carried out by a different kinase. Hence, it was of interest to extend the curcumin studies by focusing on the predicted phosphorylation sites in luminal cell-distributed 2B15, which is critical for DHT homeostasis in this key cell, which carries out DHT-occupied androgen receptor-dependent prostate-specific functions. Because 2B15 metabolizes both DHT (14) and its 5α-androstane-3α,17β-diol metabolite, similar to UGT2B17 (15), we elected to use both endogenous substrates to define the critical requirements of the isozyme. The fact that treatment of UGT2B15-expressing COS-1 cells with 5 and 10 μm curcumin led to 40 and 80% down-regulation, respectively, in 1 h with detectable recovery by 3 h suggested that the isozyme undergoes regulated phosphorylation (Fig. 2). Curcumin inhibition did not affect the 2B15 or β-actin (data not shown) protein level. Because down-regulation and recovery following exposure to 10 μm curcumin did not differ in the presence and absence of cycloheximide (Fig. 2), the results indicate that the effect was independent of protein synthesis and was likely dependent on disrupted phosphate signaling (25).
FIGURE 2.
Effect of curcumin and cycloheximide on UGT2B15 activity expressed in COS-1 cells. Seventy h after transfection with the pSVL-UGT2B15 construct and with exposure to phosphate-free medium during the last 12 h, COS-1 cells were exposed to curcumin (Cur) in DMEM containing 1% FCS with and without cycloheximide (Chx) as described under “Experimental Procedures.” Homogenates of cells treated with curcumin or curcumin/cycloheximide were assayed in vitro using DHT and 5α-androstane-3α,17β-diol. Western blot analysis was carried out with anti-UGT-1168 antibody as described. Data represent three experiments with S.E. of ±2–8%. CON, control.
Effect of Classical Protein Kinase C Inhibitors on UGT2B15 Activity
Because curcumin inhibits members of both the PKC (21, 23–25) and TK (21, 30) families, we examined the effects of classical PKC inhibitors (calphostin C (24), bisindolylmaleimide (24), Gö 6976 (31), and röttlerin (31, 32)) on 2B15 activity. Between 25 and 500 nm calphostin C inhibited activity in a concentration-dependent manner, resulting in a 90% reduction in both DHT and 5α-androstane-3α,17β-diol turnover (supplemental Fig. S1A) without an effect on the protein level of 2B15, PKCα, or β-actin. The fact that calphostin C inhibited activity by ∼90% (supplemental Fig. S1A) and that the other selected PKC inhibitors (250 nm bisindolylmaleimide, 1.0 μm Gö 6976, and 10 μm röttlerin) inhibited activity by 70–80% (supplemental Fig. S1B) provides strong evidence that 2B15 requires PKC phosphorylation that is regulated.
Effect of PKC Agonist PMA on UGT2B15 Activity
Because earlier studies demonstrated that UGT2B7 has three nonessential PKC sites (33) but two required TK phosphorylation sites, which undergo reversible down-regulation by curcumin, we examined whether the classical PKC agonist PMA (25) activates 2B15. Similar to other PKC-dependent systems (25), 2B15 was stimulated optimally by 100 nm PMA (supplemental Fig. S2). The fact that PKC effector agents inhibited or activated 2B15 activity, as predicted, is consistent with the required regulated phosphorylation of at least one of its three PKC sites.
Effect of Mutations at Predicted Phosphorylation Sites in 2B15
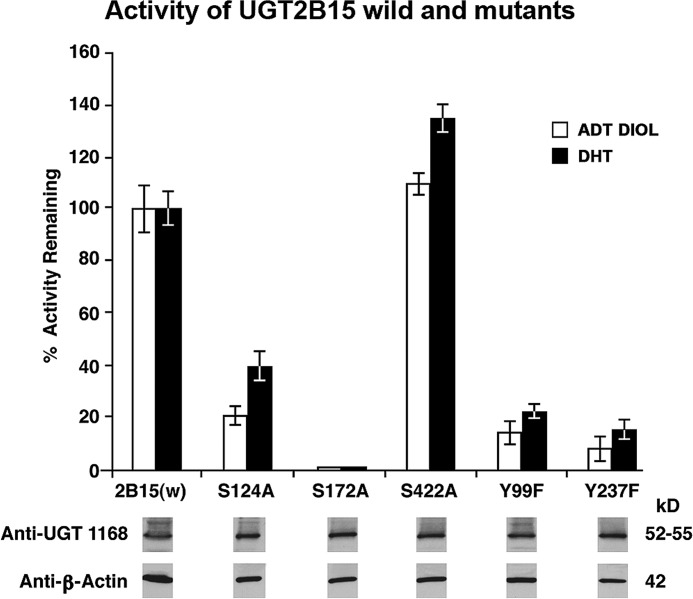
To establish whether any of the three PKC sites or two TK sites in 2B15 is required, we carried out site-directed mutagenesis of the PKC and TK phosphorylation sites in 2B15. Ala was substituted for Ser at positions 124, 172, and 422, and Phe replaced Tyr at positions 99 and 237. Examination of the mutants generated a range of effects that were highly to barely significant. The S172A mutant was null, whereas the S124A mutant was 60 and 80% inactive toward DHT and 5α-androstane-3α,17β-diol, respectively (Fig. 3). In contrast, the 2B15(S422A) mutant activity hardly changed or was ∼20% more active than wild-type 2B15 (Fig. 3). Tyrosine mutants 2B15(Y99F) and 2B15(Y237F) were 80 and 90% inactive, respectively (Fig. 3), providing the first evidence these sites in 2B15 require regulated phosphorylation, based on the reversibility of curcumin inhibition that was independent of protein synthesis. All mutants and wild-type 2B15 were under conditions of equal protein for 2B15 and β-actin as shown by Western blot analysis (Fig. 3, lower).
