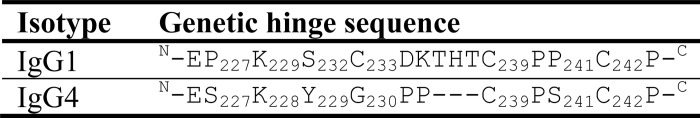Background: IgG1 and IgG4 have different inter-light chain-heavy chain disulfide bond (DSB) arrangements.
Results: IgG4 mutants with an IgG1-like DSB and a S241P hinge mutation showed increased Fab thermal stability and reduced DSB heterogeneity compared with IgG4 WT.
Conclusion: Fab domain thermal stability and DSB heterogeneity of IgG4 can be improved.
Significance: Such engineered IgG4 molecules offer potential advantages during therapeutic antibody production.
Keywords: Antibodies, Antibody Engineering, Disulfide Bond, Biotherapeutic, Protein Stability, Site-directed Mutagenesis, Chinese Hamster Ovary Cells, IgG4, Thermal Stability
Abstract
The integrity of antibody structure, stability, and biophysical characterization are becoming increasingly important as antibodies receive increasing scrutiny from regulatory authorities. We altered the disulfide bond arrangement of an IgG4 molecule by mutation of the Cys at the N terminus of the heavy chain constant domain 1 (CH1) (Kabat position 127) to a Ser and introduction of a Cys at a variety of positions (positions 227–230) at the C terminus of CH1. An inter-LC-CH1 disulfide bond is thus formed, which mimics the disulfide bond arrangement found in an IgG1 molecule. The antibody species present in the supernatant following transient expression in Chinese hamster ovary cells were analyzed by immunoblot to investigate product homogeneity, and purified product was analyzed by a thermofluor assay to determine thermal stability. We show that the light chain can form an inter-LC-CH1 disulfide bond with a Cys when present at several positions on the upper hinge (positions 227–230) and that such engineered disulfide bonds can consequently increase the Fab domain thermal stability between 3 and 6.8 °C. The IgG4 disulfide mutants displaying the greatest increase in Fab thermal stability were also the most homogeneous in terms of disulfide bond arrangement and antibody species present. Importantly, mutations did not affect the affinity for antigen of the resultant molecules. In combination with the previously described S241P mutation, we present an IgG4 molecule with increased Fab thermal stability and reduced product heterogeneity that potentially offers advantages for the production of IgG4 molecules.
Introduction
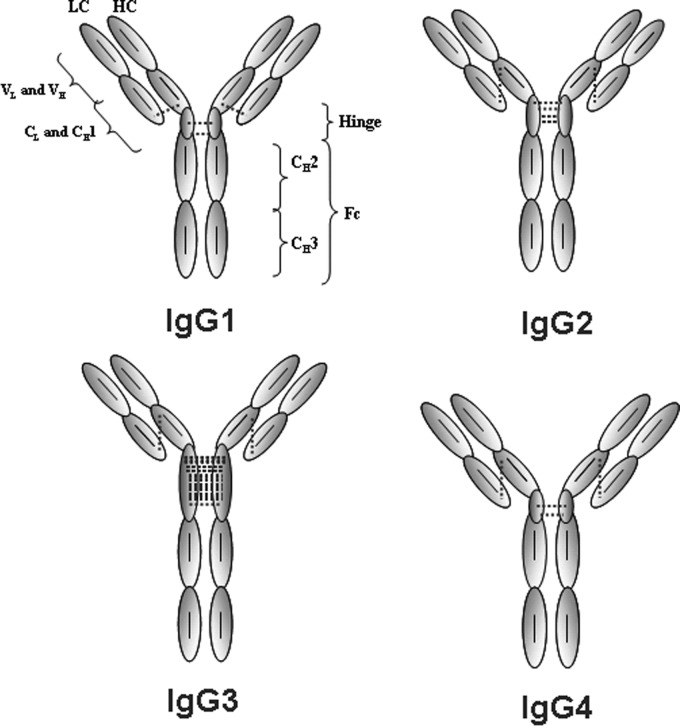
IgG is the most abundant antibody in human sera and is subdivided into four subclasses: IgG1, IgG2, IgG3, and IgG4. The Y-shape global topology consists of two heavy chains (HC),2 each covalently bonded to a light chain (LC) and to each other by inter-HC disulfide bonds (DSBs) in the hinge region. The IgG1 inter-LC-CH1 DSB is formed between Cys-214 (Kabat numbering) (1) of the LC κ and Cys-233 at the C terminus of the constant heavy chain domain 1 (CH1). In contrast, in IgG2, -3, and -4, this bond is formed between Cys-214 and Cys-127 at the N terminus of the CH1 (2, 3) (Fig. 1). In addition to differences in DSB arrangements, isotypes show the greatest sequence diversity within the hinge region and in the number of interhinge DSBs (Table 1). IgG1 and IgG4 both have two interhinge DSBs, IgG2 has four interhinge DSBs, and IgG3 has 11 interhinge DSBs. IgG1 and IgG3 are generally described as active isotypes because they perform antibody-dependent cell-mediated cytotoxicity and complement-dependent cell-mediated cytotoxicity. This is because these isotypes have the greatest affinity to Fcγ receptors (4–6), whereas complement 1q binds strongly to CH2 of these two isotypes (7, 8). Conversely, IgG2 and IgG4 bind poorly to these effector molecules, resulting in relatively low effector function induction.
FIGURE 1.
Schematic representation of the arrangement and number of DSBs of the human IgG isotypes. The dotted and solid lines indicate inter- and intra-DSBs, respectively.
TABLE 1.
Alignment of human IgG1 and IG4 genetic hinge sequences (Kabat numbering)
When employing an IgG as a biotherapeutic agent, the differential functionality described above is a key consideration in isotype selection. IgG1 is currently the most widely used as a therapeutic due to its long half-life (9) and enhanced antibody-dependent cell-mediated cytotoxicity and complement-dependent cell-mediated cytotoxicity induction. These characteristics are beneficial when employed against cancer targets (10). In cases where the target antigen only needs to be neutralized without cell killing, IgG2 or IgG4 could also be used. In addition to effector functions and stability and biophysical characteristics, analytical and regulatory aspects are important selection criteria for such monoclonal antibodies (mAbs) destined for clinical use. Further, it is well known that during manufacturing, purification, formulation, and storage, the isotypes behave differently in terms of their sensitivity to pH or temperature, aggregation propensity, and susceptibility to degradation (11–13). With regard to monitoring or predicting stability, thermal stability is increasingly accepted as a convenient surrogate measure of global stability. Garber and Demarest (14) showed that IgG isotypes can be ranked based on their CH2 thermal stabilities as follows: IgG1 > IgG2 > IgG4. Comparisons between IgG1 and IgG4 have also consistently shown that IgG4 molecules have lower Fab domain thermal stability compared with IgG1.3
IgG4 molecules are unique compared with other human IgG isotypes in that they can form functionally monovalent bispecific molecules through a mechanism called “Fab arm” or “half-molecule” exchange (15–19). The interhinge DSBs at positions 239 and 242 have been shown to be liable to form intrahinge DSBs generating half-molecules that can covalently reassociate with an IgG4 half-molecule of the same or a different variable region (20, 21). This phenomenon is known to involve CH3 (22) and the core hinge of the antibody (20), but the mechanism that drives this process is not well understood or characterized. In vivo, this may be advantageous in natural antibody responses but less desirable when IgG4 is employed as a biotherapeutic. It has been shown that the core hinge mutation S241P can greatly diminish the formation of the intrahinge DSBs (20). This mutation was also later shown to have a protective effect against freeze/thaw-induced aggregation of IgG4 (23). Domain exchange experiments of IgG4 CH3 with an IgG1 (22) or specific mutations in CH3 have been shown to further minimize the rate of Fab arm exchange (24, 25). Other stabilizing mutations of IgG4 within CH3 were described by Nobuaki and Hideaki (26) that reduced their sensitivity to low pH, resulting in an IgG4 producing less aggregate.
