Background: Retinoid dehydrogenases/reductases (RDHs) reduce retinal in rod photoreceptors.
Results: In single rod cells, RDH8 reduces retinal generated in outer segments; RDH12 reduces retinal that escapes to inner segments.
Conclusion: By detoxifying stray retinal, RDH12 acts as a barrier against intracellular aldehyde diffusion.
Significance: This protective role is consistent with the severe pathology resulting from RDH12 mutations in human disease.
Keywords: Dehydrogenase, Fluorescence, Photoreceptors, Reductase, Retinoid
Abstract
In vertebrate rod cells, retinoid dehydrogenases/reductases (RDHs) are critical for reducing the reactive aldehyde all-trans-retinal that is released by photoactivated rhodopsin, to all-trans-retinol (vitamin A). Previous studies have shown that RDH8 localizes to photoreceptor outer segments and is a strong candidate for performing this role. However, RDH12 function in the photoreceptor inner segments is also key, because loss of function mutations cause retinal degeneration in some forms of Leber congenital amaurosis. To investigate the in vivo roles of RDH8 and RDH12, we used fluorescence imaging to examine all-trans-retinol production in single isolated rod cells from wild-type mice and knock-out mice lacking either one or both RDHs. Outer segments of rods deficient in Rdh8 failed to reduce all-trans-retinal, but those deficient in Rdh12 were unaffected. Following exposure to light, a leak of retinoids from outer to inner segments was detected in rods from both wild-type and knock-out mice. In cells lacking Rdh8 or Rdh12, this leak was mainly all-trans-retinal. Wild-type rods incubated with all-trans-retinal reduced moderate loads of retinal within the cell interior, but this ability was lost by cells deficient in Rdh8 or Rdh12. Our findings are consistent with localization of RDH8 to the outer segment where it provides most of the activity needed to reduce all-trans-retinal generated by the light response. In contrast, RDH12 in inner segments can protect vital cell organelles against aldehyde toxicity caused by an intracellular leak of all-trans-retinal, as well as other aldehydes originating both inside and outside the cell.
Introduction
The vitamin A metabolite 11-cis-retinal serves as the chromophore moiety of the mammalian visual pigments, rhodopsin, and cone opsins, the primary light detectors (1). In vertebrate rod photoreceptor cells, photons absorbed by rhodopsin isomerize the 11-cis-chromophore to the all-trans-form that stabilizes an active signaling conformation of the protein, which initiates the reactions resulting in light detection (2, 3). The photoactivated rhodopsin eventually dissociates into its protein and retinoid components, releasing the toxic aldehyde all-trans-retinal into the outer segment compartment of the rod cell. Within the same compartment, all-trans-retinal is converted to all-trans-retinol by a retinoid dehydrogenase/reductase (RDH)3 that uses NADPH as a cofactor (4, 5). All-trans-retinol is then removed from the outer segment and transferred to the adjacent retinal pigment epithelium (RPE) in a process facilitated by the interphotoreceptor retinoid-binding protein (6). This sequence of reactions accomplishes the removal of all-trans-retinal from the outer segment and at the same time delivers all-trans-retinol to the RPE.
In the RPE, all-trans-retinol can be recycled to generate the 11-cis-retinal necessary for the assembly of rhodopsin. The first step involves the esterification of all-trans-retinol by lecithin:retinol acyltransferase to form all-trans retinyl esters (7, 8). Next, the retinoid isomerohydrolase activity of RPE65 acts on the retinyl ester substrates to produce 11-cis-retinol (9–11). An RDH that uses NAD+ as a cofactor then oxidizes the 11-cis-retinol to produce 11-cis-retinal (12) that is transported from the RPE to the retina by the interphotoreceptor retinoid-binding protein (13) where it combines with opsin to form rhodopsin.
The RDHs that catalyze the interconversion of retinal4 and retinol involved in rhodopsin turnover are members of the family of short chain dehydrogenase/reductases. The retina and the RPE contain several RDH isoforms, including at least three that are loci for inherited defects, RDH5, RDH10, and RDH12 (14–17). RDH isoforms 8, 11, 12, 13, and 14 and DHRS3 (retSDR1) have been localized within the photoreceptor cells (18–21). Among these, only RDH8 has been localized in the outer segment; it uses NADPH/NADP+ as cofactor, and its substrates include the trans, but not cis, isomers of retinal and retinol (18, 22). RDH12 has been localized in the photoreceptor cell body and also requires NADPH/NADP+ as cofactor, but in contrast, does not show isomer specificity (19). Although no human diseases have been associated with mutations in the gene encoding RDH8, mutations in the gene encoding RDH12 are associated with one of the forms of Leber congenital amaurosis (14, 15).
To investigate the functional roles of RDH8 and RDH12 in photoreceptor physiology, previous studies have developed knock-out mice lacking either one or both of these enzymes (23–25). Analysis of retinoid metabolism and visual performance in these animals showed that chromophore production necessary for continuous visual cycle activity does not appear to depend on the rate of reduction of all-trans-retinal in the rod outer segments. We have now further examined the roles of RDH8 and RDH12 in the reduction of all-trans-retinal using fluorescence imaging of retinoids in single living rod cells obtained from these three strains of mice. The large difference in the absorption maxima of all-trans-retinal and all-trans-retinol allowed us to discern the contribution of all-trans-retinal in the fluorescence signal by using excitation light of different wavelengths. Our studies show that RDH8 is necessary for reducing the bulk of the all-trans-retinal released by photoactivated rhodopsin in the outer segment and that RDH12 can reduce all-trans-retinal that enters the inner segment from the outer segment or from the environment outside the cell. These findings show for the first time that both enzymes are required to reduce all-trans-retinal generated after bleaching and present in both photoreceptor inner and outer segments.
EXPERIMENTAL PROCEDURES
Animals
Wild-type mice (129/Sv and C57BL/6) were from Harlan Laboratories (Indianapolis, IN). The Rpe65 protein in C57BL/6 mice has a methionine at amino acid position 450, whereas in 129/Sv mice it has a leucine. The mice that lacked Rdh12 (Rdh12−/−) had Rpe65-Leu450, whereas those that lacked Rdh8 (Rdh8−/−) or both Rdh8 and Rdh12 (Rdh8−/−;Rdh12−/−) had Rpe65-Met450. Although the kinetics of rhodopsin regeneration are significantly slower in mice with Rpe65-Met450 compared with those with Rpe65-Leu450 (26, 27), these differences in RPE enzyme activity would not be predicted to affect RDH activity present in isolated rod cells. The animals were 3–5 months old and were kept in cyclic light with a 12-h light cycle (6 a.m. to 6 p.m.). All of the animal procedures were carried out in accordance with protocols approved by the institutional animal care and use committees of the Medical University of South Carolina and the University of Michigan Medical School and with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. For experiments, the animals were dark-adapted overnight and sacrificed under dim red light, and the retinas were excised under either dim red or infrared light in mammalian Ringer's (130 mmol/liter NaCl, 5 mmol/liter KCl, 0.5 mmol/liter MgCl2, 2 mmol/liter CaCl2, 25 mmol/liter hemisodium-HEPES, 5 mmol/liter glucose, pH 7.40). Isolated rod photoreceptor cells were obtained as described previously (28).
Fluorescence Imaging
Fluorescence measurements were carried out on the stage of an inverted Zeiss Axiovert 100 microscope with a 40× oil immersion objective lens (N.A. = 1.3). The light for excitation was provided by a Xenon continuous arc light source and fluorescence images were acquired with a CCD camera. For measuring the overall kinetics of the rod outer segment fluorescence signal, fluorescence was excited with a broadband (40-nm bandwidth) 360-nm filter, and the emission was collected through a long pass 420-nm filter (emission >420 nm). For measuring the dependence of the fluorescence signal on the excitation light wavelength, fluorescence was excited with narrow band pass (10-nm bandwidth) filters centered at 340, 360, and 380 nm; emission was collected for >420 nm. For measuring the dependence of the fluorescence signal on the emitted light wavelength, fluorescence was excited with the broadband (40-nm bandwidth) 360-nm filter, and emission was measured through a 457-nm filter (50-nm bandwidth) and a 528-nm filter (38-nm bandwidth). All of the experiments were carried out at 37 °C. With the available glass optics, there is no way to avoid the mixing of the retinol and retinal signals just by using different narrow band excitation. With glass optics, 340 nm is the lowest wavelength that can be used and is actually the isosbestic point for retinal (λmax = ∼380 nm) and retinol (λmax = ∼325 nm) (supplemental Fig. S1A). Thus, the retinal contribution cannot be eliminated. The particular broadband excitation of 360 nm was selected because it allows for comparison with previously published data from a wide range of species.