FIGURE 3.
Effect of mutations at predicted PKC or TK phosphorylation sites in UGT2B15 on its activity. Mutations at Ser124, Ser172, Ser422, Tyr99, or Tyr237 were introduced as described under “Experimental Procedures.” Glucuronidation of DHT and 5α-androstane-3α,17β-diol (ADT DIOL) and Western blot analysis using anti-UGT-1168 or anti-β-actin antibody were carried out as described under “Experimental Procedures.” For analysis, 2B15-His or one of its PKC site mutants was expressed in COS-1 cells for 72 h. Triplicate determinations were carried out, and the data represent three experiments with S.E. of ±2–6%. w, wild-type.
Co-immunoprecipitation of PKCα with UGT2B15-His Its Mutants
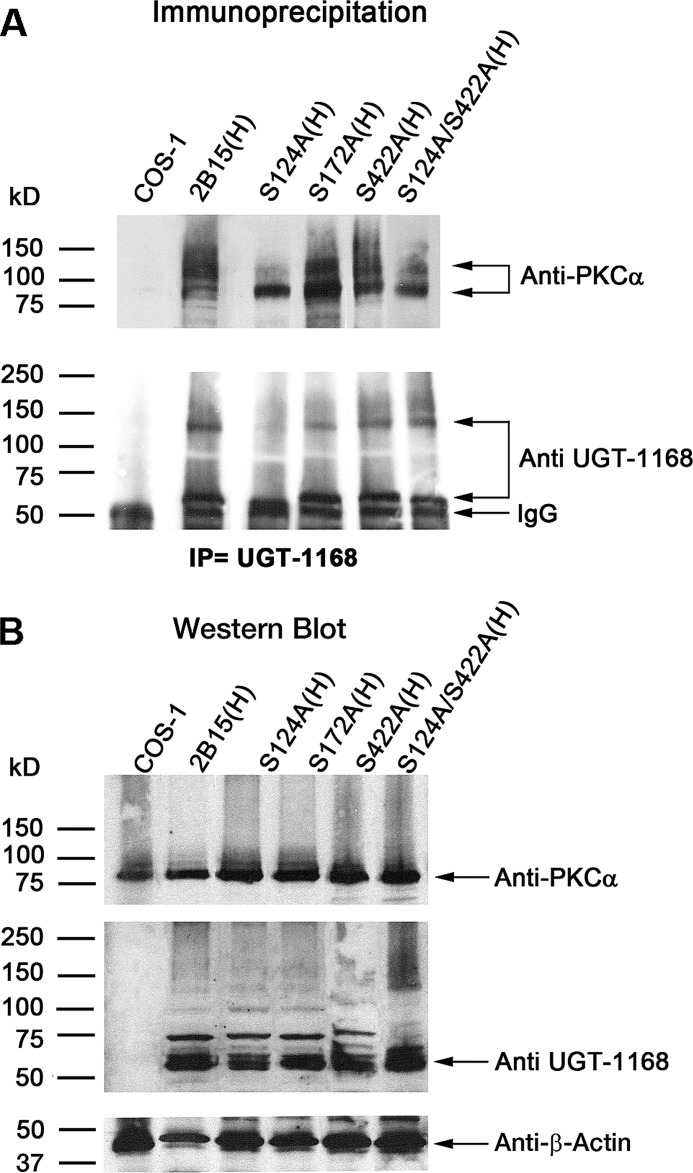
To establish the PKC isozyme(s) involved in 2B15-His phosphorylation, we carried out screens with antibodies against different PKC isozymes on a Western blot that contained co-immunoprecipitates of wild-type 2B15-His and 2B15-His mutated at different PKC sites (S124A, S172A, or S422A) and the partially inactive double mutant S124A/S422A. Immunoprecipitates were captured with anti-UGT-1168 antibody (Fig. 4A) (21, 24) under conditions of equal UGT-specific protein (Fig. 4B). The results show that 2B15-His migrated to 55–58 kDa (Fig. 4A, lower) and that PKCα migrated to 82 kDa (Fig. 4A, upper) in the gel, whereas WT 2B15-His or its mutant co-migrated as a complex with PKCα to the 130–140- and >150-kDa positions following expression of the 2B15 protein in COS-1 cells. Immunoprecipitation of PKCα-containing complexes using anti-UGT-1168 antibody indicated that PKCα was initially trapped as a complex that disassociated during electrophoresis. Hence, the results indicate that less than half of PKCα trapped with anti-UGT-1168 antibody by the mutants remained associated with the complexes during electrophoresis (Fig. 4A), except the essentially normally active 2B15(S422A)-His mutant; in contrast, there was a nearly complete association of PKCα and WT 2B15-His during electrophoresis. Although