Background: The cholesterol-dependent cytolysins (CDCs) undergo a complex set of structural transitions to form the homo-oligomeric pore complex.
Results: Structural transitions are propagated between monomers of the oligomeric complex.
Conclusion: Specific structural changes establish the geometry of the oligomeric pore complex and promote the completion of existing oligomers.
Significance: CDCs use membrane binding and ordered intermolecular interactions to drive assembly of their β-barrel pore.
Keywords: Amyloid, Bacterial Toxins, Cholesterol-binding Protein, Plasma Membrane, Protein Self-assembly, β-Barrel Pore, Membrane Attack Complex, Perforin
Abstract
The assembly of the cholesterol-dependent cytolysin (CDC) oligomeric pore complex requires a complex choreography of secondary and tertiary structural changes in domain 3 (D3) of the CDC monomer structure. A point mutation was identified in the archetype CDC, perfringolysin O, that blocks detectable D3 structural changes and traps the membrane-bound monomers in an early and reversible stage of oligomer assembly. Using this and other mutants we show that specific D3 structural changes are propagated from one membrane-bound monomer to another. Propagation of these structural changes results in the exposure of a β-strand in D3 that allows it to pair and form edge-on interactions with a second β-strand of a free membrane-bound monomer. Pairing of these strands establishes the final geometry of the pore complex and is necessary to drive the formation of the β-barrel pore. These studies provide new insights into how structural information is propagated between membrane-bound monomers of a self-assembling system and the interactions that establish the geometry of the final pore complex.
Introduction
Cholesterol-dependent cytolysins (CDCs)3 are secreted by a large number of Gram-positive bacterial pathogens and contribute to pathogenesis in a variety of ways. The soluble (CDC) monomers bind to their receptor (cholesterol or CD59) (for review, see Ref. 1) and assemble on cell membranes into large homo-oligomeric doughnut-shaped pore complexes. The CDC from Clostridium perfringens, perfringolysin O (PFO), has been used extensively as a model system to study the conformational changes that occur during the transition of the CDCs from soluble monomers to membrane-embedded pore complexes. The interaction of PFO monomers with cholesterol-rich membranes initiates a series of secondary and tertiary structural changes that result in the formation of a large ring-shaped β-barrel pore (for review, see Ref. 2) composed of 34–36 monomers (3). Prior to the formation of the β-barrel pore, the only structures that contact the membrane are three short loops and the residues of the conserved undecapeptide located at the tip of domain 4 (D4) (4–6) (Fig. 1). This interaction initiates the D3 structural changes that lead to oligomerization and pore formation.
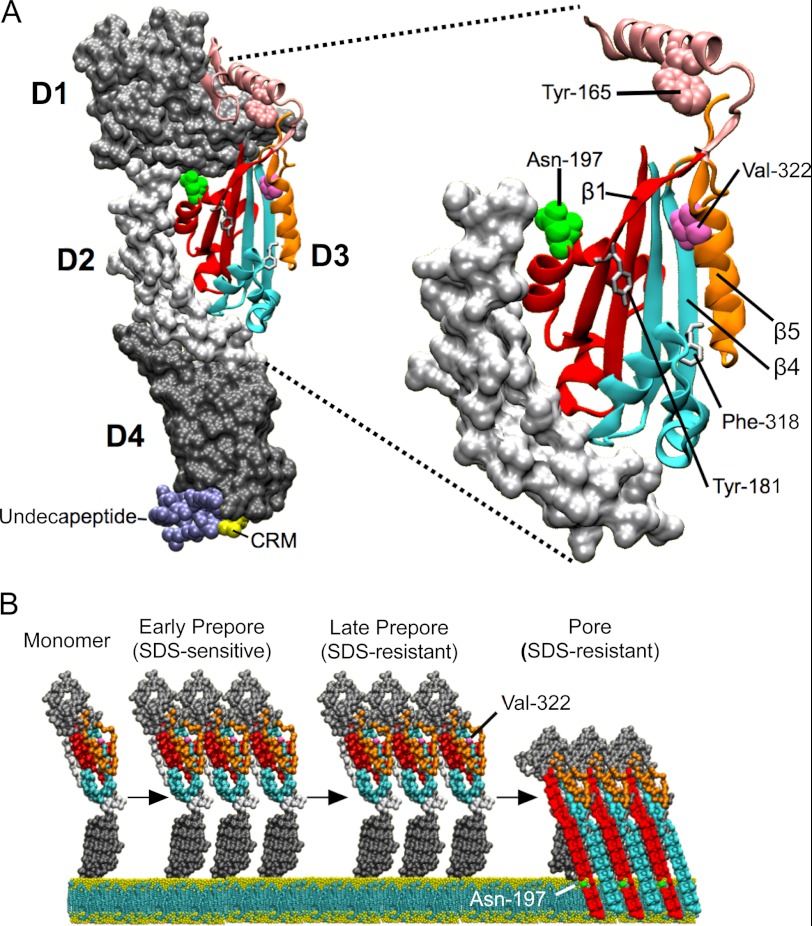
FIGURE 1.
Crystal structure of PFO and a model of pore formation. A, locations of various structures and residues pertinent to this study are shown in the soluble monomer crystal structure of PFO (11). D3 and the structure containing Trp-165 in domain 1 are shown in detail in the right panel. The D3 core β-sheet comprises the four β-strands that are contiguous with the twin α-helical bundles that ultimately unravel to form the twin TMHs that contribute to the formation the β-barrel pore. B, synopsis of the PFO pore-forming mechanism is shown. The SDS-sensitive prepore stage represents several different substates where different mutations trap PFO in an SDS-sensitive prepore structure (7, 13), including the mutation of Trp-165 reported herein. SDS resistance of the prepore structure is achieved by the formation of the intermolecular backbone hydrogen bonds between β-strands 1 and 4 and the π-stacking of interaction between Tyr-181 and Phe-318 of the core β-sheets of two monomers (7). As a point of reference, the locations of residues Asn-197 and Val-322 are also shown in the mechanism model in B when the two D3 α-helical bundles are unraveled to form the two membrane-spanning β-hairpins (8, 9). D1–D4, domains 1–4. The PFO structure was generated using Visual Molecular Dynamics (29). The structural models of the prepore and pore monomers in B were derived from the cryo-electron microscopy derived structures of the pneumolysin prepore and pore fitted with the alpha-carbon backbone of the PFO crystal structure (30).
