Background: Ca2+- and cAMP-raising agonists promote exocytosis of Weibel-Palade bodies from endothelial cells.
Results: cAMP-mediated Weibel-Palade body release depends on Rap1 activation by the exchange protein activated by cAMP (Epac).
Conclusion: The Epac-Rap1 pathway is involved in the regulation of cAMP-mediated Weibel-Palade body release.
Significance: We unraveled a new signaling cascade that regulates cAMP-mediated Weibel-Palade body exocytosis and systemic VWF levels in plasma.
Keywords: Cyclic AMP (cAMP), Endothelial Cell, Exocytosis, Protein Kinase A (PKA), Secretion, Small GTPases, von Willebrand factor, Epac, Rap1, Weibel-Palade Body
Abstract
Endothelial cells contain specialized storage organelles called Weibel-Palade bodies (WPBs) that release their content into the vascular lumen in response to specific agonists that raise intracellular Ca2+ or cAMP. We have previously shown that cAMP-mediated WPB release is dependent on protein kinase A (PKA) and involves activation of the small GTPase RalA. Here, we have investigated a possible role for another PKA-independent cAMP-mediated signaling pathway in the regulation of WPB exocytosis, namely the guanine nucleotide exchange factor Epac1 and its substrate, the small GTPase Rap1. Epinephrine stimulation of endothelial cells leads to Rap1 activation in a PKA-independent fashion. siRNA-mediated knockdown of Epac1 abolished epinephrine-induced activation of Rap1 and resulted in decreased epinephrine-induced WPB exocytosis. Down-regulation of Rap1 expression and prevention of Rap1 activation through overexpression of Rap1GAP effectively reduced epinephrine- but not thrombin-induced WPB exocytosis. Taken together, these data uncover a new Epac-Rap1-dependent pathway by which endothelial cells can regulate WPB exocytosis in response to agonists that signal through cAMP.
Introduction
Vascular endothelial cells provide a dynamic interface between circulating blood and underlying tissues that is critically involved in maintaining vascular integrity and homeostasis. The endothelium provides a surface for adhesion and subsequent extravasation of leukocytes to sites of inflammation. In addition, vascular endothelial cells are involved in the regulation of vascular tone, contribute to neo-vascularization, and mediate the formation of a platelet plug in the event of vascular damage. Rapid recruitment of bio-active components from intracellular storage pools has been shown to contribute to the critical role of endothelial cells in maintaining vascular homeostasis. A significant number of hemostatic components and inflammatory mediators originate from endothelial cell-specific, cigar-shaped organelles called Weibel-Palade bodies (WPBs)2 (1). WPBs function as storage vesicles for von Willebrand factor (VWF), a multimeric adhesive glycoprotein crucial for platelet plug formation, the leukocyte receptor P-selectin, and a number of bioactive compounds that include the chemoattractants IL-8 and eotaxin-3 (2, 3). Following stimulation with agonists that increase intracellular Ca2+ levels, such as thrombin or histamine, WPBs fuse with the plasma membrane, thereby releasing their content onto the cellular surface or into the circulation. Also agonists such as epinephrine and vasopressin that raise intracellular levels of cAMP have been shown to promote the release of WPBs (4, 5). The physiological importance of this pathway is illustrated by the rise in VWF levels in patients with von Willebrand disease and mild hemophilia A following administration of the vasopressin analog DDAVP (6) or epinephrine (7). In response to cAMP-mediated stimulation, a subset of WPBs clusters at the microtubule organizing center, which involves retrograde transport of vesicles mediated by the dynein-dynactin complex (8, 9). Previous work from our group has indicated that WPB exocytosis in response to cAMP-mediated agonists is partly controlled by a protein kinase A (PKA)-dependent signaling pathway that eventually leads to the activation of RalA, a small GTPase that co-sediments with WPBs in density gradients (10–12). In its activated form, RalA has been shown to promote exocytosis through interaction with Sec5 and Exo84 (13, 14) components of the exocyst complex and by enhancing ARF-dependent phospholipase D1 activity (15). Consistent with these findings, phospholipase D1 has recently been implicated in agonist-induced release of WPBs (16). Several reports have documented signaling pathways independent of PKA that may be involved in the regulation of cAMP-mediated secretory vesicle release. More specifically, the exchange protein activated by cAMP (Epac) has been implicated in cAMP-mediated vesicle exocytosis (17, 18). In this study, we explored a potential role for the cAMP-guanine nucleotide exchange factor Epac and its substrate Rap1 in the regulation of WPB exocytosis by human primary endothelial cells.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Culture media, trypsin, penicillin, and streptomycin were from Invitrogen. EGM-2 was from Lonza (Verviers, Belgium). Epinephrine, thrombin, forskolin, 3-isobutyl-1-methylxanthine (IBMX), anti-Myc mouse monoclonal antibody (9E10), and anti-α-tubulin mouse monoclonal antibody (DM1A) were from Sigma. The Epac-specific cAMP analog 8-pCPT-2′-O-Me-cAMP-AM (Me-cAMP-AM) (19) and the PKA-specific cAMP analog 6-Bnz-cAMP-AM (20) were from Biolog (Bremen, Germany). Glutathione-Sepharose 4B was from GE Healthcare. Anti-Rap1 rabbit polyclonal antibody (sc-121) and anti-β-catenin rabbit polyclonal antibody (sc-7199) were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-VWF mouse monoclonal antibody CLB-RAg20 has been described previously (21). The Epac1 mouse monoclonal antibody 5D3 (22) was a kind gift from Dr. J. L. Bos. Alexa 488- and Alexa 633-conjugated goat anti-mouse IgG, Alexa 568-conjugated goat anti-rabbit IgG secondary antibodies, and Alexa 488-phalloidin were from Invitrogen. Chemiluminescence blotting substrate and Complete Protease Inhibitor Mixture tablets were from Roche Diagnostics. All chemicals used were of analytical grade. The enzyme-linked immunosorbent assay (ELISA) for VWF and VWF propeptide have been described previously (23).
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical veins and cultured in EGM-2 medium. Stimulation of endothelial cells with thrombin or epinephrine was performed in the following manner: HUVECs, grown in 6-well plates, were washed two times with serum-free medium (SF medium: 50% M199, 50% RPMI 1640, 0.3 mg/ml l-glutamine, 100 units/ml penicillin, 100 mg/liter streptomycin). After washing, cells were preincubated with SF medium for 1 hour. At the beginning of stimulation, the preincubation medium was replaced by SF medium containing either 1 unit/ml thrombin, a mixture of 10 μm epinephrine, and 100 μm IBMX; a mix of 10 μm forskolin and 100 μm IBMX; 1 μm Me-cAMP-AM; or 1 μm 6-Bnz-cAMP-AM.
siRNA
All siRNAs were purchased from Dharmacon (Thermo Fisher Scientific Dharmacon Products, Lafayette, CO). For siRNA-mediated knockdown of Epac1, siGENOME SMARTpool M-007676-01 RapGEF3 was used. Individual siRNA J-007676-05 (target sequence CGUGGAACUCAUGAGAUG) derived from the ON-TARGETplus Set of 4 Upgrade, human RapGEF3 (LU-007676), was used to verify the results obtained with the siGENOME SMARTpool of RAPGEF3.