a substantial amount of PKCα and 2B15(S172A)-His co-migrated to the 130–140-kDa position, the majority of PKCα trapped by the completely inactive mutant dissociated and remained at the 82-kDa position (Fig. 4A). In contrast, the 2B15(S124A) mutant, which was more active than 2B15(S172A), was the least effective at trapping complexes. The double mutant construct 2B15(S124A/S422A) showed some recovery in the amount of the 130–140-kDa complex compared with the single 2B15(S124A) mutant (Fig. 4A). All comparisons of complex formation and stability were made under conditions of equal specific proteins as shown in Fig. 4B. Based on the results of the activity and amount/quality of UGT2B15 and PKCα complexes that were trapped by co-immunoprecipitation and that remained complexed during electrophoresis, our findings indicate that 2B15 phospho-Ser124 is the most critical for the stability of both the 130–140- and >150-kDa complexes.
FIGURE 4.
Co-immunoprecipitation of UGT2B15-His and PKCα cells using 2B15-transfected COS-1 cells. Seventy-two h after wild-type and mutant 2B15 constructs were transfected into COS-1 cells, homogenates were prepared for (A) co-immunoprecipitation (IP) and (B) Western-blotted as described under “Experimental Procedures.” Antibodies used are shown.
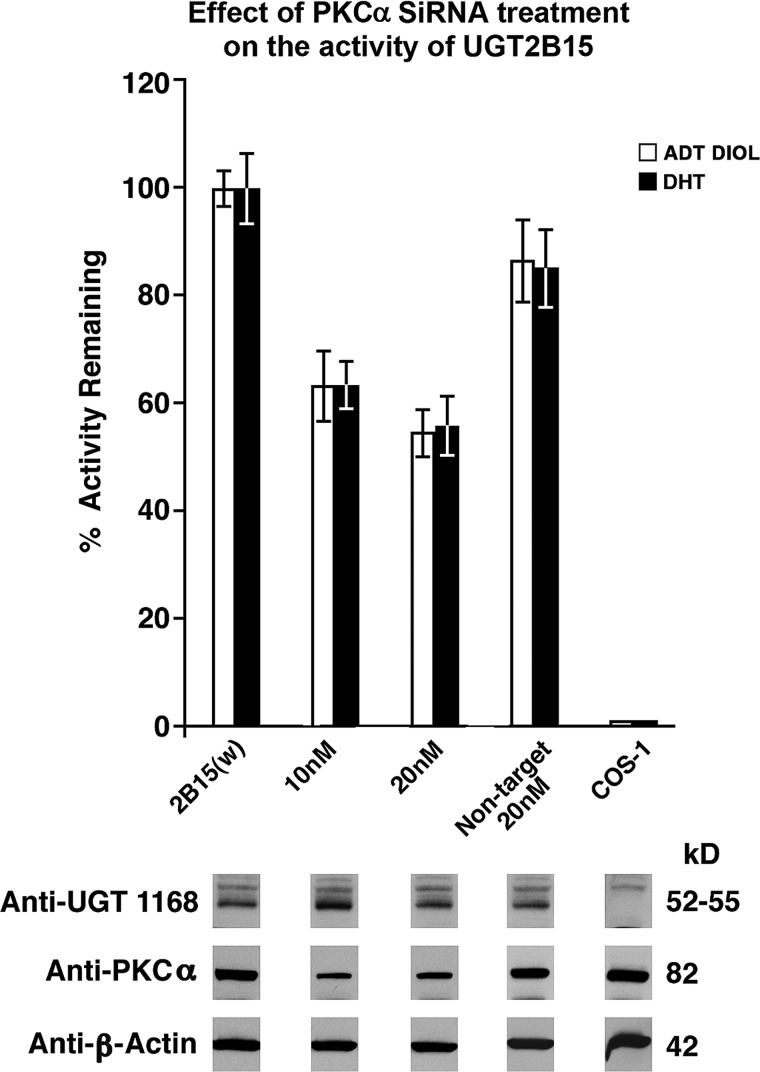
Effect of PKCα siRNA on UGT2B15-His Activity
The finding of only PKCα bound to 2B15-containing complexes in Fig. 4A out of the five different PKC isozymes examined provides strong evidence that PKCα is primarily responsible for phosphorylating PKC site(s) in 2B15. Furthermore, the dramatic reduction in 2B15 activity following treatment with increasing concentrations of PKCα siRNA (Fig. 5, upper), which mimicked the decreases in PKCα protein levels (Fig. 5, lower), is consistent with PKCα carrying out the required phosphorylation of PKC sites in 2B15. Hence, the reductions in 2B15 activity following PKCα siRNA treatment confirm that PKCα plays an important role in 2B15 activity.