After binding, membrane-bound monomers establish intermolecular contacts through the formation of backbone hydrogen bonds and π-stacking interactions (Tyr-181 and Phe-318 in PFO) (7) between β-strands 1 (β1) and 4 (β4) of the four-stranded core β-sheet of D3 (Fig. 1) (7). These interactions require that β5 rotates away from β4, thus freeing up the edge of β4 of one membrane-bound monomer to form edge-on interactions with β1 of another membrane-bound monomer. Also, two D3 α-helical bundles extend from the core β-sheet of D3 (Fig. 1) and unravel to form the twin extended transmembrane β-hairpins (TMHs), which then contribute to the formation of the large β-barrel pore (8, 9). To unfold and extend these α-helices into β-hairpins the interface between domains 3 and 2 is disrupted, the twist in the core β-sheet is relieved, and intermolecular hydrogen bonds and the π-stacking interaction are formed between the remaining residues of β-strands 1 and 4 to create the transmembrane β-barrel (Fig. 1B).
These structural changes and the oligomerization of PFO monomers into the pore complex do not occur in the absence of membrane binding: oligomeric structures of PFO that resemble the oligomeric membrane complexes are not detected in solution, even at the high protein concentrations required for crystallization (10, 11). Hence, membrane binding via D3 is necessary to initiate conformational changes in D3 that are required for oligomerization and pore formation. This mechanism is further supported by the observation that the membrane insertion of the undecapeptide tryptophan residues in D3 is conformationally coupled to the membrane insertion of the D3 TMHs (4). We have also observed a coupled rotation of D4 and D1–3 around the D2-D4 interface that disrupts some D2-D3 interactions in different crystal forms of PFO (12), which suggests a pathway for allosterically coupling membrane binding to the activation of the D3 structural transitions necessary to the formation of the oligomeric pore complex.
Although membrane binding is clearly the initiating event for pore formation, we have shown that monomer-monomer cooperativity is important in driving the assembly of the β-barrel pore (13). Here, we show that a point mutant in a conserved tryptophan residue traps PFO in an early stage of prepore formation that blocks the detectable D3 structural transitions and restricts the oligomer geometry to a linear structure rather than the typical circular structure. The study of this mutant suggests that prior to any detectable D3 structural changes the membrane-bound PFO monomers initially form weak, but specific intermolecular interactions. Our studies also suggest that these initial interactions synergistically propagate further D3 structural changes, which strengthen the interactions between monomers and drive the major structural changes in D3. These structural changes irrevocably commit the monomers to oligomerization and dictate the pore geometry, which leads to the formation of the oligomeric pore complex.
MATERIALS AND METHODS
Preparation of PFO Derivatives
The gene for PFOA459C, the cysteine-less PFO derivative in which the cysteine at residue 459 was replaced with alanine, was cloned into pTrcHisA (Invitrogen) as previously described (9). This plasmid (pRT20) was used as the template for all other amino acid residue substitutions. Various amino acid substitutions in PFO were generated by PCR QuikChange mutagenesis (Stratagene) and each derivative sequenced (Oklahoma Medical Research Foundation Core DNA sequencing facility). The expression and purification of recombinant His-tagged PFO and its derivatives from Escherichia coli were carried out as described (6). The toxin was stored in 5 mm dithiothreitol (DTT) and 10% (v/v) sterile glycerol at −80 °C until used.
Liposome Preparation
All phospholipids were obtained from Avanti Polar Lipids (Alabaster, AL). Cholesterol was obtained from Steraloids (Wilton, NH). Liposomes containing 45:55 mol % of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids) to cholesterol (Steraloids) were prepared as described previously using an Avestin, Inc. (Ottawa, ON, Canada) Liposofast extruder (9).
Hemolytic Activity
The hemolytic activity of each labeled or unlabeled PFO derivative was determined on human red blood cells (hRBCs) as described previously (14). The EC50 is defined as the effective concentration of toxin required to lyse 50% of the hRBCs. The hemolytic activity of each mutant was compared with that of recombinant wild-type PFO.
SDS-Agarose Gel Electrophoresis (SDS-AGE)
SDS-AGE was carried out as described previously (9, 14, 15). Briefly, PFO (171 nm) was incubated in the presence or absence of POPC:cholesterol liposomes for 30 min at 37 °C. Samples were solubilized with SDS sample buffer at 37 °C for 2 min, and then the monomeric and oligomeric complexes were resolved on a 1.5% SDS-agarose gel.
Modification of PFO with Fluorescent and Nonfluorescent Probes
The labeling of PFO cysteine-containing mutants at the cysteine sulfhydryl with the environmentally sensitive dye N,N′-dimethyl-N′-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)ethylenediamine (IANBD, Invitrogen) was carried out as described previously (7). The labeled protein was separated from free dye by gel filtration (Sephadex G-50; inner diameter 1.5 × 25 cm) in HBS (50 mm HEPES, pH 7.5, 100 mm NaCl). For pyrene labeling, N-(1-pyrene)maleimide (Invitrogen) in dimethyl sulfoxide was added to a 4-fold molar excess over the PFO in HBS. After 2 h at room temperature DTT was added to 5 mm to stop the reaction. The mixture was passed through a Sephadex G-50 column equilibrated with HBS buffer to separate pyrene-labeled PFO from the free dye. All labeled PFO derivatives were made 10% (v/v) in glycerol and stored at −80 °C. Prior to use the sample was spun at 21,000 × g for 10 min to remove any precipitated protein and the protein concentration determined using the Bio-Rad protein assay according to the manufacturer's instructions.
Fluorescence Measurements
All fluorescence intensity measurements were performed using an SLM-8100 photon-counting spectrofluorometer as described previously (7). The excitation wavelength and bandpass, and the emission wavelength and bandpass, were, respectively: 470, 4, 530, and 4 nm for NBD; 295, 2, 348, and 4 nm for Trp; 345, 4, 470, and 4 nm for pyrene.
Förster Resonance Energy Transfer (FRET) Analysis
FRET measurements were carried out as described previously (13) with the following changes. PFOW165T was labeled with sulfhydryl-specific maleimide derivatives of donor (D) (Alexa Fluor 488) or acceptor (A) (Alexa Fluor 568) fluorescent dyes at cysteine-substituted Asp-30. Asp-30 is located at the N terminus of mature PFO, and substitution of cysteine for this residue does not affect the activity of PFO (data not shown).
An equimolar mixture of donor (D)- and acceptor (A)-labeled PFOW165T (22 nm each; the DA sample) was stirred in HBS at 37 °C. To correct for light scattering and direct excitation of the acceptor, a sample was prepared in parallel in which unlabeled PFOW165T (U) replaced the donor-labeled PFO to create the UA sample, therefore net DA = DA − UA. Net D fluorescence in the absence of A (DU) was determined by DU-UU. Emission was determined between 500 and 600 nm (excitation at 470 nm; bandpass at 4 nm). Liposomes were then added to each sample, and the contents were mixed for 30 min at 37 °C to allow PFOW165T to bind and oligomerize on the liposomes prior to reading the emission. To determine whether the association of the D-and A-labeled PFOW165T could be disrupted, a 4 molar excess of β4-β5 disulfide-locked PFO (PFOβ4β5) (7) or prepore-locked PFO (PFOPPL) (16) were added to the oligomerized PFOW165T in both the DA and DU samples, and after an additional 30 min the net D fluorescence was determined.
