Background: CC chemokine ligand 2 (CCL2) recruits leukocytes in inflammatory tissues.
Results: Vimentin, a cytoskeletal protein, interacted with phosphorylated MAPKs, was critical for CCL2 production in mast cells activated via FϵcRI and a CC chemokine receptor.
Conclusion: Vimentin was involved in optimal CCL2 production in mast cells.
Significance: This work contributes to understanding of mechanisms for chemokine production in mast cells, which are therapeutic targets for allergic inflammation.
Keywords: Allergy, Chemokines, FC Receptors, MAP Kinases (MAPKs), Mast Cell, Vimentin
Abstract
Accumulating evidence points to cross-talk between FcϵRI and CC chemokine receptor (CCR)-mediated signaling pathways in mast cells. Here, we propose that vimentin, a protein comprising type III intermediate filament, participates in such cross-talk for CCL2/monocyte chemotactic protein 1 (MCP-1) production in mast cells, which is a mechanism for allergic inflammation. Co-stimulation via FcϵRI, using IgE/antigen, and CCR1, using recombinant CCL3/macrophage inflammatory protein-1α (MIP-1α), increased expression of phosphorylated, disassembled, and soluble vimentin in rat basophilic leukemia (RBL)-2H3 cells expressing human CCR1 (RBL-CCR1 cells) and bone marrow-derived murine mast cells, both models of mucosal type mast cells. Furthermore, co-stimulation enhanced production of CCL2 as well as phosphorylation of MAPK. Treating the cells with p38 MAPK inhibitor SB203580, but not with MEK inhibitor PD98058, reduced CCL2 production, suggesting that p38 MAPK, but not ERK1/2, plays a critical role in the chemokine production. Immunoprecipitation analysis showed that vimentin interacts with phosphorylated ERK1/2 and p38 MAPKs in the co-simulated cells. Preventing disassembly of the vimentin by aggregating vimentin filaments using β,β′-iminodipropionitrile reduced the interaction of vimentin with phosphorylated MAPKs as well as CCL2 production in the cells. Taken together, disassembled vimentin interacting with phosphorylated p38 MAPK could mediate CCL2 production in mast cells upon FcϵRI and CCR1 activation.
Introduction
Mast cells play an important role in IgE-associated allergic disorders and immune responses to parasites, and FcϵRI cross-linking is a key event in activating mast cells. The association of allergen with IgE bound to FcϵRI trigger signaling cascades leading to activation of kinases, phosphatases, and GTPases, which subsequently induce a variety of events, such as degranulation, cytoskeleton rearrangement, increased gene transcription, and cytokine/chemokine production, in the activated mast cells (1, 2).
Besides the classical FcϵRI-mediated mechanism, mast cells are also activated by chemokines (3, 4). A superfamily of small, structurally related cytokine molecules, chemokines are characterized by their ability to affect trafficking of leukocytes. Some chemokines, such as CCL2/MCP-1 (monocyte chemotactic protein 1), CCL3/MIP-1α (macrophage inflammatory protein-1α), CCL5/RANTES (regulated upon activation, normal T-cell expressed and secreted), and CCL11/eotaxin-1, have been reported to activate mouse, rat, or human mast cells (3–6). Abundant expression of these CC chemokines and accumulation of leukocytes has also been observed in allergic inflammatory tissues (7–9). It is very likely that FcϵRI and CCR5 engagements occur either simultaneously or in relatively rapid succession in mast cells in vivo.
We previously found that CCL3 acts as a co-stimulator for FcϵRI-mediated degranulation in conjunctival mast cells using CCL3-deficient mice (10). Moreover, CCL3 synergistically enhanced FcϵRI-mediated degranulation and gene expression of cytokines and chemokines (e.g. IL-6 and CCL7/MCP-3) in a rat basophilic leukemia 2H3 cell line (RBL-2H3) expressing CC chemokine receptor 1 (CCR1), a receptor for CCL3, and bone marrow-derived murine mast cells (11–14). These observations indicate that (i) the simultaneous engagement of FcϵRI and CCR1 is important for optimal activation of mast cells in vitro and physiologically relevant levels of mast cell activation in vivo, and (ii) there is a cross-talk between the FcϵRI-mediated and CCR1-mediated signaling cascades.
In this paper, we also found that CCL3 synergistically enhanced FcϵRI-mediated CCL2 production in RBL-CCR1 cells and bone marrow-derived murine mast cells (BMMCs). CCL2 is a chemoattractant to induce migration of monocytes, T cells, and eosinophils (3, 9). Increased expression of CCL2 protein in inflammatory tissues of allergic patients has been observed (3, 7–9, 15). Targeting chemokine(s) is a strategy to establish new anti-inflammatory drugs for treatment of allergenic diseases. To better understand the molecular mechanisms for chemokine production involved in the complex response of mast cell activation, we investigated the proteins involved in the cross-talk between FcϵRI-mediated and CCR1-mediated signaling pathways.
Here, we identified phosphorylated vimentin, a cytoskeletal protein, as the major protein species up-regulated in the co-stimulated mast cells with IgE/Ag and CCL3. Interestingly, vimentin interacted with mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase 1/2 (ERK1/2), and p38 MAPK. Furthermore, our findings suggest that vimentin is a component for optimal production of CCL2 in the FcϵRI- and CCR1-engaged mast cells.
EXPERIMENTAL PROCEDURES
Cell Culture
Monolayer cultures of RBL-CCR1 cells (rat basophilic leukemia RBL-2H3 cells expressing human CCR1) (16) were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 12% fetal bovine serum (FBS), 2 mm glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 1 mg/ml geneticin.
To generate primary BMMCs, low density mononuclear cells (LDMNCs) were isolated from BALB/c mice (Jackson Laboratories) and cultured in RPMI1640 containing 10% FBS, 4 mm glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.1 mm non-essential amino acids, and 50 μm 2-mercaptoethanol in the presence of 5 ng/ml of recombinant murine IL-3 (PeproTech) for 4–6 weeks (12, 17). The purity of BMMCs exceeded 80%, which was determined by flow cytometric analysis after staining c-Kit and FcϵRI on the cell surface. Tissue culture media and cell culture supplements were from Invitrogen.
Chemokine Production Assay
RBL-CCR1 cells (3.0 × 106 cells/ml) and BMMCs (1.0 × 106 cells/ml) were sensitized with 10 and 100 ng/ml anti-DNP IgE monoclonal antibody (SPE7, Sigma-Aldrich) overnight, respectively. These cells were then treated with or without the p38 MAPK inhibitor SB203580 (Calbiochem) or MEK inhibitor PD98058 (Calbiochem) for 1 h or β,β′-iminodipropionitrile (Sigma-Aldrich), inhibitor of soluble vimentin formation, for 1 h and subsequently stimulated with DNP-conjugated human serum albumin (DNP-HSA from Sigma-Aldrich) and/or human rCCL3 (R&D Systems) in DMEM containing 2% FCS for 2–6 h. The concentrations of rat and murine CCL2 in the culture supernatant were measured by ELISA (Peprotech and eBioscience, respectively).
Measurement of Phosphorylated ERK1/2 and p38 MAPK by ELISA
After sensitization with anti-DNP-IgE mAb, cells were stimulated with rCCL3 and/or DNP-HSA for 5 min and lysed in 10 mm Tris buffer, pH 7.4, containing 100 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm NaF, 20 mm Na4P2O7, 2 mm Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, and 0.5% deoxycholate. The levels of total and phosphorylated ERK and p38 kinase in cell lysates were measured by ELISA (Invitrogen) following the manufacturer's instructions.