Here we report the generation of a more thermally stable IgG4 by altering the inter-LC-CH1 DSB arrangement to mimic that of an IgG1. The mutants were analyzed for DSB heterogeneity by SDS-PAGE and for thermal stability using a thermofluor assay. We show that the Cys-214 (on the LC) is capable of being promiscuous in bonding to Cys residues in the HC at four different positions but exhibits some preference for bonding at position 229. When bonded to a Cys residue in the C terminus of the CH1, the Fab stability was shown to increase by up to 6.8 °C. In combination with the previously reported S241P mutation (20), we present an IgG4 molecule with reduced levels of product DSB heterogeneity and increased Fab domain stability compared with IgG4 WT.
EXPERIMENTAL PROCEDURES
Generation of Mutant Plasmids, Expression, and Purification
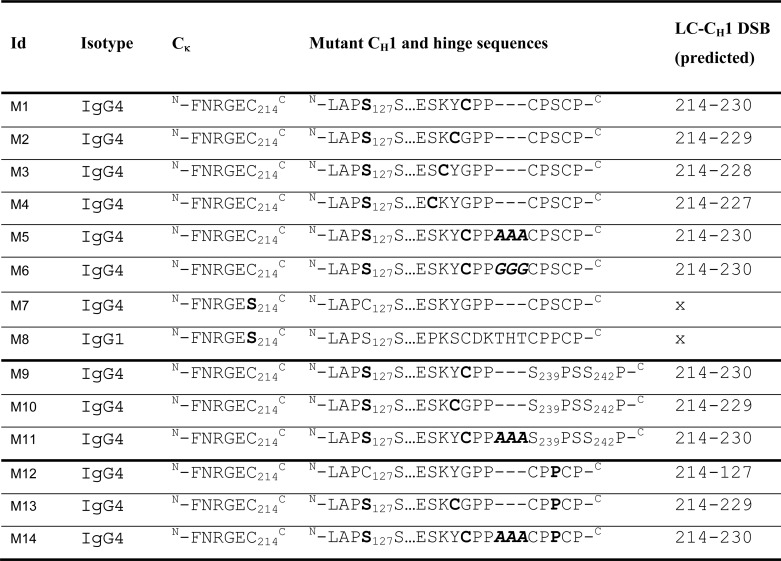
Site-directed mutagenesis was performed using the QuikChange® system (Stratagene, Wokingham, UK) according to the manufacturer's instructions. Oligonucleotides were designed to mutate C127S at the N terminus of IgG4 and substitute residues at positions 227, 228, 229, and 230 with Cys residues. To introduce a space between the introduced Cys-230 and hinge Cys-239, either a 3-residue Ala (∧AAA) or Gly (∧GGG) was introduced to the protein sequence and called spacers. In addition, hinge mutations C239S and C242S were also incorporated into selected mutants. (See Table 2 for a list of mutants.) Mutants with no inter-HC or inter-LC-CH1 DSB for IgG1 and IgG4 WT were also generated. Mammalian single gene vectors (UCB) containing either the HC or LC were co-transfected into CHO-K1 cells (ATCC, Teddington, UK) to transiently express the IgG4 mutant antibodies. Cells were cultured in CD CHO medium (Invitrogen) supplemented with 2 mm Glutamax (Invitrogen) at 37 °C and 5% CO2. To generate material for immunoblot analyses, small scale transfections were performed using Lipofectamine2000 (Invitrogen). To generate larger amounts of material for purification, antibody DNA was transfected into CHOK1 cells by electroporation. For this, HC and LC DNA were diluted to 3.2 μg/ml, and 50 μl of HC and 50 μl of LC DNA was added to 3.2 × 108 cells, which were resuspended in 700 μl of Earle's balanced salt solution (Sigma). The cell/DNA mixes were then electroporated into the cells. For each mutant antibody, three cuvettes were electroporated and added to 250 ml of CD CHO culture medium (Invitrogen), supplemented with 2 mm Glutamax (Invitrogen). In addition to transfection of IgG1 and IgG4 WT DNA, Table 2 lists the combinations of mutant HC and LC DNA that were used for transfections. On day 3, sodium butyrate was added to the cultures to a final concentration of 3 mm. Cell culture supernatants were harvested once the cells' viability dropped below 40% or on day 14 post-transfection (whichever occurred first). Supernatants were purified on a 5-ml HiTrap MabSelect SuRe protein A column (GE Healthcare). Samples were loaded onto the column at 2 ml/min. The column was then washed with PBS, pH 7.4, at 2 ml/min, and bound material was eluted with 0.1 m sodium citrate, pH 3.4, at 2 ml/min, and 1-ml fractions were collected. The eluted protein was detected by absorbance at 280 nm. Peak fractions were neutralized by adding 0.2 ml of 2 m Tris-HCl (pH 8.5) to each. Purified material was buffered exchanged into PBS, pH 7.4, and stored at 4 °C for further analysis.
TABLE 2.
Mutations (in boldface type) and insertions (boldface and italic type) made in either the heavy chain or κ chain and the predicted inter LC-CH1 DSB (Kabat numbering)
“x” indicates that no DSB is predicted to form between LC and HC.
Immunoblot Analysis
Expressed material in crude supernatants was run on Tris-Gly gels (Invitrogen) and analyzed by immunoblot. Samples were centrifuged at 1200 rpm for 5 min to pellet the cells. Using protein A biosensors, the supernatant antibody concentration was determined on an OCTET instrument (ForteBio, Menlo Park, UK). Appropriate volumes of antibody were calculated to load 30 ng onto the gel. Antibody, 5 μl of 4× LDS loading buffer (Invitrogen), 2 μl of 100 mm N-ethylmaleimide (Thermo Scientific, Basingstoke, UK), and PBS were prepared to a final volume of 20 μl. Samples were boiled for 3 min at 95 °C, and SDS-PAGE was performed. Gels were run at 150 V for 1 h. Following protein transfer (8 min) to a nitrocellulose membrane using the iBlot dry transfer system (Invitrogen), membranes were blocked for 1 h at room temperature in blocking buffer (0.1% (v/v) Tween 20, 5% (w/v) skimmed milk in PBS) with agitation. Rabbit anti-human IgG Fc HRP-conjugated antibody (Jackson Immunoresearch, catalog no. 309-001-008) and goat anti-human κ light chain HRP-conjugated antibody (Bethyl, catalog no. A80-115P) were diluted in PBS plus 0.1% Tween 20 (PBS-T) to 1:1000 and were used to probe the membranes for 1 h with agitation. The membranes were then washed three times for 5 min each with PBS-T. The migration patterns were visualized using a metal-enhanced DAB substrate kit according to the manufacturer's instructions (Pierce).
