Background: The production of the hormone renin is transcriptionally regulated.
Results: The nuclear receptor COUP-TFII binds to the renin gene promoter and is necessary for the cAMP-induced renin gene expression.
Conclusion: COUP-TFII stimulates renin gene transcription.
Significance: The molecular mechanisms controlling the expression of renin are crucial for the understanding of its role in blood pressure regulation and nephrogenesis.
Keywords: Cell Signaling, Cyclic AMP (cAMP), Gene Expression, Gene Transcription, Transcription Factors, Transgenic Mice
Abstract
This study aimed to investigate the possible involvement of the orphan nuclear receptor chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) in the regulation of renin gene expression. COUP-TFII colocalized with renin in the juxtaglomerular cells of the kidney, which are the main source of renin in vivo. Protein-DNA binding studies demonstrated that COUP-TFII binds to an imperfect direct repeat COUP-TFII recognition sequence (termed hereafter proxDR) in the proximal renin promoter. Because cAMP signaling plays a central role in the control of the renin gene expression, we suggested that COUP-TFII may modulate this cAMP effect. Accordingly, knockdown of COUP-TFII in the clonal renin-producing cell lines As4.1 and Calu-6 diminished the stimulation of the renin mRNA expression by cAMP agonists. In addition, the mutation of the proxDR element in renin promoter reporter gene constructs abrogated the inducibility by cAMP. The proxDR sequence was found to be necessary for the function of a proximal renin promoter cAMP-response element (CRE). Knockdown of COUP-TFII or cAMP-binding protein (CREB), which is the archetypal transcription factor binding to CRE, decreased the basal renin gene expression. However, the deficiency of COUP-TFII did not further diminish the renin expression when CREB was knocked down. In agreement with the cell culture studies, mutant mice deficient in COUP-TFII have lower renin expression than their control strain. Altogether our data show that COUP-TFII is involved in the control of renin gene expression.
Introduction
The renin-angiotensin system is one of the key regulatory systems that control blood pressure. The renin-angiotensin system plays a central role in the regulation of arterial tone and NaCl excretion (1). The kidney hormone renin determines the activity of the renin-angiotensin system. Renin is produced in the juxtaglomerular (JG)2 cells, which represent a small cellular population in the afferent arteriole. The renin production is precisely regulated at the transcriptional level (1, 2). The renin gene is controlled by a variety of transcription factors that interact to produce a distinctive developmental and cell-specific expression pattern. Therefore, the proper expression of renin during the embryogenesis is decisive for the kidney development (nephrogenesis). COUP-TFII is an orphan nuclear receptor transcription factor (3, 4). It belongs to a nuclear receptor subfamily that includes also the closely related COUP-TFI (5–10). COUP-TFII is involved in the organogenesis of several organs and tissues (11, 12). In addition, it is also expressed throughout the kidney, starting in the early embryonic stages (13). However, nothing is known about the possible role of COUP-TFII in the regulation of the kidney functions in the adult. Interestingly, a putative COUP-TFII recognition sequence was identified in the proximal promoter of the human renin gene (14). DNase I footprint assays demonstrated protein binding to this motif, but it remained elusive whether COUP-TFII is part of the bound complex (15). Because the proximal promoter is decisive for the control of renin transcription, we examined whether COUP-TFII is involved in the regulation of renin gene expression. Such a function of COUP-TFII is not unlikely, because a row of nuclear receptors is already known to regulate renin gene transcription (1, 2). We used for the experiments As4.1 and Calu-6 cells, which are clonal cell lines with endogenous renin production, as well as mice deficient in COUP-TFII (11, 16, 17). We found that COUP-TFII is expressed in the renin-producing cells and that it binds to the proximal renin promoter. We also show that COUP-TFII is involved in the regulation of the renin gene by cAMP signaling, which is considered to be the principal intracellular cascade regulating the synthesis of renin.
EXPERIMENTAL PROCEDURES
Cell Culture
The clonal renin-producing cell lines As4.1 and Calu-6 were already described by our group (18–24). Briefly, the As4.1 mouse cells (ATCC-CRL-2193) were propagated in Dulbecco's modified essential medium supplemented with 10% fetal calf serum, l-glutamine and sodium pyruvate, 100 units/ml penicillin, and 100 μg/ml streptomycin and incubated at 37 °C in a humidified atmosphere containing 10% CO2. The human Calu-6 cells (ATCC-HTB-56) were cultured in Eagle's minimal essential medium supplemented with 5% fetal bovine serum, sodium pyruvate, 100 units/ml penicillin, 100 μg/ml streptomycin, and 1% nonessential amino acids at 37 °C in a humidified atmosphere containing 5% CO2. The cells were treated with cAMP agonists overnight (16–20 h) where indicated. Forskolin was applied at 5 μm, the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine at 100 μm, and pituitary adenylate cyclase-activating polypeptide at 300 nm.
Western Blotting
Protein extracts were isolated from As4.1 and Calu-6 cells, and Western blotting was performed as described (20, 22, 24). Confluent cells grown in 25-cm2 flasks were lysed in protein lysis buffer (10 mm Tris, 1% SDS, 1% Nonidet P-40, 5 mm Pefabloc), and the protein concentration was measured. The samples were boiled in Laemmli sample buffer for 5 min and applied onto 10% SDS-polyacrylamide gels. The proteins were separated by electrophoresis and then transferred onto nitrocellulose membranes (Bio-Rad) in transfer buffer (48 mm Tris, 39 mm glycine, 0.037% SDS, 20% methanol). After blocking, anti-COUP-TFII, CREB, or anti-β-actin primary antibody (Perseus Proteomics, Cell Signaling, and Sigma, respectively) was applied. Bound antibody was detected with horseradish peroxidase-conjugated secondary antibody (DiaNova) and visualized by enhanced chemiluminescence.
Renal tissues were homogenized and lysed in radioimmune precipitation assay buffer (150 mm NaCl, 10 mm Tris-Cl (pH 7.5), 0.1% SDS, 0.1% Triton X-100, 1% deoxycholate, and 5 mm EDTA). Protein content of the lysates was determined by the BCA protein assay system (Pierce). The lysates were subjected to SDS-PAGE and blotted onto nitrocellulose membranes. Immunoblotting was performed using goat anti-mouse renin-1 antibody (R&D Systems) or mouse anti-β-actin antibody (Sigma). The signals were visualized by using the ECL Plus Western blotting detection system (Amersham Biosciences). Densitometry was done using ImageJ (National Institutes of Health).
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear proteins were isolated from As4.1 and Calu-6 cells following the standard protocol of our laboratory (20, 21, 23). The nuclear protein extracts were probed with a mouse or human renin COUP-TFII recognition sequence (designated proxDR) in EMSAs. To this end, we used the GelShiftTM chemiluminescent assay kit from Active Motif, following the instructions of the manufacturer. Double-stranded 3′-biotinylated oligonucleotides were used as probes (sense strand (human), 5′-ccaggggtcacagggccaagc-3′; sense strand (mouse), 5′-ctagaggttgcggggccaggc-3′ (the proxDR sequences are underlined)). The COUP-TFII antibody used for the supershifts was from Perseus Proteomics.
Chromatin Immunoprecipitation (ChIP)
DNA-protein complexes isolated from As4.1 or Calu-6 cells were analyzed in ChIP assays using the ChIP-IT kit from Active Motif (19). In brief, DNA-protein cross-linked complexes were precleared with Protein G-agarose beads (input samples) or were further precipitated with anti-COUP-TFII antibody (Perseus Proteomics). After reversal of the cross-linking and digestion of RNA and proteins, DNA was eluted and PCR-amplified for 36 cycles. The primers used for the amplification of the mouse and the human renin promoter fragment containing the COUP-TFII-binding site proxDR were as follows: 5′-ggtagggtctgagttagag-3′ (sense) and 5′-caggcatgggggtgtgggaag-3′ (antisense) (amplicon length 176 bp) and 5′-ggttgggtctgggctaggg-3′ (sense) and 5′-ggaggaaggacagctgtggg-3′ (antisense) (amplicon length 183 bp), respectively. Mouse peroxisome proliferator-activated receptor γ (PPARγ) exon 2 fragment (166 bp) and human renin exon 10 fragment (116 bp) were amplified as negative controls in As4.1 and in Calu-6 cells with the following primers: 5′-gtggatctctccgtaatg-3′ (sense) and 5′-ggtactcttgaagtttcaggtc-3′ (antisense) and 5′-gctgtgcacactggccat-3′ (sense) and 5′-ggttgttacgccgatcaaactc-3′ (antisense), respectively.