For siRNA-mediated knockdown of Rap1, siGENOME SMARTpool M-003623-02 (human Rap1a) and siGENOME SMARTpool M-010364-03 (human Rap1b) were used. siGENOME NonTargeting siRNA Pool 1 (D-001206-13-05) was used as a control in these experiments. siRNA (20 pmol per well of a 6-well plate) was delivered to HUVECs by transfection using Interferin (PolyPlus, Westburg, Leusden, the Netherlands) according to the manufacturer's instructions. Transfected HUVECs were grown on fibronectin-coated glass coverslips for 72 h before stimulation.
Production of DNA Constructs and Viral Vectors
The full-length Rap1GAP cDNA clone 5767775 was obtained from Open Biosystems (Thermo Fisher Scientific, Open Biosystems Products, Huntsville, AL). A myc tag was inserted at the amino terminus of Rap1GAP using the following oligonucleotide primers: Fw1, AATATGGAGCAGAAGCTGATCTCCGAGGAGGACCTGATTGAGAAGATGCAGGGAAAGCAGGAT, and Rev1 AATGAATTCCCTGCAGGCTAACAGCCCAGCTGGGGCATGTGCTGCT. A second PCR with forward primer Fw2, AATGGATCCGCTAGCGCCACCATGGAGCAGAAGCTGATCTCCGAGGAGGACCTGATTGAG, and Rev1 was used to introduce NheI and SbfI restriction sites that facilitated cloning in the lentiviral vector pLV-CMV (24). The final pLV-CMV-myc-Rap1GAP construct was checked by sequencing.
The lentiviral packaging system consists of three constructs encoding gag/pol (pMDL.RRE), vesicular stomatitis virus glycoprotein envelope (pCMV-VSV-G), and rev (pRSV-Rev) (25). Lentivirus was prepared essentially as described previously (25).
Rap1 Activation Assays
The Ras binding domain of RalGDS fused to a GST tag was expressed in isopropyl 1-thio-β-d-galactopyranoside-induced bacteria as described previously (26). Purified GST-Ras binding domain (100 μg/sample) was precoupled to a 30-μl/sample of glutathione-Sepharose 4B for 1 h at 4 °C. The precoupled glutathione-Sepharose was then washed three times with lysis buffer containing 15% (v/v) glycerol, 1% Nonidet P-40, 50 mm Tris-HCl (pH 7.5), 200 mm NaCl, 2.5 mm MgCl2, 10 mm benzamidine, 100 nm aprotinin, supplemented with 1 protease inhibitor tablet/50 ml. Following stimulation, cells grown in 6-well plates were lysed in 400 μl of lysis buffer. The activated GTP-bound form of Rap1 was then isolated from cell lysates by incubation of 300 μl of lysate with GST-Ras binding domain precoupled glutathione-Sepharose for 1 h at 4 °C. Finally, the Sepharose beads were washed four times with lysis buffer, and bound proteins were resuspended in Laemmli sample buffer. Proteins were run on an SDS 12.5% polyacrylamide gel and analyzed by Western blotting employing an anti-Rap1 polyclonal antibody.
RESULTS
Epac and VWF Secretion
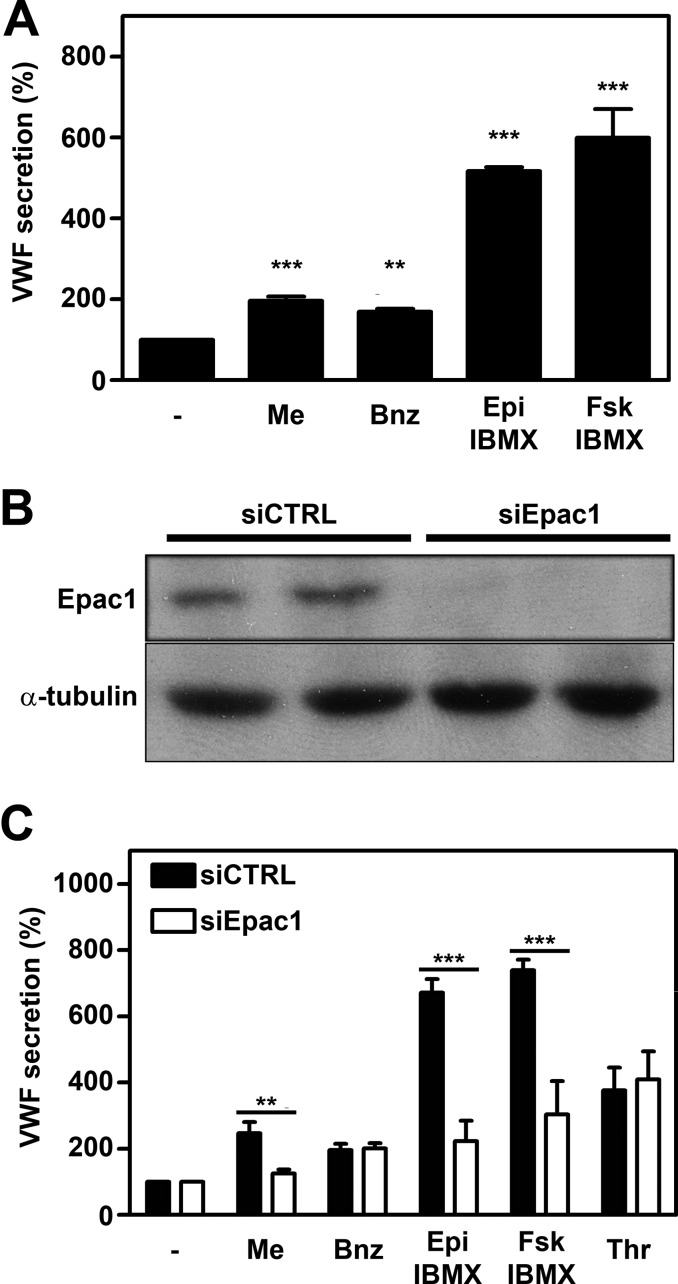
Exocytosis of WPBs occurs following triggering of G protein-coupled proteins of the Gs subtype, which elevate intracellular cAMP levels and promote PKA-dependent activation of RalA (4, 5, 12). Inhibition of PKA returns epinephrine-induced activation of RalA to basal levels and thereby abolishes VWF secretion (12). Epac, the exchange protein activated by cAMP for the small GTPases Rap1 and Rap2, is involved in regulation of endothelial barrier function (27–29) and endothelial cell adhesion (30) but also regulated secretion of insulin in pancreatic beta-cells (31). Endothelial cells selectively express Epac1 but not Epac2 (28, 29). It has been shown previously that the Epac-specific cAMP analog 8-pCPT-2′-O-Me-cAMP promotes exocytosis of WPBs (9, 32). To determine whether Epac plays a role in cAMP-mediated WPB exocytosis, we first confirmed that 1 μm 8-pCPT-2′-O-Me-cAMP-AM (Me-cAMP-AM) promotes release of VWF (Fig. 1A). Similarly, the PKA-specific agonist 6-Bnz-cAMP-AM also induced release of VWF at a concentration of 1 μm; however, Me-cAMP-AM and 6-Bnz-cAMP-AM are less potent in the induction of VWF secretion when compared with 10 μm epinephrine and 10 μm forskolin both supplemented with 100 μm of the phosphodiesterase inhibitor IBMX (Fig. 1A). When Me-cAMP-AM and 6-Bnz-cAMP-AM are used in combination at a concentration of 1 μm, an additive effect can be observed that is illustrated by a statistically significant increase of VWF secretion compared to when the stimuli are used individually, suggesting a contribution of both Epac and PKA pathways in WPB release. However, the combination of Me-cAMP-AM and 6-Bnz-cAMP-AM did not reach the level of VWF secretion induced by epinephrine + IBMX. (supplemental Fig. S1A).