Size Exclusion Chromatography (SEC) HPLC
SEC HPLC was performed to monitor covalently and non-covalently associated purified antibody. Samples were run on an analytical Superdex 200 10/30 Tricorn column (GE Healthcare) at a flow rate of 1 ml/min in PBS, pH 7.4. UV detection was set at 214 and 280 nm. IgG size was determined by using gel filtration standards (Bio-Rad).
Thermal Stability
A thermofluor assay was used to monitor the thermal stability of purified antibody. The reaction mix contained 5 μl of purified mAb at 1 mg/ml, 5 μl of 30× SYPRO® orange dye (Invitrogen), and 40 μl of PBS. 10 μl of the mix was dispensed in triplicate into a 384 PCR optical well plate and analyzed on a 7900HT fast real-time PCR system (Agilent Technologies, Wokingham, UK). This PCR system contains a heating device for accurate temperature control set at 20–99 °C at a ramp rate of 1.1 °C/min; a charged coupled device monitored the fluorescence changes in the wells.
Antigen Affinity Measurement
Antigen affinity (KD) was determined on a Biacore 3000 instrument using a CM5 sensor chip with anti-hIgG (Fc fragment-specific) immobilized on the surface, which captured recombinant antibody from the transient culture supernatant. Human cytokine was then titrated across the captured antibody surface, and binding kinetics was determined. Differences in KD of more than 2-fold compared with IgG4 WT were considered significant.
RESULTS
Generation and Expression of IgG4 Molecules
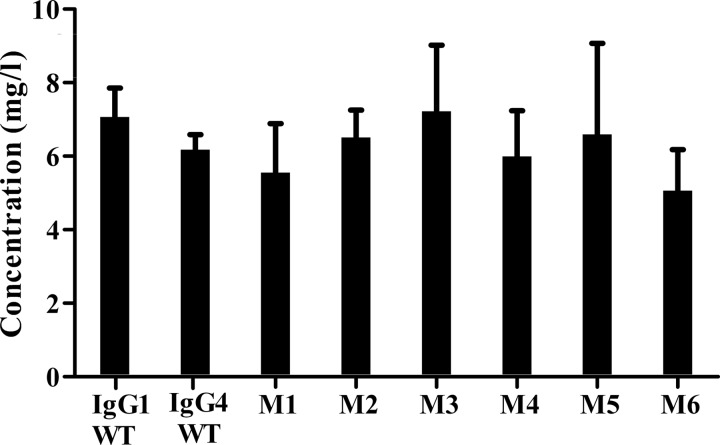
From a sequence alignment of IgG1 and IgG4 (Table 1), residues within the IgG4 CH1 were identified to investigate their ability to form an alternative inter-LC-CH1 DSB after mutation to a Cys residue (Table 2). Position 230 seemed most analogous to that found in IgG1, but positions 227–229 were also mutated. The contribution of the hinge length was also included in the analysis by introduction of either a 3-Ala or 3-Gly spacer. After sequence verification, plasmids coding for the desired mutations were transfected into CHO-K1 cells to allow transient antibody expression. Expression levels ranged from 5 to 7 μg/ml, but no differences in expression levels of antibody were observed between these mutants and WT IgG4 and IgG1 (Fig. 2).
FIGURE 2.
Expression analysis of IgG1 WT, IgG4 WT, and IgG4 mutants as determined by using protein A biosensors in an OCTET detection system (n = 3). Error bars, S.D.
Immunoblot Analysis of IgG4 Molecules with Altered Inter-LC-CH1 DSB Arrangements
Analysis by immunoblot under non-reducing conditions of the expressed material in cell culture supernatants showed a number of distinct bands. These bands were assigned to molecular forms, as shown in Table 3. Fully intact antibody was assigned (HCLC)2, HC dimer was assigned (HC)2, two HC with one LC covalently associated was assigned (HC)2LC, one HC covalently associated with one LC was assigned HCLC, free HC was assigned HC, LC dimer was assigned (LC)2, and free LC was assigned LC. The identities of these were confirmed by taking each band observed on both the anti-hFc and anti-κ blots and subjecting them to N-terminal sequencing (data not shown).
TABLE 3.
Description of antibody species observed in immunoblot analysis
Thick dark lines indicate HC, and thick gray lines indicate LC. Thin black lines indicate interchain DSBs.