Plasmids
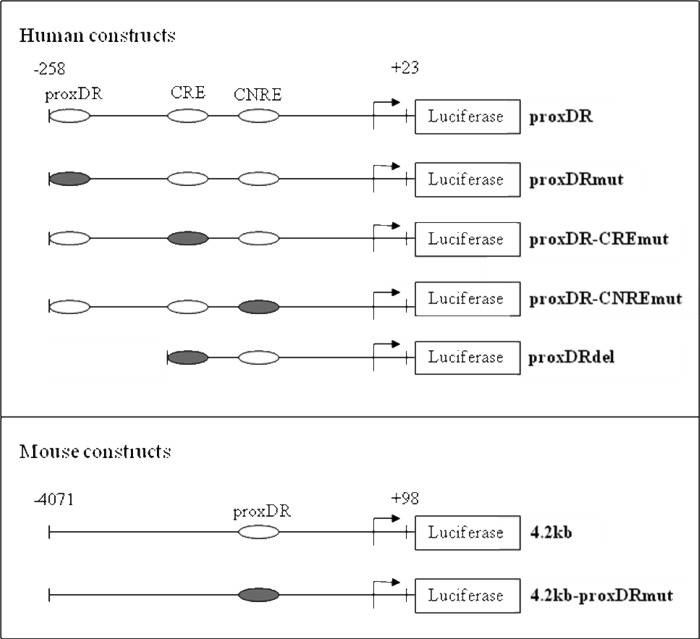
Plasmid constructs used in the cell culture experiments are shown in Table 1. Construct proxDR represents pGL3 vector (Promega) encoding firefly luciferase under the control of the human renin promoter (bases −258 to +23 relative to the transcription start site). Construct proxDRdel contained bases −228 to +23 of the human renin promoter. Constructs proxDR-CREmut and proxDR-CNREmut were obtained from construct proxDR after mutation of CRE (at −226 to −219 bp) and CNRE (at −135 to −107 bp), respectively. In construct 4.2kb, the firefly luciferase is under the control of bases −4071 to +98 of the mouse renin promoter. The COUP-TFII recognition sequence proxDR in the human (positions −255 to −242 bp) and in the mouse (positions −717 to −704 bp) renin promoter was inactivated by mutation to yield constructs proxDRmut and 4.2kb-proxDRmut, respectively.
TABLE 1.
Plasmids used for the experiments
The cis-regulatory elements are illustrated with ellipses. A filled ellipse indicates a mutation.
Transient Transfection and Luciferase Assay
Firefly luciferase reporter vector (0.2 μg) and 0.01 μg of Renilla luciferase (pRL-0 vector from Promega) were transfected with Fugene 6 (Roche Applied Science). Twenty-four h after transfection, the medium was replaced with a fresh medium with the active substances indicated. Cells were then harvested after an overnight incubation (16–20 h). Relative luciferase activity (RLA) was calculated as firefly to Renilla luciferase activity.
RNA Isolation, Reverse Transcription, and Quantitative LightCycler PCR
Total RNA was isolated with Qiagen RNeasy spin columns. A standard protocol for reverse transcription was used. Real-time PCR (Roche Applied Science) was performed as described above. The primers used are shown in Table 2.
TABLE 2.
Primers used in real-time PCR
The sequences are in 5′ to 3′ orientation.
| Gene | Sense primer | Antisense primer | Amplicon |
|---|---|---|---|
| bp | |||
| Human renin | atgaagggggtgtctgtggggtc | ggatatccatggcgtggatggcc | 304 |
| Human β-actin | cttctacaatgagctgcgtgtgg | gaggatcttcatgaggtagtca | 313 |
| Human COUP-TFI | ttcgtccgtttggtaggtaaaa | ggaagcgcccaaggtctag | 120 |
| Human COUP-TFII | ttcgtccgtttggtaggtaaaa | tctgtttcactcccccttcttattt | 131 |
| Human CREB | atgaccatggaatctggagc | gctgtactagagttacggtgg | 162 |
| Mouse renin | atgaagggggtgtctgtggggtc | atgtcggggagggtgggcacctg | 193 |
| Mouse L32 | ttaagcgaaactggcggaaac | ttgttgctcccataaccgatg | 100 |
RNA Interference
Ready-to-use double-stranded siRNAs targeting mouse or human COUP-TFII or human CREB were synthesized by Dharmacon (ON-TARGETplus siRNA SMARTpool L-063437-01, L-003422-00, and L-003619-00, respectively). Fifty nmol of double-stranded nontargeting siControl siRNA (Dharmacon; used as control) or COUP-TFII-specific (siCOUP-TFII) or CREB-specific (siCREB) siRNA were transfected into As4.1 cells using Dharmafect 3 (Dharmacon) or into Calu-6 cells using Dharmafect 2 (Dharmacon) according to the manufacturer's protocol. The cells were harvested 72 h after transfection.
Immunohistochemistry
Kidneys from adult mice were fixed by 4% paraformaldehyde and embedded in paraffin. Immunolabeling was performed on 5-μm sections incubated with chicken anti-renin IgG (diluted 1:200; Davids Biotechnologie), mouse anti-COUP-TFII IgG (diluted 1:1000; Perseus Proteomics), and goat anti-SM22 IgG (diluted 1:100; Abcam ab10135). Alternatively, tissues were incubated with mouse anti-COUP-TFII antibody or goat anti-mouse renin-1 antibody (R&D Systems). The Tyramide Signal Amplification system (Invitrogen) was applied for visualization according to the manufacturer's instructions. The images were taken by a fluorescence microscope (Zeiss) or confocal microscope (Olympus).
COUP-TFII Knock-out Mice
Mice were maintained in accordance with the National Institutes of Health standards for the care and use of experimental animals. All procedures for animal studies were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. ROSA26CREERT2; COUP-TFIIFlox/Flox mice (CreERT2CIIFF) were described previously (11). Control mice (wtCIIFF) were Cre-negative and homozygous for the floxed COUP-TFII allele. The mice were back-crossed to C57BL/6 background for at least four generations, and the comparisons were made between littermates. To induce COUP-TFII gene deletion, 2-month-old mice were intraperitoneally injected with 1 mg of tamoxifen (Sigma) for 3 consecutive days. The mice were subjected to experiments 1 month after injection.
Statistics
The results from at least two independent cell culture experiments were taken for the statistical analysis of the data. In each experiment, at least 3 samples/condition (control, treatment with different chemicals or siRNAs, or combinations of them) were assigned. Thus, the number of the cell culture samples in the groups subjected to comparison was at least 6 (n ≥ 6). Levels of significance were determined by analysis of variance and Student's unpaired t test. p < 0.05 relative to the corresponding comparison group was considered significant.
RESULTS
COUP-TFII Is Expressed in Renin-producing Cells and Binds to the Proximal Renin Promoter
COUP-TFII immunoreactivity was observed in the kidney at the vascular pole of the glomerulus, where the renin-producing JG cells are also located (13). Costaining with renin-specific antibody demonstrated that COUP-TFII is expressed in the JG cells of the mouse kidney (see supplemental Fig. S1). In addition, we found COUP-TFII immunoreactivity in the clonal renin-producing cells lines As4.1 and Calu-6 (see supplemental Fig. S2). These findings demonstrated that COUP-TFII is expressed in renin-producing cells.