Fluorescence images were acquired at different times after bleaching isolated cells with long wavelength light (1 min; >530 nm from a 150 W halogen lamp illuminator). Image acquisition and analysis were carried out using Slidebook (Intelligent Imaging Innovations, Denver, CO). For experiments with exogenous retinal and retinol, the retinoids were delivered using lipid-free BSA (1% in Ringer's) as a carrier. The emission properties of reduced pyridine nucleotide fluorescence were measured with a solution of 200 μm NADH placed in the same chambers used for cells. All of the reagents were of analytical grade.
Fluorescence image acquisition was always carried out with the same parameters, and the pixel intensity values used for data analysis were not scaled. To facilitate visual comparison of intensities in Figs. 1 and 4, images are presented with the same intensity scale: a single intensity value is used for image minimum, and a single intensity value is used for image maximum for all images.
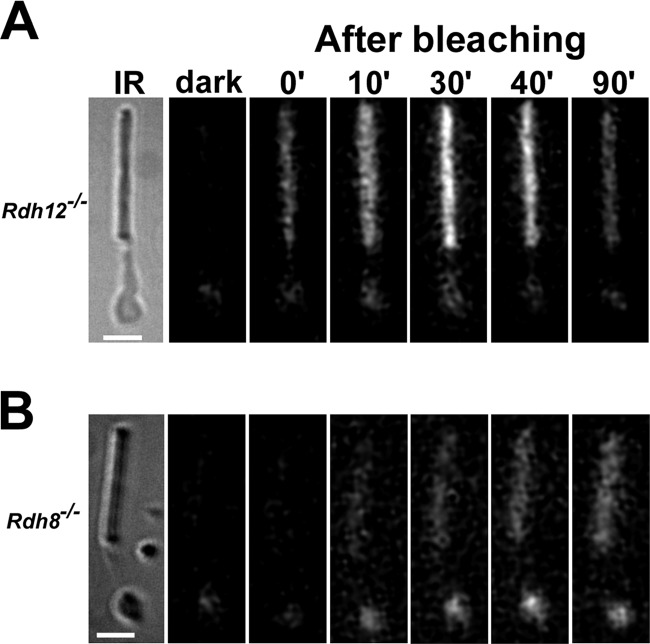
FIGURE 1.
Fluorescence increases after bleaching in the outer and inner segment of isolated rod photoreceptors from mice lacking Rdh12 (A) or Rdh8 (B). The bars are 5 μm. IR, infrared images of the cells. Fluorescence images of the cells (excitation, 360 nm; emission, >420 nm), before (dark) and at different times after bleaching, are shown. The particular fluorescence excitation filter was used to allow comparison with previously reported results from different species including mouse. All of the images are shown with the same intensity scaling. The experiments were done at 37 °C.
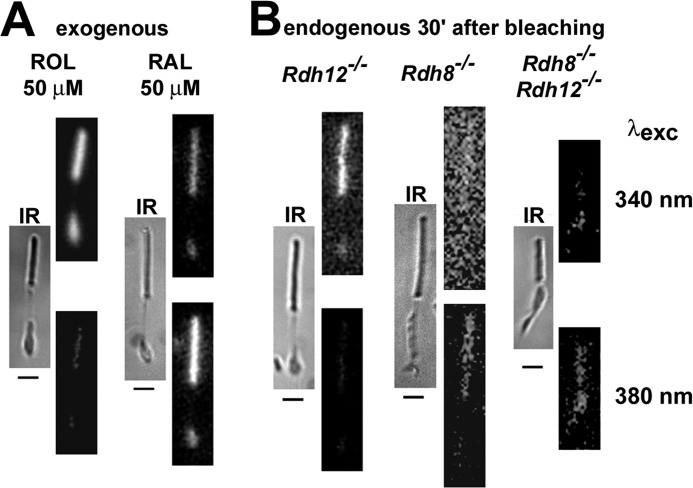
FIGURE 4.
Retinol and retinal were distinguished by exciting mouse rod photoreceptors with 340 nm (top rows of fluorescence images) and 380 nm (bottom rows) light. Fluorescence emission was collected for >420 nm. IR, infrared images. For each top and bottom pair, the images are shown at the same intensity scaling. All bars are 5 μm. A, fluorescence images 15 min after the addition of 50 μm retinol or retinal. B, fluorescence images 30 min after bleaching of Rdh12−/−, Rdh8−/−, and Rdh8−/−;Rdh12−/− cells. All of the experiments were done at 37 °C. The reason the Rdh8−/− image acquired with 340-nm excitation appears brighter is the higher intensity value of the background compared with the intensity of the background in the 380-nm excitation image; both images are displayed with the same offset.
Retinol Fraction and the Fex-380/Fex-340 Fluorescence Ratio
The contributions of retinal and retinol to the fluorescence signal were assessed from the ratio Fex-380/Fex-340 of the fluorescence excited by 380- and 340-nm light (emission collected for >420 nm). Retinal and retinol have different absorption spectra (29) (supplemental Fig. S1A). If RAL and ROL stand for retinal and retinol respectively, ε is the extinction coefficient at wavelengths 340 and 380 nm, and Q is the fluorescence quantum yield (independent of excitation wavelength), then:
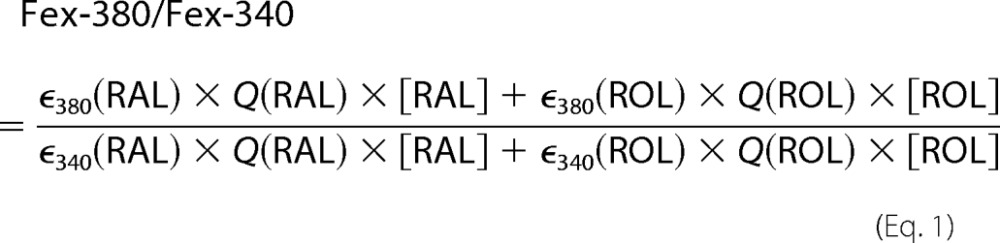 |
To find the dependence of the Fex-380/Fex-340 ratio on the fraction of retinol, if r = [ROL]/([RAL] + [ROL]) is the fraction of retinol, Equation 1 can be written as follows.
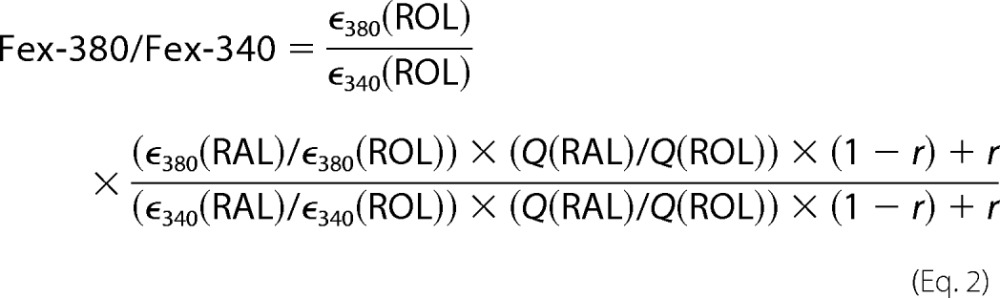 |
When all of the chromophore is retinol, r = 1, and Fex-380/Fex-340 = ε380(ROL)/ε340(ROL). When all of the chromophore is retinal, r = 0, and Fex-380/Fex-340 = ε380(RAL)/ε340(RAL).