FIGURE 5.
Effect of PKCα siRNA on UGT2B15 expressed in COS-1 cells. Twenty-four h after transfection with pSVL-UGT2B15, cells were treated with different concentrations of either PKCα siRNA or non-target siRNA for 44 h as described under “Experimental Procedures.” ADT DIOL, 5α-androstane-3α,17β-diol; w, wild-type.
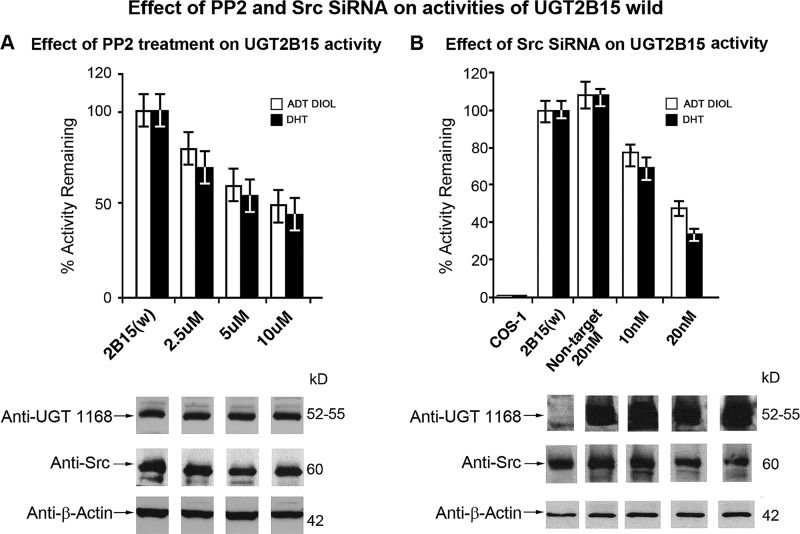
Effects of Src Kinase Effector Agents 1,25-Dihydroxyvitamin D3, Src-specific PP2 Inhibitor, and Src Kinase siRNA on UGT2B15 Expressed in COS-1 Cells
To assess whether Src has a positive effect on 2B15 activity, we treated 2B15-expressing COS-1 cells with the Src activator (27, 28) 1,25-dihydroxyvitamin D3. The results show that the agent (2 and 5 nm) caused the typical rapid increase in 2B15 activity, reaching 1.5- and 2.0-fold between 2 and 5 min, without affecting 2B15, Src, or β-actin protein levels (supplemental Fig. S3). Using other typical Src effector agents, we determined that the Src-specific inhibitor PP2 inhibited 2B15 activity in a concentration-dependent manner that reached 50% at 10 μm (Fig. 6A, upper) without affecting general or specific protein levels (Fig. 6A, lower). Finally, treatment of 2B15-transfected COS-1 cells with increasing concentrations of Src siRNA versus non-target siRNA caused a 50–70% decrease in 2B15 activity (Fig. 6B, upper) in parallel with diminution of Src protein but without affecting 2B15 or β-actin protein (Fig. 6B, lower). These combined results clearly demonstrate that Src kinase has positive effect(s) on 2B15 activity.
FIGURE 6.
Effect of Src-specific inhibitor PP2 or Src siRNA on UGT2B15 expressed in COS-1 cells. A, 70 h after transfection with pSVL-UGT2B15, cells were treated with different concentrations of PP2 before harvesting to assay for DHT and 5α-androstane-3α,17β-diol (ADT DIOL). Western blot analysis was carried out with anti-UGT-1168, anti-Src, and anti-β-actin antibodies as described under “Experimental Procedures.” B, effect of Src siRNA on UGT2B15. Twenty-four h after transfection with pSVL-UGT2B15, cells were treated with different concentrations of either Src siRNA or non-target siRNA for 44 h as described under “Experimental Procedures.”