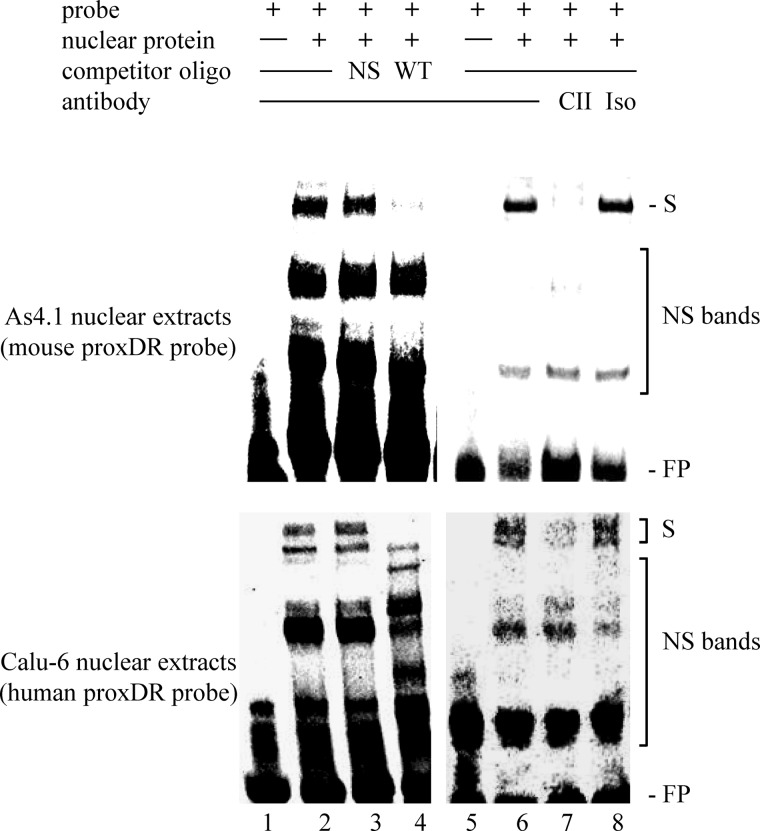
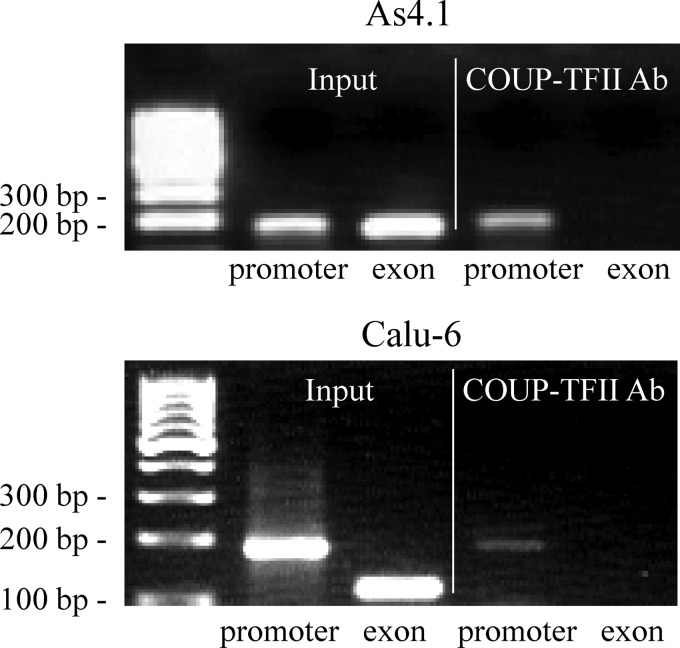
An imperfect direct repeat (DR) of the canonical nuclear receptor DNA-binding sequence 5′-(A/G)GGTCA-3′ (termed hormone response element (HRE)) with high similarity to the COUP-TFII consensus motif was identified in the proximal human renin promoter (14). This element (termed proxDR) is evolutionary conserved because the mouse renin proxDR is highly homologous to the human sequence (Table 3). We performed EMSA and ChIP with nuclear extracts isolated from the renin-producing cell lines As4.1 and Calu-6 (mouse and human, respectively) to study whether COUP-TFII binds to proxDR. The mouse proxDR sequence binds a single specific protein complex as detected by EMSA (Fig. 1, top, lane 2). This protein complex contains COUP-TFII because it disappears when a COUP-TFII-specific antibody is added (Fig. 1, top, lane 7). Interestingly, the human proxDR sequence binds two specific protein complexes as detected by EMSA (Fig. 1, bottom, lane 2). Both complexes appeared to contain COUP-TFII, because the addition of COUP-TFII antibody strongly interfered with their binding to the human proxDR probe (Fig. 1, bottom, lane 7). The interaction of the COUP-TFII antibody with the proxDR-bound complexes was specific in both cell types, because the addition of an unrelated antibody (negative control; Fig. 1, lane 8) did not affect the protein-DNA interaction. The binding of COUP-TFII to an ∼200-bp native DNA region containing proxDR in either cell type was confirmed by ChIP (Fig. 2). COUP-TFII did not bind to an unrelated exon sequence that served as negative control (Fig. 2). This confirmed the specificity of the COUP-TFII interaction with the proximal renin promoter.
TABLE 3.
Comparison of canonical, human renin, and mouse renin COUP-TFII binding sequences
| COUP-TFII binding sequences | |
|---|---|
| Canonical | GTGTGA aAGGTCA |
| Human renin | GGGTCAcaGGGCCA |
| Mouse renin | AGGTTGcgGGGCCA |
FIGURE 1.
COUP-TFII binds to the COUP-TFII recognition sequence proxDR in the renin promoter. Nuclear extracts from As4.1 (top) or Calu-6 (bottom) cells were probed with mouse (top) or human (bottom) proxDR oligonucleotide in EMSAs. The samples in lanes 1 and 5 contained only the corresponding labeled probe. Nonspecific unlabeled oligonucleotide (NS) was added in 100-fold molar excess to the samples in lane 3 to demonstrate the specificity of the binding. Non-labeled probe (WT) was added in 100-fold molar excess to the samples in lane 4 for competition analysis. Anti-COUP-TFII (CII) antibody or isotype control (Iso) antibody (2 μl of each) was added to the samples in lanes 7 and 8, respectively. S, shifted protein complexes; NS bands, nonspecific bands; FP, free probe.
FIGURE 2.
COUP-TFII binds to the proximal renin promoter in its native context. Cross-linked nuclear extracts isolated from As4.1 (top) or Calu-6 cells (bottom) were used in ChIP with COUP-TFII antibody (COUP-TFII Ab) followed by isolation of the precipitated DNA. DNA isolated from non-precipitated samples was used as positive control (Input). The input and the COUP-TFII antibody-precipitated samples were subjected to PCR with a primer pair amplifying a proximal renin promoter fragment containing the COUP-TFII recognition sequence proxDR (promoter) or with a primer pair amplifying a non-related exon sequence that served as a negative control (exon).