Estimates for ε380(ROL)/ε340(ROL) were obtained from the inner and outer segments of cells loaded with 50 μm retinol, and estimates for ε380(RAL)/ε340(RAL) from the inner and outer segments of Rdh8−/−;Rdh12−/− cells loaded with 5 and 50 μm retinal (see Fig. 5). Therefore,
The isosbestic point for retinal and retinol is ∼340–350 nm (supplemental Fig. S1A). Therefore,
If
is the ratio of fluorescence quantum yields for retinol and retinal, and we define
then, after substituting the values from Equations 3–7 into Equation 2, we obtain
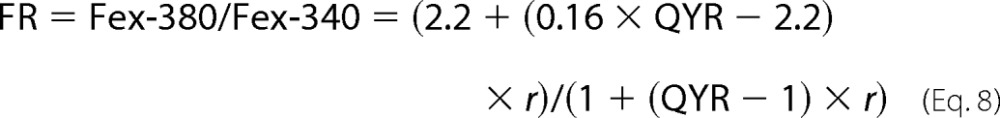 |
or
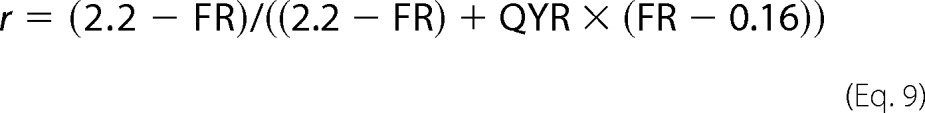 |
The relative quantum yields for retinal and retinol were estimated in two ways: (a) by loading broken off rod outer segments with 50 μm retinal or retinol for 10 min and measuring the relative fluorescence using 340-nm excitation, which is the isosbestic point (supplemental Fig. S1B); this gave QYR = 5.1; or (b) by adding known amounts of retinol or retinal (0.8 mm) to a solution of purified unbleached bovine rod outer segment membranes (containing 60 μm rhodopsin, purified according to Ref. 30), placing them in the same chambers used for cells and measuring the fluorescence using 340-nm excitation (supplemental Fig. S1C). The membranes without added retinoid were used to measure background fluorescence. This gave QYR = 15.7.
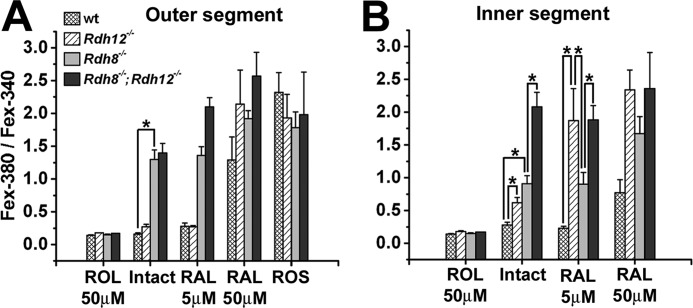
FIGURE 5.
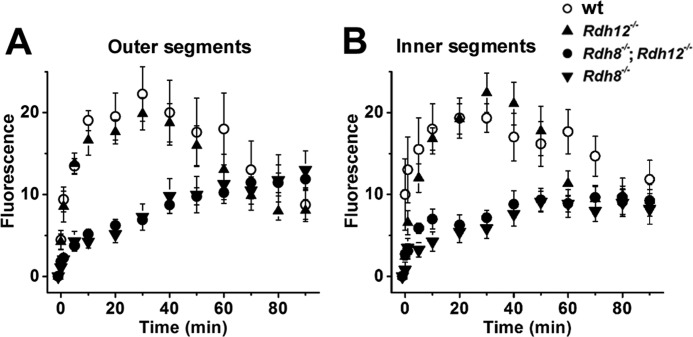
The ratio Fex-380/Fex-340 of the fluorescence intensities excited by 380- and 340-nm light is indicative of the retinol fraction in the outer (A) and inner (B) segments of rod photoreceptors. The Fex-380/Fex-340 ratio characteristic of retinol was obtained by loading intact cells with 50 μm retinol (ROL). The Fex-380/Fex-340 ratio characteristic of retinal was obtained by loading intact cells deficient in both Rdh8 and Rdh12 with 50 μm retinal (RAL). The fluorescence of wild-type (wt) outer and inner segments, 30 min after bleaching, has a ratio consistent with high retinol. The ratio shifts to higher values, indicative of high retinal, for both outer and inner segments of all types of cells loaded with high concentrations of retinal (50 μm). Broken off rod outer segments (ROS) of all types of cells also have a high Fex-380/Fex-340 ratio, again indicating retinal. The outer segments of rods that contain Rdh8 (wild type and Rdh12−/−) exhibit a ratio indicative of high retinol, even when loaded with moderate concentrations of retinal (RAL 5 μm). Rdh8 deficiency results in higher outer segment Fex-380/Fex-340 ratios, indicating high retinal, even with the endogenous chromophore only. The inner segments of cells that lack Rdh8 also exhibit higher ratios as well. Rdh12 deficiency results in higher inner segment Fex-380/Fex-340 ratios, indicating higher retinal, even with the endogenous chromophore only. The data shown are the means ± S.E., with n ≥ 5 cells. The temperature was 37 °C.
The values for QYR measured in this manner were several times smaller than the value in ethanol (∼50) (31) but were broadly consistent with the relative levels of fluorescence intensity attributed to endogenous retinal that have been measured in broken off rod outer segments (32). The relation between the ratio Fex-380/Fex-340 and the fraction of total retinoid that is in the form of retinol, r, is plotted in supplemental Fig. S1D for the two values of QYR. The Fex-380/Fex-340 ratio allows for assessing the contributions of ROL and RAL to the fluorescence signal. However, it provides information only on the ratio ROL/RAL and not on the total retinoid amount present. Thus, the total fluorescence signal is also needed to provide a measure of the total retinoid present.
Statistical Analysis
Analysis of variance was used to test for statistical significance. In the figures, statistically significant differences are indicated with asterisks.
RESULTS
Rdh8-dependent Light-induced Increases in Rod Cell Fluorescence
After mouse or other vertebrate rod and cone photoreceptors are exposed to light, there is an increase in the outer segment fluorescence that has been attributed to the formation of all-trans-retinol (28, 31, 33). Fig. 1 shows that a similar increase in fluorescence (excitation, 360 nm; emission, >420 nm) was observed in isolated mouse rods deficient in Rdh12 (Rdh12−/−) (Fig. 1A), but the increase was significantly suppressed in rods deficient in Rdh8 (Rdh8−/−) (Fig. 1B). This increase in rod outer segment fluorescence was accompanied by an increase in inner segment fluorescence (Fig. 1), as previously observed in wild-type (see Fig. 3A in Ref. 28) and Abca4-deficient (34) mouse rods. For Rdh12−/− cells that retain Rdh8, the changes in outer and inner segment fluorescence were indistinguishable from those in 129/Sv wild-type cells (Fig. 2). On the other hand, a lack of Rdh8 resulted in strong suppression of the fluorescence change in both the outer and the inner segment. In addition, there was no discernible difference between cells deficient in both Rdh8 and Rdh12 (Rdh8−/−;Rdh12−/−) from those deficient in only Rdh8 (Fig. 2). The strong suppression of the fluorescence increase in the outer segments of Rdh8−/− cells resembled the suppression observed in broken off rod outer segments. Broken off rod outer segments have been separated from the rest of the cell and do not have access to the NADPH supplied by metabolic pathways of the inner segment and as a result cannot reduce all-trans-retinal to retinol. For wild-type cells, the modest increase in fluorescence in broken off rod outer segments after bleaching has been shown to be due to the accumulation of all-trans-retinal (32). As supplemental Fig. S2 shows, the increase in fluorescence after bleaching in the outer segments of Rdh8−/− cells was virtually the same as in broken off rod outer segments from wild-type mice, suggesting that the observed increase in fluorescence was due to increased all-trans-retinal.