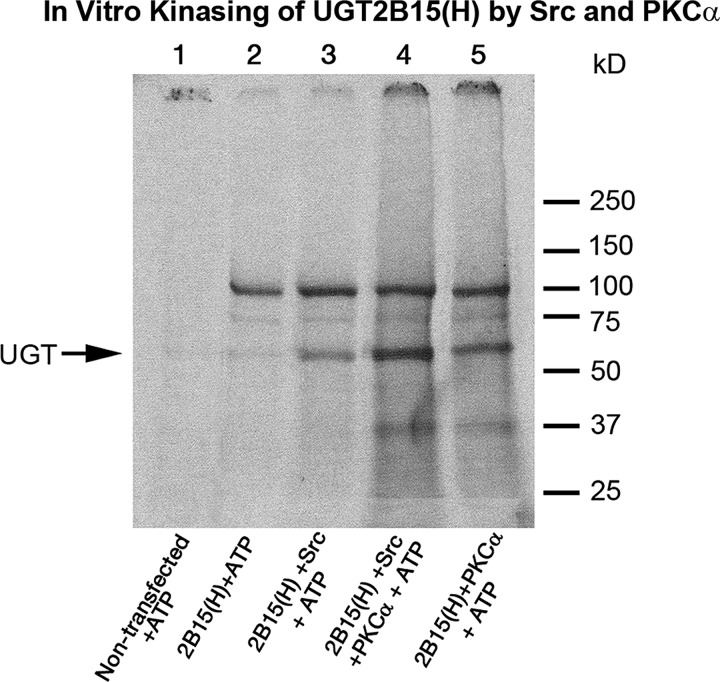
In Vitro Evidence That PKCα and Src Phosphorylate UGT2B15-His
To assess whether PKCα and/or Src directly phosphorylates 2B15-His, we solubilized 2B15-His-transfected or non-transfected SYF−/− cell homogenates and subjected them to phosphorylation by adding [γ-33P]ATP or buffer as described under “Experimental Procedures” and previously (21, 25). Following phosphorylation and His affinity purification as described previously (21, 25), samples were resolved in a 4–15% gradient SDS-PAGE system. PKCα incorporated >2-fold greater phosphate in 2B15-His compared with Src kinase (Fig. 7). Moreover, the combination of the two kinases incorporated additive levels of [γ-33P]ATP-dependent label into 2B15-His (Fig. 7). There was very little phosphorylation without the addition of either kinase to the cell homogenate (Fig. 7, lane 2). Hence, the pattern indicates that the two kinases bring about additive phosphorylation of 2B15 predictably involving the phosphorylation sites already identified. The fact that the four mutants at Tyr99, Ser124, Ser172, and Tyr237 lost activity suggests that each of the phosphorylated sites positively affects activity compared with the S422A mutant, which was unchanged or was barely up-regulated, suggesting that it moderately down-regulates 2B15 activity.
FIGURE 7.
In vitro phosphorylation of UGT2B15 expressed in SYF−/− cells by PKCα and Src kinase. Solubilized non-transfected and 2B15-transfected SYF−/− fibroblast cells were prepared for kinase reaction. [γ-33P]ATP was included all reactions, and kinase buffer, PKCα, Src kinase, or both kinases were added as described under “Experimental Procedures.” Reactions proceeded for 30 min at 30 °C. Experiments were carried out three times. Samples were purified by His affinity chromatography and subjected to resolution in a 4–15% gradient SDS-PAGE system. Gels were dried and exposed for photographs. H, His.
DISCUSSION
For the first time, we have evidence to indicate that a UGT isozyme undergoes regulated phosphorylation by two different kinases. Both PKCα and Src kinase phosphorylate UGT2B15, which is consistent with the fact that the isozyme contains three PKC and two TK phosphorylation sites by computer analysis. Inhibition of 2B15 activity by typical PKC inhibitors (curcumin, calphostin C, bisindolylmaleimide, Gö 6976, and röttlerin) and activation by PMA support the claim that PKC phosphorylates the isozyme. The fact that individual mutations in the 2B15 isozyme at two of the three predicted PKC phosphorylation sites or at two of the two TK sites ranged from null to 30% active provides strong evidence that 2B15 requires both PKC and TK phosphorylation. Moreover, the reversible inhibition by curcumin that was unaffected by cycloheximide co-treatment indicates that 2B15 undergoes regulated phosphorylation that involves signaling.