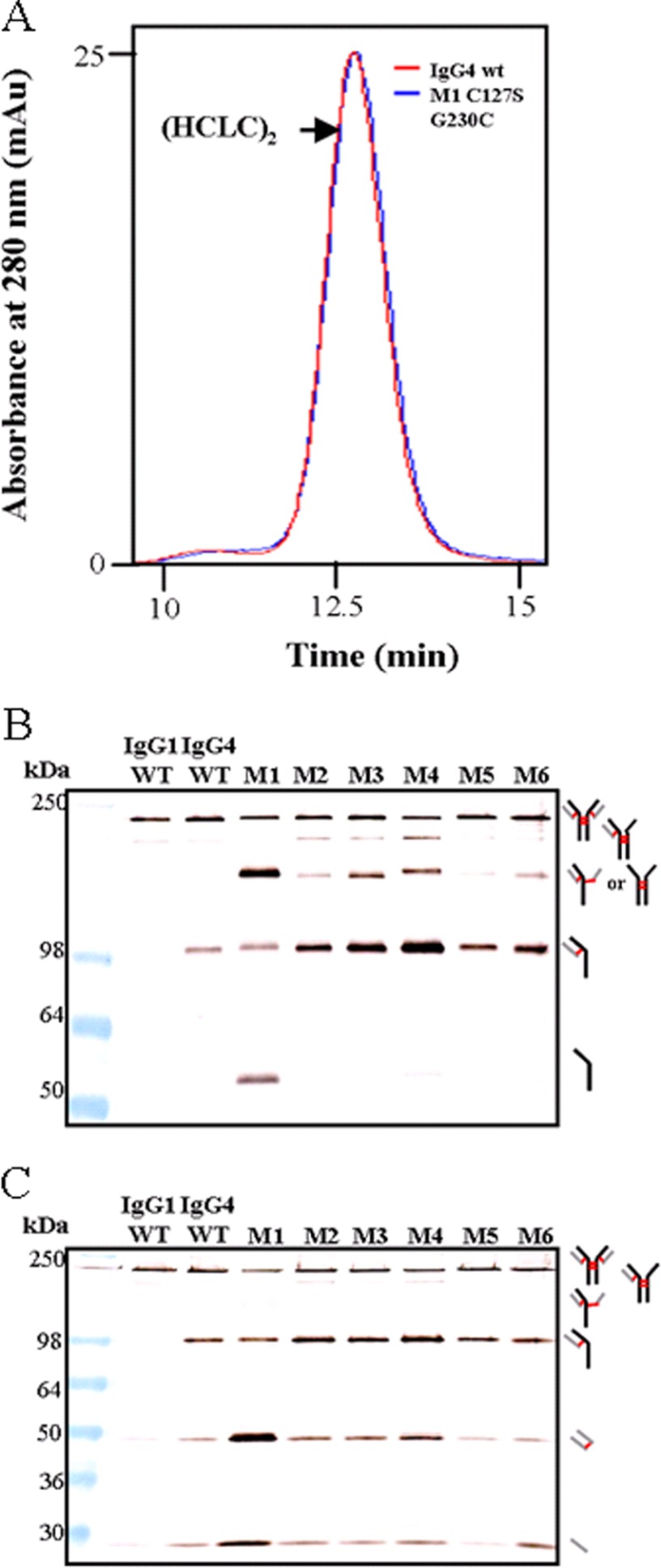
Fig. 3A shows an overlay of the IgG4 WT and mutant 1 (M1, C127S/G230C) SEC traces. No difference was observed between the two samples, which means that despite the multiple banding patterns observed after immunoblotting, the parent molecular species formed intact (HCLC)2 in solution, but the polypeptides were not exclusively covalently bonded. Fig. 3, B and C, shows the migration pattern of IgG1 WT (lane 1), IgG4 WT (lane 2), and mutants 1–6 (M1–M6). IgG1 WT has the simplest banding pattern, consisting largely of intact (HCLC)2, with low levels of (HC)2LC, (LC)2, and free LC. In comparison, IgG4 WT had substantially more HCLC and increased levels of (LC)2 and free LC. M1 (C127S/G230C) showed a greater DSB heterogeneity than IgG4 WT, and increased amounts of (HC)2, HC, (LC)2, and LC were also present. M2 (C127S/Y229C) had a banding pattern similar to that of IgG4 WT except for an additional (HC)2 band that was observed and increased levels of HCLC. Compared with IgG4 WT, M3 (C127S/K228C) also had an additional (HC)2 band, and the band intensity of the HCLC band was much greater. In M4 (C127S/S227C), the additional (HC)2 band was also observed alongside some free HC. The HCLC band was considerably more intense compared with IgG4 WT for mutants M3 and M4. The M5 mutant (C127S/G230C ∧AAA) samples had a banding pattern most similar in its migration pattern to the IgG4 WT pattern. However, low levels of (HC)2 were also present in M5 (C127S/G230C ∧AAA) as well as increased levels of HCLC. M6 (C127S/G230C ∧GGG) samples also contained the additional (HC)2 band, but it was more intense than M5, as was the HCLC band. Overall, these data show that the molecules with the simplest banding pattern are the M2 (C127S/Y229C) and M5 (C127S/G230C ∧AAA) mutants.
FIGURE 3.
SEC HPLC and immunoblot of IgG4 molecules with altered DSB arrangements toward an IgG1-like DSB, including mutants with a 3-residue spacer within the hinge region. All mutants include C127S in addition to the mutation noted. A, SEC HPLC trace comparison of IgG4 WT and M1 (C127S/G230C). B, comparison of IgG4 WT with mutants that had a Cys introduced at different positions in the CH1. Samples were run under non-reducing conditions and probed with an anti-human Fc antibody. C, comparison of the same samples, also run under non-reducing conditions but probed with an anti-human κ antibody. For both A and B, thick black lines indicate HC, thick gray lines indicate LC, and red lines indicate DSBs.
Thermal Stability Analyses of IgG4 Molecules with Altered Inter-LC-CH1 DSB Arrangements
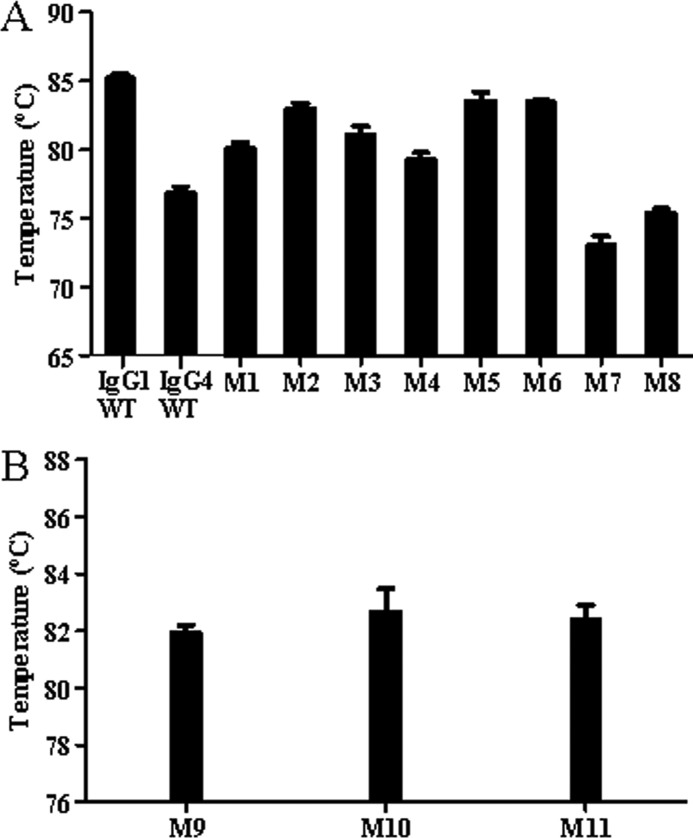
A thermofluor assay can be used to determine thermal unfolding events of IgG domains and was performed to determine the contribution of the LC-CH1 DSB to the thermal stability of the Fab domain. In the case of a mixed population of antibody disulfide isoforms in solution, an average of the thermal stabilities of the different species is necessarily calculated. The IgG4 WT Fab stability (76.8 °C) was more than 8 °C lower than the IgG1 Fab stability (85.2 °C) (Fig. 4). An increase in Fab stability of 3.3 °C was observed between IgG4 WT and the M1 (C127S/G230C) mutant. This improvement was even greater at 6.2 °C when comparing the M2 (C127S/Y229C) mutant to IgG4 WT. Alternative positions of the Cys on the CH1 resulted in a much smaller increase in Fab stability of 4.3 and 2.5 °C for M3 (C127S/K228C) and M4 (C127S/S227C), respectively, compared with the IgG4 WT Fab stability. The Fab stabilities of M5 (C127S/G230C ∧AAA) and M6 (C127S/G230C ∧GGG) showed an increase above the IgG4 WT Fab stability of almost 7 °C. To investigate the intrinsic stability of both IgG1 and IgG4 stability, mutants that have no (predicted) inter-LC-CH1 DSB due to the mutation of both LC and CH1 Cys to Ser were investigated. The IgG4 molecule with no inter-LC-CH1 DSB (M7) had a lower Fab stability compared with IgG4 WT by 3 °C at 73 °C, whereas the IgG1 Fab stability decreased by 10 °C when no inter-LC-CH1 DBS is present (M8) to 75.4 °C. Fab stability of the IgG1 molecule remained greater than that of the IgG4 molecule by ∼2 °C. These results in combination with the results observed when altering the DSB arrangement of the IgG4 molecule show that it is the DSB arrangement that very substantially contributes to the increased Fab stability of IgG1 compared with IgG4. Introduction of an IgG1-like DSB arrangement increased the stability of the Fab domain of an IgG4 but to a varying extent dependent on both position of the Cys in CH1 and the length of the upper hinge.