Electron Microscopy of PFO Oligomeric Complexes
To form the oligomeric complexes, PFO protein solutions (∼0.1 mg/ml) in buffer (20 mm HEPES, pH 7.0) were pipetted into Teflon wells as 13-μl droplets and coated with 1 μl of a 0.5 mg/ml lipid containing a 1:1 molar mixture of cholesterol and 1,2 dioleoyl-sn-glycero-3-phosphocholine. After incubation at room temperature for 30 min, the oligomeric PFO (or its derivatives) structures were transferred to carbon support films on electron microscopy (EM) grids and negatively stained with 1% (w/v) uranyl acetate and observed with a CM200 FEG microscope operating at 120 kV, under low dose conditions. Images were taken with a 2K × 2K Teitz CCD camera.
RESULTS
Cytolytic Activity of Trp-165 Mutants
A cysteine scan of PFO revealed that substitution of Trp-165 resulted in a cytolytically inactive PFO derivative. Trp-165 is conserved in all known CDCs, suggesting that it is located at a structurally sensitive site in the CDC structure and likely requires an aromatic residue at this location. This was confirmed by a series of mutants containing either aromatic or non-aromatic side chains. As shown in Table 1 substitutions with aromatic residues retained between 19 and 100% of the cytolytic activity of wild-type PFO, whereas mutants that contained non-aromatic residues did not exhibit detectable cytolytic activity.
TABLE 1.
Relative hemolytic activity of Trp-165 mutants
The EC50 (effective concentration for 50% lysis) was determined for wild-type PFO and the PFOT165 substitution mutants. Shown is the relative hemolytic activity of each mutant compared with WT toxin (% activity = (WT EC50/mutant EC50) × 100). ND, hemolytic activity was not detectable in the range of concentrations using in the assay. All assays were performed in triplicate, and in all cases the S.D. was <5%.
| PFO mutant | Hemolytic activity |
|---|---|
| % WT | |
| WT | 100 |
| W165Y | 100 |
| W165H | 19 |
| W165A, C, E, I, K, L, M, N, S, T, V | ND |
Membrane Oligomer Formation by the Trp-165 Mutants
Membrane-bound monomers of PFO oligomerize to form characteristic prepore and pore structures that are visualized by SDS-AGE (17). SDS-AGE analysis of the Trp-165 mutants showed that aromatic substitutions formed SDS-resistant oligomers similar to wild-type PFO. The non-aromatic substitution mutants, however, did not form detectable SDS-resistant oligomers as exemplified by the Cys and Thr substitutions (Fig. 2).
FIGURE 2.
Oligomer formation by wild-type PFO and PFOW165 mutants. Formation of SDS-resistant and SDS-sensitive oligomers by mutants derived from the three classes of PFO Trp-165 mutants is shown by SDS-AGE. Lane 1, soluble monomeric PFO. Lanes 2–6, native PFO, PFOW165Y, PFOW165H, PFOW165C, and PFOW165T incubated with cholesterol-rich liposomes.
We have previously characterized oligomer-forming mutants, which are sensitive to dissociation with SDS but still form the typical doughnut-shaped rings of the CDC oligomers as visualized by EM (13, 16). The mutants containing an aromatic residue for the native tryptophan exhibited the typical ring-shaped pore complexes (Fig. 3). The oligomers of both aromatic substitutions were slightly larger than native PFO by 1–2 monomers based on the size of the monomer crystal structure and the ring size demonstrated by EM. The Thr and Cys mutants, however, formed linear oligomers (Fig. 3) that were representative of other non-aromatic substitution mutants of Trp-165 (data not shown).
FIGURE 3.
EM images of membrane-bound oligomers of wild-type and PFOW165 mutants. Oligomeric structures of PFO or individual PFO mutants were formed on cholesterol lipid layers, subsequently transferred onto EM grids, and negatively stained for electron microscopic analysis. Scale bars, 50 nm.
Membrane Binding by PFOW165T
The linear geometry and SDS-sensitive phenotype of the non-aromatic-substituted mutants suggest that they are defective in one or more stages of oligomer assembly, which prevents the formation of the typical circular complexes (17). To elucidate the structural basis of this defect each stage of assembly of the oligomeric pore complex in the PFOW165T mutant was evaluated. We first evaluated membrane binding by monitoring the change in the intrinsic fluorescence of the native tryptophans of the undecapeptide at the base of D3 as they enter the nonpolar bilayer upon binding (4, 5, 18). Upon the addition of cholesterol-rich liposomes to either PFO or PFOW165T a similar increase in the intrinsic tryptophan fluorescence intensity was observed for both proteins (Fig. 4A), showing that native PFO and PFOW165T bound and inserted their undecapeptide tryptophans similarly into the membrane. Therefore, the W165T mutation did not affect the interaction of D4 with the membrane.
FIGURE 4.
Domain 3 conformational changes are blocked in PFOW165T. Membrane binding and the D3 structural changes normally associated with assembly and formation of the oligomeric β-barrel pore were detected by placing fluorophores at specific locations and comparing the emission intensities of native PFO and PFOW165T derivatives in the absence (solid line) and presence (dashed line) of liposomes. A, membrane binding of PFO and PFOW165T was detected by comparing the emission intensities of undecapeptide tryptophans of wild-type PFO (WT) and PFOW165T in the absence and presence of liposomal membranes (4). B, disruption of the domain D2-D3 interface was detected by monitoring the aqueous exposure (and resulting decrease in the emission intensity) of the buried NBD, located at cysteine-substituted Asn-197, as D3 swings away from D2 (9). C, disengagement of β5 from β4 was detected by monitoring the decrease in the emission of NBD positioned at cysteine-substituted Val-322, which is buried beneath β5. As β4 and β5 separate, NBD moves from a nonpolar to polar environment, and its emission intensity decreases (7). D, intermolecular association of β1 and β4 from adjacent membrane-bound monomers is shown by the π-stacking of pyrene dyes attached to Cys-substituted Thr-179 (located in β1) and Val-322 (located in β4) and the resultant broad excimer emission near 470 nm (7). Pyrene emission spectra are shown for an equimolar mixture of pyrene-derivatized PFOT179C and PFOV322C in wild-type (WT) and PFOW165T backgrounds in the absence (solid line) and presence (dashed line) of liposomes.