Concerning the PKC isozyme(s) responsible for phosphorylating 2B15, the fact that anti-UGT-1168 antibody co-immunoprecipitated wild-type 55–58-kDa 2B15-His and 82-kDa PKCα in 130–140- and >150-kDa complexes in solubilized 2B15-His-transfected COS-1 cells, but not in non-transfected cells, provides strong evidence that PKCα phosphorylates 2B15. In addition, the major decreases in 2B15 activity upon PKCα siRNA treatment support our claim that PKCα phosphorylates 2B15. Contrariwise, finding that 1,25-dihydroxyvitamin D3 treatment of 2B15-expressing COS-1 cells elicited the typical rapid activation of Src-TK (27, 28) manifested as increases in 2B15-dependent DHT metabolism provides the first evidence that Src also phosphorylates 2B15. Notably, the 1,25-dihydroxyvitamin D3 activation of 2B15 is similar to the results in our earlier study in which treatment of LS180 cells with 1,25-dihydroxyvitamin D3 elicited a rapid activation of 2B7,5 which represents the first indication that 2B7 requires regulated phosphorylation by Src. (The study showed that Src participates in dictating 2B7 endogenous substrate selections (21).) Again considering the predicted 2B15 TK phosphorylation sites, inhibition or down-regulation of 2B15 activity by Src-specific effector agents (PP2 and Src siRNA) provides the first strong evidence that 2B15 also undergoes TK phosphorylation by Src. Hence, the fact that 2B15 undergoes inhibition by both sets of inhibitors and that it is down-regulated by both PKCα and Src kinase siRNAs again confirms that PKCα and Src separately or combined phosphorylate 2B15. In vitro phosphorylation of 2B15 here confirmed that PKCα and Src can independently phosphorylate 2B15. We note, however, that although 2B7 requires only Src-dependent phosphorylation, 1A7, 1A10, and 1A1 require only PKC-dependent phosphorylation compared with 2B15. Hence, we have now discovered the first UGT (2B15) that requires regulated phosphorylation at both PKC and TK sites.
Control of 2B15 activity by phosphorylation at both PKCα and Src TK sites suggests that the requirement signifies a complex signaling pathway(s) that involves activation and/or inhibition coordinated via cross-talk (34). Whereas the major 2B15 substrate, DHT, is the active steroid that drives prostate specific functions, it is necessary that 2B15 is subjected to careful regulation to avoid DHT insufficiencies or deleterious excesses. Hence, it is possible that complex 2B15 regulation directly anchors metabolism of the active androgen so as to prevent rapid deleterious changes in DHT levels in either direction. This is reminiscent of pathways involving both PKC and Src in relationship to DHT control of prostate cell proliferation (35). Although it is not possible to determine the contribution of each phosphorylated site in 2B15, we have demonstrated that phospho-Ser124 is involved in the stability of 2B15.6
Considering the publications cited (5–7) concerning DHT synthesis in the prostate (see Fig. 1A), the distribution of 5α-SR-2 (7) in luminal cells to reduce testosterone to DHT for occupation of the androgen receptor to carry out prostate-specific functions is likely made possible by 2B15-regulated phosphorylation that imposes low turnover to make DHT homeostasis possible. Providing transport is not encumbered, it is possible that excess DHT is transported via gap junctions into basal cells to undergo rapid glucuronidation by 2B17.7 Importantly, 5α-SR-2 reduction of testosterone to generate DHT appears necessary to prevent castration-resistant prostate cancer development compared with 4-androstenedione reduction by 5α-SR-1 in the recently described testosterone bypass pathway (Fig. 1B) (9, 10).
Also, we recall the preventive treatment studies cited in the Introduction (11–13) that demonstrated that lowering DHT levels in males by lowering the “rate” of testosterone reduction to DHT by inhibitors of 5α-SR-2 significantly reduced the incidences of prostate malignancies, as well as BPH. The finding that luminal cell-distributed 2B15, which is evidently relevant in maintaining constitutive DHT levels for prostate-specific functions, is controlled by an evident complex set of factors involving two different kinases suggests that it is essential to determine the pivotal regulatory cross-talk that dictates the rate of DHT glucuronidation. Moreover, previous studies (6, 7) provide evidence that the stratified prostate epithelial cell types are structurally interconnected, enabling participation of the robust basal cell-distributed 2B17 activity. Whether this connectivity is viable is also relevant.
Supplementary Material
This work was supported, in whole or in part, by the NICHD Intramural Research Program.

This article contains supplemental Figs. S1–S3.
S. K. Chakraborty, N. K. Basu, S. Jana, M. Basu, A. Raychoudhuri, and I. S. Owens, unpublished data.
S. Jana, N. K. Basu, A. Raychoudhuri, and I. S. Owens, manuscript in preparation.
M. Basu, S. Jana, N. K. Basu, and I. S. Owens, manuscript in preparation.
- DHT
- 5α-dihydrotestosterone
- DHEA
- dehydroepiandrosterone
- 5α-SR-2
- 5α-steroid reductase 2
- BPH
- benign prostate hyperplasia
- UGT
- UDP-glucuronosyltransferase
- TK
- tyrosine kinase
- PMA
- phorbol 12-myristate 13-acetate.