FIGURE 4.
Comparison of the thermal stability of the Fab domains of selected mutants determined by the thermofluor assay (n = 3). Error bars, S.D.
Contribution of the S241P Core Hinge Mutation to DSB Heterogeneity and Fab Thermal Stability
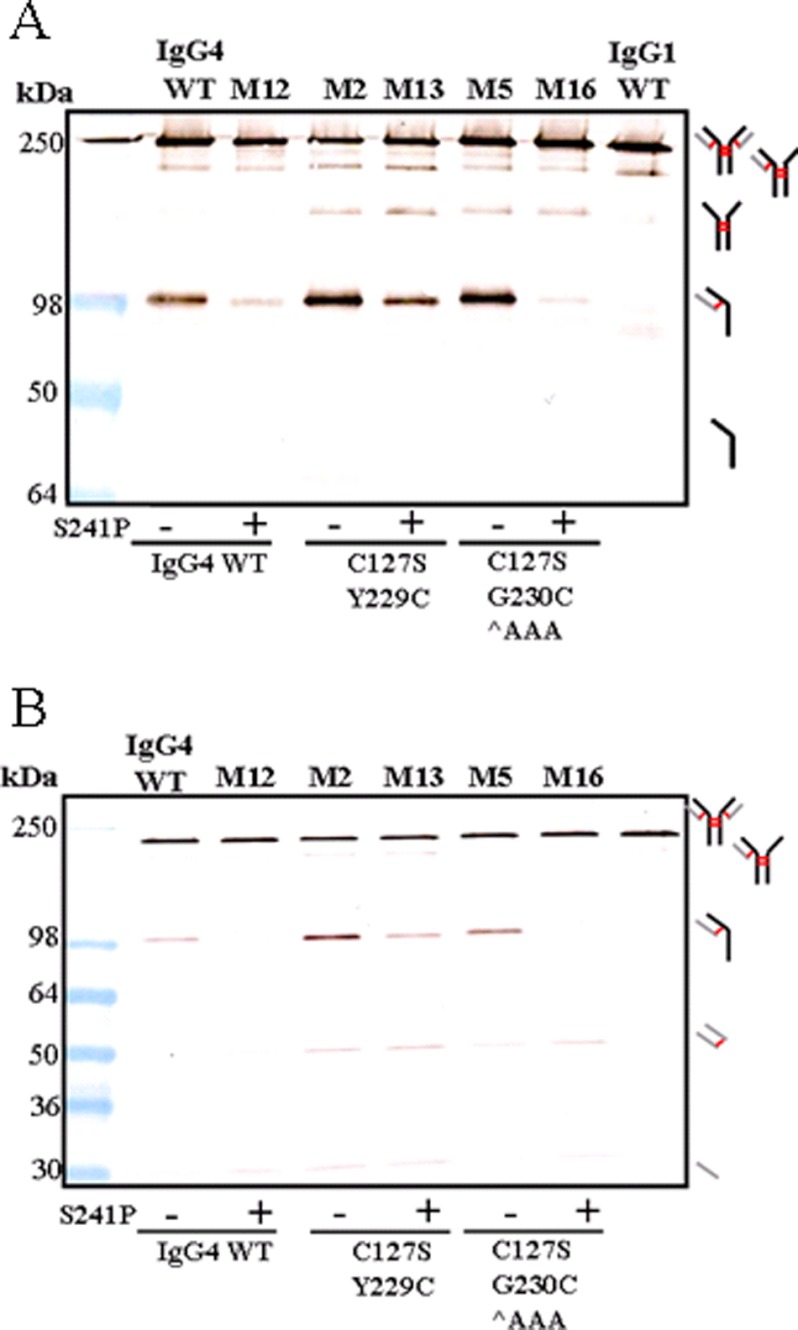
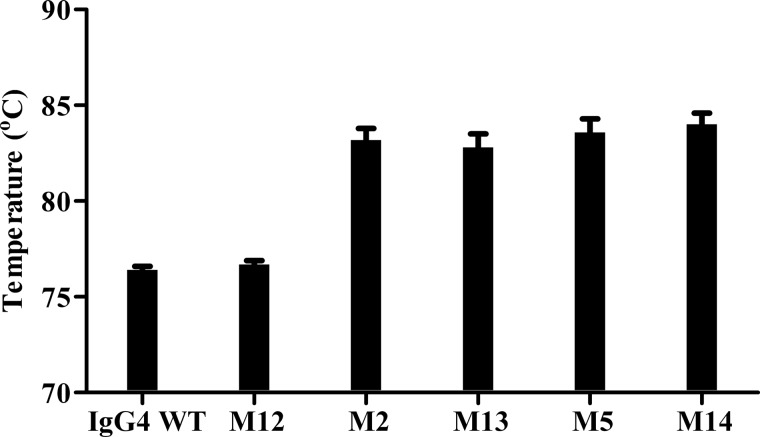
Fig. 5, A and B, shows that the incorporation of the S241P mutation reduced the level of HCLC observed when samples were analyzed by immunoblotting under non-reducing conditions. This is in agreement with previous studies (20). Comparison of the IgG4 WT banding pattern to mutant M12 (IgG4 S241P) showed a large reduction in the level of HCLC. Compared with IgG4 WT, M2 (C127S/Y229C) had increased levels of (HC)2 and HCLC. Introduction of the S241P mutation to this mutant (M13, C127S/Y229C/S241P) reduced the level of HCLC but not to the same extent as observed between IgG4 WT and M12. The introduction of an Ala spacer (M5, C127S/G230C ∧AAA) increased the relative amounts of HCLC compared with IgG4 WT, but upon the introduction of S241P (M14), the levels of HCLC observed after immunoblotting were similar to M12 (IgG4 S241P). Thermal stability analyses (Fig. 6) showed no significant difference in Fab stability of IgG4 WT and selected mutants with or without the S241P mutation. Fab domain stability of M12 (IgG4 S241P) remained the same at 76 °C compared with IgG4 WT. Fab domain stability of M2 (C127S/Y229C) increased by ∼6 °C compared with IgG4 WT, but introduction of the S241P mutation (M13) did not alter this increase in Fab thermal stability. Similarly, an increase of 6 °C was also observed when comparing M5 (C127S/G230C ∧AAA) to IgG4 WT, and the further introduction of S241P (M14) did not affect the increased stability.