Two-dimensional Gel Electrophoresis
RBL-CCR1 cells (1.5 × 106 cells/ml) were sensitized with anti-DNP IgE mAb overnight and stimulated with 10 ng/ml DNP-HSA and 100 ng/ml rCCL3 for 5 min. The cells were washed with cold PBS and lysed in a buffer containing urea (8 m), CHAPS (4%, w/v), DTE (65 mm), resolytes 3.5–10 (2%, v/v), 2.5 μg/ml DNase I, 2.5 μg/ml RNase, 50 mm NaF, 1 mm Na3VO4, protease inhibitors (Complete, Roche Applied Science), and a trace of bromphenol blue. The total lysates were loaded on the first dimensional separation with a sigmoidal immobilized pH gradient (IPG) from pH 4.0 to 7.0. After equilibration, the IPG gel strips were transferred onto the second dimension vertical gradient slab gels and run with the Laemmli SDS-discontinuous system. Proteins were detected using Coomassie staining. Two-dimensional gel electrophoresis and gel staining were performed by the Proteomics Core Facility at the University of Geneva.
Protein Identification by MALDI-TOF MS
Protein spots were excised from the polyacrylamide gel, reduced with dithiothreitol, treated with iodoacetamide (Sigma-Aldrich) for carboxyamidation of the cysteine residues, and digested in situ with trypsin (sequencing grade; Roche Applied Science), according to the method of Shevchenko et al. (18, 19). An aliquot of the liquid surrounding the gel pieces was mixed with an equal volume of matrix solution (60% (v/v)) acetonitrile, 0.5% (v/v) trifluoroacetic acid (Applied Biosystems), 6 mg/ml α-cyano-4-hydroxycinnamic acid (Bruker Daltonics GmbH, Bremen, Germany). One microliter of the mixture was spotted immediately onto a 384-well stainless steel MALDI-TOF target and allowed to dry. Peptide calibration standard solution (Bruker Daltonics) was spotted in a similar manner adjacent to the samples.
The mass spectra were recorded on an Ultraflex I (Bruker Daltonics) MALDI mass spectrometer. Peptides were selected for fragmentation analysis by LIFT-MS/MS sequencing (20). The spectra were interpreted using FlexAnalysis and Biotools software (Bruker Daltonics), and the data were searched against non-redundant protein sequence databases using the program MASCOT (21). Proteins were identified with 0.2-Da accuracy and a minimum of four matching peptides.
Extraction of Soluble Vimentin
The method of Valgeirsdottir et al. (22) for extracting soluble vimentin was slightly modified. Briefly, after washing with ice-cold PBS, cells were lysed in PBS (pH 7.4) containing 1% Nonidet P-40, 0.5% sodium deoxycholate, 50 mm NaF, 1 mm Na3VO4, and protease inhibitors.
Immunoprecipitation
For immunoprecipitation to detect phosphorylation of tyrosine, serine, and threonine residues in vimentin, the cells were lysed in PBS containing 5 mm EDTA, 2% SDS, 10% glycerol, 2.5 μg/ml DNase I, 2.5 μg/ml RNase, 50 mm NaF, 1 mm Na3VO4, and protease inhibitors. The lysates were diluted 20-fold with PBS containing 1% Nonidet P-40, 5 mm EDTA, 50 mm NaF, 1 mm Na3VO4, and protease inhibitors. The lysates were incubated with protein A/G Plus-agarose (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and rabbit anti-vimentin mAb (clone H54; Santa Cruz Biotechnology). Proteins complexed to the agarose gels were recovered, resuspended in sample buffer, and analyzed by immunoblotting. For immunoprecipitation to detect proteins associated with vimentin, the cells were lysed in a buffer used for extracting soluble vimentin. The lysates were incubated with protein G-agarose (Santa Cruz Biotechnology, Inc.) and monoclonal mouse anti-vimentin mAb V9 (Sigma-Aldrich).
Immunoblotting
The lysates or the proteins immunoprecipitated with anti-vimentin antibodies were suspended in sample buffer, loaded onto 12% polyacrylamide gels, and transferred to polyvinylidene difluoride membranes. The membranes were probed with primary antibodies, detected using appropriate secondary antibodies conjugated with horseradish peroxidase (Santa Cruz Biotechnology, Inc.), and enhanced with a chemiluminescent kit (Pierce). Mouse anti-vimentin phospho-Ser-55 mAb (4A4) (23), rabbit anti-vimentin phospho-Ser-71 mAb (TM71) (24), mouse anti-vimentin phospho-Ser-6 (MO6) or vimentin phospho-Ser-82 mAb (MO82) (25), rabbit anti-vimentin mAb (H54), rabbit anti-phospho-ERK1/2 mAb (Cell Signaling Technology), and rabbit anti-phospho-p38 MAPK mAb (Cell Signaling Technology) were used as the primary antibodies.
RESULTS
CCL2 Production and MAPK Activation Are Enhanced in FcϵRI- and CCR1-activated RBL-CCR1 Cells
CCL2 plays a critical role in activation and accumulation of leukocytes in allergic inflammatory tissues (3, 7–9, 15). To examine whether cross-talk between FcϵRI- and CCR-mediated signaling pathways in mast cells is involved in expression of this chemokine, we used RBL-2H3 cells expressing human CCR1, a model cell line of mucosal type mast cells.
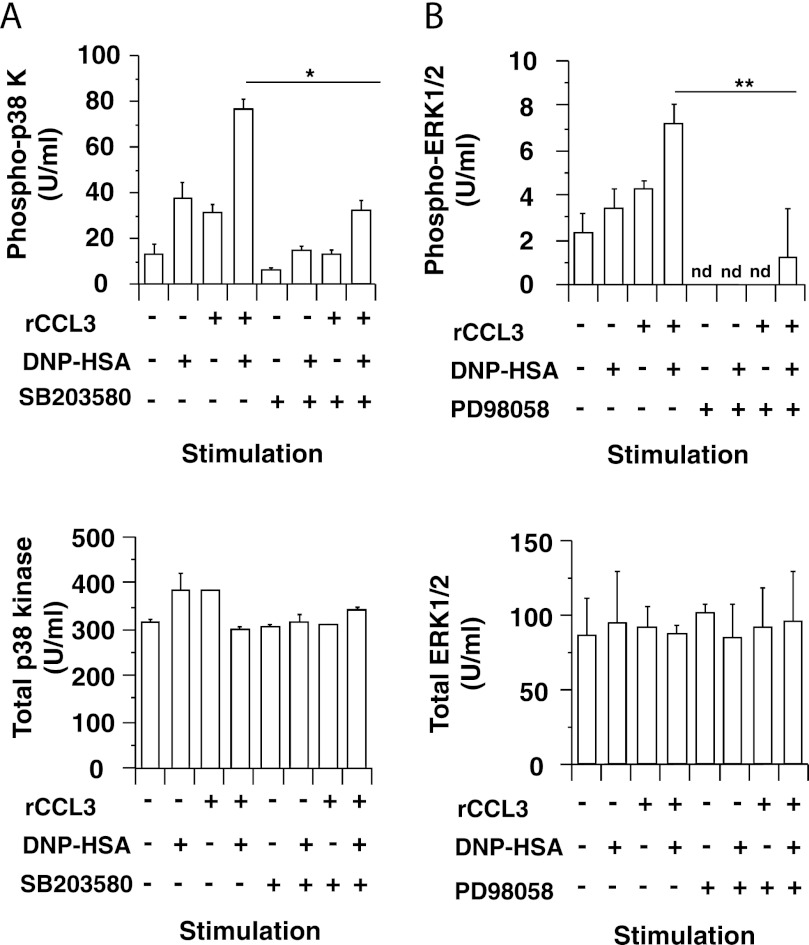
We found that co-stimulation with IgE/Ag (DNP-HSA) and rCCL3 synergistically enhanced CCL2 production in RBL-CCR1 cells (Fig. 1A). However, this CCL2 production was attenuated by the p38 MAPK inhibitor SB203580 (Fig. 1B) but not by the MEK inhibitor PD98058, which inhibits activation of ERK1/2 (Fig. 1C). These results suggest that p38 MAPK but not ERK1/2 kinase plays a role in FcϵRI- and CCR1-mediated CCL2 production in RBL-CCR1 cells. Co-stimulation with IgE/Ag and rCCL3 also enhanced phosphorylation of p38 MAPK and ERK1/2 in the cells, but SB203580 and PD98058 abolished this stimulation, respectively (Fig. 2). The results indicate that cross-talk between FcϵRI- and CCR1-mediated signaling pathways synergistically enhances MAPK activation as well as subsequent CCL2 production in RBL-CCR1 cells.