COUP-TFII and the proxDR Sequence Are Involved in the cAMP-dependent Stimulation of Renin Gene Expression
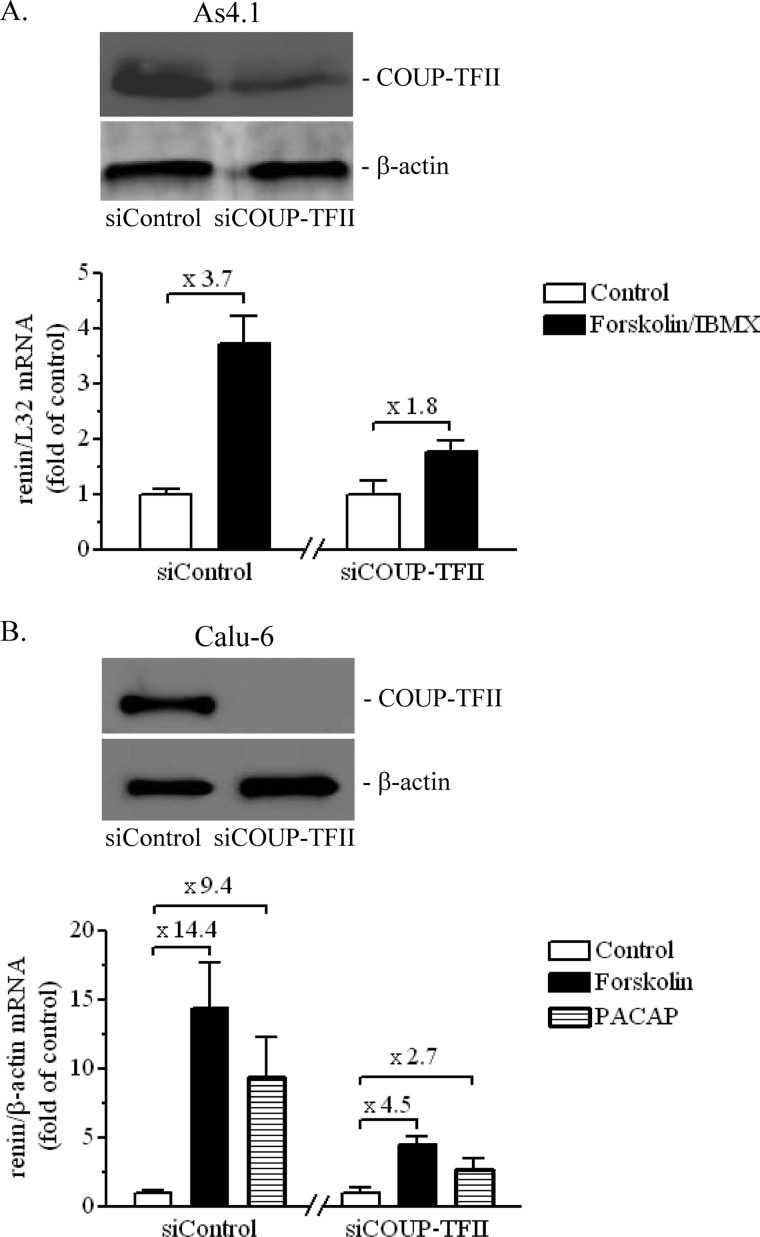
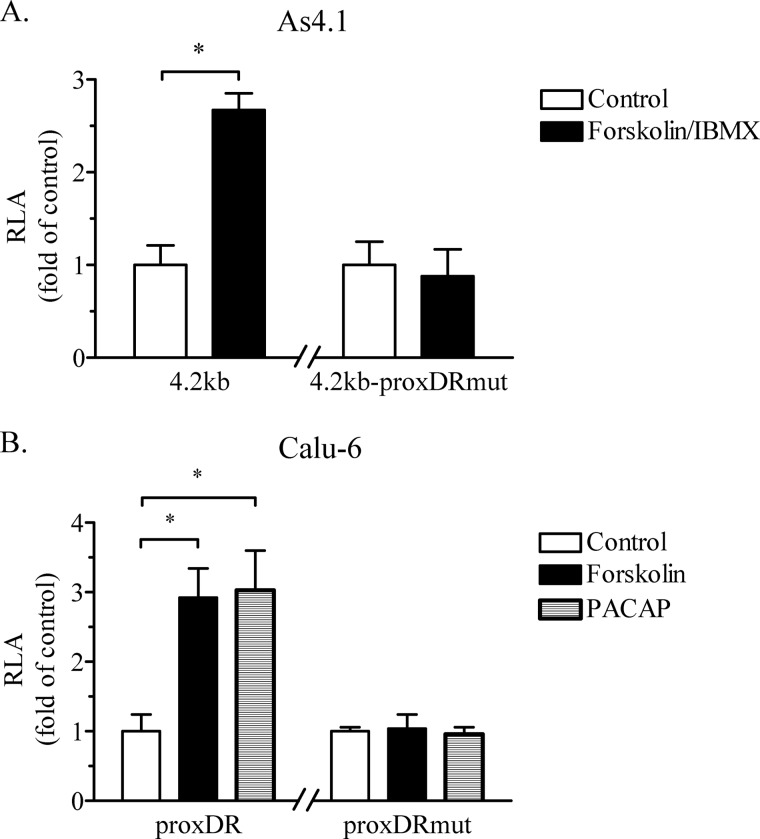
The protein-DNA binding experiments provided evidence suggesting that COUP-TFII is involved in the transcriptional control of the renin gene. However, COUP-TFII is an orphan nuclear receptor, and therefore little is known about how its transcriptional activity is regulated. The signaling cascade downstream of the second messenger cAMP is regarded as the most important intracellular pathway that controls the renin synthesis (1). Therefore, we examined the possible role of COUP-TFII and the proxDR sequence in the cAMP-dependent stimulation of the renin gene expression. To this end, the adenylate cyclase activator forskolin, in combination with the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine, was applied to the As4.1 cells, whereas forskolin or the neuropeptide pituitary adenylate cyclase-activating polypeptide was applied to Calu-6 cells. These maneuvers are well known to increase the intracellular cAMP levels and to stimulate the renin gene expression (18, 19, 25). Knockdown of COUP-TFII by siRNA attenuated the cAMP-induced increase of the renin mRNA levels in As4.1 and Calu-6 cells (Fig. 3). To examine the role of the COUP-TFII-binding site proxDR in the cAMP-dependent control of renin gene transcription, we used mouse and human renin promoter constructs in reporter gene studies. The mutation of the proxDR site in the mouse and the human renin promoter abolished their cAMP-induced transactivation (Fig. 4). We concluded that COUP-TFII and its binding sequence proxDR are involved in the regulation of renin gene expression by cAMP.
FIGURE 3.
COUP-TFII is necessary for the cAMP-induced increase of the renin mRNA expression in As4.1 (A) and Calu-6 cells (B). COUP-TFII was knocked down with sequence-specific siRNA (siCOUP-TFII). Control cells were transfected with nontargeting siRNA (siControl). The top panels in A and B show the efficacy of the knockdown in As4.1 and Calu-6 cells, respectively. Protein extracts isolated from the corresponding cell lines were probed in Western blots with COUP-TFII and β-actin (internal control) antibodies. The bottom panels in A and B show the effect of the COUP-TFII deficiency on the cAMP-stimulated renin mRNA expression in As4.1 and Calu-6 cells, respectively. Renin, human β-actin (internal control), and mouse ribosomal L32 (internal control) mRNA levels were quantified by real-time PCR. The numbers above the columns indicate the -fold stimulation relative to control. IBMX, 3-isobutyl-1-methylxanthine. The data shown are means ± S.E. (error bars).
FIGURE 4.
The COUP-TFII-binding site proxDR in the proximal renin promoter is necessary for the cAMP-induced transactivation of the renin gene in As4.1 (A) and Calu-6 cells (B). Mutation of the COUP-TFII recognition sequence proxDR in a 4.2-kb mouse promoter (construct 4.2kb-proxDRmut; A) or in a 258-bp human proximal promoter proxDR (construct proxDRmut; B) abrogates the cAMP inducibility of the renin promoter. IBMX, 3-isobutyl-1-methylxanthine. The data shown are means ± S.E. (error bars). *, p < 0.05.