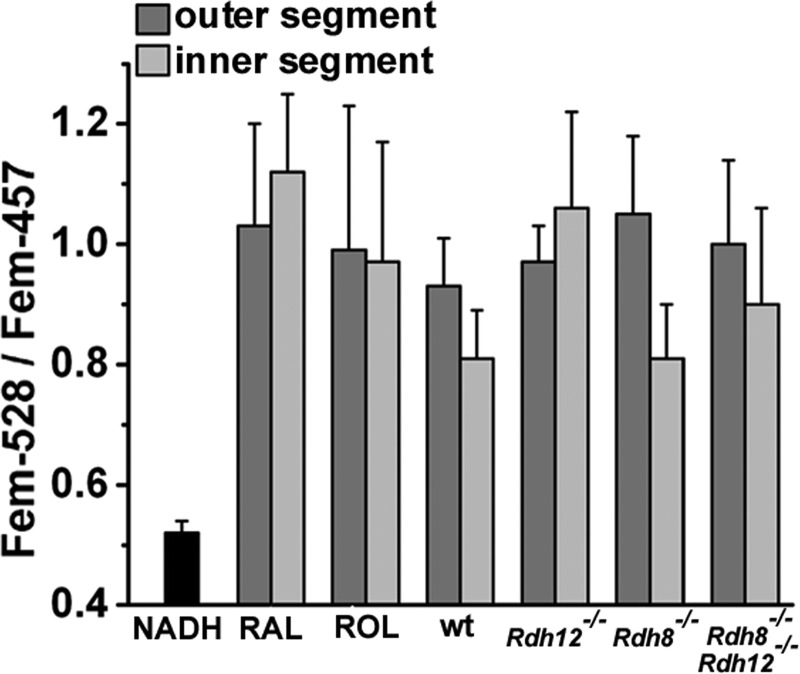
FIGURE 3.
The emission properties of the fluorescence that appears in mouse rod photoreceptor outer and inner segments after bleaching are consistent with those of retinoids and are different from those of NADH. Ratios of the emission at 528 nm over that at 457 nm (Fem-528/Fem-457)) for the outer and inner segment fluorescence changes for different mouse strains, wild type (wt, n = 7 cells), Rdh12−/− (n = 8), Rdh8−/− (n = 9), and Rdh8−/−;Rdh12−/− (n = 9), were measured 30 min after bleaching of dark-adapted intact cells. The ratios for retinal (RAL, n = 8) and retinol (ROL, n = 9) were measured 15 min after adding 50 μm of exogenous retinoid to intact Rdh8−/−;Rdh12−/− cells. The ratio for NADH was measured with a solution of 200 μm NADH (n = 4). Error bars, S.E. All of the experiments were done at 37 °C.
FIGURE 2.
Suppression of the increase in fluorescence after bleaching in the outer (A) and inner (B) segments of isolated rod photoreceptors deficient in Rdh8. Lack of Rdh12 does not affect the change in fluorescence in either compartment. Fluorescence was excited with 360-nm light and emission collected >420 nm. This fluorescence signal was due to an unknown mix of retinol and retinal but was dominated by retinol in wild type and Rdh12−/− and by retinal in Rdh8−/− and Rdh8−/−;Rdh12−/− (see Fig. 4). The change in fluorescence is plotted as a function of time after bleaching for 129/Sv wild-type (○, n = 6), Rdh12-deficient (▴, n = 11), and Rdh8-deficient (▾, n = 12) mice and mice deficient in both Rdh8 and Rdh12 (●, n = 14). Error bars, S.E. All of the experiments were done at 37 °C.
Rod Cell Fluorescence Is Distinct from That of Pyridine Nucleotides
Previous work with mouse and other vertebrate photoreceptors has shown that the increase in outer segment fluorescence after bleaching is attributable to retinol and retinal (32, 35). Special attention has been paid to the potential contribution of the reduced forms of the pyridine nucleotides NADH and NADPH whose excitation and emission spectra have significant overlap with those of retinol and retinal. There is, however, sufficient difference in their emission spectra to permit distinguishing the origin of the fluorescence signal. For fluorescence excited with 360-nm light, the emissions at 528 and 457 nm differ significantly between NADH/NADPH and retinol/retinal, and the ratio Fem-528/Fem-457 has shown that the increase in outer segment fluorescence after bleaching is due to retinoids and not to pyridine nucleotides (32, 35). Fig. 3 compares the Fem-528/Fem-457 ratio of the fluorescence increase after bleaching in the outer, as well as the inner segment, of the different mouse strains in our studies, with the ratios for retinal, retinol, and NADH. The emission properties of the fluorescence signals obtained were consistent with retinol and retinal and not with NADH or NADPH.
Increased Retinal/Retinol Ratios in Rod Cells Deficient in Rdh8 or Rdh12
Retinal and retinol have virtually identical fluorescence emission spectra and indistinguishable Fem-528/Fem-457 emission ratios (Fig. 3). Because of this similarity, it is not possible to distinguish between the two retinoids using fluorescence emission. On the other hand, retinal and retinol do have a large difference in excitation spectra, with λmax values of ∼380 nm for retinal and 325 nm for retinol. The ratio Fex-380/Fex-340 of the fluorescence (emission > 420 nm) excited with 380- and 340-nm light differs dramatically between retinal and retinol (Fig. 4A). Thus, this ratio can be used as an indicator of the retinoid generated after bleaching in the outer and inner segments of rod photoreceptors from different strains of mice. In Rdh8−/− cells, the fluorescence signal was similar to that of retinal (Fig. 4B), whereas in Rdh12−/− cells it was similar to retinol (Fig. 4B). For exogenously added retinal and retinol, the fluorescence signal was fairly bright for both retinoids, because large amounts accumulated in the outer and inner segment during the 15-min loading period (Fig. 4A). However, for endogenously generated retinoids, the fluorescence signal was much dimmer in the case of retinal compared with retinol (Fig. 4B), because retinal has a much lower fluorescence quantum yield.
Relation between Fluorescence Ratios and Retinol/Retinal Fraction
The data in Fig. 5 provide an accurate representation of the roles of Rdh12 and Rdh8. However, they do so only in a semiquantitative manner, because the fluorescence signal ratio Fex-380/Fex-340 is not proportional to the fraction of total retinoid that is in the form of either retinal or retinol. This is due to the large difference in quantum yields between retinal and retinol, such that even at relatively low fractions of the total, retinol dominates the fluorescence signal. For high concentrations of retinol, the ratio was 0.14–0.18 and indistinguishable from the ratio of the endogenous retinoid at 30 min after bleaching, 0.16 ± 0.02 (n = 16) for outer segments of intact wild-type cells (Fig. 5), which is known to be due to ∼80% retinol (28). For high concentrations of retinal, achieved either in broken off rod outer segments or by loading the cells with 50 μm retinal, the ratio was on the order of 1.8–2.6 (Fig. 5). Conversion of the Fex-380/Fex-340 ratios to retinol/retinal fractions, which provide an intuitively familiar measure of enzyme activity, requires knowledge of the fluorescence quantum yield ratio, QYR of Equation 6. Measurements using single broken off mouse rod outer segments loaded with the same concentrations of retinal or retinol for the same length of time (supplemental Fig. S1B) gave a QYR value of ∼5.1. This approach has the advantage of measuring QYR from rod outer segments; however, it does not ensure that the outer segments have accumulated equal concentrations of retinal and retinol. This was addressed in separate measurements that used purified unbleached bovine rod outer segment membranes to which known concentrations of retinal or retinol were added (supplemental Fig. S1C). These gave a QYR value of ∼15.7. Assuming that the absorption spectra of the two retinoids are the same in cells as in solution, the relation between the fluorescence ratio and the fraction of retinol present was obtained (Equation 9) and plotted in supplemental Fig. S1D for the two values of QYR. The ratio becomes very sensitive to changes in retinol when retinol is present as a small fraction of the total retinoid. The conversion of the ratios from Fig. 5 to fractions of retinol is provided in supplemental Table S1 for the two different values of QYR, both of which showed the same trends. For simplicity of presentation, the value of QYR = 5.1 is used in the rest of the paper. For ratios higher than ∼0.5, the fraction of retinol drops below 0.5; ratios of 0.75, 1.0, and 1.5 correspond to retinol fractions of ∼0.3, 0.2, and 0.1, respectively.