Disengagement of PFOW165T Domains 2 and 3
Two major D3 structural transitions occur that lead to the formation of the β-barrel pore. One of these transitions is the disruption of the interface between D2 and D3 that is necessary to extend and transform the two D3 α-helical bundles into the twin TMHs, which then contribute to the formation of the β-barrel pore (8, 9). Asn-197 is located in TMH1 and is buried in the nonpolar D2-D3 interface. Upon pore formation Asn-197 makes the transition to the polar environment of the pore lumen as the α-helical bundle unravels to form TMH1, which, along with TMH2, extends across the bilayer (9). Therefore the disruption of the D2-D3 interface is monitored by placement of the environmentally sensitive probe NBD on a cysteine substituted for Asn-197 and then measuring the change in emission intensity as it makes this transition. As expected, we observed a decrease in NBD emission intensity for PFON197C-NBD as it moves from the nonpolar D2-D3 interface to the pore lumen (Fig. 4B). In contrast, little change in the environment of the NBD probe was seen for PFOW165T/N197C-NBD (Fig. 4B), indicating that Asn-197 remains buried in the D2-D3 interface.
Disengagement of β-Strand 5 from β-Strand 4
The other major D3 structural transition that occurs along with the disruption of the D2-D3 interface is the disengagement of the D3 β-strand 5 (β5) from β-strand 4 (β4) (7, 13). This transition is necessary to free up the edge of β4 so that it is available to form the intermolecular backbone hydrogen bonds with β1 of another membrane-bound monomer. This interaction contributes to the formation of SDS-resistant oligomers. Val-322 is buried beneath β5 in the PFO monomer but is exposed to the aqueous environment when β5 rotates away from β4 (7). Therefore, an NBD probe positioned at this location also undergoes a transition from a nonpolar to polar environment, which is reflected by a decrease in its fluorescence intensity. The expected decrease in fluorescence intensity of the NBD probe in the functional PFOV322C-NBD was observed as β5 rotates away from β4, whereas only a small change in the environment of the probe was observed for PFOW165T/V322C-NBD (Fig. 4C). Therefore, β4 still remains buried under β5 in PFOW165T thereby preventing β4 from pairing with β1 of another membrane-bound monomer.
To confirm that the intermolecular pairing of β1-β4 between monomers of PFOW165T was not taking place we measured excimer formation resulting from the π-stacking of pyrene probes placed on cysteine-substituted residues Thr-179 in β1 of one monomer and Val-322 on β4 of a second monomer. If β1 and β4 are paired then these pyrenes will be oriented in such a way that will allow the stacking interaction to take place, which results in the formation of the excimer emission (7). As expected, the pyrenes in the membrane-bound PFO monomers formed a π-stacking interaction, as evidenced by the appearance of a broad excimer emission centered at about 470 nm (Fig. 4D). No excimer emission was observed for the PFOW165T mutant, demonstrating that the pyrenes in β1 and β4 were unable to stack and hence that β1 and β4 in adjacent monomers were not properly juxtaposed in the oligomers formed by PFOW165T.
The combined data shown in Fig. 4 demonstrate that substitution of Trp-165 with threonine does not affect membrane binding of the monomer D4, but prevents both the detectable structural transitions within D3 and the intermolecular pairing of β1 and β4 between membrane-bound monomers.
Monomer-Monomer Interactions of PFOW165T Are Reversible
Native PFO monomers form membrane oligomers that are resistant to dissociation by SDS and cannot freely dissociate once they are incorporated into the native oligomer (16, 17). Because the oligomers formed by PFOW165T are SDS-sensitive we determined whether they could freely exchange with monomers added after the PFOW165T linear oligomers were allowed to form. This was accomplished by determining whether FRET between the monomers of donor (D) and acceptor (A) fluorophore-labeled PFOW165T, which are incorporated into preformed linear oligomers, could be disrupted by the subsequent addition of various unlabeled PFO derivatives. If PFOW165T monomers were in equilibrium between the monomer and oligomer state on the membrane then the addition of unlabeled monomers would exchange with the D- and A-labeled monomers in the oligomer and disrupt the FRET-dependent A quenching of the D fluorescence.
As expected, we observed FRET-dependent donor quenching as D- and A-labeled PFOW165T interacted to form the linear oligomers (Fig. 5, compare DA with DU; U = unlabeled PFOW165T). We then determined whether PFOW165T monomers incorporated into the preformed oligomers could be exchanged by the addition of unlabeled mutants of PFO, which are trapped at various stages of pore assembly. These included unlabeled versions of PFOβ4β5, which contains an engineered disulfide between β4 and β5 that prevents the interaction of β1 and β4 of adjacent monomers and is trapped at an early SDS-sensitive stage of the prepore, (see the last section under “Results”) (7); PFOPPL, which can oligomerize into an SDS-resistant prepore oligomer but cannot insert the β-barrel due to an engineered disulfide (16), and PFOW165T itself.
FIGURE 5.
PFOW165T is in equilibrium between the membrane monomer and oligomer. A–C, the ability of unlabeled PFOβ4β5, PFOPPL, or PFOW165T to disrupt FRET between donor (or D)- and acceptor (or A)-labeled PFOW165T in preformed membrane oligomers was monitored by changes in A-dependent D emission. DA (solid line) or D and unlabeled (U) PFOW165T (dotted line) were allowed to form oligomers (30 min at 37 °C) on liposome membranes. After emission intensities were measured, a 4-fold molar excess of unlabeled PFOβ4β5 (A), PFOPPL (B), or PFOW165T (C) was then added to the PFOW165T DA samples and incubated for an additional 30 min before a second emission scan was performed (dashed line). D, the same experiment was performed as in A with PFOPPL in place of the donor- and acceptor-labeled PFOW165T to demonstrate that prepore locked PFO oligomers were not in equilibrium with monomers. E, the same experiment was performed as in panel A except a 4-fold molar excess of the β4β5-locked version of the related S. pneumoniae CDC, pneumolysin (PLYβ4β5) was mixed with the preformed PFOW165T oligomer.
As shown in Fig. 5, A–C, the addition of unlabeled PFOβ4β5, PFOPPL, or PFOW165T disrupted FRET between the D- and A-labeled PFOW165T. A 4-fold molar excess of each PFO derivative nearly eliminated FRET, indicating that the monomers in the PFOW165T oligomer were exchangeable after it formed the linear oligomers. The fact that a 4-fold molar excess of each unlabeled PFO derivative could nearly completely abolish FRET between D- and A-labeled PFOW165T indicates that the exchange with the unlabeled variants completely randomized their distribution within the linear oligomers. On average, inserting four monomers between each pair of D- and A-labeled PFOW165T would increase their separation by more than 100 Å, which would decrease the FRET efficiency to near zero. PFOβ4β5 and PFOPPL acted similarly to PFOW165T to disrupt FRET between the PFOW165T DA pair, suggesting that they formed interactions with PFOW165T similar to those it forms with itself. PFOPPL has been shown to form SDS-resistant oligomers; and when the same experiment was performed with the donor- and acceptor-labeled PFOPPL, the FRET could not be disrupted by the addition of unlabeled PFOPPL after the oligomers had formed (Fig. 5D). Therefore, unlike PFOW165T the monomers of the PFOPPL oligomeric complex cannot be exchanged once they interact to form the oligomer. As described below in the last section under “Results,” the same is true for the interaction of the PFOβ4β5 monomers, which cannot form the intermolecular β1-β4 interaction.