REFERENCES
- 1. Russell D. W., Wilson J. D. (1994) Steroid 5α-reductase: two genes/two enzymes. Annu. Rev. Biochem. 63, 25–61 [DOI] [PubMed] [Google Scholar]
- 2. Cunha G. R., Lung B. (1978) The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J. Exp. Zool. 205, 181–193 [DOI] [PubMed] [Google Scholar]
- 3. Cunha G. R., Ricke W., Thomson A., Marker P. C., Risbridger G., Hayward S. W., Wang Y. Z., Donjacour A. A., Kurita T. (2004) Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J. Steroid Biochem. Mol. Biol. 92, 221–236 [DOI] [PubMed] [Google Scholar]
- 4. Labrie F., Belanger A., Luu-The V., Labrie C., Simard J., Lin S.-X. (2000) in Dehydroepiandrosterone (DHEA): Biochemical, Physiological, and Clinical Aspects (Kalimi M., Regelson W., eds) pp. 299–393, Walter de Guyter and Co., New York [Google Scholar]
- 5. Arlt W. (2004) Dehydroepiandrosterone and aging. Best Pract. Res. Clin. Endocrinol. Metab. 18, 363–380 [DOI] [PubMed] [Google Scholar]
- 6. El-Alfy M., Pelletier G., Hermo L. S., Labrie F. (2000) Unique features of the basal cells of human prostate epithelium. Microsc. Res. Tech. 51, 436–446 [DOI] [PubMed] [Google Scholar]
- 7. Pelletier G. (2008) Expression of steroidogenic enzymes and sex steroid receptors in human prostate. Best Pract. Res. Clin. Endocrinol. Metab. 22, 223–228 [DOI] [PubMed] [Google Scholar]
- 8. Morley M., Jones C., Sidhu M., Gupta V., Bernier S. M., Rushlow W. J., Belliveau D. J. (2010) PKC inhibitor increases gap junction intercellular communication and cell adhesion in human neuroblastoma. Cell Tissue Res. 340, 229–242 [DOI] [PubMed] [Google Scholar]
- 9. Luu-The V. (2011) Assessment of steroidogenic pathways that do not require testosterone as intermediate. Horm. Mol. Biol. Clin. Invest. 5, 161–165 [DOI] [PubMed] [Google Scholar]
- 10. Chang K. H., Li R., Papari-Zareei M., Watumull L., Zhao Y. D., Auchus R. J., Sharifi N. (2011) Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc. Natl. Acad. Sci. U.S.A. 108, 13728–13733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mostaghel E. A., Geng L., Holcomb I., Coleman I. M., Lucas J., True L. D., Nelson P. S. (2010) Variability in the androgen response of prostate epithelium to 5α-reductase inhibition: implications for prostate cancer chemoprevention. Cancer Res. 70, 1286–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parsons J. K., Palazzi-Churas K., Bergstrom J., Barrett-Connor E. (2010) Prospective study of serum dihydrotestosterone and subsequent risk of benign prostate hyperplasia in community dwelling men: the Rancho Bernardo study. J. Urol. 184, 1040–1044 [DOI] [PubMed] [Google Scholar]
- 13. Andriole G. L., Bostwick D. G., Brawley O. W., Gomella L. G., Marberger M., Montorsi F., Pettaway C. A., Tammela T. L., Teloken C., Tindall D. J., Somerville M. C., Wilson T. H., Fowler I. L., Rittmaster R. S. (2010) Effect of dutasteride on the risk of prostate cancer. N. Engl. J. Med. 362, 1192–1202 [DOI] [PubMed] [Google Scholar]
- 14. Chen F., Ritter J. K., Wang M. G., McBride O. W., Lubet R. A., Owens I. S. (1993) Characterization of a cloned human dihydrotestosterone/androstanediol UDP-glucuronosyltransferase and its comparison to other steroid isoforms. Biochemistry 32, 10648–10657 [DOI] [PubMed] [Google Scholar]
- 15. Beaulieu M., Lévesque E., Hum D. W., Bélanger A. (1996) Isolation and characterization of a novel cDNA encoding a human UDP-glucuronosyltransferase active on C19 steroids. J. Biol. Chem. 271, 22855–22862 [DOI] [PubMed] [Google Scholar]
- 16. Chouinard S., Pelletier G., Bélanger A., Barbier O. (2004) Cellular specific expression of the androgen-conjugating enzymes UGT2B15 and UGT2B17 in the human prostate epithelium. Endocr. Res. 30, 717–725 [DOI] [PubMed] [Google Scholar]
- 17. Nakamura A., Nakajima M., Yamanaka H., Fujiwara R., Yokoi T. (2008) Expression of UGT1A and UGT2B mRNA in human normal tissues and variable cell lines. Drug Metab. Dispos. 36, 1461–1464 [DOI] [PubMed] [Google Scholar]
- 18. Ritter J. K., Sheen Y. Y., Owens I. S. (1990) Cloning and expression of human liver UDP-glucuronosyltransferase in COS-1 cells. 3,4-Catechol estrogens and estriol as primary substrates. J. Biol. Chem. 265, 7900–7906 [PubMed] [Google Scholar]