Retinol Fraction in Rod Inner and Outer Segments from Wild-type and Rdh8- and Rdh12-deficient Retinas
The sensitivity of the Fex-380/Fex-340 ratio to the presence of small fractions of retinol was used to assess the ability of the outer and inner segments of rod photoreceptors deficient in either Rdh8 or Rdh12, or both, to convert retinal to retinol (Fig. 5). Because of the lack of a sufficient supply of NADPH and regardless of their strain of origin, broken off rod outer segments could not reduce endogenous retinal. Hence, the fluorescence of bleached broken off rod outer segments had a high Fex-380/Fex-340 ratio, consistent with the presence of retinal (Fig. 5A). The Fex-380/Fex-340 ratio for retinol was obtained by loading the cells with a high concentration (50 μm) of retinol for 15 min (Fig. 5); this ratio was much lower than that for retinal.
In wild-type rods, the endogenously generated fluorescence in outer segments was overwhelmingly caused by the presence of retinol, having a ratio of 0.16 ± 0.02 (n = 16) that corresponds to a retinol fraction >80%. For the inner segments, the endogenously generated fluorescence had a ratio of 0.28 ± 0.04 (n = 12), corresponding to a retinol fraction of ∼0.75. Both the outer and inner segments of wild-type rods could efficiently reduce moderate loads (5 μm) of exogenous retinal, as shown by the low values of the fluorescence ratio, 0.28 ± 0.05 (n = 10) and 0.23 ± 0.03 (n = 8) for the outer and inner segments, respectively. In contrast, in Rdh8−/−;Rdh12−/− cells deficient in both Rdh8 and Rdh12, the fluorescence generated by endogenous or exogenous retinal (5 or 50 μm) was mostly due to retinal, as judged by the high Fex-380/Fex-340 ratio.
In Rdh12−/− cells that lacked Rdh12 but retained wild-type Rdh8, the fluorescence generated in the outer segments by endogenous retinoids, as well as by moderate loads (5 μm) of exogenous retinal, had Fex-380/Fex-340 ratios of 0.27 ± 0.04 (n = 23) and 0.27 ± 0.02 (n = 6), corresponding to large fractions of retinol (∼0.75). In the inner segment of these cells, however, retinal made a large contribution to the endogenously generated fluorescence signal, as judged by an Fex-380/Fex-340 ratio of 0.62 ± 0.08 (n = 21), corresponding to a retinol fraction of ∼0.4. This ratio was significantly higher (p < 0.004) than the ratio of 0.28 ± 0.04 (n = 12) for wild-type cells, corresponding to a retinol fraction of ∼0.75. For moderate loads (5 μm) of exogenous retinal, virtually all of the fluorescence appearing in the inner segments of Rdh12−/− cells was due to retinal, having an Fex-380/Fex-340 ratio of 1.87 ± 0.49 (n = 6), significantly higher (p < 0.002) than wild type.
The importance of Rdh12 was most strikingly demonstrated in the absence of Rdh8, in comparisons of the Fex-380/Fex-340 ratios for Rdh8−/− and Rdh8−/−;Rdh12−/− inner segments (Fig. 5B). For endogenous retinal (Intact), loss of Rdh12 resulted in an increase in the ratio from 0.91 ± 0.12 (n = 15) to 2.08 ± 0.22 (n = 15) (p < 0.0001), corresponding to a drop in the retinol fraction from 0.25 to 0.01. For exogenous retinal (5 μm), a loss of Rdh12 resulted in an increase in the ratio from 0.90 ± 0.18 (n = 8) to 1.88 ± 0.22 (n = 13) (p < 0.006).
For Rdh8−/− cells that lacked Rdh8 but retained wild-type Rdh12, the endogenously generated fluorescence in outer segments had an Fex-380/Fex-340 ratio of 1.30 ± 0.14 (n = 25), corresponding to a retinol fraction of ∼0.13. This fluorescence ratio was much higher (p < 0.001) than the ratio of 0.16 ± 0.02 (n = 16) for wild-type cells, which exhibit retinol fractions >80%. The outer segments of Rdh8−/− cells also failed to reduce even moderate loads (5 μm) of exogenous retinal, resulting in a ratio of 1.36 ± 0.13 (n = 7). In the inner segments of Rdh8−/− cells, the endogenously generated fluorescence contained a large contribution by retinal, as indicated by an Fex-380/Fex-340 ratio of 0.91 ± 0.12 (n = 15), equivalent to a retinol fraction of only ∼0.25; this ratio was significantly higher (p < 0.001) than the ratio of 0.28 ± 0.04 (n = 12) for wild-type cells that corresponds to a retinol fraction of ∼0.75. Importantly, for moderate (5 μm) loads of exogenous retinal, the retinol contribution to inner segment fluorescence was significantly greater in Rdh8−/− cells that contained Rdh12 than in the Rdh12−/− cells (p < 0.04).
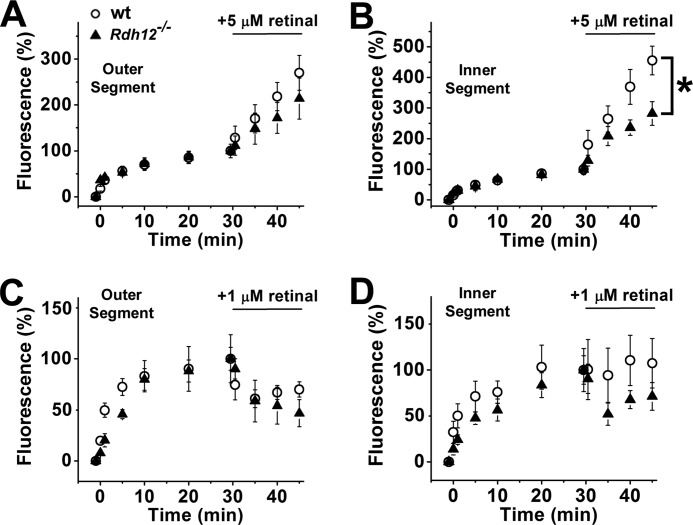
Fluorescence Increases in Rod Cells from Rdh12-deficient Mice Exposed to Exogenous Retinal
The results from Fig. 5 indicate that the inner segments of Rdh12−/− cells have a defect affecting the conversion of retinal to retinol. Because of the importance of this enzyme in the function of the human retina, we examined in more detail the response of Rdh12−/− cells to loads of retinal. For this, isolated rods were exposed to moderate (1 and 5 μm) concentrations of exogenous retinal at 30 min after bleaching, the time at which the fluorescence of retinol generated from endogenous retinal reaches its peak. Upon exposure of cells to 5 μm retinal, there was a severalfold increase in fluorescence (excitation, 360 nm; emission, >420 nm) in both their outer and inner segments. For outer segments, this fluorescence increase was the same regardless of the presence or absence of Rdh12 (Fig. 6A). For inner segments, fluorescence increased ∼2-fold slower (p < 0.03) in those of Rdh12−/− rods compared with 129/Sv wild type (Fig. 6B). For exposures to lower concentrations of exogenous retinal (1 μm), the fluorescence of the outer segment declined (Fig. 6C). This is because of the robust removal of the endogenous retinol by the 1% BSA that was used as a carrier for the exogenous retinal; the delivery and reduction of the exogenously supplied retinal proceeds at a much slower rate than the removal of the endogenous retinol, resulting in the decline of fluorescence. As with the exposures to 5 μm retinal, the outer segment fluorescence changes were the same in Rdh12−/− cells as in 129/Sv wild type (Fig. 6C). For inner segments of Rdh12−/− cells, the fluorescence declined after exposure to 1 μm retinal-containing solution, whereas for 129/Sv wild-type cells it remained approximately stable (Fig. 6D), although at this concentration of retinal the difference between wild-type and Rdh12−/− cells was not statistically significant. Again, the BSA that is used as a carrier removed the endogenous retinol, but in the case of the wild-type cells, the inner segment retinal reductase activity converted sufficient amounts of the exogenously supplied retinal to retinol to maintain the level of fluorescence.
FIGURE 6.