We also showed that this interaction was not the result of a weak, nonspecific interaction of the membrane-bound monomers, but that it was a specific interaction of the PFO monomers. The addition of a 4-fold molar excess of pneumolysin (PLYβ4β5), a CDC from Streptococcus pneumoniae that exhibits about 40% identity with PFO, did not disrupt of the FRET between the PFOW165T monomers (Fig. 5E).
These combined data show three important features of the PFOW165T monomer-monomer interaction: (i) PFOW165T appears to be trapped at an early stage of oligomerization that is reversible; (ii) the complementary interfaces mediating this interaction are intact in the various disulfide-locked mutants of PFO; and (iii) the interaction is specific to PFO and its derivatives.
Functional PFO Drives D3 Structural Transitions in PFOW165T
The above studies showed that disulfide-locked PFO derivatives could interact with PFOW165T. We therefore asked whether the interaction of PFOPPL with PFOW165T could drive the PFOW165T monomers to undergo the D3 structural transitions that were blocked by this mutation. When the disulfide of PFOPPL is in the oxidized state (PFOPPLox) it still can form β1-β4 intermolecular interactions but cannot insert its TMHs into the membrane until the disulfide is reduced (16). Therefore, we determined whether the interaction of PFOPPLox with PFOW165T could drive further the disengagement of β5 from β4 in PFOW165T (16). The disengagement of β5 from β4 was measured by monitoring a NBD probe located at V322C in β4 in PFOW165T oligomers as described in Fig. 4C. The rotation of β5 away from β4 exposures the NBD probe to the polar environment, and the emission of the probe is quenched by water.
The fluorescence emission of the NBD probe was determined for the PFOW165T/V322C-NBD monomers before and after the addition of liposomes to allow the formation of the linear oligomers (Fig. 6A, compare dotted and dashed lines). Following formation of the PFOW165T oligomers a 4-fold molar excess of unlabeled PFOPPLox was introduced and allowed to intercalate into the PFOW165T linear oligomers. The fluorescence emission of the NBD probe was again measured (Fig. 6A, solid gray line). The disulfide in PFOPPLox was then reduced (PFOW165Tred), and an emission scan was again performed (Fig. 6A, solid black line). Oxidized and reduced PFOPPL further drove the disengagement of β5 from β4 in PFOW165T as evidenced by the increased quenching of the NBD emission, signifying its transition to an increasingly more polar environment as β5 rotates away to open up the edge of β4 (Fig. 6A). The magnitude of the decrease in the NBD fluorescence emission after the reduction of the disulfide of PFOPPL was similar to that observed in PFO (Fig. 4C). A parallel experiment was performed in which additional unlabeled PFOW165T was added in place of the PFOPPL (Fig. 6B). No change in the fluorescence emission of the NBD occurs after the initial 30-min incubation period or after the addition of reducing agent. Hence, the increased polarity of the environment of the NBD in PFOW165T/V322C-NBD after the addition of the PFOPPL in its oxidized and reduced states resulted from the propagation of structural changes from PFOPPL to D3 of PFOW165T.
FIGURE 6.

Functional PFO drives conformational changes in D3 of PFOW165T. A, the disruption of the β4-β5 interaction in PFOW165T by functional derivatives of PFO in mixed oligomers was monitored as described in Fig. 4C. The relative emission of the NBD is shown for soluble PFOW165T/V322C-NBD monomer (dotted line), liposome bound PFOW65T/V322C-NBD after 30 min at 37 °C (dashed line) and then after a 4-fold molar excess of unlabeled PFOPPLox was added and incubated for an additional 30 min (gray line). The PFOPPLox disulfide was then reduced to allow the insertion of its TMHs for an additional 30 min (solid black line). B, a second experiment was performed with the PFOW165T/V322C-NBD alone, and emission scans were performed on the soluble monomer (dotted line), after addition of liposomes 30 min (dashed line), 60 min (gray line), and 90 min (black line). C, PFOW165T was labeled with NBD at position 215 in TMH1 to monitor its insertion into the membrane (9). Soluble (solid line) and membrane-bound PFOW165T/A215C-NBD (dotted line) are indicated. A 4-fold molar excess of unlabeled PFOPPLox was then added to the membrane-bound PFOW165T/A215C-NBD sample, incubated for an additional 30 min, reduced to unlock the TMHs of PFOPPLox, and after 15 min an emission scan was performed (dashed line).
We then determined whether reduction of the PFOPPL disulfide also drove the membrane insertion of PFOW165T TMHs. To detect membrane insertion of PFOW165T TMHs we positioned the environmentally sensitive NBD probe at cysteine-substituted Ala-215 in TMH1 of PFOW165T. Ala-215 is a membrane-facing residue in TMH1 that we have used extensively to monitor membrane insertion of the PFO TMHs (8, 9, 13, 16, 17, 19). The reduction of the PFOPPLox disulfide allows it to complete the transition to the membrane pore by inserting its β-barrel pore into the membrane. In the mixed oligomers of PFOPPL and PFOW165T/A215C-NBD where the disulfide of PFOPPL was reduced it drove the membrane insertion of the PFOW165T TMHs, as shown by the increased emission intensity of the NBD probe in PFOW165T/A215C-NBD as it entered the membrane (Fig. 6C).
These results show that the oxidized and reduced forms of PFOPPL could drive the necessary D3 structural transitions and membrane insertion of the TMHs of PFOW165T. The interactions between functional PFO molecules and adjacent PFOW165T molecules therefore provided the energy and direction necessary to drive the D3 structural transitions required for pore formation. Because PFOW165T can be transformed from its inactive trapped conformation to the active state by the cooperative effect of adjacent functional PFO molecules, its inactive conformation appears to be an intermediate in the pathway of PFO structural changes that create a pore.
PFOβ4β5 Monomers Interact on the Membrane Surface
The rotation of β5 away from β4 is a critical step in the formation of SDS-resistant oligomers and is blocked in both the PFOW165T mutant and in the disulfide-locked PFO β4β5 mutant (7). Previous studies of the PFOβ4β5 mutant suggested that it either remained in the monomer state or formed an SDS-sensitive oligomer (7). We observed that it could disrupt FRET between monomers of PFOW165T (Fig. 5A), which suggested that it also was forming an oligomer. Analysis of the PFOβ4β5ox-treated liposomal membranes by EM revealed the presence of short curvilinear arcs (Fig. 7A) that were converted to the typical ring- and arc-shaped structures when the engineered disulfide between β-strands 4 and 5 was reduced prior to their addition to the liposomes (Fig. 7B).