- 19. Bélanger G., Beaulieu M., Marcotte B., Lévesque E., Guillemette C., Hum D. W., Bélanger A. (1995) Expression of transcripts encoding steroid UDP-glucuronosyltransferases in human prostate hyperplastic tissue and the LNCaP cell line. Mol. Cell. Endocrinol. 113, 165–173 [DOI] [PubMed] [Google Scholar]
- 20. Barbier O., Bélanger A. (2008) Inactivation of androgens by UDP-glucuronosyltransferases in the human prostate. Best Pract. Res. Clin. Endocrinol. Metab. 22, 259–270 [DOI] [PubMed] [Google Scholar]
- 21. Mitra P. S., Basu N. K., Basu M., Chakraborty S., Saha T., Owens I. S. (2011) Regulated phosphorylation of a major UDP-glucuronosyltransferase isozyme by tyrosine kinases dictates endogenous substrate selection for detoxification. J. Biol. Chem. 286, 1639–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Basu N. K., Kubota S., Meselhy M. R., Ciotti M., Chowdhury B., Hartori M., Owens I. S. (2004) Gastrointestinally distributed UDP-glucuronosyltransferase 1A10, which metabolizes estrogens and nonsteroidal anti-inflammatory drugs, depends upon phosphorylation. J. Biol. Chem. 279, 28320–28329 [DOI] [PubMed] [Google Scholar]
- 23. Basu N. K., Kole L., Owens I. S. (2003) Evidence for phosphorylation requirement for human bilirubin UDP-glucuronosyltransferase (UGT1A1) activity. Biochem. Biophys. Res. Commun. 303, 98–104 [DOI] [PubMed] [Google Scholar]
- 24. Basu N. K., Kovarova M., Garza A., Kubota S., Saha T., Mitra P. S., Banerjee R., Rivera J., Owens I. S. (2005) Phosphorylation of a UDP-glucuronosyltransferase regulates substrate specificity. Proc. Natl. Acad. Sci. U.S.A. 102, 6285–6290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Basu N. K., Kole L., Basu M., Chakraborty K., Mitra P. S., Owens I. S. (2008) The major chemical-detoxifying system of UDP-glucuronosyltransferases requires regulated phosphorylation supported by protein kinase C. J. Biol. Chem. 283, 23048–23061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Basu N. K., Kole L., Basu M., McDonagh A. F., Owens I. S. (2007) Targeted inhibition of glucuronidation markedly improves drug efficacy in mice: a model. Biochem. Biophys. Res. Commun. 360, 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sitrin M. D., Bissonnette M., Bolt M. J., Wali R., Khare S., Scaglione-Sewell B., Skarosi S., Brasitus T. A. (1999) Rapid effects of 1,25(OH)2-vitamin D3 on signal transduction systems in colonic cells. Steroids 64, 137–142 [DOI] [PubMed] [Google Scholar]
- 28. Sakamaki Y., Inaba Y., Yoshimoto N., Yamamoto K. (2010) Potent antagonist for the vitamin D receptor: vitamin D analogues with simple side chain structure. J. Med. Chem. 53, 5813–5826 [DOI] [PubMed] [Google Scholar]
- 29. Lévesque E., Beaulieu M., Green M. D., Tephly T. R., Bélanger A., Hum D. W. (1997) Isolation and characterization of UGT2B15(Y85): a UDP-glucuronosyltransferase encoded by a polymorphic gene. Pharmacogenetics 7, 317–325 [DOI] [PubMed] [Google Scholar]
- 30. Leu T. H., Su S. L., Chuang Y. C., Maa M. C. (2003) Direct inhibitory effect of curcumin on Src and focal adhesion kinase activity. Biochem. Pharmacol. 66, 2323–2331 [DOI] [PubMed] [Google Scholar]
- 31. Perry C., Baker O. J., Reyland M. E., Grichtchenko I. I. (2009) PKCαβγ- and PKCδ-dependent endocytosis of NBCe1-A and NBCe1-B in salivary parotid acinar cells. Am. J. Physiol. Cell Physiol. 297, C1409–C1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu S. Z. (2007) Rottlerin induces calcium influx and protein degradation in cultured lenses independent of effects on protein kinase Cδ. Basic Clin. Pharmacol. Toxicol. 101, 459–464 [DOI] [PubMed] [Google Scholar]
- 33. Mitra P. S., Basu N. K., Owens I. S. (2009) Src supports UDP-glucuronosyltransferase 2B7 detoxification of catechol estrogens associated with breast cancer. Biochem. Biophys. Res. Commun. 382, 651–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hornia A., Lu Z., Sukezane T., Zhong M., Joseph T., Frankel P., Foster D. A. (1999) Antagonistic effects of protein kinase C α and δ on both transformation and phospholipase D activity mediated by the epidermal growth factor receptor. Mol. Cell. Biol. 19, 7672–7680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sen A., O'Malley K., Wang Z., Raj G. V., Defranco D. B., Hammes S. R. (2010) Paxillin regulates androgen- and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells. J. Biol. Chem. 285, 28787–28795 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.