FIGURE 1.
Involvement of Epac in cAMP-mediated WPB release. A, HUVECs were incubated with SF medium (−), supplemented with 1 μm Me-cAMP-AM (Me), 1 μm 6-Bnz-cAMP-AM (Bnz), 10 μm epinephrine and 100 μm IBMX (Epi), or 10 μm forskolin and 100 μm IBMX (Fsk). After 60 min, the amount of VWF secreted in the medium was measured by ELISA. Basal VWF secretion (unstimulated) was set to 100%. (n = 5, ***, p < 0.001; **, p < 0.01; one-way ANOVA followed by Dunnett post hoc test.) Error bars show S.E. B, HUVECs were transfected with a control siRNA SMARTpool (siCTRL) or an siRNA SMARTpool targeting Epac1 (siEpac1). Western blot analysis at 72 h post-transfection showed down-regulation of Epac1 expression. Levels of α-tubulin are shown as a protein loading control. C, siCTRL- and siEpac1-treated HUVECs were incubated for 60 min with SF medium (−), supplemented with 1 μm Me-cAMP-AM (Me), 1 μm 6-Bnz-cAMP-AM (Bnz), 10 μm epinephrine and 100 μm IBMX (Epi), 10 μm forskolin and 100 μm IBMX (Fsk) or 1 unit/ml thrombin (Thr). The amount of VWF secreted in the medium was measured by ELISA. Unstimulated VWF secretion was not affected by the treatment and was set to 100%. (n = 3; ***, p < 0.001; **, p < 0.01; two-way ANOVA followed by Bonferroni post hoc test for selected comparison.) Error bars show S.E.
To further substantiate the involvement of Epac in the regulated exocytosis of WPBs in endothelial cells, we used siRNA-mediated silencing of Epac1 (Fig. 1B), followed by stimulation with different agonists. Depletion of Epac1 did not notably alter the number or distribution of WPBs (supplemental Fig. S2). In the absence of Epac1, epinephrine- and forskolin-induced release of WPBs was decreased as evidenced by the decline in released VWF (Fig. 1C). In contrast, thrombin-induced release of WPBs, which is not mediated by cAMP but uses Ca2+ as a second messenger, was not affected by knockdown of Epac1 (Fig. 1C). As expected, the Me-cAMP-AM-induced VWF release was also dependent on Epac1, whereas the 6-Bnz-cAMP-AM-induced VWF release was not (Fig. 1C). Stimulation with these agonists is accompanied by changes in cytoskeletal organization. Epinephrine and Me-cAMP-AM led to an increase in cortical actin in an Epac1-dependent manner, in line with earlier reports (29), whereas thrombin induced the formation of stress fibers independent of Epac1 (supplemental Fig. S3).
A pool of four siRNAs targeting Epac1 was used for these experiments; assessment of an individual siRNA targeting Epac1 yielded similar results (supplemental Fig. S1B). Together, our findings show that epinephrine- and forskolin-induced release of WPBs requires Epac1.
Rap1 Activation
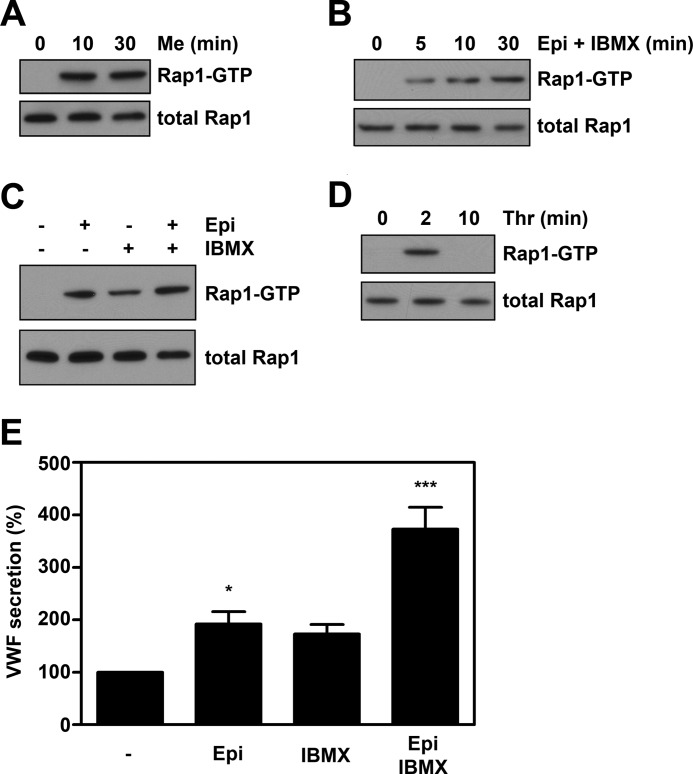
Activation of the small GTPase Rap1 by Me-cAMP and forskolin in HUVEC proceeds in an Epac-dependent manner (27, 28). To confirm that agonists are able to activate the Epac/Rap1 pathway, we performed Rap1 activity assays. Similarly as when challenged with Me-cAMP-AM (Fig. 2A) or the cAMP-elevating compound forskolin (data not shown), endothelial cells activate Rap1 when stimulated with epinephrine and IBMX (Fig. 2B); after 5 min a sustained increase in the amount of Rap1-GTP was observed. These results indicate that triggering of WPB exocytosis with the cAMP-elevating agonist epinephrine is accompanied by the activation of Rap1. As in the original study of Vischer et al. (5), we routinely added the phosphodiesterase inhibitor IBMX to prevent degradation of cAMP to potentiate cAMP-mediated processes (12). IBMX has been reported to increase intracellular cAMP in endothelial cells, and it has been shown to increase the amount of active Rap1 in several cellular systems. To test whether the cAMP rise within endothelial cells induced by IBMX alone would suffice for WPB release, we measured VWF release from endothelial cells treated for 45 min with either 100 μm IBMX, 10 μm epinephrine, or both. Only when challenged with epinephrine in combination with IBMX were we able to detect a significant increase in VWF secretion (Fig. 2E). In agreement with previous findings (5), IBMX on its own only induced a modest increase in WPB release, despite its ability to activate Rap1 activation (Fig. 2C). As reported previously, incubation with epinephrine alone resulted in only a modest increase in VWF release (Fig. 2E) (5). Also under these conditions, an increase in Rap1 activation was observed (Fig. 2C). Following incubation with thrombin (1 unit/ml), a sharp but transient increase in the amount of active Rap1 (Fig. 2D) was observed, confirming earlier findings by Cullere et al. (27). The amount of active Rap1 was maximal after 2 min of thrombin stimulation and decreased to background levels after 10 min. Currently, the identity of the exchange factor responsible for the thrombin-induced Rap1 activation in endothelial cells is unclear; however, the Ca2+ and diacylglycerol-activated exchange factor for Rap1, CalDAG-GEFI, has been shown to induce Rap1 activation in platelets in response to thrombin (33). This raises the possibility that guanine exchange factors of the CalDAG-GEF family are involved in thrombin-induced Rap1 activation in endothelial cells. These findings show that both epinephrine- and thrombin-induced exocytoses of WPB coincide with the activation of the small GTPase Rap1. Remarkably, the kinetics of Rap1 activation in response to thrombin and epinephrine are very similar to that observed for the activation of the small GTPase RalA (12, 34), suggesting that these two GTPases are activated in a coordinated fashion.