Role of COUP-TFII and the proxDR Sequence in cAMP-dependent Control of Renin Gene Transcription
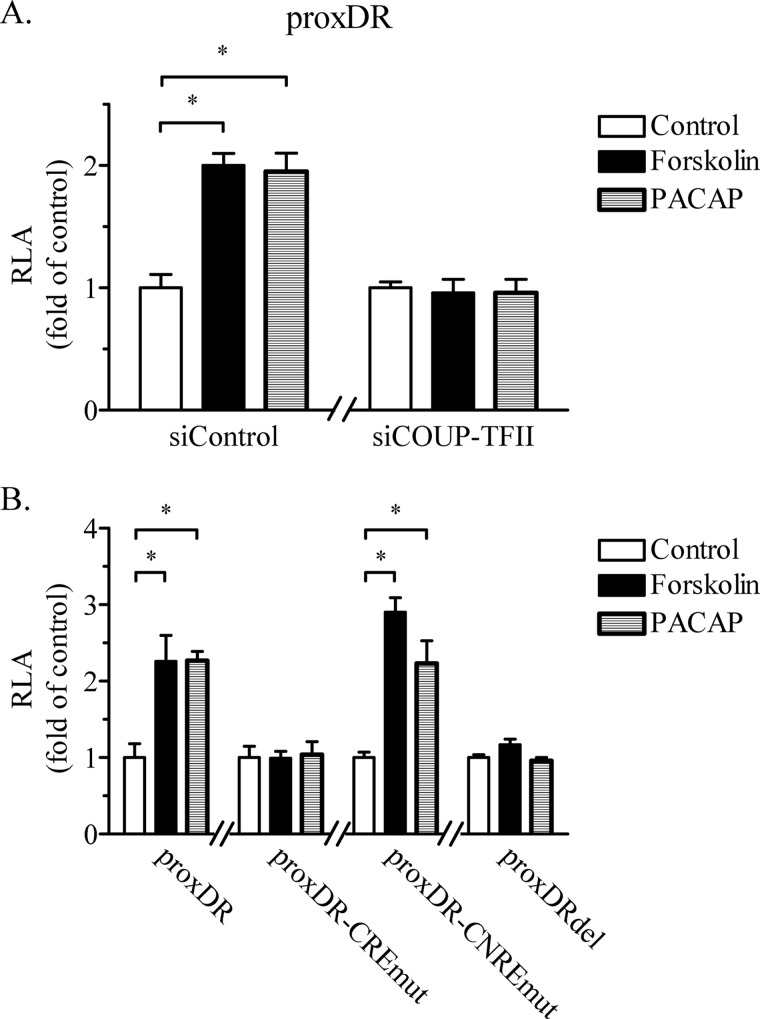
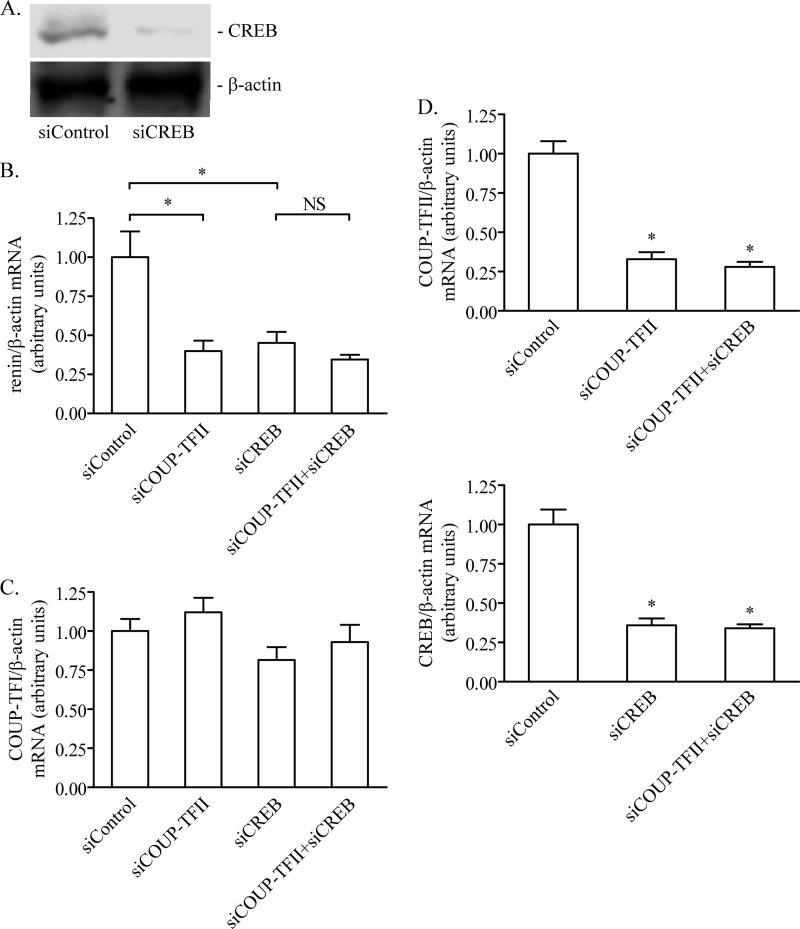
We were further interested in studying the molecular mechanisms of the interaction of COUP-TFII with cAMP signaling to the renin gene promoter. Using the Calu-6 cells as a model, we found that the knockdown of COUP-TFII abolished the stimulatory effect of cAMP on the transcriptional activity of the proximal human renin promoter containing the proxDR sequence (Fig. 5A). Because we had already seen that the mutation of the COUP-TFII-binding proxDR site abrogated the stimulation of the renin transcription by cAMP (Fig. 4), we assumed that the proxDR sequence is necessary for the function of the cAMP target sequences within the renin gene. There are two cAMP target sites in the proximal renin promoter construct proxDR: CRE at −226 to −219 bp and cAMP and CNRE at −135 to −107 bp (26, 27). Mutation of CRE eliminated, whereas mutation of CNRE did not affect, the stimulation of the renin promoter activity by the cAMP agonists (Fig. 5B). Thus, the proximal CRE sequence seems to mediate the stimulation of the renin promoter activity by cAMP. Importantly, the deletion of proxDR to yield a shorter renin promoter reporter construct (proxDRdel) resulted in a loss of the cAMP-induced stimulation, demonstrating that the proximal CRE alone is not sufficient to mediate the effect of cAMP on renin gene transcription (Fig. 5B). These results indicated that the COUP-TFII-binding sequence proxDR cooperates with the proximal promoter CRE site in the cAMP-dependent transactivation of the renin gene. CREB is the archetypal transcription factor targeted by cAMP. Earlier experiments with Calu-6 cells have demonstrated that CREB binds to the renin proximal promoter CRE and is necessary for the renin gene expression (27). In agreement with these previous data, we found that selective deficiency of either COUP-TFII or CREB decreased the renin gene expression (Fig. 6, B and C). The combined deficiency of COUP-TFII and CREB did not further decrease the renin gene expression as compared with the situation when a single transcription factor was knocked down (Fig. 6B). Moreover, the knockdown rate of COUP-TFII as well as of CREB was comparable when only one of them or when simultaneously both were targeted by sequence-specific siRNAs (Fig. 6D). Thus, COUP-TFII and CREB appear to share a common mechanism to regulate renin gene expression. Altogether, the last data strongly suggest that COUP-TFII bound to proxDR synergizes with CREB bound to CRE in the cAMP-dependent control of the renin gene.
FIGURE 5.
COUP-TFII and the proxDR sequence are necessary for the cAMP-induced expression of the renin gene mediated by a CRE site in the proximal promoter. A, COUP-TFII is necessary for the cAMP-induced renin gene transcription in Calu-6 cells. COUP-TFII was knocked down with sequence-specific siRNA (siCOUP-TFII). Control cells were transfected with nontargeting siRNA (siControl). Calu-6 cells were transfected with the 258-bp human proximal promoter construct proxDR containing the COUP-TFII binding site, and the effect of cAMP agonists on its transcriptional activity was tested. B, Calu-6 cells were transfected with the proxDR construct, with construct proxDR-CREmut carrying the mutated cAMP target site CRE, with construct proxDR-CNREmut carrying the mutated cAMP target site CNRE, or with construct proxDRdel in which proxDR was deleted and the effect of cAMP agonists on the transcriptional activity was tested. The data shown are means ± S.E. (error bars). *, p < 0.05.
FIGURE 6.
COUP-TFII cooperates with CREB in the control of renin gene expression. Calu-6 cells were transfected with nontargeting siRNA as control (siControl) or with COUP-TFII sequence-specific siRNA (siCOUP-TFII), CREB sequence-specific siRNA (siCREB), or a combination of COUP-TFII and CREB siRNAs (siCOUP-TFII+siCREB). A, efficacy of the CREB knockdown. Protein extracts were probed in Western blots with CREB and β-actin (internal control) antibodies. See Fig. 3B for the efficacy of the COUP-TFII knockdown. B, effect of the deficiency of COUP-TFII, CREB, or both on renin gene expression. Renin and human β-actin (internal control) mRNA levels were quantified by real-time PCR. C, specificity of the knockdown. COUP-TFI and human β-actin (internal control) mRNA levels were quantified by real-time PCR. D, top, COUP-TFII expression is similarly diminished when COUP-TFII alone or COUP-TFII and CREB are knocked down. Bottom, CREB expression is similarly diminished when CREB alone or COUP-TFII and CREB are knocked down. COUP-TFII, CREB, and human β-actin (internal control) mRNA levels were quantified by real-time PCR. The data shown are means ± S.E. (error bars). *, p < 0.05. NS, not significant.