The inner segments (B and D) Rdh12-deficient mice have a lower capacity to reduce exogenously added retinal compared with wild-type mice; their outer segments (A and C) have similar capacity as wild type. A, fluorescence increase in the outer segments of rod photoreceptors from 129/Sv (○, n = 7) and Rdh12-deficient (▴, n = 9) mice, at different times after bleaching. All-trans-retinal (5 μm) was added at 30 min after bleaching. B, fluorescence in the inner segment of the cells shown in A. C, fluorescence increase in the outer segments of rod photoreceptors from 129/Sv (○, n = 9) and Rdh12-deficient (▴, n = 4) mice, at different times after bleaching. All-trans-retinal (1 μm) was added at 30 min after bleaching. D, fluorescence in the inner segments of the cells shown in C. The fluorescence values have been normalized to that immediately before the addition of retinal. Error bars, S.E. All of the experiments were done at 37 °C.
Increased Levels of Short Chain Aldehydes Do Not Contribute to Changes in Rod Cell Fluorescence
It is important to show that the results in Fig. 6 reflect the conversion of exogenous retinal to retinol and not the reduction of other RDH12 substrates. Indeed, the addition of 5 μm decanal, a substrate of the family of short chain dehydrogenases/reductases to which RDH12 belongs, did not result in a fluorescence increase in either the outer or the inner segment (supplemental Fig. S3). Instead, there was a decrease caused by the removal of endogenously generated retinol by the BSA present as a carrier. The decrease in fluorescence was the same in wild-type and Rdh12−/− cells. This further corroborates the participation of a robust inner segment RDH12 activity in the conversion of retinal to retinol. This activity was the same in rod photoreceptors from 129/Sv and C57BL/6 mice (supplemental Fig. S4), which are known to have different rates of rhodopsin regeneration because of differences in the isomerase activity of Rpe65.
DISCUSSION
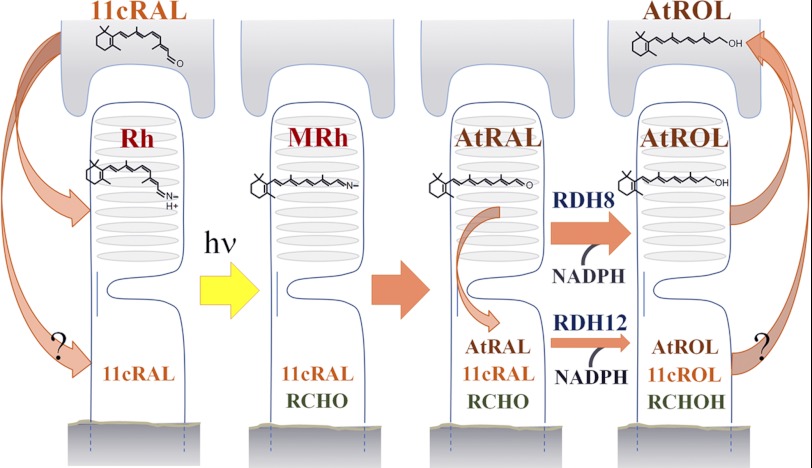
After exposure of a rod photoreceptor to light, our studies now show that the generation of all-trans-retinal and retinol in mouse rod outer segments is accompanied by a leak of these retinoids into the inner segments that can be quantified by assaying the intrinsic fluorescence of these compounds. Because of the presence of mitochondrial and nuclear DNA in the inner segment, and the possibilities for protein damage and buildup of metabolic by-products, photoreceptor cells are vulnerable to irreversible damage from retinal, a reactive aldehyde. Protection against retinal necessitates its effective clearance, which we find requires the combined action of RDH8 and RDH12. A model showing the proposed roles played by these two enzymes in the reduction and cycling of retinal and retinol between the RPE and rod photoreceptors is presented in Fig. 7.
FIGURE 7.
Schematic of retinal reductase activity in rod photoreceptor cells and retinoid flow to and from the RPE. A single RPE cell is represented above a single photoreceptor cell below. The scheme includes the flow of 11-cis-retinal into the inner segment. At the present time, the signals that regulate the flow of 11-cis-retinal from the retinal pigment epithelium to the rod photoreceptor are not well understood, and no carrier has been identified that would facilitate its migration to the inner segment. The lack of isomeric specificity for RDH12 raises the possibility that the role of the enzyme could include protection against a potential 11-cis-retinal leak as well. 11cRAL, 11-cis-retinal; 11cROL, 11-cis-retinol; AtRAL, all-trans-retinal; AtROL, all-trans-retinol; RCHO, short chain aldehyde; RCHOH, short chain alcohol; Rh, rhodopsin; MRh, metarhodopsin.
In wild-type mouse rods, Rdh8 and Rdh12 reduce most of the retinal generated by the bleaching of the full complement of the visual pigment (>80% in the outer segment and ∼75% in the inner segment), as shown by the Fex-380/Fex-340 fluorescence ratios at 30 min after bleaching. This would indicate fairly powerful reductase activity, given that the concentration of the visual pigment (36), and thus the concentration of released retinal after an exhaustive bleach, is ∼3 mm in the outer segment, perhaps reaching even higher levels in bright continuous light. This is further supported by the significant conversion of moderate loads (5 μm) of exogenous retinal to retinol; 75 and 84% in the outer and inner segment, respectively. Thus, these two enzymes appear to account for most of the retinoid reductase activity in rod photoreceptors. In their absence, any residual activity is overwhelmed, with Rdh8−/−;Rdh12−/− cells failing to reduce substantial amounts of endogenous retinal, achieving only ∼11% conversion in the outer segment and ∼1% in the inner segment.
The results obtained in our study are consistent with the previous localization of Rdh8 in the outer segment (23) and Rdh12 in the inner segment (24, 38) of rod photoreceptors cells. We find that Rdh8 activity is essential for reducing the majority of the retinal generated in the outer segment. Rods that lack Rdh8 show strong suppression of fluorescence increases seen in wild-type cells after rhodopsin bleaching. Rdh8−/− outer segments convert only a small fraction, ∼13%, of their total chromophore to retinol, indicating that Rdh8 accounts for most of the outer segment retinal reductase activity. In contrast, outer segments that have Rdh8 can convert most of their endogenous retinal to retinol, even in the absence of Rdh12 (∼75% in Rdh12−/− cells; >80% in wild type). Furthermore, rods without Rdh12 can effectively process moderate loads (5 μm) of exogenously supplied retinal (retinol fraction of ∼75% in both Rdh12−/− and wild-type cells). Previous studies have shown that the reduction of retinal is less in the eyes and retinas of Rdh8−/− mice but that there is substantial reduction of retinal, and formation of retinol, even in its absence (25). Our studies suggest that this occurs as a result of leakage of retinal out of rod outer segments and into rod inner segments (discussed below).
A loss of Rdh12 activity results in a lower fraction of endogenous retinol (∼40%, compared with ∼75% in wild type) in rod inner segments after bleaching. The effect of Rdh12 deficiency becomes even more evident in the handling of higher loads of retinal. When rods are incubated with 5 μm exogenous retinal, the conversion of retinal to retinol in outer segments is not significantly diminished by the loss of Rdh12. However, the fluorescence levels in inner segments deficient in Rdh12 are significantly lower than in wild-type inner segments, with the retinol fraction dropping from 84% in wild type, to just 4% in Rdh12−/− cells. These differences are clearly due to the conversion of retinal to retinol, because they disappear when decanal is used instead. Evidence for a significant leak of endogenous retinoid from photoreceptor-outer to -inner segments is seen in the absence of Rdh12, when the fraction of retinol present in the inner segments after bleaching drops to ∼40%, compared with ∼75% in wild type. Furthermore, in cells that lack Rdh8, the fraction of retinol in the inner segment drops from 25% in Rdh8−/− cells to 1% in Rdh8−/−;Rdh12−/− cells, indicating a nearly complete loss of the ability of double knock-out animals to reduce retinal present in the inner segment. Thus, our data do not support the notion that any other RDH isoforms contribute significantly to the retinal reductase activity of rod inner segments. This finding is of interest, in that a closely related isoform, Rdh11, having substrate specificity similar to that of Rdh12, is also expressed in mouse inner segments (19) at ∼7-fold lower levels than Rdh12 (39), but apparently contributes relatively little to the retinal reductase activity present.