FIGURE 5.
Investigating the effect of introducing the S241P mutation within the core hinge of IgG4 WT of selected mutants. A, immunoblot analysis under non-reducing conditions with (+) or without (−) the S241P mutation. The membrane was probed with an anti-human FC antibody. B, the membrane was probed with an anti-human κ antibody. For both A and B, thick black lines indicate HC, thick gray lines indicate LC, and red lines indicate DSBs.
FIGURE 6.
Thermal stability of the Fab domains of IgG4 and mutant IgG4 with (+) or without (−) the S241P mutation as determined by the thermofluor assay (n = 3). Error bars, S.D.
Thermal Stability Analyses of IgG4 Molecules with Mutated Interhinge Arrangements
To investigate the intrinsic Fab domain stability of an IgG4 with an altered DSB arrangement, hinge Cys residues were mutated to Ser residues to prevent interhinge or alternative LC-HC DSB formation. Upon SDS-PAGE analysis of such mutants, only HCLC was observed (data not shown). Fig. 4B shows Fab thermal stabilities of previously described mutants M1, M2, and M5 lacking interhinge DSBs. An increase in Fab stability was observed when comparing M1 (C127S/G230C) with its corresponding mutant M9 (C127S/G230C/C239S/C242S) from 80 to 82 °C. However, when comparing the stability of M2 (C127S/Y229C) and M5 (C127S/G230C ∧AAA) with the stability of their respective hinge mutants, M10 (C127S/Y229C/C239S/C242S) and M11 (C127S/G230C ∧AAA C239S/C242S), the stabilities were comparable. M2 and M10 had respective Fab thermal stabilities of 83 and 82.7 °C. M5 and M11 had Fab stabilities of 83.6 and 82.4 °C, respectively.
Antigen Affinity
Antigen affinity analysis was performed to ensure that mutation of the IgG4 DSB arrangement did not affect the affinity to the target antigen. A less than 2-fold change in affinity was regarded as insignificant using this assay. The results showed that the affinity of IgG4 WT to antigen was 114 pm, and the affinities of the mutants were 87, 121, and 131 pm for M1, M2, and M5, respectively (Table 4). These fall within the range of error for the BIAcore assay; hence, the altered DSB arrangements investigated were considered not to affect affinity to the antigen.
TABLE 4.
Antigen affinity of IgG1 WT, IgG4 WT, and selected IgG4 mutants to their cytokine
| IgG | ka | kd | KD |
|---|---|---|---|
| m−1s−1 | s−1 | pm | |
| IgG1 WT | 2.77E+06 | 3.54E−04 | 128 |
| IgG4 WT | 2.87E+06 | 3.26E−04 | 114 |
| M1 (C127S/G230C) | 3.13E+06 | 2.73E−04 | 87 |
| M2 (C127S/Y229C) | 2.07E+06 | 2.50E−04 | 121 |
| M5 (C127S/G230C ∧AAA) | 2.16E+06 | 2.82E−04 | 131 |
DISCUSSION
We have altered the DSB arrangement of an IgG4 to mimic that of IgG1, and our results show that the LC Cys-214 residue can form a DSB with the HC if the HC Cys is at position 227, 228, 229, or 230. In addition, we have also shown that such an altered DSB arrangement can increase the Fab domain thermal stability of an IgG4 molecule. These results are in agreement with those of Heads et al.,3 who demonstrated an improvement of Fab thermal stability when replacing the IgG4 hinge with the IgG1 hinge. In addition, replacement of the IgG1 hinge with that of an IgG4 resulted in a decrease in IgG1 Fab thermal stability.3
For all of the different mutants described and investigated in this study, the manipulation of an IgG4 molecule to form an inter-LC-CH1 DSB at position 229 (M2) or at position 230 in combination with an Ala spacer (M5) showed the least product heterogeneity when samples were analyzed by non-reducing SDS-PAGE. M2 (C127S/Y229C), M5 (C127S/G230C ∧AAA), and M6 (C127S/G230C ∧GGG) also benefit from the greatest increase in Fab domain thermal stability compared with the IgG4 WT molecule, as we might expect from the more homogeneous populations. Improved stabilities of Fab domains or of CH2 have been correlated to improved yields in Escherichia coli; higher levels of properly folded, functional protein; and increased levels of monomer (27–29). It is possible that the increase in Fab domain stability that we observe for the modified IgG4 molecules could potentially decrease the propensity to form aggregate that may be valuable in antibody production processes and long term storage.
IgG4 has also been shown to be liable to undergo Fab arm exchange, and the presence of HCLC after running IgG4 on SDS-PAGE has been well described (15, 17, 18, 20). The hinge flexibility and its tendency to form intra-DSBs can be limited by the introduction of S241P to the core hinge (20, 21, 30). The addition of N-ethylmaleimide during sample preparation can further minimize the formation of HCLC by preventing DSB scrambling (32, 33). Our results are consistent with these findings, and the level of HCLC observed on a gel when introduced to either IgG4 WT or mutants thereof (M2 or M5) was decreased. However, for M2, this decrease is somewhat less than for other S241P mutants, and we suggest that the S241P mutation in the context of an altered DSB arrangement at position 229 diminishes the positive impact of S241P because of a possible slight conformational change to the IgG4 molecule.
Our data also show that LC can form DSBs with Cys residues at several positions on the HC. IgG1 is unique in its DSB arrangement because LC Cys-214 forms an inter-LC-CH1 DSB with HC Cys-233, whereas this DSB is formed with Cys-127 in IgG2, -3, and -4 (3). Although these Cys residues are at different positions in linear sequence, spatially they are in close proximity to one another (29). By introducing Cys residues at different positions on the HC of an IgG4 molecule, we investigated if LC Cys-214 has a preference in bonding to an alternative Cys position. Previous work performed on IgG2 demonstrated that this isotype can form functional isoforms by LC Cys-214, forming DSBs with Cys residues at both the C and N termini of the HC (31, 34, 35). In the context of an IgG4 molecule, we suggest, based on the level of (HCLC)2 and the simplicity of each mutant's banding pattern, that one can rank the preference of the LC Cys-214 to form a DSB with a Cys on the HC in the order 127 > 230 (if a spacer is present) > 229 > 228 > 227 > 230. An increase in Fab domain thermal stability of ∼6.5 °C over IgG4 WT was observed for M2 (C127S/Y229C), and we suggest that the preferential bonding of LC Cys-214 to Cys-229 compared with 228 and 227 is related to the improved Fab domain thermal stability.