COUP-TFII Is Involved in the Control of Renin Production in Vivo
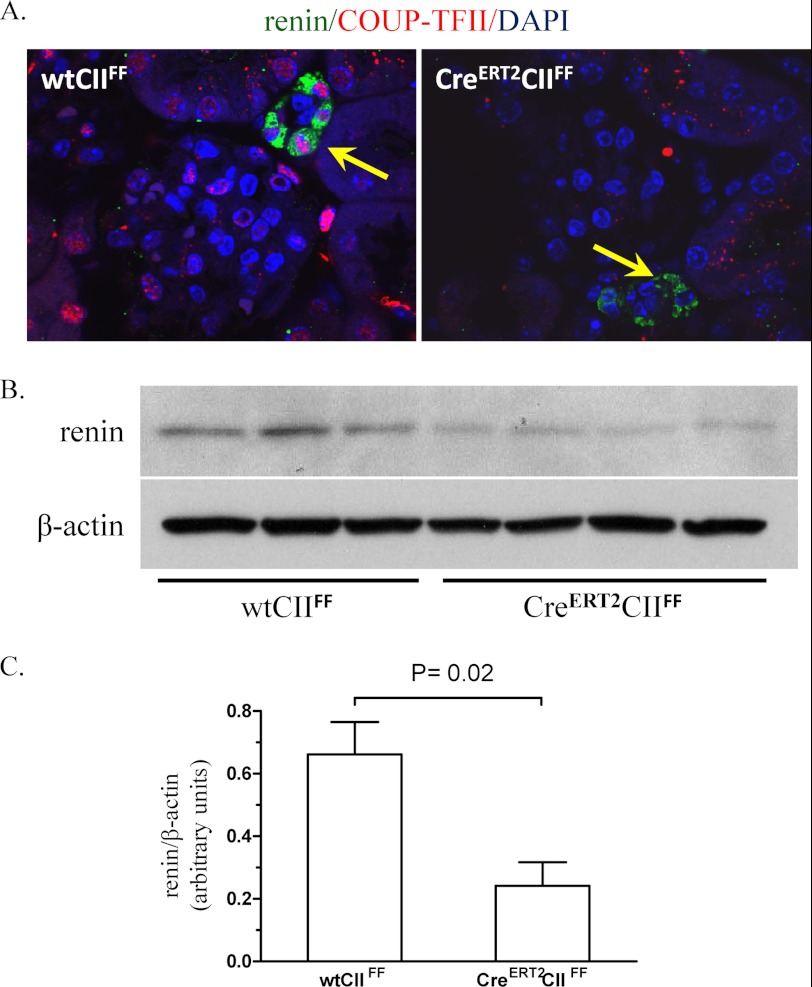
To study the relevance of COUP-TFII for the control of the renin gene expression in vivo, we generated inducible COUP-TFII knock-out mice. The deletion of COUP-TFII was proven to be efficient in the kidneys of the induced adult animals (Fig. 7A). The COUP-TFII deficiency was accompanied by decreased renal expression of renin (Fig. 7, B and C). This finding fits to the paradigm that cAMP is decisive for renin production in vivo (1, 28). Furthermore, it is in agreement with our cell culture data, which have shown a synergism between COUP-TFII and the cAMP signaling in the control of renin gene expression. Thus, the in vivo data confirmed that COUP-TFII is involved in the control of renin expression.
FIGURE 7.
COUP-TFII knock-out mice have decreased renin expression. A, efficiency of the knockout. Shown are renin (green), COUP-TFII (red), and DAPI (nuclear marker) (blue) co-immunostaining in control (wtCIIFF) and COUP-TFII knock-out (CreERT2CIIFF) mouse kidneys. Arrows, renin immunoreactivity. B and C, renin expression is decreased in the COUP-TFII knock-out mice (CreERT2CIIFF, n = 3) compared with the control mice (wtCIIFF, n = 4). Renin and β-actin (internal control) in mouse kidneys are detected by immunoblotting (B) and quantified by densitometry (C). The data shown are means ± S.E. (error bars).
DISCUSSION
The main purpose of this study was to examine the role of the orphan nuclear receptor COUP-TFII in the regulation of the renin gene expression. We used two different renin-producing cell types and a mutant mouse line with deletion of COUP-TFII in the adult. The combined data clearly demonstrated that COUP-TFII is involved in the transcriptional control of the renin gene. We first detected COUP-TFII in the renin-producing JG cells of the mouse kidney. COUP-TFII was also expressed in the mouse As4.1 and the human Calu-6 cell lines. Thus, COUP-TFII colocalizes with renin species-independently, and in such a way, it could be essential for the regulation of the renin production in humans as well. We next provided evidence that COUP-TFII binds to the mouse and the human proximal promoter region containing a sequence (termed proxDR) that is homologous to the consensus COUP-TFII-binding site. Notably, COUP-TFII bound to the proxDR element as a part of a single protein complex in EMSA with nuclear extracts isolated from As4.1 cells and as a part of two protein complexes with nuclear extracts isolated from Calu-6 cells. It remains to be clarified whether this discrepant binding pattern is species-, sequence-, or cell-specific. In any case, the further data (Figs. 3 and 4) imply that this discrepancy has no significant functional impact on the role of COUP-TFII in the control of renin gene transcription. Similarly to many other nuclear receptors, COUP-TFII binds to a DR of the canonical HRE (5′-(A/G)GGTCA-3′) (29, 30). The originally identified COUP-TF-binding site in the chicken ovalbumin promoter is an imperfect DR with a 1-bp spacer (31). Mouse and human renin proxDRs are also imperfect repeats, but they are separated by 2 bp (Table 3). This is in agreement with earlier reports demonstrating that COUP-TFs bind to DRs with diverse spacings (30). Mouse and human renin proxDRs are over 70% homologous with highest degree of discrepancy in the 3′-flanking region of the first repeat and completely conserved second repeat (Table 3). Whereas the human proxDR element is located at −255 to −242 bp, the mouse proxDR is located at −717 to −704 bp relative to the transcription start site of the renin gene. This positional discrepancy is due to a 476-bp insertion at −68 bp in the mouse renin gene (32). This mouse-specific fragment is flanked by 14-bp imperfect repeats. Therefore, it was suggested that the 476-bp sequence represents a mobile element that has arisen since the divergence of mouse and rat renin genes (32). A conserved DR of the HRE sequence binding Ear2/retinoic acid receptor/retinoid X receptor/PPARγ exists also in the distal (kidney) enhancer of the renin gene (20, 21, 33, 34). Interestingly, COUP-TFII was identified as a yeast one-hybrid screen hit with the enhancer HRE and As4.1 cell nuclear extracts (33). Recently, the interaction of COUP-TFII with the renin enhancer HRE repeat was confirmed (35). COUP-TFII is an orphan nuclear receptor that could regulate the expression of its target genes either positively or negatively (29). In addition, it modulates the transactivating capacity of other transcription factors. Although nuclear receptors, such as retinoic acid receptors, retinoid X receptors, vitamin D receptor, liver X receptor α, or PPARγ, are known to be involved in the transcriptional control of the renin gene (20, 34, 36, 37), we did not look for their possible interactions with COUP-TFII. Instead, we investigated the role of COUP-TFII in the cAMP-dependent regulation of renin expression. In vitro and in vivo studies consistently demonstrated that cAMP signaling is one of the most important intracellular cascades regulating the renin gene expression (25, 28, 38–40). The combined data from As4.1 and Calu-6 cells showed that COUP-TFII and its binding sequence proxDR are necessary for the cAMP-dependent increase of the renin mRNA levels and promoter activity. Although the COUP-TFII deficiency only attenuated the mRNA increase, the mutation of proxDR completely abrogated the transactivation of the promoter. These findings are in agreement with the knowledge that cAMP induces the renin gene transcription and at the same time stabilizes the renin mRNA (25, 41, 42). Therefore, the residual cAMP-dependent increase of the renin mRNA levels after COUP-TFII knockdown is most probably due to a prolonged half-life of the mRNA.