FIGURE 1.
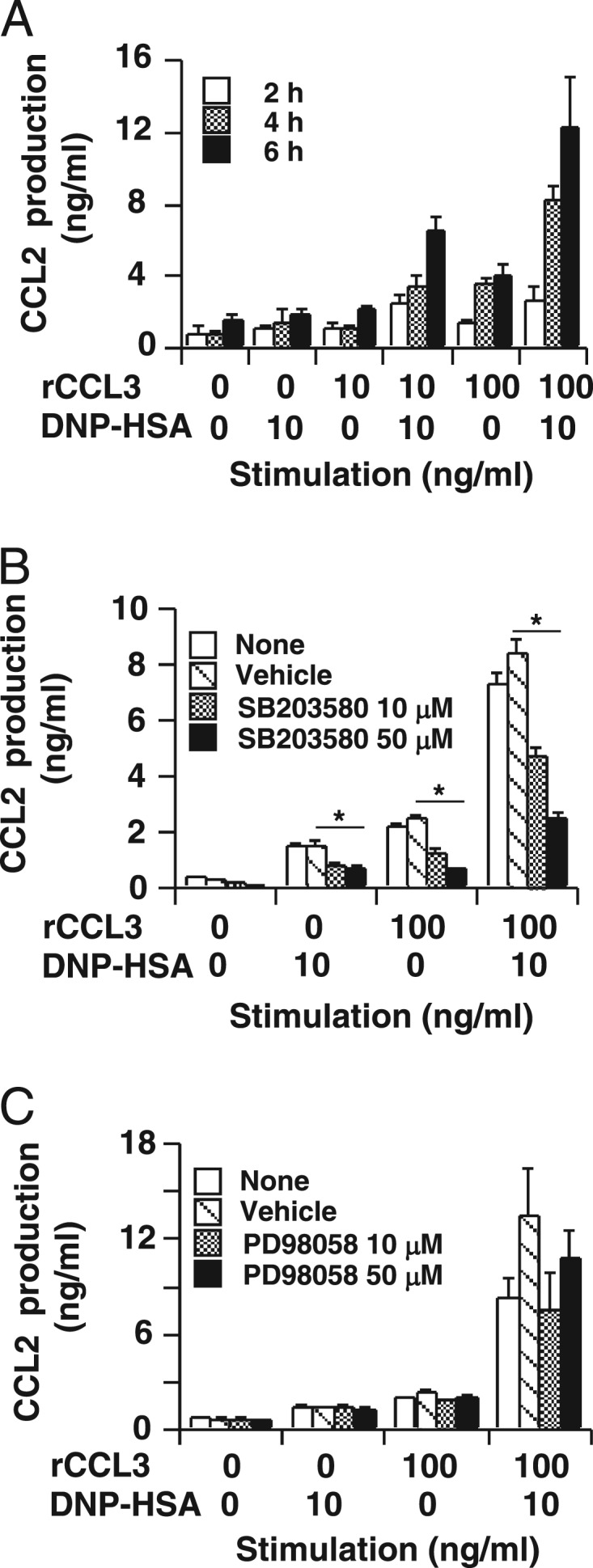
Synergistic CCL2 production in RBL-CCR1 cells co-stimulated via FcϵRI and CCR1. A, after sensitization with anti-DNP-IgE mAb, RBL-CCR1 cells were stimulated with 10 ng/ml DNP-HSA and/or various concentrations of rCCL3 for 2, 4, or 6 h before determining CCL2 production by ELISA. B, to inhibit p38 MAPK activation, the cells were incubated with 10 or 50 μm SB203580 for the last 1 h of sensitization. C, to inhibit ERK1/2 activation, the cells were incubated with 10 or 50 μm PD98058 for the last 1 h of sensitization. The cells were then stimulated with 10 ng/ml DNP-HSA and/or 100 ng/ml rCCL3 for 6 h. The plotted data of mean CCL2 concentrations in culture supernatants measured by ELISA are representative of three independent experiments. Error bars, S.D. *, p < 0.01.
FIGURE 2.
Increased phosphorylation of p38 MAPK and ERK1/2 in RBL-CCR1 cells stimulated via FcϵRI and CCR1. After sensitization with anti-DNP-IgE mAb, RBL-CCR1 cells were stimulated with 10 ng/ml DNP-HSA and/or 100 ng/ml rCCL3 for 5 min. To inhibit p38 kinase and ERK1/2 activation, the cells were incubated with 50 μm SB203580 or 50 μm PD98058 for the last 1 h of sensitization. Levels of phosphorylated and total p38 kinase (A) and ERK1/2 (B) protein in total cell lysates were measured by ELISA. The data are representative for three independent experiments. nd, not detectable (<1.0 unit (U)/ml). Error bars, S.D. *, p < 0.01; **, p < 0.05.
Protein Expression Analysis of FcϵRI- and CCR1-activated RBL-CCR1 Cells Revealed Vimentin Proteins
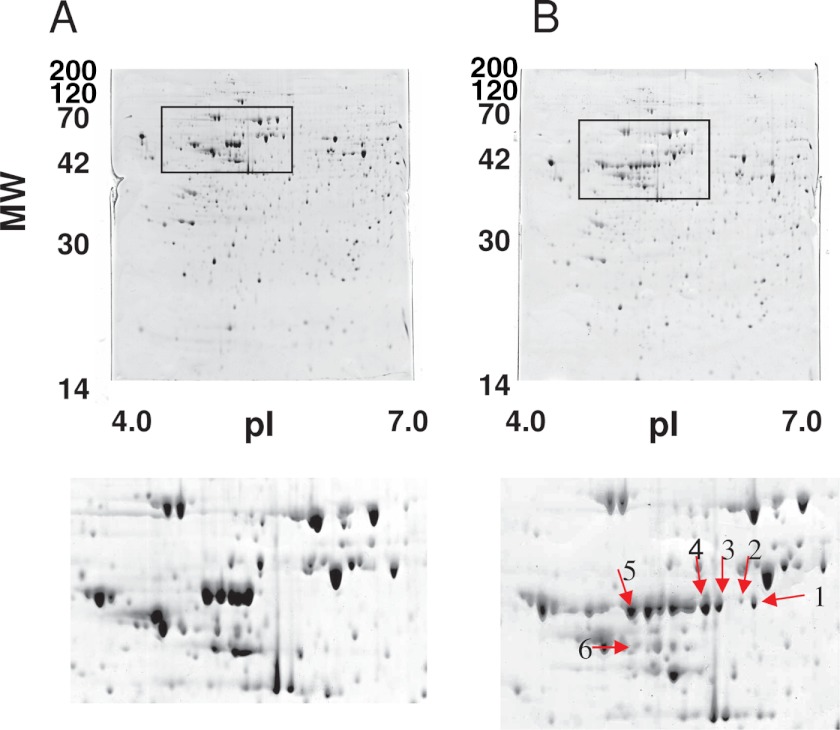
CCL2 is a therapeutic target in allergic diseases (9, 26). To elucidate the molecular mechanisms for CCL2 production in mast cells, we set out to identify proteins involved in the cross-talk between FcϵRI- and CCR1-mediated signaling pathways. Therefore, we analyzed total cell lysates of RBL-CCR1 cells non-stimulated or co-stimulated with IgE/Ag plus rCCL3 by two-dimensional electrophoresis. We observed clear up-regulation of six protein species (Fig. 3) and identified rat vimentin (accession number P31000) as the major component of all of these spots using MALDI-TOF MS (Table 1). Vimentin has multiple phosphorylation sites, which would explain the various isoforms observed migrating in a horizontal line across the gel. It is likely that spot 6, with a lower molecular weight, is a truncated fragment of vimentin.