Our findings provide physiological confirmation of the presence of Rdh12 in rod inner segments and further indicate that it plays a central role in protecting against the leak of retinal from outer segments during light exposure. In addition, the broad substrate specificity of Rdh12, along with its robust enzymatic activity, as evidenced by its ability to reduce moderate loads of exogenous retinal, suggests that Rdh12 plays a generally important protective role. This could include reduction of all-trans-retinal released from rhodopsin newly formed in the inner segment, as well as by-products of outer segment lipid peroxidation (40). Furthermore, our studies showing significant uptake of exogenous all-trans-retinal into rod inner segments suggest that 11-cis-retinal, with a potential toxicity equivalent to that of all-trans-retinal, may also enter this compartment. Although retinaldehyde toxicity was not experimentally addressed in the present study, aldehyde cytotoxicity is well established (41–43). Thus, a lack of protection against both retinaldehydes and short chain aldehydes would be predicted to lead to irreversible damage to the mitochondria, the nucleus, and the integrity of cellular proteins in general. This could explain the association of RDH12 mutations with the presence of severe retinal pathology in Leber congenital amaurosis patients (14, 15) and suggests that therapeutic interventions to reduce aldehyde toxicity may be useful in treating this disease (44). The notable absence of a retinal degeneration phenotype in Rdh12−/− mice suggests the presence of important biological differences that make human photoreceptors more susceptible to RDH12 deficiency. However, differences relative to the expression and localization of RDH isoforms, including RDH11, are not likely to be the reason why Rdh12−/− mice exhibit a less severe phenotype than individuals with RDH12 loss of function mutations. One important difference that could make human photoreceptors more susceptible to RDH12 deficiencies would be the need for a faster regeneration of rhodopsin after bleaching, which would require a higher rate of 11-cis-retinal delivery and result in higher fluxes of retinaldehyde through the cell (45).
The finding that the two enzymes Rdh8 and Rdh12 account for most of the retinal reductase activity in mouse rod photoreceptors and that there is very limited retinol formation in their absence has important implications for the mechanism of rhodopsin regeneration. Because the rate of recovery of 11-cis-retinal levels after bleaching is not decreased in mice deficient in Rdh8, Rdh12, or both (25), the reduction of all-trans-retinal to retinol in the rod outer segment is not required for rhodopsin regeneration, at least within the time frame of these experiments. The retinyl ester pool in the RPE corresponds to only a fraction of the rhodopsin amount in the retina (for example, Ref. 46) and therefore is not sufficient to generate the full amount of 11-cis-retinal needed for rhodopsin regeneration after a large bleach or during continuous illumination. One possible source for the needed retinol is the circulation. Another possibility is that retinal generated in the rod outer segment is transported to the RPE carried by the interphotoreceptor retinoid-binding protein or as a consequence of phagocytic uptake of shed photoreceptor membranes (37) and converted to retinol there. The present experiments with isolated rod photoreceptor cells cannot distinguish between these possibilities, but a combination of both mechanisms seems likely.
In summary, using fluorescence microscopy to quantitate relative levels of retinal and retinol, we have shown that Rdh8 and Rdh12 provide most of the retinal reductase activity present in mouse rod cells. We have also verified that the physiological function of each RDH in living cells is consistent with their known localization in the outer and inner segment, respectively. Rdh8 is responsible for reducing the bulk of the all-trans-retinal released from photoactivated rhodopsin after light exposure, but some retinal leaks to the inner segment, even in the presence of Rdh8. Rdh12 can protect the inner segment from this retinal leak and has sufficient activity to reduce additional aldehydes as well. Our findings represent a significant advance in understanding the role of RDHs in protecting against the toxic effects of retinaldehydes and other short chain aldehydes generated in light-exposed photoreceptor cells and provide important insight into the pathological mechanisms responsible for severe retinal degeneration in individuals with RDH12 mutations.
Supplementary Material
Acknowledgments
We thank Austra Liepa for expert technical assistance, Christian Huebner for Rdh12-deficient mice, and Krzysztof Palczewski for Rdh8-deficient mice.
This work was supported, in whole or in part, by National Institutes of Health Grants P30-EY07003 and R01-EY014850. This work was also supported by the Foundation Fighting Blindness, Research to Prevent Blindness. This work was conducted in a facility constructed with support from National Institutes of Health Grant C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources.

This article contains supplemental Table S1 and Figs. S1–S4.
When the isomer is not specified, retinal and retinol refer to the all-trans-isomers.
- RDH
- retinoid dehydrogenase/reductase
- RPE
- retinal pigment epithelium
- QYR
- ratio of fluorescence quantum yield of retinol over that of retinal.
REFERENCES
- 1. Wald G. (1968) Molecular basis of visual excitation. Science 162, 230–239 [DOI] [PubMed] [Google Scholar]
- 2. Hargrave P. A. (2001) Rhodopsin structure, function, and topography the Friedenwald lecture. Invest. Ophthalmol. Vis. Sci. 42, 3–9 [PubMed] [Google Scholar]
- 3. Ebrey T., Koutalos Y. (2001) Vertebrate photoreceptors. Prog. Retin. Eye Res. 20, 49–94 [DOI] [PubMed] [Google Scholar]
- 4. Futterman S., Hendrickson A., Bishop P. E., Rollins M. H., Vacano E. (1970) Metabolism of glucose and reduction of retinaldehyde in retinal photoreceptors. J. Neurochem. 17, 149–156 [DOI] [PubMed] [Google Scholar]
- 5. Saari J. C. (2000) Biochemistry of visual pigment regeneration. The Friedenwald lecture. Invest. Ophthalmol. Vis. Sci. 41, 337–348 [PubMed] [Google Scholar]
- 6. Okajima T. I., Pepperberg D. R., Ripps H., Wiggert B., Chader G. J. (1989) Interphotoreceptor retinoid-binding protein. Role in delivery of retinol to the pigment epithelium. Exp. Eye Res. 49, 629–644 [DOI] [PubMed] [Google Scholar]
- 7. Saari J. C., Bredberg D. L., Farrell D. F. (1993) Retinol esterification in bovine retinal pigment epithelium. Reversibility of lecithin:retinol acyltransferase. Biochem. J. 291, 697–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruiz A., Winston A., Lim Y. H., Gilbert B. A., Rando R. R., Bok D. (1999) Molecular and biochemical characterization of lecithin retinol acyltransferase. J. Biol. Chem. 274, 3834–3841 [DOI] [PubMed] [Google Scholar]
- 9. Redmond T. M., Poliakov E., Yu S., Tsai J. Y., Lu Z., Gentleman S. (2005) Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc. Natl. Acad. Sci. U.S.A. 102, 13658–13663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moiseyev G., Chen Y., Takahashi Y., Wu B. X., Ma J. X. (2005) RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc. Natl. Acad. Sci. U.S.A. 102, 12413–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin M., Li S., Moghrabi W. N., Sun H., Travis G. H. (2005) Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell 122, 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simon A., Hellman U., Wernstedt C., Eriksson U. (1995) The retinal pigment epithelial-specific 11-cis retinol dehydrogenase belongs to the family of short chain alcohol dehydrogenases. J. Biol. Chem. 270, 1107–1112 [PubMed] [Google Scholar]
- 13. Okajima T. I., Pepperberg D. R., Ripps H., Wiggert B., Chader G. J. (1990) Interphotoreceptor retinoid-binding protein promotes rhodopsin regeneration in toad photoreceptors. Proc. Natl. Acad. Sci. U.S.A. 87, 6907–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Janecke A. R., Thompson D. A., Utermann G., Becker C., Hübner C. A., Schmid E., McHenry C. L., Nair A. R., Rüschendorf F., Heckenlively J., Wissinger B., Nürnberg P., Gal A. (2004) Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat. Genet. 36, 850–854 [DOI] [PubMed] [Google Scholar]