We used the Calu-6 cells and the proximal human renin promoter to examine the molecular mechanisms of the involvement of COUP-TFII in the cAMP-mediated transactivation of the renin gene. The renin production is better inducible by cAMP in the Calu-6 cells than in the As4.1 cells (25, 41). Furthermore, the proximal human renin promoter containing the proxDR site is shorter than the orthologous mouse sequence because of the 476-bp insertion (see above), thus allowing us to precisely map the cis-acting elements. We found that COUP-TFII and proxDR play a role in the cAMP-induced regulation of the renin gene. proxDR is necessary for the cAMP-dependent control of a proximal CRE at −225 to −219 which is known to bind CREB (27). Moreover, COUP-TFII and CREB seem to regulate the renin gene expression through a common transcriptional mechanism. In accordance with our data, it has already been reported that the HRE repeat in the distal renin enhancer, which is another COUP-TFII binding site, cooperates with a distal renin enhancer CRE site in the cAMP-dependent stimulation of the renin promoter activity (25). An activating effect of COUP-TF in the context of several transcription factor binding sites, including CRE, is also known for the phosphoenolpyruvate carboxykinase gene (43). Based on our data, a model of the cAMP-dependent regulation of the renin gene transcription could be proposed in which the transcription factors bound to proxDR and proximal CRE (COUP-TFII and CREB, respectively) cooperate within one transcription initiation protein complex (enhaceosome) at the proximal renin promoter. Interestingly, the proximal renin CRE was not active in a shorter promoter fragment lacking proxDR, whereas a consensus CRE (which differs from the proximal renin CRE by only two swapped bases) was still functional in the same construct (18). These findings imply that the enhanceosome complex built at the proximal renin promoter upon cAMP-induced transactivation is highly specific. At last, we also provided evidence that the effect of COUP-TFII on the renin expression is relevant in vivo. Transgenic mouse strains with defects in cAMP signaling to the renin gene are, as a rule, renin-deficient (28, 40, 44). Because, on the other hand, we found that COUP-TFII is necessary for the cAMP-dependent control of the renin gene expression in vitro, it could be predicted that mice deficient in COUP-TFII should have reduced renin production. Consistent with this assumption, the knockout of COUP-TFII in mice resulted in decreased renin expression. In agreement with the cell culture data, the COUP-TFII knock-out mice were not completely devoid of renin, because the cAMP signaling also stabilizes the renin mRNA independently on its transcriptional effect (also see above for discussion). Surprisingly, there was no significant difference in the plasma renin concentration between control and COUP-TFII-deficient mice (data not shown). It is noteworthy that the renin expression and plasma renin concentration do not obligatorily change in parallel in genetically engineered mice (45, 46).3 One possible explanation for this phenomenon is that it is an artifact of the genomic manipulation. One further scenario is that the generalized COUP-TFII deficiency may modulate either the processing of the renin protein or the mechanisms of renin release. We are currently working on this issue by generating mice with inducible COUP-TFII deletion restricted to the renin-producing JG cells of the kidney.
The overall data presented here show that COUP-TFII is essentially involved in the molecular mechanisms of the stimulation of renin gene expression and that this effect is relevant in vivo. Because the COUP-TFII expression, similarly to the renin expression, is tightly regulated during embryogenesis, we provide the first evidence for a novel nuclear receptor transcription factor, which could be important for the developmental control of the renin gene.
Supplementary Material
Acknowledgments
We thank Dr. Anelia Todorova and Anna M'Bangui for competent technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants DK45641 (to M. J. T.), HL76448 (to S. Y. T.), and DK59820 and DK62434 (to M. J. T. and S. Y. T.). This work was also supported by Deutsche Forschungsgemeinschaft Grants SFB 699/B1 and TO 679/1-1 (to V. T. T.) and MO 1695/4-1 (to H. M.).

This article contains supplemental Figs. S1 and S2.
V. Todorov, unpublished observations.
- JG
- juxtaglomerular
- COUP-TF
- chicken ovalbumin upstream promoter transcription factor
- DR
- direct repeat
- CRE
- cAMP-response element
- CNRE
- cAMP and overlapping negative response element
- RLA
- relative luciferase activity
- HRE
- hormone response element
- CREB
- cAMP-response element-binding protein
- PPAR
- peroxisome proliferator-activated receptor.
REFERENCES
- 1. Castrop H., Höcherl K., Kurtz A., Schweda F., Todorov V., Wagner C. (2010) Physiology of kidney renin. Physiol. Rev. 90, 607–673 [DOI] [PubMed] [Google Scholar]
- 2. Pan L., Gross K. W. (2005) Transcriptional regulation of renin. An update. Hypertension 45, 3–8 [DOI] [PubMed] [Google Scholar]
- 3. Ladias J. A., Karathanasis S. K. (1991) Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science 251, 561–565 [DOI] [PubMed] [Google Scholar]
- 4. Wang L. H., Ing N. H., Tsai S. Y., O'Malley B. W., Tsai M. J. (1991) The COUP-TFs compose a family of functionally related transcription factors. Gene Expr. 1, 207–216 [PMC free article] [PubMed] [Google Scholar]
- 5. Li L., Xie X., Qin J., Jeha G. S., Saha P. K., Yan J., Haueter C. M., Chan L., Tsai S. Y., Tsai M. J. (2009) The nuclear orphan receptor COUP-TFII plays an essential role in adipogenesis, glucose homeostasis, and energy metabolism. Cell Metab. 9, 77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin F. J., Chen X., Qin J., Hong Y. K., Tsai M. J., Tsai S. Y. (2010) Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J. Clin. Invest. 120, 1694–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin F. J., Qin J., Tang K., Tsai S. Y., Tsai M. J. (2011) Coup d'etat. An orphan takes control. Endocr. Rev. 32, 404–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takamoto N., You L. R., Moses K., Chiang C., Zimmer W. E., Schwartz R. J., DeMayo F. J., Tsai M. J., Tsai S. Y. (2005) COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development 132, 2179–2189 [DOI] [PubMed] [Google Scholar]
- 9. Wang L. H., Tsai S. Y., Cook R. G., Beattie W. G., Tsai M. J., O'Malley B. W. (1989) COUP transcription factor is a member of the steroid receptor superfamily. Nature 340, 163–166 [DOI] [PubMed] [Google Scholar]
- 10. You L. R., Lin F. J., Lee C. T., DeMayo F. J., Tsai M. J., Tsai S. Y. (2005) Suppression of Notch signaling by the COUP-TFII transcription factor regulates vein identity. Nature 435, 98–104 [DOI] [PubMed] [Google Scholar]
- 11. Qin J., Chen X., Xie X., Tsai M. J., Tsai S. Y. (2010) COUP-TFII regulates tumor growth and metastasis by modulating tumor angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 3687–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pereira F. A., Qiu Y., Zhou G., Tsai M. J., Tsai S. Y. (1999) The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 13, 1037–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suh J. M., Yu C. T., Tang K., Tanaka T., Kodama T., Tsai M. J., Tsai S. Y. (2006) The expression profiles of nuclear receptors in the developing and adult kidney. Mol. Endocrinol. 20, 3412–3420 [DOI] [PubMed] [Google Scholar]
- 14. Konoshita T., Makino Y., Wakahara S., Ido K., Yoshida M., Kawai Y., Miyamori I. (2004) Candidate cis-elements for human renin gene expression in the promoter region. J. Cell Biochem. 93, 327–336 [DOI] [PubMed] [Google Scholar]