FIGURE 3.
Differential protein expression analysis in RBL-CCR1 cells stimulated via FcϵRI and CCR1. After sensitization with anti-DNP-IgE, RBL-CCR1 cells were stimulated with or without 10 ng/ml DNP-HSA and 100 ng/ml rCCL3 for 5 min. Total cell lysates of the unstimulated cells (A) or total cell lysates of the stimulated cells (B) were subjected to two-dimensional PAGE and stained with Coomassie Brilliant Blue. Protein spots up-regulated in a gel are shown with the red arrows. MW, molecular weight.
TABLE 1.
Identified proteins in RBL-CCR1 cells co-stimulated via FcϵRI and CCR1
| Spot no. | Protein name | Species | NCBI accession no. | Theoretical pI | Theoretical mass | Experimental pI | Experimental mass | Sequence coverage |
|---|---|---|---|---|---|---|---|---|
| kDa | kDa | % | ||||||
| 1 | Vimentin | Rat | 14389299 | 5.1 | 53.77 | 5.35 | 51.56 | 56 |
| 2 | Vimentin | Rat | 14389299 | 5.1 | 53.77 | 5.39 | 51.25 | 61 |
| 3 | Vimentin | Rat | 14389299 | 5.1 | 53.77 | 5.26 | 50.378 | 73 |
| 4 | Vimentin | Rat | 14389299 | 5.1 | 53.77 | 5.2 | 50.78 | 74 |
| 5 | Vimentin | Rat | 14389299 | 5.1 | 53.77 | 4.97 | 50.78 | 75 |
| 6 | Vimentin | Rat | 14389299 | 5.1 | 53.77 | 4.98 | 42.04 | 56 |
Vimentin Was Disassembled in FcϵRI- and CCR1-activated RBL-CCR1 Cells
Vimentin is a major structural component of intermediate filaments that create cell rigidity and shape (27). Upon phosphorylation, vimentin regulates the disassembly of these intermediate filaments (28–32). Vimentin also organizes signaling process in a phosphorylation-dependent manner (33, 34). To obtain insights into the roles of vimentin in mast cell activation, we examined phosphorylation of vimentin in FcϵRI- and/or CCR1-activated RBL-CCR1 cells.
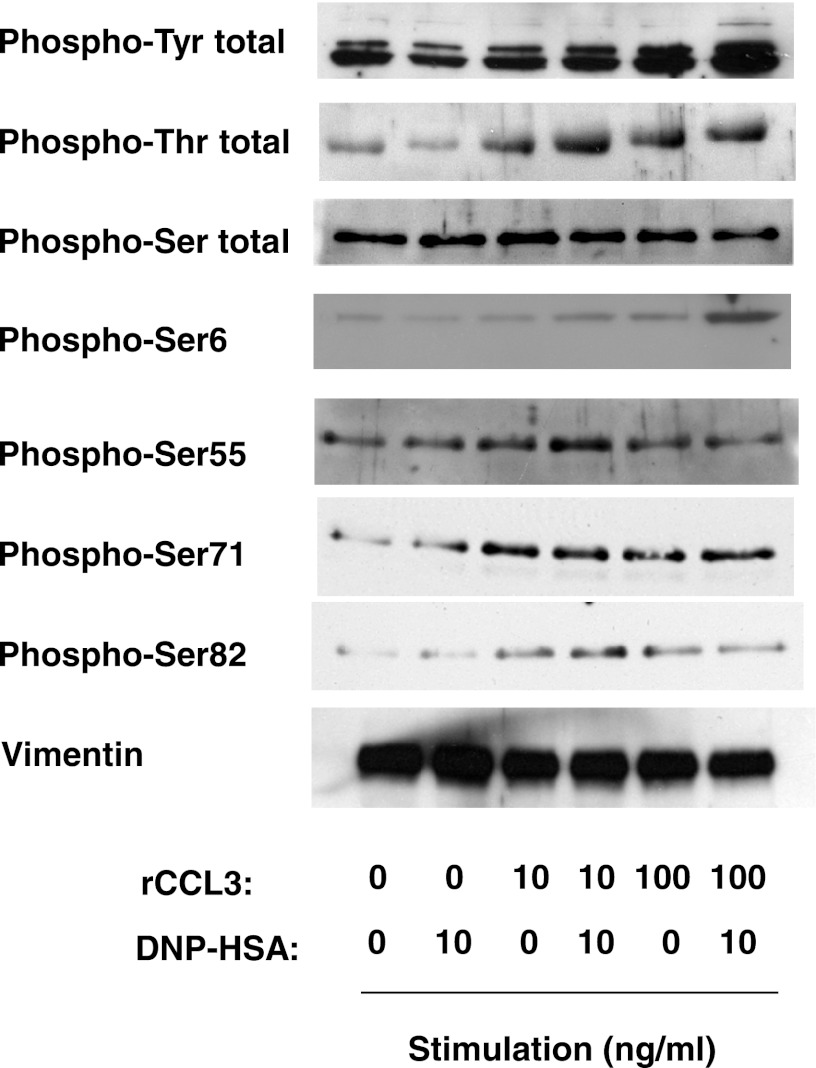
We therefore extracted total proteins of unstimulated and stimulated cells using a lysis buffer containing 2% SDS and 10% glycerol and diluted it using a buffer containing 1% Nonidet P-40 for immunoprecipitation. We then analyzed the phosphorylation status of vimentin by immunoprecipitating the protein and detecting different phosphospecies by immunoblotting with specific anti-phosphotyrosine, phosphothreonine, and phosphoserine antibodies. The total tyrosine phosphorylation of vimentin in RBL-CCR1 cells was enhanced in an rCCL3 concentration-dependent manner, when the cells were co-stimulated with the chemokine and IgE/Ag. In contrast, high levels of serine and threonine phosphorylation were observed in the cells co-stimulated with 10 ng/ml rCCL3 and/or 10 ng/ml IgE/Ag (Fig. 4). The highest levels of phosphorylation at serines 55, 71, and 82 in the N terminus of vimentin were detected when the cells were stimulated with 10 ng/ml rCCL3 and 10 ng/ml IgE/Ag. Interestingly, appreciable levels of phosphorylation at Ser-6 required co-stimulation with 100 ng/ml rCCL3 and 10 ng/ml IgE/Ag (Fig. 4).
FIGURE 4.
Phosphorylation of vimentin in RBL-CCR1 cells stimulated via FcϵRI and CCR1. After sensitization with anti-DNP-IgE mAb, RBL-CCR1 cells were stimulated with 10 ng/ml DNP-HSA and/or various concentrations of rCCL3 for 5 min. Total cell lysates of non-stimulated or the stimulated cells were subjected to immunoprecipitation with anti-vimentin mAb. The immunoprecipitated proteins were analyzed by immunoblotting using antibodies against phosphotyrosine; phosphothreonine; phosphoserine; phosphorylated vimentin at Ser-6, -55, -71, or -82; or vimentin. The data are representative of two independent experiments.