FIGURE 2.
Stimulus-induced WPB exocytosis is accompanied by activation of the small GTPase Rap1. Cells were preincubated with SF-medium for 1 h. Subsequently, cells were stimulated with 1 μm Me-cAMP-AM (A), 10 μm epinephrine and 100 μm IBMX (B), 10 μm epinephrine alone, 10 μm epinephrine and 100 μm IBMX or 100 μm IBMX alone (C), or 1 units/ml thrombin (D) for the indicated periods. Activation of Rap1 in HUVECs was determined using a Rap1-GTP specific pulldown. Western blots of activated Rap1 demonstrate the activation of Rap1 by cAMP- and Ca2+-mediated agonists. The amount of total Rap1 shown in the lower panels is used as loading control. E, HUVEC were prestimulated with SF medium for 1 h and then treated for 45 min with SF medium (−), 10 μm epinephrine (Epi), 100 μm IBMX (IBMX), or 10 μm epinephrine and 100 μm IBMX together (Epi IBMX). VWF secretion in the medium was assessed by VWF ELISA. (n = 6; ***, p < 0.001; *, p < 0.05 by one-way ANOVA followed by Dunnett post hoc test.) Error bars show S.E.
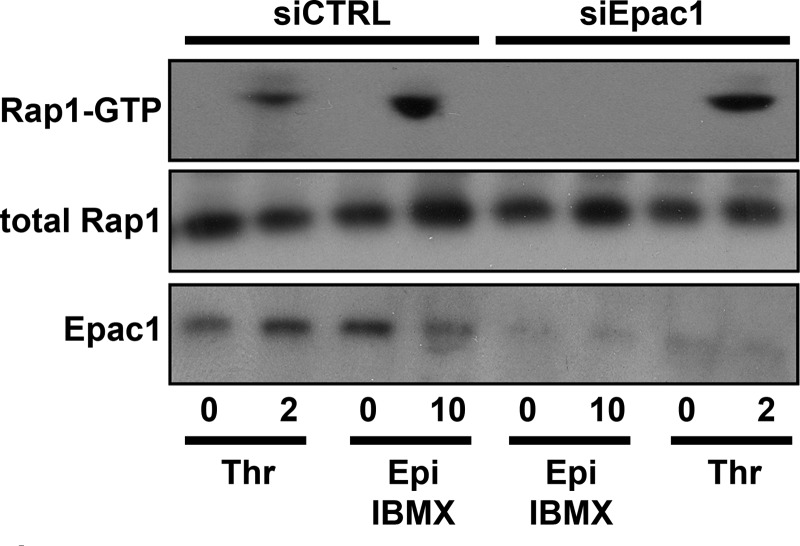
Subsequently, we addressed whether siRNA-mediated knockdown of Epac1 abolished epinephrine-induced activation of Rap1. In agreement with previous findings (28), knockdown of Epac1 did abolish the activation of the small GTPase Rap1 by epinephrine but not by thrombin (Fig. 3). These findings confirm that Rap1 is activated in an Epac-dependent manner in endothelial cells upon stimulation with cAMP- but not Ca2+-elevating agonists (27, 28, 35).
FIGURE 3.
Impaired epinephrine-stimulated Rap1 activation in endothelial cells after down-regulation of Epac1. HUVECs treated with Epac1 siRNA (siEpac1) or control siRNA (siCTRL) were preincubated with SF medium for 1 h. Subsequently, cells were stimulated with 10 μm epinephrine and 100 μm IBMX (Epi) or 1 unit/ml thrombin (Thr) for the indicated minutes. Activation of Rap1 in HUVECs was determined using a Rap1-GTP specific pulldown. Western blots of Rap1-GTP illustrate the inability of Epac1 down-regulated endothelial cells to activate Rap1 in response to epinephrine, although thrombin-induced Rap1 activation remains unaffected. The total amount of Rap1 shown in the middle panel is used as loading control.
Rap1 Activation and VWF Release
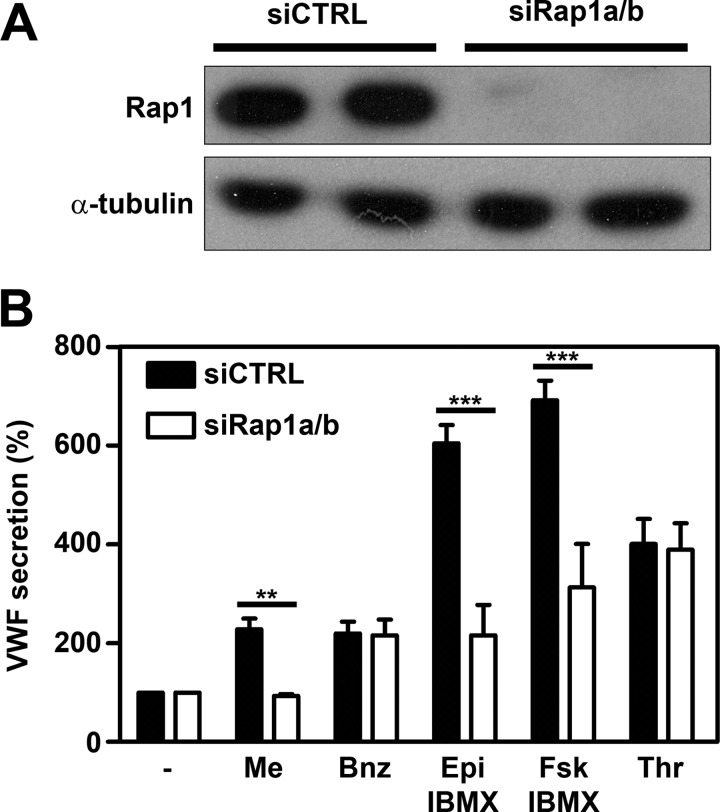
To further establish whether the Epac-Rap1 pathway is involved in the regulation of WPB exocytosis by the cAMP-raising agonist, we used two strategies to down-regulate endogenous Rap1 activity as follows: the siRNA-mediated knockdown of Rap1a and Rap1b and the ectopic expression of Rap1GAP. The Rap1 antibody used in this study cannot discriminate between Rap1a and Rap1b. Silencing of either Rap1a or Rap1b did not result in a complete lack of Rap1 expression (supplemental Fig. S4) suggesting that both isoforms are expressed in endothelial cells. Co-administration of Rap1a and Rap1b siRNA resulted in efficient silencing of Rap1 (Fig. 4A; supplemental Fig. S4). Silencing of Rap1 did not affect steady state WPB numbers or distribution and also its effects on cytoskeletal remodeling were comparable with down-regulation of Epac1; cytoskeletal remodeling induced by epinephrine or Me-cAMP-AM was abrogated; however, actin stress fiber formation induced by thrombin was unaffected (supplemental Fig. S3). Under these conditions, both epinephrine- and forskolin-induced releases of VWF were strongly reduced, but thrombin-induced release was not affected (Fig. 4B). As expected, Me-cAMP-AM-induced VWF release was also completely abolished, whereas 6-Bnz-cAMP-AM-induced release was not affected (Fig. 4B). This indicates that Rap1 is required for epinephrine-induced release of WPBs from endothelial cells. It also suggests that the role of Epac in WPB exocytosis depends on its ability to catalyze the GDP/GTP exchange of Rap1.
FIGURE 4.