- 15. Konoshita T., Fuchs S., Makino Y., Wakahara S., Miyamori I. (2007) A proximal direct repeat motif characterized as a negative regulatory element in the human renin gene. J. Cell Biochem. 102, 1043–1050 [DOI] [PubMed] [Google Scholar]
- 16. Lang J. A., Yang G., Kern J. A., Sigmund C. D. (1995) Endogenous human renin expression and promoter activity in CALU-6, a pulmonary carcinoma cell line. Hypertension 25, 704–710 [DOI] [PubMed] [Google Scholar]
- 17. Sigmund C. D., Okuyama K., Ingelfinger J., Jones C. A., Mullins J. J., Kane C., Kim U., Wu C. Z., Kenny L., Rustum Y. (1990) Isolation and characterization of renin-expressing cell lines from transgenic mice containing a renin-promoter viral oncogene fusion construct. J. Biol. Chem. 265, 19916–19922 [PubMed] [Google Scholar]
- 18. Desch M., Harlander S., Neubauer B., Gerl M., Germain S., Castrop H., Todorov V. T. (2011) cAMP target sequences enhCRE and CNRE sense low-salt intake to increase human renin gene expression in vivo. Pflugers Arch. 461, 567–577 [DOI] [PubMed] [Google Scholar]
- 19. Desch M., Schubert T., Schreiber A., Mayer S., Friedrich B., Artunc F., Todorov V. T. (2010) Mol. Endocrinol. 24, 2139–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Todorov V. T., Desch M., Schmitt-Nilson N., Todorova A., Kurtz A. (2007) Peroxisome proliferator-activated receptor-γ is involved in the control of renin gene expression. Hypertension 50, 939–944 [DOI] [PubMed] [Google Scholar]
- 21. Todorov V. T., Desch M., Schubert T., Kurtz A. (2008) The Pal3 promoter sequence is critical for the regulation of human renin gene transcription by peroxisome proliferator-activated receptor-γ. Endocrinology 149, 4647–4657 [DOI] [PubMed] [Google Scholar]
- 22. Todorov V., Müller M., Schweda F., Kurtz A. (2002) Tumor necrosis factor-α inhibits renin gene expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R1046–R1051 [DOI] [PubMed] [Google Scholar]
- 23. Todorov V. T., Völkl S., Müller M., Bohla A., Klar J., Kunz-Schughart L. A., Hehlgans T., Kurtz A. (2004) Tumor necrosis factor-α activates NFκB to inhibit renin transcription by targeting cAMP-responsive element. J. Biol. Chem. 279, 1458–1467 [DOI] [PubMed] [Google Scholar]
- 24. Todorov V., Müller M., Kurtz A. (2001) Kidney Blood Press. Res. 24, 75–78 [DOI] [PubMed] [Google Scholar]
- 25. Klar J., Sandner P., Muller M. W., Kurtz A. (2002) Cyclic AMP stimulates renin gene transcription in juxtaglomerular cells. Pflugers Arch. 444, 335–344 [DOI] [PubMed] [Google Scholar]
- 26. Horiuchi M., Pratt R. E., Nakamura N., Dzau V. J. (1993) Distinct nuclear proteins competing for an overlapping sequence of cyclic adenosine monophosphate and negative regulatory elements regulate tissue-specific mouse renin gene expression. J. Clin. Invest. 92, 1805–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ying L., Morris B. J., Sigmund C. D. (1997) Transactivation of the human renin promoter by the cyclic AMP/protein kinase A pathway is mediated by both cAMP-responsive element binding protein-1 (CREB)-dependent and CREB-independent mechanisms in Calu-6 cells. J. Biol. Chem. 272, 2412–2420 [DOI] [PubMed] [Google Scholar]
- 28. Chen L., Kim S. M., Oppermann M., Faulhaber-Walter R., Huang Y., Mizel D., Chen M., Lopez M. L., Weinstein L. S., Gomez R. A., Briggs J. P., Schnermann J. (2007) Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gαs in juxtaglomerular cells. Am. J. Physiol. Renal Physiol. 292, F27–F37 [DOI] [PubMed] [Google Scholar]
- 29. Park J. I., Tsai S. Y., Tsai M. J. (2003) Molecular mechanism of chicken ovalbumin upstream promoter-transcription factor (COUP-TF) actions. Keio J. Med. 52, 174–181 [DOI] [PubMed] [Google Scholar]
- 30. Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. (1992) Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol. Cell Biol. 12, 4153–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sagami I., Tsai S. Y., Wang H., Tsai M. J., O'Malley B. W. (1986) Identification of two factors required for transcription of the ovalbumin gene. Mol. Cell Biol. 6, 4259–4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burt D. W., Nakamura N., Kelley P., Dzau V. J. (1989) Identification of negative and positive regulatory elements in the human renin gene. J. Biol. Chem. 264, 7357–7362 [PubMed] [Google Scholar]
- 33. Liu X., Huang X., Sigmund C. D. (2003) Identification of a nuclear orphan receptor (Ear2) as a negative regulator of renin gene transcription. Circ. Res. 92, 1033–1040 [DOI] [PubMed] [Google Scholar]
- 34. Shi Q., Gross K. W., Sigmund C. D. (2001) Retinoic acid-mediated activation of the mouse renin enhancer. J. Biol. Chem. 276, 3597–3603 [DOI] [PubMed] [Google Scholar]
- 35. Weatherford E. T., Liu X., Sigmund C. D. (2012) Regulation of renin expression by the orphan nuclear receptors Nr2f2 and Nr2f6. Am. J. Physiol. Renal Physiol. 302, F1025–F1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Y. C., Kong J., Wei M., Chen Z. F., Liu S. Q., Cao L. P. (2002) 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Invest. 110, 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tamura K., Chen Y. E., Horiuchi M., Chen Q., Daviet L., Yang Z., Lopez-Ilasaca M., Mu H., Pratt R. E., Dzau V. J. (2000) LXRα functions as a cAMP-responsive transcriptional regulator of gene expression. Proc. Natl. Acad. Sci. U.S.A. 97, 8513–8518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neubauer B., Machura K., Chen M., Weinstein L. S., Oppermann M., Sequeira-Lopez M. L., Gomez R. A., Schnermann J., Castrop H., Kurtz A., Wagner C. (2009) Development of vascular renin expression in the kidney critically depends on the cyclic AMP pathway. Am. J. Physiol. Renal Physiol. 296, F1006–F1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pan L., Black T. A., Shi Q., Jones C. A., Petrovic N., Loudon J., Kane C., Sigmund C. D., Gross K. W. (2001) Critical roles of a cyclic AMP-responsive element and an E-box in regulation of mouse renin gene expression. J. Biol. Chem. 276, 45530–45538 [DOI] [PubMed] [Google Scholar]
- 40. Kim S. M., Chen L., Faulhaber-Walter R., Oppermann M., Huang Y., Mizel D., Briggs J. P., Schnermann J. (2007) Regulation of renin secretion and expression in mice deficient in β1- and β2-adrenergic receptors. Hypertension 50, 103–109 [DOI] [PubMed] [Google Scholar]
- 41. Lang J. A., Ying L. H., Morris B. J., Sigmund C. D. (1996) Transcriptional and posttranscriptional mechanisms regulate human renin gene expression in Calu-6 cells. Am. J. Physiol. 271, F94–F100 [DOI] [PubMed] [Google Scholar]
- 42. Morris B. J., Adams D. J., Beveridge D. J., van der Weyden L., Mangs H., Leedman P. J. (2004) cAMP controls human renin mRNA stability via specific RNA-binding proteins. Acta Physiol. Scand. 181, 369–373 [DOI] [PubMed] [Google Scholar]
- 43. Scott D. K., Mitchell J. A., Granner D. K. (1996) The orphan receptor COUP-TF binds to a third glucocorticoid accessory factor element within the phosphoenolpyruvate carboxykinase gene promoter. J. Biol. Chem. 271, 31909–31914 [DOI] [PubMed] [Google Scholar]
- 44. Yang T., Endo Y., Huang Y. G., Smart A., Briggs J. P., Schnermann J. (2000) Renin expression in COX-2-knockout mice on normal or low salt diets. Am. J. Physiol. Renal Physiol. 279, F819–F825 [DOI] [PubMed] [Google Scholar]
- 45. Duan S. Z., Ivashchenko C. Y., Whitesall S. E., D'Alecy L. G., Duquaine D. C., Brosius F. C., 3rd, Gonzalez F. J., Vinson C., Pierre M. A., Milstone D. S., Mortensen R. M. (2007) Hypotension, lipodystrophy, and insulin resistance in generalized PPARγ-deficient mice rescued from embryonic lethality. J. Clin. Invest. 117, 812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mercure C., Prescott G., Lacombe M. J., Silversides D. W., Reudelhuber T. L. (2009) Chronic increases in circulating prorenin are not associated with renal or cardiac pathologies. Hypertension 53, 1062–1069 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.