FIGURE 7.

PFOβ4β5 forms SDS-sensitive oligomers on membranes. A and B, EMs of membrane-bound PFOβ4β5 in its oxidized state (A) and reduced (pore-forming) state (B). Scale bars, 50 Å. The intermolecular interaction of monomers was determined by FRET between donor- and acceptor-labeled PFOβ4β5. C, FRET-dependent donor quenching measured in its oxidized membrane-bound form. Compare the DA and DU samples. After PFOβ4β5 had been allowed to interact and donor quenching was at its maximum we added unlabeled PFOPPL (dashed line) to determine whether the interaction of the PFOβ4β5 was reversible, like that observed for PFOW165T (Fig. 5B).
We then used FRET to determine whether monomers of PFOβ4β5 in these oligomeric membrane complexes were also in equilibrium between monomer and oligomer states on the membrane, similar to that observed for PFOW165T. As expected from the EM analysis, FRET-dependent quenching of the D emission is seen when the D- and A-labeled PFOβ4β5 are added to the membrane. However, no change in the quenching of the donor emission was observed when a 4 molar excess of unlabeled PFOPPL was subsequently added to the preformed PFOβ4β5 oligomers (Fig. 7C, dashed line). Unlike the interaction of PFOW165T monomers, the interaction of membrane-bound PFOβ4β5 monomers is not reversible, which indicates that they have formed additional contacts that prevent the reversal of the interaction of monomers within the SDS-sensitive PFOβ4β5 oligomer.
DISCUSSION
The coordination among membrane binding, monomer-monomer interaction, and the D3 structural transitions in PFO that results in assembly of the oligomeric pore complex is a critical, though complex, process. The studies described here provide key insights into this process by showing that monomer-monomer interactions are obligatorily required to drive the D3 conformational changes necessary for the formation of the oligomer and β-barrel pore. Our studies suggest that prior to D3 structural changes the membrane-bound monomers initially form weak transient, but specific, interactions. Through normal thermal fluctuations a fraction of these interactions form stable interactions via the intermolecular pairing of β-strands 1 and 4, which are propagated to other monomers that interact with the growing oligomer. These interactions set the intermolecular geometry of monomer-monomer interaction that establishes the pore size and irreversibly commits the monomers to the assembly of the oligomeric prepore and pore complexes. Hence, the intermolecular interaction of membrane-bound monomers drives changes in the molecular structure of the CDCs that determine the pore size and leads to the completion of the oligomeric pore complex.
The intermolecular pairing of the D3 β-strands 1 and 4 between two membrane-bound monomers is a critical interaction that leads to the formation of the ring-shaped oligomeric complex of PFO. This interaction was lost in PFOW165T and is also blocked in the disulfide-locked mutant PFOβ4β5, which contains an engineered disulfide that restricts rotation of β5 away from β4 unless the disulfide is reduced. Both PFOW165T and PFOβ4β5 form oligomers that are sensitive to SDS and exhibit an increased curvature of radius. In contrast, PFOPPL forms the intermolecular β1-β4 interaction, and its oligomers exhibit architecture similar to that of the native pore complex (3, 16). When the disulfide of PFOβ4β5 was reduced prior to addition to the membranes to allow the formation of the β1-β4 intermolecular interaction, its oligomers exhibited an architecture that was indistinguishable from that of native PFO. Hence, the intermolecular β1-β4 interaction locks membrane-bound monomers into their final intermolecular geometry and establishes the final size of the pore.
We also showed that in mixed oligomers that PFOPPLox could further drive the disruption of the β4 and β5 interaction in PFOW165T thereby showing that it propagated this change to the latter. The reduction of the engineered disulfide in PFOPPL further drove the disruption of the β4-β5 interaction and the membrane insertion of the PFOW165T TMHs. These results show that D3 structural changes can be propagated from one monomer to another. Regulating the D3 structural transitions by monomer-monomer contact may be important to establish the proper environment for unfolding and assembling the twin TMHs from the D3 α-helical bundles (8, 9). We have shown that if the D2-D3 interface is disrupted in soluble CDC monomers it causes the D3 α-helical bundles to unfurl prematurely and results in the rapid and irreversible loss of cytolytic activity and aggregation of the soluble monomers (20). Therefore, if membrane binding alone were sufficient to initiate the unfurling of the D3 α-helical bundles the membrane surface would be populated by individual monomers with their α-helical bundles unfolded to different extents, thereby making them susceptible to nonspecific, off-pathway interactions with other monomers and proteins on the membrane surface. Our studies suggest that monomer-monomer contact drives the D3 structural transitions that lead to transition of the α-helical bundles to the TMHs, which would minimize the residence time of the TMHs in the disordered state and therefore minimize possible off-pathway interactions.
Our studies also suggest an explanation for the nonstochastic assembly of the PFO oligomer observed by Shepard et al. (17). They observed that at the earliest stages of assembly of the PFO oligomer the only detectable species were the monomer and the terminal prepore oligomer, which suggests that cooperative completion of oligomers was favored over the initiation of new oligomers. Based on the studies herein, we propose that the energy barrier to the D3 structural changes that leads to stabilizing intermolecular interactions (i.e. intermolecular pairing of β1 and β4) is met in only a fraction of the encounters between membrane-bound monomers (shown schematically in Fig. 8). Transient higher energy states of D3 due to normal thermal fluctuations would result in the partial disruption of the β4-β5 hydrogen bonds. Although these states are transient they would be sampled many times by the interactions between membrane-bound monomers typified by those observed for PFOW165T. In a fraction of these encounters where the free edge of β4 is exposed by these thermal fluctuations it could pair with β1 of another monomer. Because β1 can form more potential backbone hydrogen bonds with β4 (eight) than β4 forms with β5 (four) the reversal of this interaction would be energetically unfavorable, thus stabilizing the dimer (see B in Fig. 8). The formation of this stable interface would induce structural changes in the second monomer that would disrupt the β4-β5 interaction at the free interface of the dimer, similar to what we observed when oxidized and reduced forms of PFOPPL interacted with PFOW165T. This would free up β4 on the open interface of the dimer (see C in Fig. 8), or, at least, increase the probability that β4 was in an open state and available to pair with β1 of another monomer (see D in Fig. 8). Therefore, unlike the formation of the stable dimer pair, the addition of monomers to the growing end of the oligomer would not be subject to the same energetic constraints facing dimer formation because the β4 of the dimer, or higher order oligomer, would exhibit a higher probability of being in the open conformation, which could then pair with β1 of a free membrane-bound monomer. Hence, the D3 structural changes that are initiated in the stable dimer would be propagated cooperatively to each monomer as they contacted the growing end of the oligomer, a process that would favor the extension of oligomers over the initiation of new oligomers.