The more complex banding pattern observed for M1 (C127S/G230C) compared with IgG4 WT suggests that Cys-214 (on κ LC) forms a DSB with other HC Cys residues and that there is a mixed population of covalently associated IgG4, non-covalently associated “correct” IgG4, and a small percentage of covalently associated IgG4 with incomplete or unusual DSB arrangements expressed with this mutant. One possible arrangement is that the LC Cys-214 forms a DSB with Cys-239 (in the core hinge) instead of the introduced Cys-230; hence, the introduced Cys-230 may form an inter-HC DSB with another HC Cys-230. Alternatively, Cys-230 and both hinge Cys residues all form DSBs with their corresponding residues on another HC, evident from the more intense (HC)2 band observed on a gel. In this case, the LC must be non-covalently associated with the HC in solution to form (HCLC)2, as is illustrated by the SEC data (Fig. 3A). The increase in Fab thermal stability for M1 (C127S/G230C) to 80.5 °C compared with IgG4 WT (76.4 °C) is an average of the proposed disulfide isoforms in solution. One possible disulfide LC-CH1 isoform that increased the average Fab thermal stability is between Cys-214 (LC) and introduced Cys-230 (HC). IgG4 mutant M9 (C127S/G230C/C239S/C242S) contains a LC-CH1 DSB directed to this position and has a Fab thermal stability of 82 °C (Fig. 4B).
Inappropriate LC-CH1 DSB formation of M1 (C127S/G230C) was apparently improved by the introduction of a three-residue spacer, as observed from the reduced DSB heterogeneity of M5 (C127S/G230C ∧AAA) and M6 (C127S/G230C ∧GGG). We hypothesize that the introduced residues generate a distance between Cys-230 and Cys-239, reducing the ability of LC Cys-214 to form an inappropriate DSB with Cys-239. Again such an increase in the DSB homogeneity resulted in a concomitant increase in Fab domain stability. The increased intensities of lower molecular weight species in M6 (C127S/G230C ∧GGG) compared with M5 (C127S/G230C ∧AAA) show that the type of amino acid residue used in the spacer can also contribute to antibody DSB formation and HC and LC assembly.
Although we have demonstrated that the altered DSB arrangement of IgG4 can substantially improve the Fab thermal stability relative to IgG1 Fab thermal stability, the largest increase in stability (M2, M5, and M6) of the most stable IgG4 mutants is still ∼2 °C less than that of the IgG1 WT. The relative smaller increases in Fab thermal stability of M3 (C127S/K228C) and M4 (C127S/S227C) at 81 and 79 °C, respectively, suggest that the positioning of the DSB is a contributing factor to Fab domain stability. In addition, M10 (C127S/Y229C/C239S/C242S) and M11 (C127S/G230C ∧AAA C239S/C242S) show that the Fab thermal stability is not influenced by the interhinge DSBs if a relatively homogenous disulfide isoform population is expressed. Furthermore, work at UCB has shown that the 7 residues that differ between IgG1 and IgG4 in the CH1 do not contribute to Fab thermal stability.3 We have also shown a difference of 3 °C in the intrinsic Fab stability of IgG4 and IgG1 with no inter-LC-CH1 DSBs (M7 and M8), which could support the apparent discrepancy of 2 °C observed between IgG1 and IgG4 mutants with an IgG1-like DSB arrangement.
In summary, we have shown that it is possible to increase the Fab stability of an IgG4 by altering the DSB architecture of the molecule. However, such changes can have an effect on the final product heterogeneity, and it was by placing a Cys at position 229 as a single mutation or at position 230 in combination with a 3-Ala spacer that a more homogenous antibody population was observed when expressed from CHO cells. Data from antigen binding studies confirmed that the alternative DSB arrangements investigated do not influence affinity to antigen. These findings emphasize the importance of the spatial position, orientation, and microenvironment for complete DSB formation during IgG assembly. We propose that these mutations and this antibody format can be used during the manufacturing of IgG4 molecules for biotherapeutic processes to yield a more homogeneous and stable product.
Acknowledgments
We thank S. Heywood, R. Adams, and J. Heads for helpful discussions and technical assistance.
J. T. Heads, R. Adams, L. E. D'Hooghe, D. P. Humphreys, A. G. Popplewell, A. D. G. Lawson, and A. J. Henry, submitted for publication.
- HC
- heavy chain
- LC
- light chain
- CH1
- CH2, and CH3, heavy chain constant domain 1, 2, and 3, respectively
- DSB
- disulfide bond
- SEC
- size exclusion chromatography
- M1–M14
- mutants 1–14, respectively.
REFERENCES
- 1. Kabat E. A., Wu T. T., Gottesman K. S., Foeller C. (1991) Sequences of Proteins of Immunological Interest, 5th. Ed., United States Public Health Service, National Institutes of Health, Bethesda, MD [Google Scholar]
- 2. Frangione B., Milstein C., Pink J. R. (1969) Structural studies of immunoglobulin G. Nature 221, 145–148 [DOI] [PubMed] [Google Scholar]
- 3. Schur P. H. (1972) Human γ-g subclasses. Prog. Clin. Immunol. 1, 71–104 [PubMed] [Google Scholar]
- 4. Fries L. F., Hall R. P., Lawley T. J., Crabtree G. R., Frank M. M. (1982) Monocyte receptors for the Fc portion of IgG studies with monomeric human IgG1. Normal in vitro expression of Fc γ receptors in HLA-B8/Drw3 subjects with defective Fc γ-mediated in vivo clearance. J. Immunol. 129, 1041–1049 [PubMed] [Google Scholar]
- 5. Kurlander R. J., Batker J. (1982) The binding of human immunoglobulin G1 monomer and small, covalently cross-linked polymers of immunoglobulin G1 to human peripheral blood monocytes and polymorphonuclear leukocytes. J. Clin. Invest. 69, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woof J. M., Partridge L. J., Jefferis R., Burton D. R. (1986) Localization of the monocyte-binding region on human immunoglobulin G. Mol. Immunol. 23, 319–330 [DOI] [PubMed] [Google Scholar]
- 7. Bindon C. I., Hale G., Brüggemann M., Waldmann H. (1988) Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J. Exp. Med. 168, 127–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brüggemann M., Williams G. T., Bindon C. I., Clark M. R., Walker M. R., Jefferis R., Waldmann H., Neuberger M. S. (1987) Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J. Exp. Med. 166, 1351–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Correia I. R. (2010) Stability of IgG isotypes in serum. MAbs 2, 221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salfeld J. G. (2007) Isotype selection in antibody engineering. Nat. Biotechnol. 25, 1369–1372 [DOI] [PubMed] [Google Scholar]