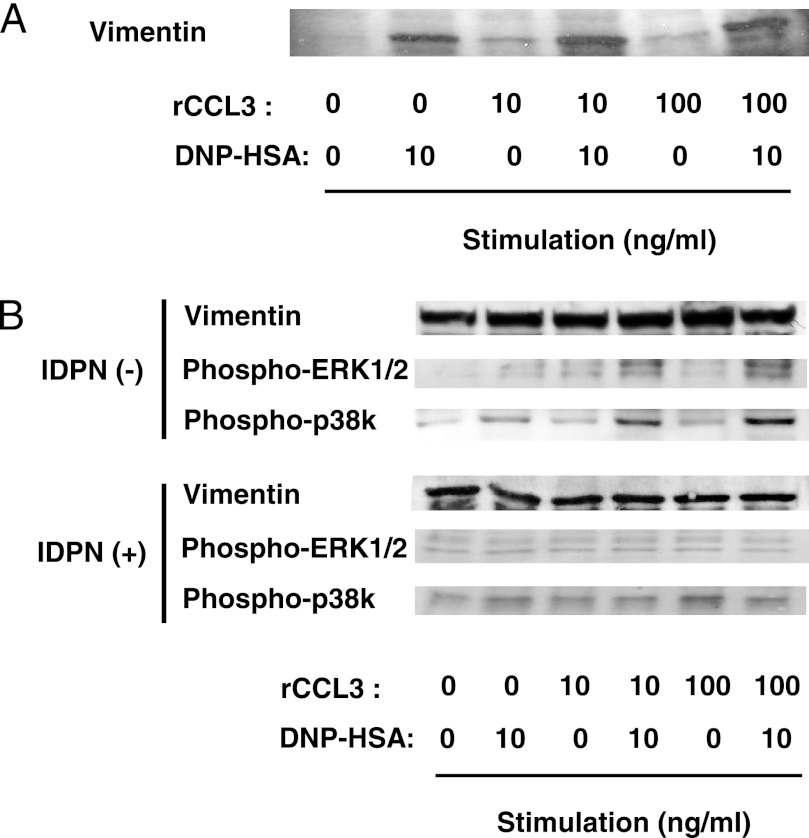
Serine phosphorylation at the N terminus appears to regulate the disassembly of vimentin filaments (28–32). It has also been shown that disassembly of vimentin filaments convert the protein into a soluble form (29–32). Vimentin reportedly interacts with phosphorylated MAPK in neurons and adipocytes (35–37). Hence, we hypothesized that (i) FcϵRI and CCR1 activation induces vimentin disassembly and that (ii) the disassembled and soluble vimentin interacts with activated MAPKs and is thus involved in CCL2 production in mast cells. To test these hypotheses, we examined levels of vimentin filament disassembly by detecting amounts of soluble vimentin in activated RBL-CCR1 cells. Cell lysates were prepared with lysis buffer containing 1% Nonidet P-40, which extracts soluble forms of vimentin (22). Indeed, co-stimulation with rCCL3 and IgE/Ag enhanced formation of soluble vimentin in RBL-CCR1 cells (Fig. 5A). Taken together, FcϵRI and CCR1 engagement induces vimentin phosphorylation, which in turn increases soluble vimentin levels in these activated RBL-CCR1 cells.
FIGURE 5.
Interaction of vimentin and phosphorylated MAPK in RBL-CCR1 cells stimulated via FcϵRI and CCR1. After sensitization with anti-DNP-IgE, RBL-CCR1 cells were stimulated with 10 ng/ml DNP-HSA and/or various concentrations of rCCL3 for 5 min. A, after the stimulation, soluble forms of vimentin were extracted and analyzed by immunoblotting using anti-vimentin Abs. B, the cells were incubated with β,β′-iminodipropionitrile (IDPN (+)) to promote aggregation of vimentin intermediate filaments for the last 1 h of sensitization. Soluble forms of vimentin in the cells were extracted and subjected to immunoprecipitation using anti-vimentin mAb and analyzed by immunoblotting using anti-phosphorylated ERK1/2 mAb, anti-phosphorylated p38 kinase mAb, or anti-vimentin Abs. The data are representative of three independent experiments.
Vimentin Interacted with Phosphorylated MAPKs in FcϵRI- and CCR1- activated RBL-CCR1 Cells
Next, we examined whether vimentin interacts with MAPKs and is involved in CCL2 production in RBL-CCR1 cells upon FcϵRI and CCR1 stimulation. Immunoprecipitation with anti-vimentin antibody was performed using extracts of the cells stimulated with IgE/Ag and/or rCCL3, which contained soluble vimentin. The immunoprecipitated samples were separated on one-dimensional SDS-PAGE followed by Western blotting with anti-phospho-p38 MAPK or anti-phospho-ERK1/2 mAb. We detected association of vimentin with both phosphorylated p38 kinase and ERK1/2 in the extract from the stimulated cells (Fig. 5B). These results suggest that vimentin may interact with phosphorylated p38 MAPK and ERK1/2 in activated RBL-CCR1 cells.
CCL2 Production in CCR1- and FcϵRI- activated RBL-CCR1 Cells Was Decreased by Preventing Formation of Soluble Vimentin
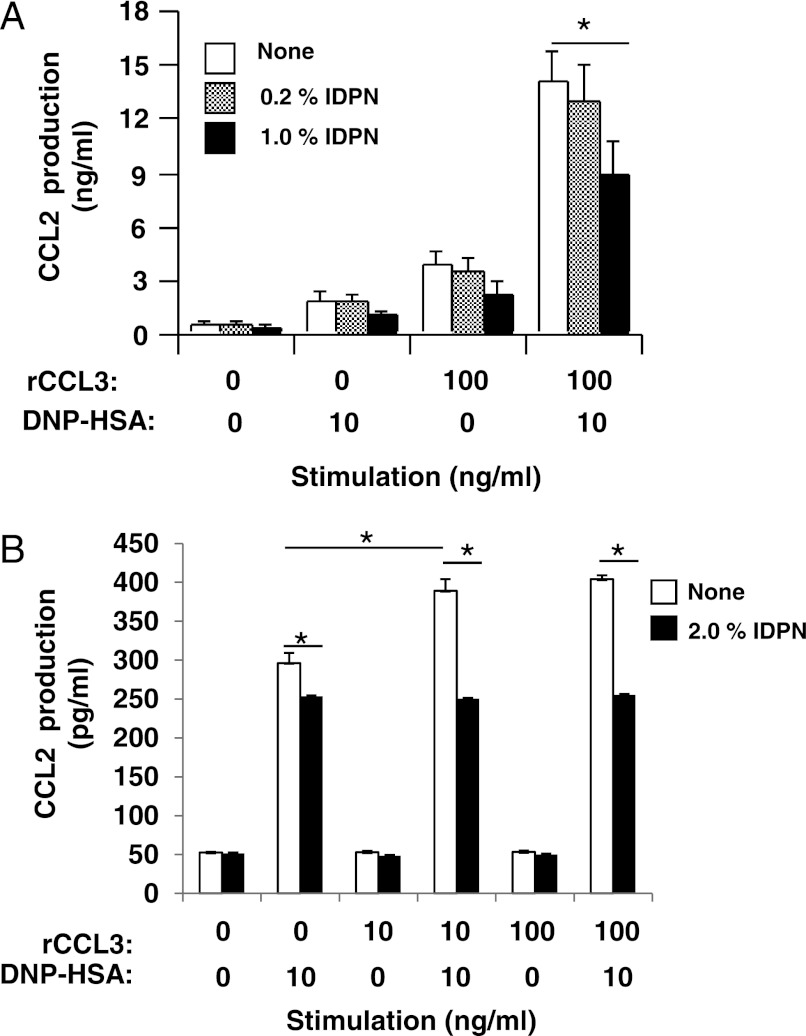
To further examine whether vimentin filaments are required for the phosphorylation of MAPKs and production of CCL2, RBL-CCR1 cells were treated with β,β′-iminodipropionitrile (IDPN), which induces aggregation of vimentin intermediate filaments and prevents formation of soluble vimentin (37, 38). The IDPN-treated cells were then stimulated with rCCL3 and/or IgE/Ag. As expected, soluble vimentin amounts decreased in the stimulated RBL-CCR1 cells after treatment with IDPN (Fig. 5B). Notably, FcϵRI- and CCR1-stimulated phosphorylation of p38 MAPK and ERK1/2 was not reduced in the IDPN-treated RBL-CCR1 cells (supplemental Fig. S1). Due to the low amount of soluble vimentin, the levels of associated MAPKs with the vimentin were lower in the IDPN-treated cells (Fig. 5B). Furthermore, IDPN treatment reduced CCL2 production in FcϵRI- and CCR1-activated RBL-CCR1 cells (Fig. 6A), suggesting involvement of soluble vimentin in the chemokine production.