FIGURE 8.
Model for oligomeric complex assembly. Our data indicate that membrane-bound PFO monomers initially collide and form weak, reversible interactions (A), similar to what we observed for PFOW165T. Monomers collide many times, and a fraction of these collisions result in the formation of a stable dimer if the β4-β5 interaction of monomer 1 happens to be in the open conformation (B), as a result of normal random thermal fluctuations in the D3 structure. β4 of the core β-sheet of monomer 1 can then form a stable interaction with β1 of the core β-sheet of a second monomer (C). Once this interaction is formed its reversal is less likely because β1 is capable of forming eight backbone hydrogen bonds with β4 whereas only four are formed between β4 and β5 in the monomer (11). Also, Tyr-181 in β1 and Phe-318 in β4 form a π-stacking interaction, which further stabilizes this interaction and results in a SDS-stable prepore complex (7, 13). The formation of the stable β1-β4 interaction induces structural changes in D3 that also disrupt the hydrogen bonds between the β4-β5 pair of monomer 2 (D), similar to that observed when PFOPPL is incubated with PFOW165T (Fig. 6B). β4 on the open interface of the dimer pair (D) is then free to interact with β1 of another monomer. The D3 structural changes are propagated to each successive monomer as it is added, thus exposing β4 on the growing end of the oligomer. Because β4 is already exposed in the elongating oligomer the interaction of β4 with the β1 of each new monomer is not subject to the same energy barrier that exists during the formation of a stable dimer (B). Therefore, the addition of free monomers to established oligomers is preferred over the initiation of new oligomers.
It is important to note that the intermediate stages of oligomer assembly exhibited by PFOW165T and PFOβ4β5 would not normally be observed in native PFO. These two mutants trap PFO at what would otherwise be transient stages of the monomer-monomer interaction: in native PFO once two monomers establish a stable dimer by the intermolecular β1-β4 pairing the intermolecular geometry between the monomers is also established. Hence, neither the long linear oligomers of PFOW165T nor the short curvilinear oligomers of PFOβ4β5 will be observed in native PFO due to their transient nature. We should also note that although we do not detect the major structural changes in D3 of PFOW165T that lead to the formation of the prepore and pore complexes, it is likely that membrane binding leads to some small structural changes in PFOW165T that facilitate the interaction of the monomers, interactions that are not observed in the soluble monomers.
The membrane attack complex/perforin (MACPF) protein family has recently been shown to exhibit a D3-like structure, and it has been proposed that they may exhibit features of the CDC pore-forming mechanism (21–26). They also contain a β4 analog, and some exhibit a β5 analog suggesting that some MACPF proteins may employ a mechanism similar to the CDCs to regulate assembly of their pore. Aspects of the assembly of the CDC oligomeric pore complex are also reminiscent of the mechanism suggested for the initiation of fibril formation in protein folding diseases such as Alzheimer disease. Protein folding diseases are typified by the conversion of soluble functional globular proteins into β-strand containing fibrillar aggregates (27, 28). Chiti and Dobson (28) have suggested that the amyloidogenic state is accessible through the normal thermal fluctuations of the native proteins, which results in the formation of intermolecular edge-on backbone hydrogen bond interactions between β-strands and subsequent formation of β-sheet-rich fibrils. This mechanistic model is not unlike the mechanism of assembly for the CDCs suggested by the studies herein.
In summary, mutation of the conserved Trp-165 blocks all detectable D3 structural transitions and reveals that its membrane-bound monomers interact in a low affinity and reversible mode that does not allow adjacent monomers to adopt a functional conformation. The blockage was overcome when functional PFO derivatives in the hybrid oligomers induced PFOW165T to undergo the structural transitions that culminated in the rotation of β5 away from β4 and the formation and membrane insertion of the TMHs. Thus, early monomer-monomer interactions promote conformational changes that most likely align specific structures between monomers that increase the number of intermolecular interactions and thereby strengthening the association even more. These stabilizing structural changes are then propagated to each monomer as it encounters the growing end of the oligomer. This interaction triggers the conformational changes in D3 that establish the geometry of the ring complex and irreversibly commit the membrane-bound monomers to the formation of the oligomeric pore structure.
This work was supported, in whole or in part, by National Institutes of Health Grant AI037657 through the NIAID. This work was also supported by the Robert A. Welch Foundation Chair Grant BE-0017 and by the Victorian Government Operational Infrastructure Support Scheme to St. Vincent's Institute.
- CDC
- cholesterol-dependent cytolysin
- AGE
- agarose gel electrophoresis
- PFO
- perfringolysin O
- PPL
- prepore-locked
- NBD
- N,N′-dimethyl-N-(iodoacetyl)-N′-(7-nitrobenz-2-oxa-1,3-diazolyl)ethylenediamine
- PPLox
- PPL in the oxidized state
- PPLred
- PPL in the reduced state
- TMH
- transmembrane β-hairpin.