- 11. Hari S. B., Lau H., Razinkov V. I., Chen S., Latypov R. F. (2010) Acid-induced aggregation of human monoclonal IgG1 and IgG2. Molecular mechanism and the effect of solution composition. Biochemistry 49, 9328–9338 [DOI] [PubMed] [Google Scholar]
- 12. Ishikawa T., Ito T., Endo R., Nakagawa K., Sawa E., Wakamatsu K. (2010) Influence of pH on heat-induced aggregation and degradation of therapeutic monoclonal antibodies. Biol. Pharm. Bull. 33, 1413–1417 [DOI] [PubMed] [Google Scholar]
- 13. Sahin E., Grillo A. O., Perkins M. D., Roberts C. J. (2010) Comparative effects of pH and ionic strength on protein-protein interactions, unfolding, and aggregation for IgG1 antibodies. J. Pharm. Sci. 99, 4830–4848 [DOI] [PubMed] [Google Scholar]
- 14. Garber E., Demarest S. J. (2007) A broad range of Fab stabilities within a host of therapeutic IgGs. Biochem. Biophys. Res. Commun. 355, 751–757 [DOI] [PubMed] [Google Scholar]
- 15. Aalberse R. C., Schuurman J. (2002) IgG4 breaking the rules. Immunology 105, 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aalberse R. C., Stapel S. O., Schuurman J., Rispens T. (2009) Immunoglobulin G4. An odd antibody. Clin. Exp. Allergy 39, 469–477 [DOI] [PubMed] [Google Scholar]
- 17. Labrijn A. F., Buijsse A. O., van den Bremer E. T., Verwilligen A. Y., Bleeker W. K., Thorpe S. J., Killestein J., Polman C. H., Aalberse R. C., Schuurman J., van de Winkel J. G., Parren P. W. (2009) Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat. Biotechnol. 27, 767–771 [DOI] [PubMed] [Google Scholar]
- 18. Schuurman J., Van Ree R., Perdok G. J., Van Doorn H. R., Tan K. Y., Aalberse R. C. (1999) Normal human immunoglobulin G4 is bispecific. It has two different antigen-combining sites. Immunology 97, 693–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schuurman J., Perdok G. J., Gorter A. D., Aalberse R. C. (2001) The inter-heavy chain disulfide bonds of IgG4 are in equilibrium with intra-chain disulfide bonds. Mol. Immunol. 38, 1–8 [DOI] [PubMed] [Google Scholar]
- 20. Angal S., King D. J., Bodmer M. W., Turner A., Lawson A. D., Roberts G., Pedley B., Adair J. R. (1993) A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol. Immunol. 30, 105–108 [DOI] [PubMed] [Google Scholar]
- 21. Bloom J. W., Madanat M. S., Marriott D., Wong T., Chan S. Y. (1997) Intrachain disulfide bond in the core hinge region of human IgG4. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Protein Sci. 6, 407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Neut Kolfschoten K. M., Schuurman J., Losen M., Bleeker W. K., Martínez-Martinez P., Vermeulen E., den Bleker T. H., Wiegman L., Vink T., Aarden L. A., De Baets M. H., van de Winkel J. G., Aalberse R. C., Parren P. W. (2007) Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317, 1554–1557 [DOI] [PubMed] [Google Scholar]
- 23. Lu Y., Harding S. E., Rowe A. J., Davis K. G., Fish B., Varley P., Gee C., Mulot S. (2008) The effect of a point mutation on the stability of IgG4 as monitored by analytical ultracentrifugation. J. Pharm. Sci. 97, 960–969 [DOI] [PubMed] [Google Scholar]
- 24. Labrijn A. F., Rispens T., Meesters J., Rose R. J., den Bleker T. H., Loverix S., van den Bremer E. T., Neijssen J., Vink T., Lasters I., Aalberse R. C., Heck A. J., van de Winkel J. G., Schuurman J., Parren P. W. (2011) Species-specific determinants in the IgG CH3 domain enable Fab-arm exchange by affecting the noncovalent CH3-CH3 interaction strength. J. Immunol. 187, 3238–3246 [DOI] [PubMed] [Google Scholar]
- 25. Rose R. J., Labrijn A. F., van den Bremer E. T., Loverix S., Lasters I., van Berkel P. H., van de Winkel J. G., Schuurman J., Parren P. W., Heck A. J. (2011) Quantitative analysis of the interaction strength and dynamics of human IgG4 half molecules by native mass spectrometry. Structure 19, 1274–1282 [DOI] [PubMed] [Google Scholar]
- 26. Nobuaki T., Hideaki Y. (25 July 2007) EPO Patent EP1810979
- 27. Chennamsetty N., Voynov V., Kayser V., Helk B., Trout B. L. (2009) Design of therapeutic proteins with enhanced stability. Proc. Natl. Acad. Sci. U.S.A. 106, 11937–11942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Demarest S. J., Chen G., Kimmel B. E., Gustafson D., Wu J., Salbato J., Poland J., Elia M., Tan X., Wong K., Short J., Hansen G. (2006) Engineering stability into Escherichia coli secreted Fabs leads to increased functional expression. Protein Eng. Des. Sel. 19, 325–336 [DOI] [PubMed] [Google Scholar]
- 29. Saphire E. O., Stanfield R. L., Crispin M. D., Parren P. W., Rudd P. M., Dwek R. A., Burton D. R., Wilson I. A. (2002) Contrasting IgG structures reveal extreme asymmetry and flexibility. J. Mol. Biol. 319, 9–18 [DOI] [PubMed] [Google Scholar]
- 30. Roux K. H., Strelets L., Michaelsen T. E. (1997) Flexibility of human IgG subclasses. J. Immunol. 159, 3372–3382 [PubMed] [Google Scholar]
- 31. Wypych J., Li M., Guo A., Zhang Z., Martinez T., Allen M. J., Fodor S., Kelner D. N., Flynn G. C., Liu Y. D., Bondarenko P. V., Ricci M. S., Dillon T. M., Balland A. (2008) Human IgG2 antibodies display disulfide-mediated structural isoforms. J. Biol. Chem. 283, 16194–16205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu H., Gaza-Bulseco G., Chumsae C., Newby-Kew A. (2007) Characterization of lower molecular weight artifact bands of recombinant monoclonal IgG1 antibodies on non-reducing SDS-PAGE. Biotechnol. Lett. 29, 1611–1622 [DOI] [PubMed] [Google Scholar]
- 33. Taylor F. R., Prentice H. L., Garber E. A., Fajardo H. A., Vasilyeva E., Blake Pepinsky R. (2006) Suppression of sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample preparation artifacts for analysis of IgG4 half-antibody. Anal. Biochem. 353, 204–208 [DOI] [PubMed] [Google Scholar]
- 34. Dillon T. M., Ricci M. S., Vezina C., Flynn G. C., Liu Y. D., Rehder D. S., Plant M., Henkle B., Li Y., Deechongkit S., Varnum B., Wypych J., Balland A., Bondarenko P. V. (2008) Structural and functional characterization of disulfide isoforms of the human IgG2 subclass. J. Biol. Chem. 283, 16206–16215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinez T., Guo A., Allen M. J., Han M., Pace D., Jones J., Gillespie R., Ketchem R. R., Zhang Y., Balland A. (2008) Disulfide connectivity of human immunoglobulin G2 structural isoforms. Biochemistry 47, 7496–7508 [DOI] [PubMed] [Google Scholar]