FIGURE 6.
Reduced CCL2 production by β,β′-iminodipropionitrile treatment in RBL-CCR1 cells and BMMCs stimulated via FcϵRI and CCR1. RBL-CCR1 cells (A) and BMMCs (B) were sensitized with anti-DNP-IgE and were incubated with IDPN for the last 1 h of sensitization. The cells were then stimulated with 10 ng/ml DNP-HSA and/or 10 or 100 ng/ml rCCL3. Culture supernatants were harvested after stimulation. Concentrations of CCL2 in the supernatant were measured by ELISA. The plotted CCL2 concentration data are representative of three independent experiments. Error bars, S.D. *, p < 0.01.
Finally, we verified the involvement of vimentin in FcϵRI/CCR1-stimulated CCL2 production in BMMCs, a model of primary mast cells. Stimulating BMMCs with IgE/Ag plus rCCL3 enhanced formation of soluble vimentin, phosphorylation of MAPKs, and CCL2 production (Fig. 6B and supplemental Fig. S2). Furthermore, pretreatment of BMMCs with IDPN reduced CCL2 production upon IgE/Ag and rCCL3 stimulation (Fig. 6B).
DISCUSSION
Here, we identified vimentin as a component involved in the cross-talk between FcϵRI- and CCR1-mediated signaling pathways in mast cells. FcϵRI and/or CCR1 activation of RBL-CCR1 cells induced phosphorylation and subsequent disassembly of vimentin. Importantly, the disassembled and soluble vimentin interacted with phosphorylated ERK1/2 and p38 MAPKs in the stimulated RBL-CCR1 cells. p38 MAPK plays a role in FcϵRI- and CCR1-mediated CCL2 production in RBL-CCR1 cells. Conversely, inducing aggregation of vimentin filaments by IDPN reduced the levels of disassembled vimentin and hence the interactions with phosphorylated MAPKs and production of CCL2 in mast cells. These results suggest that vimentin could play a role in optimal CCL2 production in mast cells.
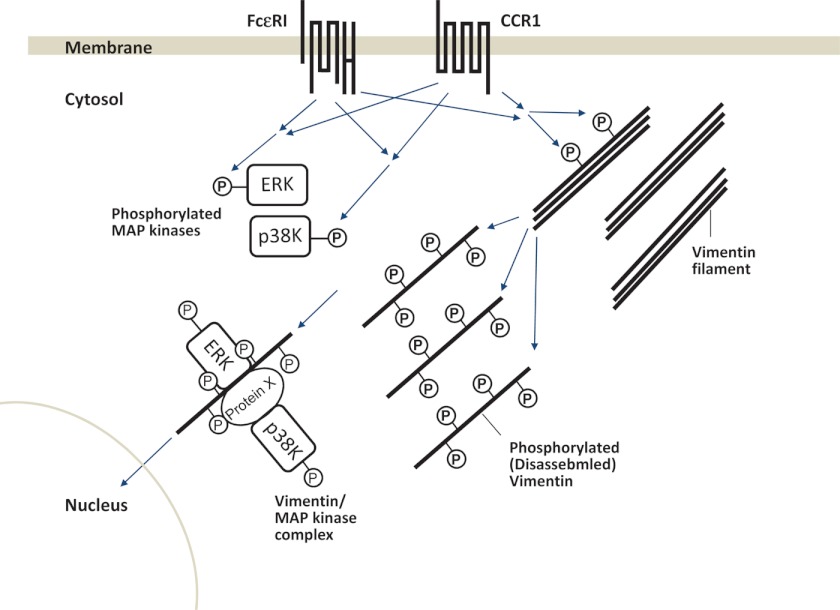
Vimentin has been shown to interact with phosphorylated ERK1/2 in several cells, such as neurons and adipocytes (35, 37). Perlson et al. (36) observed that vimentin binds directly to phosphorylated ERK1/2 but not to phosphorylated p38 MAPK and non-phosphorylated forms of these MAPKs. We observed that soluble vimentin interacted not only with phosphorylated ERK1/2 but also with phosphorylated p38 MAPK in FcϵRI- and CCR1-activated RBL-CCR1 cells. Because vimentin is capable of interacting with a variety of proteins involved in cell signaling cascades (31, 39), phosphorylated p38 MAPK might indirectly interact with vimentin by binding to other vimentin-binding proteins. In neurons, it has been shown that the complex of vimentin and phosphorylated ERK1/2 moves from the cytoplasm to the nucleus (35). Translocation of MAPKs into the nucleus is a mechanism for the gene expression of cytokines and chemokines. Disassembled vimentin, which is generated by FcϵRI and/or CCR1 stimulation, may act as a shuttle protein for the MAPKs to enter into the nucleus in mast cells, as observed in neurons (see the hypothetical model in Fig. 7).
FIGURE 7.
Hypothetical model for FcϵRI and CCR1 mediated signaling pathways in mast cells. FcϵRI and CCR1 activation synergistically induces phosphorylation of ERK1/2, p38 MAPK and vimentin in mast cells. Phosphorylation of vimentin induces disassembly of the filament protein. The disassembled vimentin interacts with phosphorylated ERK1/2 directly or with phosphorylated p38 MAPK indirectly via other vimentin-binding protein(s) (protein X). Vimentin may then act as a shuttle protein for the activated MAPKs to translocate them into the nucleus.
p38 MAPK has been shown to play a critical role in calcium ionophore-, cytokine-, or FcϵRI-mediated CCL2 production in cultured mast cells, such as BMMC and a human leukemic mast cell line, HMC-1 (40–42). Consistent with these previous studies, p38 MAPK is apparently involved in FcϵRI- and CCR1-mediated CCL2 production in RBL-CCR1 cells because inhibiting p38 MAPK reduced CCL2 production. Co-stimulation with rCCL3 and IgE/Ag enhanced phosphorylation of ERK1/2 and p38 MAPK in RBL-CCR1 cells and BMMCs. Such enhanced phosphorylation of p38 kinase and the association of the activated kinase with the disassembled vimentin could lead to its translocation into the nucleus and the enhanced CCL2 production we observed. Although vimentin interacts with phosphorylated ERK1/2, only p38 MAPK is required for CCL2 production in RBL-CCR1 cells. This may be due to enhancer and promoter regions in the CCL2 gene that only p38 MAPK may regulate. It has been shown that ERK1/2 and p38 MAPK activate a different set of substrates and transcription factors (43). Future studies on the enhancer/promoter region in the CCL2 gene would elucidate a detailed molecular mechanism for p38 MAPK-mediated chemokine production in mast cells.