Down-regulation of Rap1 expression inhibits epinephrine-induced WPB release. A HUVECs were transfected with a control siRNA SMARTpool (siCTRL) or an siRNA SMARTpool targeting Rap1a and Rap1b (siRap1a + siRap1b). Western blot analysis 72 h post-transfection showed down-regulation of Rap1 expression. Levels of α-tubulin are shown as a protein loading control. B, control siRNA and Rap1 siRNA (siRap1a + siRap1b)-treated HUVECs were incubated for 60 min with SF medium (−), supplemented with 1 μm Me-cAMP-AM (Me), 1 μm 6-Bnz-cAMP-AM (Bnz), 10 μm epinephrine and 100 μm IBMX (Epi), 10 μm forskolin and 100 μm IBMX (Fsk), or 1 unit/ml thrombin (Thr). The amount of VWF released is expressed as percentage relative to the amount of VWF released in the absence of a stimulus. (n = 3; ***, p < 0.001; **, p < 0.01; by two-way ANOVA followed by Bonferroni post hoc test for selected comparison.) Error bars show S.E.
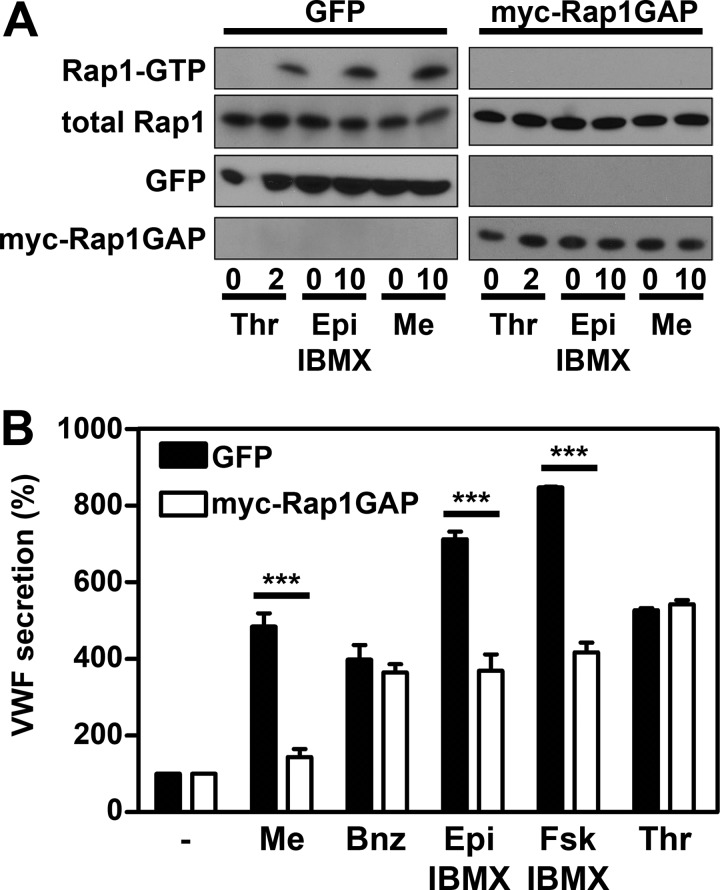
Ectopic expression of Myc-tagged Rap1GAP was used to explore the potential role of Rap1 activation in regulated exocytosis of WPBs. Rap1GAP is a GTPase-activating protein specific for Rap1 but with no GAP activity toward related GTPases such as Rap2 or Ras (36). Earlier reports have shown that the amount of active Rap1 was strongly reduced in cells overexpressing Rap1GAP (37). HUVECs were transduced with lentiviruses encoding GFP, as negative control, or myc-Rap1GAP. Confluent monolayers of HUVECs were stimulated with agonists, and the activation of Rap1 was determined. Overexpression of Rap1GAP prevented both thrombin- and epinephrine-induced activations of Rap1, whereas overexpression of GFP did not affect Rap1 activation (Fig. 5A). This shows that Rap1GAP overexpression is an efficient method to inhibit endogenous Rap1 activity in HUVECs. We also measured VWF release in endothelial cells overexpressing Rap1GAP. A strong decline in epinephrine- and forskolin-induced release of VWF was observed in cells overexpressing Rap1GAP, whereas thrombin-induced release of VWF was not affected (Fig. 5B). As expected, expression of GFP did not prevent epinephrine- or forskolin-induced VWF release. Also, Me-cAMP-AM-induced VWF release was abolished by Rap1GAP, whereas 6-Bnz-cAMP-AM induced release was not affected (Fig. 5B). These findings show that activation of Rap1 is required for cAMP-dependent exocytosis of WPBs.
FIGURE 5.
Activation of Rap1 is crucial for epinephrine-induced WPB release. HUVECs were transduced with GFP (negative control) or Rap1GAP carrying a Myc tag (myc-Rap1GAP) and were grown for 72 h. A, cells were incubated with 1 unit/ml thrombin (Thr), 10 μm epinephrine and 100 μm IBMX (Epi), or 1 μm Me-cAMP-AM (Me) for the indicated minutes. Activation of Rap1 in HUVECs was determined using a Rap1-GTP specific pulldown. The total amount of Rap1 shown in the 2nd panels is used as loading control. GFP and myc-Rap1GAP expression are shown in the lower panels. B, transduced HUVECs were incubated with SF medium (−), supplemented with 1 μm Me-cAMP-AM (Me), 1 μm 6-Bnz-cAMP-AM (Bnz), 10 μm epinephrine and 100 μm IBMX (Epi), 10 μm forskolin and 100 μm IBMX (Fsk), or 1 unit/ml thrombin (Thr). After 60 min, the medium was collected, and VWF secretion was measured by ELISA. VWF release is expressed in percentages relative to the amount of VWF released without stimulation. (n = 3; ***, p < 0.001; by two-way ANOVA followed by Bonferroni post hoc test for selected comparison.) Error bars show S.E.
DISCUSSION
Agonist-induced release of WPBs allows for the rapid mobilization of adhesion molecules, chemokines, and vasoactive compounds from endothelial cells (3). At present, two intracellular signaling pathways have been described that promote release of WPBs. Agonists like histamine and thrombin mediate WPB release by raising intracellular Ca2+ levels, whereas cAMP-dependent signaling pathways control epinephrine and vasopressin-induced release of WPBs. Previous studies have supported the concept that both signaling pathways converge at the level of the guanine exchange factor RalGDS (11). Subsequent activation of the small GTPase Ral promotes assembly of the exocyst complex and mediates phospholipase D1-facilitated fusion of WPBs with the plasma membrane (16, 34). In this study, we show that Epac1, through the activation of the small GTPase Rap1, is crucial for epinephrine- but not for thrombin-induced release of WPBs. In agreement with previous findings, we observed that Rap1 is activated in an Epac-independent manner following stimulation of endothelial cells with thrombin. Overexpression of Rap1GAP does not interfere with thrombin-induced release of WPBs indicating that activation of Rap1 is not critical for Ca2+-mediated WPB release. Thrombin is a prothrombotic and pro-inflammatory agonist that elicits a rapid activation of Ral (12, 34). We anticipate that the massive activation of Ral and the rise of intracellular Ca2+ by Ca2+-mediated agonists such as thrombin may be sufficient to promote WPB exocytosis. In contrast, the slow but sustained activation of Ral by cAMP-raising agonists like epinephrine may require concomitant activation of Rap1 to induce WPB release. Previously, we have shown that the Epac-specific cAMP analog 8-CPT-2′-O-Me-cAMP can promote release of WPBs independent of Ral activation (12). This observation suggests that apart from Rap1 other downstream effectors of Epac may also contribute to WPB exocytosis. In response to G protein-coupled receptor activation, Epac also catalyzes the activation of the small GTPase R-Ras, which in turn can also promote phospholipase D activity (38). Under these conditions, WPB exocytosis may occur in the absence of detectable Ral activation.