REFERENCES
- 1. Hotze E. M., Tweten R. K. (2012) Membrane assembly of the cholesterol-dependent cytolysin pore complex V. Biochim. Biophys. Acta 1818, 1028–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tweten R. K., Parker M. W., Johnson A. E. (2001) in Pore-Forming Toxins (van der Goot G., ed) pp. 15–33, Springer-Verlag, Heidelberg [Google Scholar]
- 3. Czajkowsky D. M., Hotze E. M., Shao Z., Tweten R. K. (2004) Vertical collapse of a cytolysin prepore moves its transmembrane β-hairpins to the membrane. EMBO J. 23, 3206–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heuck A. P., Hotze E. M., Tweten R. K., Johnson A. E. (2000) Mechanism of membrane insertion of a multimeric β-barrel protein: perfringolysin O creates a pore using ordered and coupled conformational changes. Mol. Cell 6, 1233–1242 [DOI] [PubMed] [Google Scholar]
- 5. Ramachandran R., Heuck A. P., Tweten R. K., Johnson A. E. (2002) Structural insights into the membrane-anchoring mechanism of a cholesterol-dependent cytolysin. Nat. Struct. Biol. 9, 823–827 [DOI] [PubMed] [Google Scholar]
- 6. Soltani C. E., Hotze E. M., Johnson A. E., Tweten R. K. (2007) Structural elements of the cholesterol-dependent cytolysins that are responsible for their cholesterol-sensitive membrane interactions. Proc. Natl. Acad. Sci. U.S.A. 104, 20226–20231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramachandran R., Tweten R. K., Johnson A. E. (2004) Membrane-dependent conformational changes initiate cholesterol-dependent cytolysin oligomerization and intersubunit β-strand alignment. Nat. Struct. Mol. Biol. 11, 697–705 [DOI] [PubMed] [Google Scholar]
- 8. Shatursky O., Heuck A. P., Shepard L. A., Rossjohn J., Parker M. W., Johnson A. E., Tweten R. K. (1999) The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins. Cell 99, 293–299 [DOI] [PubMed] [Google Scholar]
- 9. Shepard L. A., Heuck A. P., Hamman B. D., Rossjohn J., Parker M. W., Ryan K. R., Johnson A. E., Tweten R. K. (1998) Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an α-helical to β-sheet transition identified by fluorescence spectroscopy. Biochemistry 37, 14563–14574 [DOI] [PubMed] [Google Scholar]
- 10. Feil S. C., Rossjohn J., Rohde K., Tweten R. K., Parker M. W. (1996) Crystallization and preliminary x-ray analysis of a thiol-activated cytolysin. FEBS Lett. 397, 290–292 [DOI] [PubMed] [Google Scholar]
- 11. Rossjohn J., Feil S. C., McKinstry W. J., Tweten R. K., Parker M. W. (1997) Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell 89, 685–692 [DOI] [PubMed] [Google Scholar]
- 12. Rossjohn J., Polekhina G., Feil S. C., Morton C. J., Tweten R. K., Parker M. W. (2007) Structures of perfringolysin O suggest a pathway for activation of cholesterol-dependent cytolysins. J. Mol. Biol. 367, 1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hotze E. M., Heuck A. P., Czajkowsky D. M., Shao Z., Johnson A. E., Tweten R. K. (2002) Monomer-monomer interactions drive the prepore to pore conversion of a β-barrel-forming cholesterol-dependent cytolysin. J. Biol. Chem. 277, 11597–11605 [DOI] [PubMed] [Google Scholar]
- 14. Soltani C. E., Hotze E. M., Johnson A. E., Tweten R. K. (2007) Specific protein-membrane contacts are required for prepore and pore assembly by a cholesterol-dependent cytolysin. J. Biol. Chem. 282, 15709–15716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hotze E., Tweten R. K. (2002) in Perspectives in Molecular Toxinology (Ménez A., ed) pp. 23–37, John Wiley & Sons, West Sussex, England [Google Scholar]
- 16. Hotze E. M., Wilson-Kubalek E. M., Rossjohn J., Parker M. W., Johnson A. E., Tweten R. K. (2001) Arresting pore formation of a cholesterol-dependent cytolysin by disulfide trapping synchronizes the insertion of the transmembrane β-sheet from a prepore intermediate. J. Biol. Chem. 276, 8261–8268 [DOI] [PubMed] [Google Scholar]
- 17. Shepard L. A., Shatursky O., Johnson A. E., Tweten R. K. (2000) The mechanism of pore assembly for a cholesterol-dependent cytolysin: formation of a large prepore complex precedes the insertion of the transmembrane β-hairpins. Biochemistry 39, 10284–10293 [DOI] [PubMed] [Google Scholar]
- 18. Nakamura M., Sekino N., Iwamoto M., Ohno-Iwashita Y. (1995) Interaction of θ-toxin (perfringolysin O), a cholesterol-binding cytolysin, with liposomal membranes: change in the aromatic side chains upon binding and insertion. Biochemistry 34, 6513–6520 [DOI] [PubMed] [Google Scholar]
- 19. Giddings K. S., Johnson A. E., Tweten R. K. (2003) Redefining cholesterol's role in the mechanism of the cholesterol-dependent cytolysins. Proc. Natl. Acad. Sci. U.S.A. 100, 11315–11320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schuerch D. W., Wilson-Kubalek E. M., Tweten R. K. (2005) Molecular basis of listeriolysin O pH dependence. Proc. Natl. Acad Sci. U.S.A. 102, 12537–12542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hadders M. A., Beringer D. X., Gros P. (2007) Structure of C8α-MACPF reveals mechanism of membrane attack in complement immune defense. Science 317, 1552–1554 [DOI] [PubMed] [Google Scholar]
- 22. Rosado C. J., Buckle A. M., Law R. H., Butcher R. E., Kan W. T., Bird C. H., Ung K., Browne K. A., Baran K., Bashtannyk-Puhalovich T. A., Faux N. G., Wong W., Porter C. J., Pike R. N., Ellisdon A. M., Pearce M. C., Bottomley S. P., Emsley J., Smith A. I., Rossjohn J., Hartland E. L., Voskoboinik I., Trapani J. A., Bird P. I., Dunstone M. A., Whisstock J. C. (2007) A common fold mediates vertebrate defense and bacterial attack. Science 317, 1548–1551 [DOI] [PubMed] [Google Scholar]
- 23. Rosado C. J., Kondos S., Bull T. E., Kuiper M. J., Law R. H., Buckle A. M., Voskoboinik I., Bird P. I., Trapani J. A., Whisstock J. C., Dunstone M. A. (2008) The MACPF/CDC family of pore-forming toxins. Cell. Microbiol. 10, 1765–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slade D. J., Lovelace L. L., Chruszcz M., Minor W., Lebioda L., Sodetz J. M. (2008) Crystal structure of the MACPF domain of human complement protein C8α in complex with the C8γ subunit. J. Mol. Biol. 379, 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Law R. H., Lukoyanova N., Voskoboinik I., Caradoc-Davies T. T., Baran K., Dunstone M. A., D'Angelo M. E., Orlova E. V., Coulibaly F., Verschoor S., Browne K. A., Ciccone A., Kuiper M. J., Bird P. I., Trapani J. A., Saibil H. R., Whisstock J. C. (2010) The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature 468, 447–451 [DOI] [PubMed] [Google Scholar]
- 26. Lovelace L. L., Cooper C. L., Sodetz J. M., Lebioda L. (2011) Structure of human C8 protein provides mechanistic insight into membrane pore formation by complement. J. Biol. Chem. 286, 17585–17592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chiti F., Dobson C. M. (2006) Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 [DOI] [PubMed] [Google Scholar]
- 28. Chiti F., Dobson C. M. (2009) Amyloid formation by globular proteins under native conditions. Nat. Chem. Biol. 5, 15–22 [DOI] [PubMed] [Google Scholar]
- 29. Humphrey W., Dalke A., Schulten K. (1996) VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38, 27–28 [DOI] [PubMed] [Google Scholar]
- 30. Tilley S. J., Orlova E. V., Gilbert R. J., Andrews P. W., Saibil H. R. (2005) Cell 121, 247–256 [DOI] [PubMed] [Google Scholar]