Vimentin seems to be involved in mast cell degranulation (39). It was hypothesized that phosphorylation of vimentin induces disassembly of the intermediate filaments, subsequently increasing the mobility of granules (39, 44, 45). Supporting this hypothesis, degranulation of BMMCs was enhanced by vimentin deficiency (39). In this study, we found that the levels of vimentin disassembly (i.e. levels of soluble vimentin) were not associated with degranulation levels in FcϵRI and/or CCR1-activated RBL-CCR1 cells (not shown). Degranulation of RBL-CCR1 cells was previously shown to be synergistically enhanced by rCCL3 and IgE/Ag stimulation in a CC chemokine dose-dependent manner; around 55 or 80% degranulation was induced by 10 or 100 ng/ml rCCL3, respectively, together with IgE/Ag (11). However, the level of soluble vimentin in the stimulated cells was almost the same (Fig. 5A). These results suggest that the disassembly of vimentin filaments does not determine the level of degranulation in mast cells.
Vimentin has many phosphorylation sites (27, 28, 31). FcϵRI and CCR1 co-stimulation in RBL-CCR1 cells enhanced phosphorylation of tyrosine, serine, and threonine residues of vimentin. It has been shown that phosphorylations at serines 55, 71, 72, and 82 of vimentin are coordinated by several kinases (i.e. Rho kinase and Aurora B) and induce disassembly of the protein filaments in mitotic cells (9, 23–25, 33). Here, co-stimulation of the cells with 10 ng/ml rCCL3 plus 10 ng/ml IgE/Ag induced the highest levels of phosphorylation at serines 55, 71, and 82. Interestingly, the levels of soluble vimentin in the cells stimulated with 10 or 100 ng/ml rCCL3 plus 10 ng/ml IgE/antigen were comparable. These results suggest that not only serines 55, 71, 82 but also other phosphorylation sites could regulate the disassembly of vimentin in activated RBL-CCR1 cells. Further study will be necessary to elucidate the mechanisms and roles of vimentin phosphorylation in the signaling cascades and function of mast cells and basophils.
In summary, our results suggest that vimentin could be a component inducing optimal CCL2 production in mast cells. Because increased expression of CCL2 has been observed in tissues of allergic patients, our findings could provide clues to unraveling detailed molecular mechanisms underlying allergic inflammation.
Acknowledgment
We thank Dr. Silja Wessler (Salzburg University) for helpful discussion.
This work was supported, in whole or in part, by National Institutes of Health (NIH) Grant R01-EY-019630-01 and NIH, NIAID, Grant AI-38910. This work was also supported by GlaxoSmithKline Pharmaceutical PLC.

This article contains supplemental Figs. S1 and S2.
- CCR
- CC chemokine receptor
- Ag
- antigen
- BMMC
- bone marrow-derived murine mast cell
- DNP
- dinitrophenyl
- DNP-HSA
- DNP-conjugated human serum albumin
- IDPN
- β,β′-iminodipropionitrile
- RBL
- rat basophilic leukemia
- IPG
- immobilized pH gradient
- rCCL3
- recombinant CCL3.
REFERENCES
- 1. Abramson J., Pecht I. (2007) Regulation of the mast cell response to the type 1 Fc ϵ receptor. Immunol. Rev. 217, 231–254 [DOI] [PubMed] [Google Scholar]
- 2. Gilfillan A. M., Tkaczyk C. (2006) Integrated signaling pathways for mast cell activation. Nat. Rev. Immunol. 6, 218–230 [DOI] [PubMed] [Google Scholar]
- 3. Ono S. J., Nakamura T., Miyazaki D., Ohbayashi M., Dawson M., Toda M. (2003) Chemokines. Roles in leukocyte development, trafficking, and effector function. J. Allergy Clin. Immunol. 111, 1185–1199; quiz 1200 [DOI] [PubMed] [Google Scholar]
- 4. Beer F., Kuo C. H., Morohoshi K., Goodliffe J., Munro P., Aye C. C., Dawson M., Richardson R. M., Jones L. H., Ikeda Y., Nakamura T., Toda M., Flynn T., Ohbayashi M., Miyazaki D., Ono S. J. (2007) Role of β-chemokines in mast cell activation and type I hypersensitivity reactions in the conjunctiva. In vivo and in vitro studies. Immunol. Rev. 217, 96–104 [DOI] [PubMed] [Google Scholar]
- 5. Tominaga T., Miyazaki D., Sasaki S., Mihara S., Komatsu N., Yakura K., Inoue Y. (2009) Blocking mast cell-mediated type I hypersensitivity in experimental allergic conjunctivitis by monocyte chemoattractant protein-1/CCR2. Invest. Ophthalmol. Vis. Sci. 50, 5181–5188 [DOI] [PubMed] [Google Scholar]
- 6. Campbell E. M., Charo I. F., Kunkel S. L., Strieter R. M., Boring L., Gosling J., Lukacs N. W. (1999) Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2−/− mice. The role of mast cells. J. Immunol. 163, 2160–2167 [PubMed] [Google Scholar]
- 7. Sousa A. R., Lane S. J., Nakhosteen J. A., Yoshimura T., Lee T. H., Poston R. N. (1994) Increased expression of the monocyte chemoattractant protein-1 in bronchial tissue from asthmatic subjects. Am. J. Respir. Cell Mol. Biol. 10, 142–147 [DOI] [PubMed] [Google Scholar]
- 8. Holgate S. T., Bodey K. S., Janezic A., Frew A. J., Kaplan A. P., Teran L. M. (1997) Release of RANTES, MIP-1α, and MCP-1 into asthmatic airways following endobronchial allergen challenge. Am. J. Respir. Crit. Care Med. 156, 1377–1383 [DOI] [PubMed] [Google Scholar]
- 9. Toda M., Nakamura T., Ohbayashi M., Ikeda Y., Dawson M., Aye C. C., Miyazaki D., Ono S. J. (2007) Mechanisms of leukocyte trafficking in allergic diseases. Insights into new therapies targeting chemokines and chemokine receptors. Expert Rev. Clin. Immunol. 3, 351–364 [DOI] [PubMed] [Google Scholar]
- 10. Miyazaki D., Nakamura T., Toda M., Cheung-Chau K. W., Richardson R. M., Ono S. J. (2005) Macrophage inflammatory protein-1α as a costimulatory signal for mast cell-mediated immediate hypersensitivity reactions. J. Clin. Invest. 115, 434–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Toda M., Dawson M., Nakamura T., Munro P. M., Richardson R. M., Bailly M., Ono S. J. (2004) Impact of engagement of FcϵRI and CC chemokine receptor 1 on mast cell activation and motility. J. Biol. Chem. 279, 48443–48448 [DOI] [PubMed] [Google Scholar]
- 12. Fifadara N. H., Aye C. C., Raghuwanshi S. K., Richardson R. M., Ono S. J. (2009) CCR1 expression and signal transduction by murine BMMC results in secretion of TNF-α, TGFβ-1, and IL-6. Int. Immunol. 21, 991–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laffargue M., Calvez R., Finan P., Trifilieff A., Barbier M., Altruda F., Hirsch E., Wymann M. P. (2002) Phosphoinositide 3-kinase γ is an essential amplifier of mast cell function. Immunity 16, 441–451 [DOI] [PubMed] [Google Scholar]
- 14. Aye C. C., Toda M., Morohoshi K., Ono S. J. (2012) Identification of genes and proteins specifically regulated by costimulation of mast cell Fcϵ receptor I and chemokine receptor 1. Exp. Mol. Pathol. 92, 267–274 [DOI] [PubMed] [Google Scholar]