In earlier reports we and others have shown that PKA is involved in cAMP-mediated release of WPBs, through the exchange factor RalGDS (4, 11, 12). From the findings documented in this study, it now appears that activation of Rap1 through Epac is also required for this process. Apparently, two parallel signaling pathways are needed for cAMP-mediated exocytosis of WPBs in endothelial cells. Epac1 and the holoenzyme PKA have a similar affinity for cAMP allowing them to respond to similar concentrations of intracellular cAMP (39). At present, limited knowledge is available regarding how activation of Epac and PKA is coordinated in endothelial cells. Integration of cAMP effector pathways by protein kinase A anchoring proteins (AKAPs) has been documented previously in different biological systems (40). Phosphodiesterases have also been shown to interact with different members of the AKAP family allowing for further fine-tuning of cAMP-dependent signaling. Interestingly, Epac1 has been demonstrated to interact with and regulate the activity of mAKAP-bound PDE4D3 in cardiomyocytes (41). In endothelial cells, Epac1 binds to AKAP9, thereby integrating microtubule dynamics and barrier function (42).
Recently, a study by Nightingale and co-workers (43) has implicated the Rab27a effector myosin-VIIa- and Rab-interacting protein (MyRIP) in WPB release. From their study, it appears that the Rab27a-MyRIP complex acts as a negative regulator of exocytosis, probably by trapping Rab27a-coated WPBs in the actin filaments via the actin motor protein myosin-Va (44). As was also suggested by Nightingale et al. (43), an additional role may lie in its capacities to act as an AKAP and interact with Sec6 and Sec8, bringing together PKA with members of the exocyst complex (45). We speculate that this would facilitate the assembly of a signaling module on WPBs that focuses the cAMP-Epac/PKA pathways to their downstream effectors in secretory granule exocytosis. Based on these finding, we anticipate that AKAP scaffolds are also involved in regulation of cAMP-mediated WPB release in endothelial cells.
In this study we show that activation of Rap1 is essential for cAMP-mediated release of WPBs. Rap1 has been implicated in exocytosis of secretory granules in a number of cellular systems (46). Multiple downstream effectors of Rap1 have been identified (47). Binding of Rap1 to the Rac1 guanine exchange factors Vav2 or Tiam promotes cell spreading (48). Rac1 has also been identified as a downstream effector of Epac/Rap1 in the release of amyloid precursor protein from transfected CHO cells (49). Subsequent studies revealed that Rap1 interacts with the Rac1-specific guanine exchange factor STEF in this system (50). Interestingly, Rac1 has been implicated in release of WPBs (51). These observations raise the possibility that Rap1 controls the activation of Rac1 thereby coordinating cytoskeletal rearrangements that facilitate WPB exocytosis.
In summary, the data presented in this work have uncovered a novel pathway by which endothelial cells can regulate WPB exocytosis in response to agonists that signal through cAMP (Fig. 6). Our findings implicate Epac1 as an important regulator of β2-adrenergic and vasopressin-2 receptor-mediated WPB exocytosis and suggest that Epac1 may perform a prominent role in the systemic regulation of VWF levels in plasma.
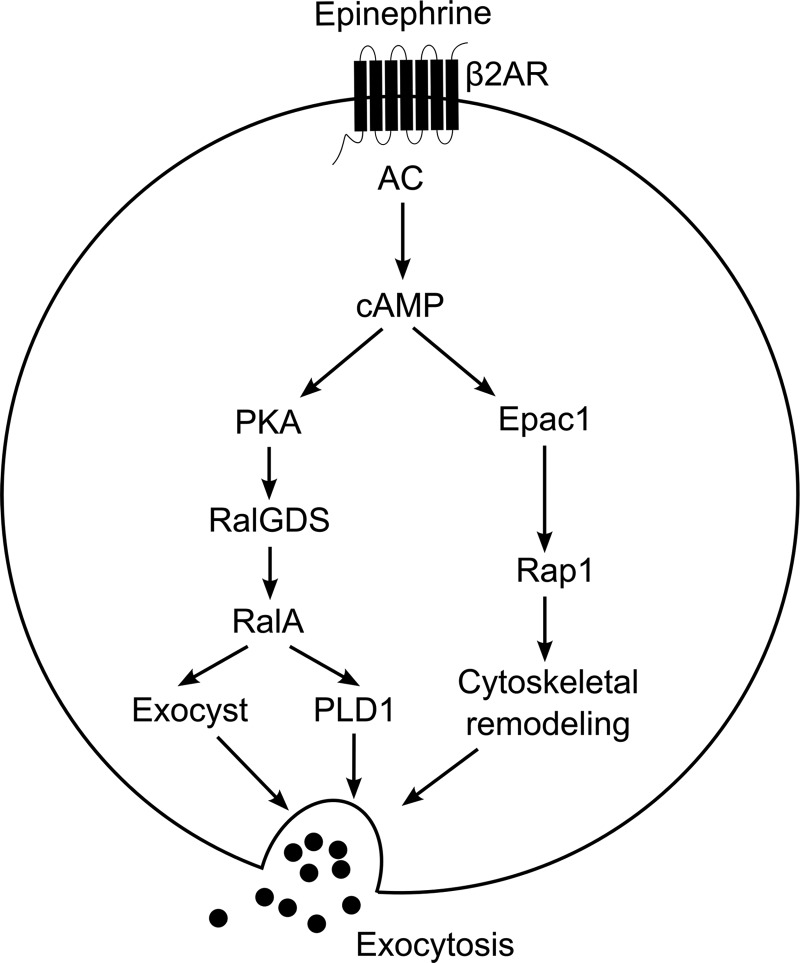
FIGURE 6.
Signaling pathways that regulate cAMP-mediated WPB exocytosis. Stimulation of the β2-adrenergic receptor (β2AR) by epinephrine stimulates cAMP production by Gs-activated adenylate cyclase (AC). Emanating from cAMP, a PKA-dependent pathway induces the activation of the small GTPase RalA by RalGDS, while simultaneously Epac1 activates Rap1. Activated RalA promotes PLD1 activity as well as assembly of the exocyst complex. In parallel, activated Rap1 facilitates WPB exocytosis, possibly by inducing cytoskeletal rearrangements.
Supplementary Material
Acknowledgments
We thank Dr. J. L. Bos for providing 5D3 antibody and Dr. Matthijs R. H. Kooistra and Drs. Anouk van Veen for helpful discussions.
This work was supported by the Netherlands Heart Foundation Grant 2002.B187, Landsteiner Foundation for Blood Transfusion Research Grant LSBR 08.19, and Dutch Organization for Scientific Research NWO TOP-Grant 91209006.

This article contains supplemental Figs. S1–S4.
- WPB
- Weibel-Palade body
- 6-Bnz-cAMP-AM
- N6-benzoyladenosine-3′,5′-cyclic monophosphate, acetoxymethyl ester
- AKAP
- A-kinase anchoring protein
- DDAVP
- 1-deamino-8-d-arginine vasopressin
- Epac
- exchange protein activated by cAMP
- HUVEC
- human umbilical vein endothelial cell
- IBMX
- 3-isobutyl-1-methylxanthine
- Me-cAMP
- 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate
- Me-cAMP-AM
- 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate, acetoxymethyl ester
- MyRIP
- myosin-VIIa- and Rab-interacting protein
- VWF
- von Willebrand factor
- ANOVA
- analysis of variance.