- 15. Perrault I., Hanein S., Gerber S., Barbet F., Ducroq D., Dollfus H., Hamel C., Dufier J. L., Munnich A., Kaplan J., Rozet J. M. (2004) Retinal dehydrogenase 12 (RDH12) mutations in leber congenital amaurosis. Am. J. Hum. Genet. 75, 639–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sandell L. L., Sanderson B. W., Moiseyev G., Johnson T., Mushegian A., Young K., Rey J. P., Ma J. X., Staehling-Hampton K., Trainor P. A. (2007) RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 21, 1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamamoto H., Simon A., Eriksson U., Harris E., Berson E. L., Dryja T. P. (1999) Mutations in the gene encoding 11-cis retinol dehydrogenase cause delayed dark adaptation and fundus albipunctatus. Nat. Genet. 22, 188–191 [DOI] [PubMed] [Google Scholar]
- 18. Rattner A., Smallwood P. M., Nathans J. (2000) Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J. Biol. Chem. 275, 11034–11043 [DOI] [PubMed] [Google Scholar]
- 19. Haeseleer F., Jang G. F., Imanishi Y., Driessen C. A., Matsumura M., Nelson P. S., Palczewski K. (2002) Dual-substrate specificity short chain retinol dehydrogenases from the vertebrate retina. J. Biol. Chem. 277, 45537–45546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belyaeva O. V., Korkina O. V., Stetsenko A. V., Kedishvili N. Y. (2008) Human retinol dehydrogenase 13 (RDH13) is a mitochondrial short-chain dehydrogenase/reductase with a retinaldehyde reductase activity. FEBS J. 275, 138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haeseleer F., Huang J., Lebioda L., Saari J. C., Palczewski K. (1998) Molecular characterization of a novel short-chain dehydrogenase/reductase that reduces all-trans-retinal. J. Biol. Chem. 273, 21790–21799 [DOI] [PubMed] [Google Scholar]
- 22. Palczewski K., Jäger S., Buczyłko J., Crouch R. K., Bredberg D. L., Hofmann K. P., Asson-Batres M. A., Saari J. C. (1994) Rod outer segment retinol dehydrogenase. Substrate specificity and role in phototransduction. Biochemistry 33, 13741–13750 [DOI] [PubMed] [Google Scholar]
- 23. Maeda A., Maeda T., Imanishi Y., Kuksa V., Alekseev A., Bronson J. D., Zhang H., Zhu L., Sun W., Saperstein D. A., Rieke F., Baehr W., Palczewski K. (2005) Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J. Biol. Chem. 280, 18822–18832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurth I., Thompson D. A., Rüther K., Feathers K. L., Chrispell J. D., Schroth J., McHenry C. L., Schweizer M., Skosyrski S., Gal A., Hübner C. A. (2007) Targeted disruption of the murine retinal dehydrogenase gene Rdh12 does not limit visual cycle function. Mol. Cell Biol. 27, 1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maeda A., Maeda T., Sun W., Zhang H., Baehr W., Palczewski K. (2007) Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc. Natl. Acad. Sci. U.S.A. 104, 19565–19570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Danciger M., Lyon J., Worrill D., LaVail M. M., Yang H. (2003) A strong and highly significant QTL on chromosome 6 that protects the mouse from age-related retinal degeneration. Invest. Ophthalmol. Vis. Sci. 44, 2442–2449 [DOI] [PubMed] [Google Scholar]
- 27. Wenzel A., Reme C. E., Williams T. P., Hafezi F., Grimm C. (2001) The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J. Neurosci. 21, 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen C., Blakeley L. R., Koutalos Y. (2009) Formation of all-trans retinol after visual pigment bleaching in mouse photoreceptors. Invest. Ophthalmol. Vis. Sci. 50, 3589–3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hubbard R., Brown P. K., Bownds D. (1971) Methodology of vitamin A and visual pigments. Methods Enzymol. 18C, 615–653 [Google Scholar]
- 30. Kwok-Keung Fung B., Stryer L. (1980) Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc. Natl. Acad. Sci. U.S.A. 77, 2500–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsina E., Chen C., Koutalos Y., Ala-Laurila P., Tsacopoulos M., Wiggert B., Crouch R. K., Cornwall M. C. (2004) Physiological and microfluorometric studies of reduction and clearance of retinal in bleached rod photoreceptors. J. Gen. Physiol. 124, 429–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen C., Koutalos Y. (2010) Rapid formation of all-trans retinol after bleaching in frog and mouse rod photoreceptor outer segments. Photochem. Photobiol. Sci. 9, 1475–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ala-Laurila P., Kolesnikov A. V., Crouch R. K., Tsina E., Shukolyukov S. A., Govardovskii V. I., Koutalos Y., Wiggert B., Estevez M. E., Cornwall M. C. (2006) Visual cycle. Dependence of retinol production and removal on photoproduct decay and cell morphology. J. Gen. Physiol. 128, 153–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blakeley L. R., Chen C., Chen C. K., Chen J., Crouch R. K., Travis G. H., Koutalos Y. (2011) Rod outer segment retinol formation is independent of Abca4, arrestin, rhodopsin kinase, and rhodopsin palmitylation. Invest. Ophthalmol. Vis. Sci. 52, 3483–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen C., Tsina E., Cornwall M. C., Crouch R. K., Vijayaraghavan S., Koutalos Y. (2005) Reduction of all-trans retinal to all-trans retinol in the outer segments of frog and mouse rod photoreceptors. Biophys. J. 88, 2278–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lem J., Krasnoperova N. V., Calvert P. D., Kosaras B., Cameron D. A., Nicolò M., Makino C. L., Sidman R. L. (1999) Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc. Natl. Acad. Sci. U.S.A. 96, 736–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Travis G. H., Sanfilippo C., Roybal C. N. (2011) Visual chromophore in rhodopsin re-enters the visual cycle following phagocytosis of outer segments by RPE cells. ARVO Meeting Abstracts 52, 3357 [Google Scholar]
- 38. Maeda A., Maeda T., Imanishi Y., Sun W., Jastrzebska B., Hatala D. A., Winkens H. J., Hofmann K. P., Janssen J. J., Baehr W., Driessen C. A., Palczewski K. (2006) Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J. Biol. Chem. 281, 37697–37704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kanan Y., Wicker L. D., Al-Ubaidi M. R., Mandal N. A., Kasus-Jacobi A. (2008) Retinol dehydrogenases RDH11 and RDH12 in the mouse retina. Expression levels during development and regulation by oxidative stress. Invest. Ophthalmol. Vis. Sci. 49, 1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marchette L. D., Thompson D. A., Kravtsova M., Ngansop T. N., Mandal M. N., Kasus-Jacobi A. (2010) Retinol dehydrogenase 12 detoxifies 4-hydroxynonenal in photoreceptor cells. Free Radic. Biol. Med. 48, 16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beauchamp R. O., Jr., Andjelkovich D. A., Kligerman A. D., Morgan K. T., Heck H. D. (1985) A critical review of the literature on acrolein toxicity. Crit. Rev. Toxicol. 14, 309–380 [DOI] [PubMed] [Google Scholar]
- 42. Esterbauer H., Schaur R. J., Zollner H. (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11, 81–128 [DOI] [PubMed] [Google Scholar]
- 43. Kehrer J. P., Biswal S. S. (2000) The molecular effects of acrolein. Toxicol. Sci. 57, 6–15 [DOI] [PubMed] [Google Scholar]
- 44. Maeda A., Golczak M., Chen Y., Okano K., Kohno H., Shiose S., Ishikawa K., Harte W., Palczewska G., Maeda T., Palczewski K. (2012) Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat. Chem. Biol. 8, 170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lamb T. D., Pugh E. N., Jr. (2004) Dark adaptation and the retinoid cycle of vision. Prog. Retin Eye Res. 23, 307–380 [DOI] [PubMed] [Google Scholar]
- 46. Saari J. C., Nawrot M., Kennedy B. N., Garwin G. G., Hurley J. B., Huang J., Possin D. E., Crabb J. W. (2001) Visual cycle impairment in cellular retinaldehyde binding protein (CRALBP) knockout mice results in delayed dark adaptation. Neuron 29, 739–748 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.