- 15. Gaga M., Ong Y. E., Benyahia F., Aizen M., Barkans J., Kay A. B. (2008) Skin reactivity and local cell recruitment in human atopic and nonatopic subjects by CCL2/MCP-1 and CCL3/MIP-1α. Allergy 63, 703–711 [DOI] [PubMed] [Google Scholar]
- 16. Richardson R. M., Pridgen B. C., Haribabu B., Snyderman R. (2000) Regulation of the human chemokine receptor CCR1. Cross-regulation by CXCR1 and CXCR2. J. Biol. Chem. 275, 9201–9208 [DOI] [PubMed] [Google Scholar]
- 17. Groschwitz K. R., Ahrens R., Osterfeld H., Gurish M. F., Han X., Abrink M., Finkelman F. D., Pejler G., Hogan S. P. (2009) Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc. Natl. Acad. Sci. U.S.A. 106, 22381–22386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shevchenko A., Wilm M., Vorm O., Mann M. (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 19. Wilm M., Shevchenko A., Houthaeve T., Breit S., Schweigerer L., Fotsis T., Mann M. (1996) Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature 379, 466–469 [DOI] [PubMed] [Google Scholar]
- 20. Suckau D., Resemann A., Schuerenberg M., Hufnagel P., Franzen J., Holle A. (2003) A novel MALDI LIFT-TOF/TOF mass spectrometer for proteomics. Anal. Bioanal. Chem. 376, 952–965 [DOI] [PubMed] [Google Scholar]
- 21. Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
- 22. Valgeirsdottir S., Claesson-Welsh L., Bongcam-Rudloff E., Hellman U., Westermark B., Heldin C. H. (1998) J. Cell Sci. 111, 1973–1980 [DOI] [PubMed] [Google Scholar]
- 23. Tsujimura K., Ogawara M., Takeuchi Y., Imajoh-Ohmi S., Ha M. H., Inagaki M. (1994) Visualization and function of vimentin phosphorylation by cdc2 kinase during mitosis. J. Biol. Chem. 269, 31097–31106 [PubMed] [Google Scholar]
- 24. Yasui Y., Goto H., Matsui S., Manser E., Lim L., Nagata K., Inagaki M. (2001) Protein kinases required for segregation of vimentin filaments in mitotic process. Oncogene 20, 2868–2876 [DOI] [PubMed] [Google Scholar]
- 25. Ogawara M., Inagaki N., Tsujimura K., Takai Y., Sekimata M., Ha M. H., Imajoh-Ohmi S., Hirai S., Ohno S., Sugiura H. (1995) Differential targeting of protein kinase C and CaM kinase II signalings to vimentin. J. Cell Biol. 131, 1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDonald J. R., Finck B. K., McIntosh L. M., Wilson S. E. (2011) Anti-inflammatory approaches that target the chemokine network. Recent Pat. Inflamm. Allergy Drug Discov. 5, 1–16 [DOI] [PubMed] [Google Scholar]
- 27. Chang L., Goldman R. D. (2004) Intermediate filaments mediate cytoskeletal cross-talk. Nat. Rev. Mol. Cell Biol. 5, 601–613 [DOI] [PubMed] [Google Scholar]
- 28. Inagaki M., Nishi Y., Nishizawa K., Matsuyama M., Sato C. (1987) Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature 328, 649–652 [DOI] [PubMed] [Google Scholar]
- 29. Eriksson J. E., He T., Trejo-Skalli A. V., Härmälä-Braskén A. S., Hellman J., Chou Y. H., Goldman R. D. (2004) Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J. Cell Sci. 117, 919–932 [DOI] [PubMed] [Google Scholar]
- 30. Li Q. F., Spinelli A. M., Wang R., Anfinogenova Y., Singer H. A., Tang D. D. (2006) Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J. Biol. Chem. 281, 34716–34724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sihag R. K., Inagaki M., Yamaguchi T., Shea T. B., Pant H. C. (2007) Role of phosphorylation in the structural dynamics and function of types III and IV intermediate filaments. Exp. Cell Res. 313, 2098–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chou Y. H., Opal P., Quinlan R. A., Goldman R. D. (1996) The relative roles of specific N- and C-terminal phosphorylation sites in the disassembly of intermediate filament in mitotic BHK-21 cells. J. Cell Sci. 109, 817–826 [DOI] [PubMed] [Google Scholar]
- 33. Ivaska J., Pallari H. M., Nevo J., Eriksson J. E. (2007) Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 313, 2050–2062 [DOI] [PubMed] [Google Scholar]
- 34. Helfand B. T., Chou Y. H., Shumaker D. K., Goldman R. D. (2005) Intermediate filament proteins participate in signal transduction. Trends Cell Biol. 15, 568–570 [DOI] [PubMed] [Google Scholar]
- 35. Perlson E., Michaelevski I., Kowalsman N., Ben-Yaakov K., Shaked M., Seger R., Eisenstein M., Fainzilber M. (2006) Vimentin binding to phosphorylated Erk sterically hinders enzymatic dephosphorylation of the kinase. J. Mol. Biol. 364, 938–944 [DOI] [PubMed] [Google Scholar]
- 36. Perlson E., Hanz S., Ben-Yaakov K., Segal-Ruder Y., Seger R., Fainzilber M. (2005) Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron 45, 715–726 [DOI] [PubMed] [Google Scholar]
- 37. Kumar N., Robidoux J., Daniel K. W., Guzman G., Floering L. M., Collins S. (2007) Requirement of vimentin filament assembly for β3-adrenergic receptor activation of ERK MAP kinase and lipolysis. J. Biol. Chem. 282, 9244–9250 [DOI] [PubMed] [Google Scholar]
- 38. Galigniana M. D., Scruggs J. L., Herrington J., Welsh M. J., Carter-Su C., Housley P. R., Pratt W. B. (1998) Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol. Endocrinol. 12, 1903–1913 [DOI] [PubMed] [Google Scholar]
- 39. Nahm D. H., Tkaczyk C., Fukuishi N., Colucci-Guyon E., Gilfillan A. M., Metcalfe D. D. (2003) Identification of Fyn-binding proteins in MC/9 mast cells using mass spectrometry. Biochem. Biophys. Res. Commun. 310, 202–208 [DOI] [PubMed] [Google Scholar]
- 40. Teshima R., Onose J., Okunuki H., Sawada J. (2000) Effect of Ca2+ ATPase inhibitors on MCP-1 release from bone marrow-derived mast cells and the involvement of p38 MAP kinase activation. Int. Arch. Allergy Immunol. 121, 34–43 [DOI] [PubMed] [Google Scholar]
- 41. Wong C. K., Tsang C. M., Ip W. K., Lam C. W. (2006) Molecular mechanisms for the release of chemokines from human leukemic mast cell line (HMC)-1 cells activated by SCF and TNF-α. Roles of ERK, p38 MAPK, and NF-κB. Allergy 61, 289–297 [DOI] [PubMed] [Google Scholar]
- 42. Gonzalez-Espinosa C., Odom S., Olivera A., Hobson J. P., Martinez M. E., Oliveira-Dos-Santos A., Barra L., Spiegel S., Penninger J. M., Rivera J. (2003) Preferential signaling and induction of allergy-promoting lymphokines upon weak stimulation of the high affinity IgE receptor on mast cells. J. Exp. Med. 197, 1453–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cargnello M., Roux P. P. (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 75, 50–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sahara N., Siraganian R. P., Oliver C. (1990) Morphological changes induced by the calcium ionophore A23187 in rat basophilic leukemia (2H3) cells. J. Histochem. Cytochem. 38, 975–983 [DOI] [PubMed] [Google Scholar]
- 45. Izushi K., Fujiwara Y., Tasaka K. (1992) Identification of vimentin in rat peritoneal mast cells and its phosphorylation in association with histamine release. Immunopharmacology 23, 153–161 [DOI] [PubMed] [Google Scholar]