REFERENCES
- 1. Weibel E. R., Palade G. E. (1964) New cytoplasmic components in arterial endothelia. J. Cell Biol. 23, 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Metcalf D. J., Nightingale T. D., Zenner H. L., Lui-Roberts W. W., Cutler D. F. (2008) Formation and function of Weibel-Palade bodies. J. Cell Sci. 121, 19–27 [DOI] [PubMed] [Google Scholar]
- 3. Rondaij M. G., Bierings R., Kragt A., van Mourik J. A., Voorberg J. (2006) Dynamics and plasticity of Weibel-Palade Bodies in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26, 1002–1007 [DOI] [PubMed] [Google Scholar]
- 4. Kaufmann J. E., Oksche A., Wollheim C. B., Günther G., Rosenthal W., Vischer U. M. (2000) Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP. J. Clin. Invest. 106, 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vischer U. M., Wollheim C. B. (1997) Epinephrine induces von Willebrand factor release from cultured endothelial cells. Involvement of cyclic AMP-dependent signaling in exocytosis. Thromb. Haemost. 77, 1182–1188 [PubMed] [Google Scholar]
- 6. Mannucci P. M., Ruggeri Z. M., Pareti F. I., Capitanio A. (1977) 1-Deamino-8-d-arginine vasopressin. A new pharmacological approach to the management of hemophilia and von Willebrand diseases. Lancet 8017, 869–872 [DOI] [PubMed] [Google Scholar]
- 7. Rickles F. R., Hoyer L. W., Rick M. E., Ahr D. J. (1976) The effects of epinephrine infusion in patients with von Willebrand disease. J. Clin. Invest. 57, 1618–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Romani de Wit T., Rondaij M. G., Hordijk P. L., Voorberg J., van Mourik J. A. (2003) Real time imaging of the dynamics and secretory behavior of Weibel-Palade bodies. Arterioscler. Thromb. Vasc. Biol. 23, 755–761 [DOI] [PubMed] [Google Scholar]
- 9. Rondaij M. G., Bierings R., Kragt A., Gijzen K. A., Sellink E., van Mourik J. A., Fernandez-Borja M., Voorberg J. (2006) Dynein-dynactin complex mediates protein kinase A-dependent clustering of Weibel-Palade Bodies in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 26, 49–55 [DOI] [PubMed] [Google Scholar]
- 10. de Leeuw H. P., Wijers-Koster P. M., van Mourik J. A., Voorberg J. (1999) Small GTP-binding protein RalA associates with Weibel-Palade bodies in endothelial cells. Thromb. Haemost. 82, 1177–1181 [PubMed] [Google Scholar]
- 11. Rondaij M. G., Bierings R., van Agtmaal E. L., Gijzen K. A., Sellink E., Kragt A., Ferguson S. S., Mertens K., Hannah M. J., van Mourik J. A., Fernandez-Borja M., Voorberg J. (2008) Guanine exchange factor RalGDS mediates exocytosis of Weibel-Palade bodies from endothelial cells. Blood 112, 56–63 [DOI] [PubMed] [Google Scholar]
- 12. Rondaij M. G., Sellink E., Gijzen K. A., ten Klooster J. P., Hordijk P. L., van Mourik J. A., Voorberg J. (2004) Small GTP-binding protein Ral is involved in cAMP-mediated release of von Willebrand factor from endothelial cells. Arterioscler. Thromb. Vasc. Biol. 24, 1315–1320 [DOI] [PubMed] [Google Scholar]
- 13. Moskalenko S., Tong C., Rosse C., Mirey G., Formstecher E., Daviet L., Camonis J., White M. A. (2003) Ral GTPases regulate exocyst assembly through dual subunit interactions. J. Biol. Chem. 278, 51743–51748 [DOI] [PubMed] [Google Scholar]
- 14. Sugihara K., Asano S., Tanaka K., Iwamatsu A., Okawa K., Ohta Y. (2002) The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat. Cell Biol. 4, 73–78 [DOI] [PubMed] [Google Scholar]
- 15. Vitale N., Mawet J., Camonis J., Regazzi R., Bader M. F., Chasserot-Golaz S. (2005) The small GTPase RalA controls exocytosis of large dense core secretory granules by interacting with ARF6-dependent phospholipase D1. J. Biol. Chem. 280, 29921–29928 [DOI] [PubMed] [Google Scholar]
- 16. Disse J., Vitale N., Bader M. F., Gerke V. (2009) Phospholipase D1 is specifically required for regulated secretion of von Willebrand factor from endothelial cells. Blood 113, 973–980 [DOI] [PubMed] [Google Scholar]
- 17. Branham M. T., Mayorga L. S., Tomes C. N. (2006) Calcium-induced acrosomal exocytosis requires cAMP acting through a protein kinase A-independent, Epac-mediated pathway. J. Biol. Chem. 281, 8656–8666 [DOI] [PubMed] [Google Scholar]
- 18. Kang G., Joseph J. W., Chepurny O. G., Monaco M., Wheeler M. B., Bos J. L., Schwede F., Genieser H. G., Holz G. G. (2003) Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J. Biol. Chem. 278, 8279–8285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vliem M. J., Ponsioen B., Schwede F., Pannekoek W. J., Riedl J., Kooistra M. R., Jalink K., Genieser H. G., Bos J. L., Rehmann H. (2008) 8-pCPT-2′-O-Me-cAMP-AM. An improved Epac-selective cAMP analog. ChemBioChem. 9, 2052–2054 [DOI] [PubMed] [Google Scholar]
- 20. Poppe H., Rybalkin S. D., Rehmann H., Hinds T. R., Tang X. B., Christensen A. E., Schwede F., Genieser H. G., Bos J. L., Doskeland S. O., Beavo J. A., Butt E. (2008) Cyclic nucleotide analogs as probes of signaling pathways. Nat. Methods 5, 277–278 [DOI] [PubMed] [Google Scholar]
- 21. Stel H. V., Sakariassen K. S., Scholte B. J., Veerman E. C., van der Kwast T. H., de Groot P. G., Sixma J. J., van Mourik J. A. (1984) Characterization of 25 monoclonal antibodies to factor VIII-von Willebrand factor. Relationship between ristocetin-induced platelet aggregation and platelet adherence to subendothelium. Blood 63, 1408–1415 [PubMed] [Google Scholar]
- 22. Price L. S., Hajdo-Milasinovic A., Zhao J., Zwartkruis F. J., Collard J. G., Bos J. L. (2004) Rap1 regulates E-cadherin-mediated cell-cell adhesion. J. Biol. Chem. 279, 35127–35132 [DOI] [PubMed] [Google Scholar]
- 23. Borchiellini A., Fijnvandraat K., ten Cate J. W., Pajkrt D., van Deventer S. J., Pasterkamp G., Meijer-Huizinga F., Zwart-Huinink L., Voorberg J., van Mourik J. A. (1996) Quantitative analysis of von Willebrand factor propeptide release in vivo. Effect of experimental endotoxemia and administration of 1-deamino-8-d-arginine vasopressin in humans. Blood 88, 2951–2958 [PubMed] [Google Scholar]
- 24. Kootstra N. A., Munk C., Tonnu N., Landau N. R., Verma I. M. (2003) Abrogation of post-entry restriction of HIV-1-based lentiviral vector transduction in simian cells. Proc. Natl. Acad. Sci. U.S.A. 100, 1298–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dull T., Zufferey R., Kelly M., Mandel R. J., Nguyen M., Trono D., Naldini L. (1998) A third-generation lentivirus vector with a conditional packaging system. J. Virol. 72, 8463–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Franke B., Akkerman J. W., Bos J. L. (1997) Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J. 16, 252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cullere X., Shaw S. K., Andersson L., Hirahashi J., Luscinskas F. W., Mayadas T. N. (2005) Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105, 1950–1955 [DOI] [PubMed] [Google Scholar]
- 28. Kooistra M. R., Corada M., Dejana E., Bos J. L. (2005) Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 579, 4966–4972 [DOI] [PubMed] [Google Scholar]
- 29. Sehrawat S., Cullere X., Patel S., Italiano J., Jr., Mayadas T. N. (2008) Role of Epac1, an exchange factor for Rap GTPases, in endothelial microtubule dynamics and barrier function. Mol. Biol. Cell 19, 1261–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen A., Dong L., Leffler N. R., Asch A. S., Witte O. N., Yang L. V. (2011) Activation of GPR4 by acidosis increases endothelial cell adhesion through the cAMP/Epac pathway. PLoS ONE 6, e27586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chepurny O. G., Leech C. A., Kelley G. G., Dzhura I., Dzhura E., Li X., Rindler M. J., Schwede F., Genieser H. G., Holz G. G. (2009) Enhanced Rap1 activation and insulin secretagogue properties of an acetoxymethyl ester of an Epac-selective cyclic AMP analog in rat INS-1 cells. Studies with 8-pCPT-2′-O-Me-cAMP-AM. J. Biol. Chem. 284, 10728–10736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Øynebråten I., Barois N., Hagelsteen K., Johansen F. E., Bakke O., Haraldsen G. (2005) Characterization of a novel chemokine-containing storage granule in endothelial cells. Evidence for preferential exocytosis mediated by protein kinase A and diacylglycerol. J. Immunol. 175, 5358–5369 [DOI] [PubMed] [Google Scholar]
- 33. Crittenden J. R., Bergmeier W., Zhang Y., Piffath C. L., Liang Y., Wagner D. D., Housman D. E., Graybiel A. M. (2004) CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat. Med. 10, 982–986 [DOI] [PubMed] [Google Scholar]
- 34. de Leeuw H. P., Fernandez-Borja M., Reits E. A., Romani de Wit T., Wijers-Koster P. M., Hordijk P. L., Neefjes J., van Mourik J. A., Voorberg J. (2001) Small GTP-binding protein Ral modulates regulated exocytosis of von Willebrand factor by endothelial cells. Arterioscler. Thromb. Vasc. Biol. 21, 899–904 [DOI] [PubMed] [Google Scholar]
- 35. Fukuhara S., Sakurai A., Sano H., Yamagishi A., Somekawa S., Takakura N., Saito Y., Kangawa K., Mochizuki N. (2005) Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol. Cell. Biol. 25, 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rubinfeld B., Munemitsu S., Clark R., Conroy L., Watt K., Crosier W. J., McCormick F., Polakis P. (1991) Molecular cloning of a GTPase-activating protein specific for the Krev-1 protein p21rap1. Cell 65, 1033–1042 [DOI] [PubMed] [Google Scholar]
- 37. de Bruyn K. M., Zwartkruis F. J., de Rooij J., Akkerman J. W., Bos J. L. (2003) The small GTPase Rap1 is activated by turbulence and is involved in integrin αIIbβ3-mediated cell adhesion in human megakaryocytes. J. Biol. Chem. 278, 22412–22417 [DOI] [PubMed] [Google Scholar]
- 38. López De Jesús M., Stope M. B., Oude Weernink P. A., Mahlke Y., Börgermann C., Ananaba V. N., Rimmbach C., Rosskopf D., Michel M. C., Jakobs K. H., Schmidt M. (2006) Cyclic AMP-dependent and Epac-mediated activation of R-Ras by G protein-coupled receptors leads to phospholipase D stimulation. J. Biol. Chem. 281, 21837–21847 [DOI] [PubMed] [Google Scholar]
- 39. Dao K. K., Teigen K., Kopperud R., Hodneland E., Schwede F., Christensen A. E., Martinez A., Døskeland S. O. (2006) Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity, but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J. Biol. Chem. 281, 21500–21511 [DOI] [PubMed] [Google Scholar]
- 40. Dodge-Kafka K. L., Soughayer J., Pare G. C., Carlisle Michel J. J., Langeberg L. K., Kapiloff M. S., Scott J. D. (2005) The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 437, 574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McConnachie G., Langeberg L. K., Scott J. D. (2006) AKAP signaling complexes. Getting to the heart of the matter. Trends Mol. Med. 12, 317–323 [DOI] [PubMed] [Google Scholar]
- 42. Sehrawat S., Ernandez T., Cullere X., Takahashi M., Ono Y., Komarova Y., Mayadas T. N. (2011) AKAP9 regulation of microtubule dynamics promotes Epac1-induced endothelial barrier properties. Blood 117, 708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nightingale T. D., Pattni K., Hume A. N., Seabra M. C., Cutler D. F. (2009) Rab27a and MyRIP regulate the amount and multimeric state of VWF released from endothelial cells. Blood 113, 5010–5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rojo Pulido I., Nightingale T. D., Darchen F., Seabra M. C., Cutler D. F., Gerke V. (2011) Myosin Va acts in concert with Rab27a and MyRIP to regulate acute von Willebrand factor release from endothelial cells. Traffic 12, 1371–1382 [DOI] [PubMed] [Google Scholar]
- 45. Goehring A. S., Pedroja B. S., Hinke S. A., Langeberg L. K., Scott J. D. (2007) MyRIP anchors protein kinase A to the exocyst complex. J. Biol. Chem. 282, 33155–33167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. D'Silva N. J., Jacobson K. L., Ott S. M., Watson E. L. (1998) β-Adrenergic-induced cytosolic redistribution of Rap1 in rat parotid acini: role in secretion. Am. J. Physiol. 274, C1667–C1673 [DOI] [PubMed] [Google Scholar]
- 47. Kooistra M. R., Dubé N., Bos J. L. (2007) Rap1. A key regulator in cell-cell junction formation. J. Cell Sci. 120, 17–22 [DOI] [PubMed] [Google Scholar]
- 48. Arthur W. T., Quilliam L. A., Cooper J. A. (2004) Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J. Cell Biol. 167, 111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maillet M., Robert S. J., Cacquevel M., Gastineau M., Vivien D., Bertoglio J., Zugaza J. L., Fischmeister R., Lezoualc'h F. (2003) Cross-talk between Rap1 and Rac regulates secretion of sAPPα. Nat. Cell Biol. 5, 633–639 [DOI] [PubMed] [Google Scholar]
- 50. Zaldua N., Gastineau M., Hoshino M., Lezoualc'h F., Zugaza J. L. (2007) Epac signaling pathway involves STEF, a guanine nucleotide exchange factor for Rac, to regulate APP processing. FEBS Lett. 581, 5814–5818 [DOI] [PubMed] [Google Scholar]
- 51. Yang S. X., Yan J., Deshpande S. S., Irani K., Lowenstein C. J. (2004) Rac1 regulates the release of Weibel-Palade bodies in human aortic endothelial cells. Chin. Med. J. 117, 1143–1150 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.