Neuroanatomists from Cajal on (1) have searched in the cerebral cortex for units of structural organization that transcend the laminar pattern visible even to the untutored eye in Nissl-stained preparations. Many have commented on the vertical column-like arrays of cell bodies running orthogonal to the horizontal laminae that are particularly conspicuous in the temporal cortex of humans and other primates (Fig. 1). These columns have been promoted in the past as the morphological correlates of the functional columnarity of the cortex, known from physiological studies (2). The hypothesis of the column as the fundamental processing unit of the cerebral cortex was formulated by Mountcastle (3) from studies of cells responding to tactile stimuli in the somatosensory cortex of the cat. The hypothesis requires that nerve cells in middle layers of the cortex, in which thalamic afferents terminate, should be joined by narrow vertical connections to cells in layers lying superficial and deep to them, so that all cells in the column are excited by incoming stimuli with only small latency differences. The columns form a series of repeating units across the horizontal extent of the cortex.
Figure 1.
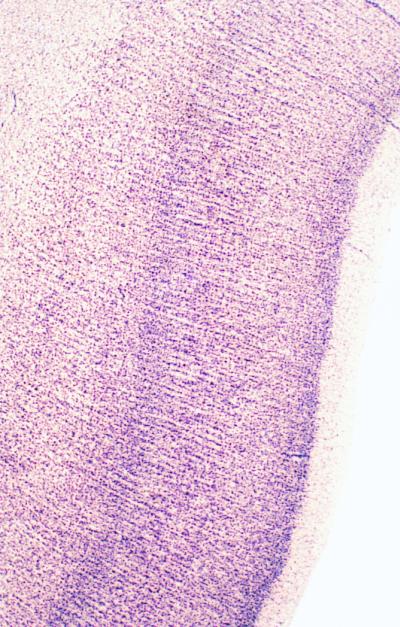
Nissl-stained section of the upper bank of the superior temporal sulcus from a human brain, showing microcolumns of nerve cells (×40).
The verticality of cell–cell connections in the cortex has never been in doubt, but determining the minimal unit of such connectivity and the extent to which it is based on morphologically definable arrays of cells continues to exercise investigators. One problem inherent in the different experimental models customarily invoked as demonstrative of cortical columns is that they differ in scale. A column defined by neurons responding to peripheral stimulation as a vertically oriented microelectrode descends through the cortical layers, is narrower than the layer IV barrels of the rodent somatosensory cortex, and the barrels differ in turn from the ocular dominance columns demonstrable in the monkey visual cortex by the alternating terminations of bundles of left and right eye-specific thalamic axons. Although united by the common thread of periodicity across the horizontal extent of the cortex and by the implication of vertical connectivity through its thickness, there are clear differences of scale and function here. It is not surprising, therefore, that scientists have sought to identify an elemental processing unit that characterizes cortex qua cortex and independent of its areal specializations.
Buldyrev et al. (4) applied quantitative methods derived from condensed matter physics to demonstrate that the vertical chains of cells, eyeballed by past generations of neuroanatomists in the human superior temporal cortex, form repeating units that they call microcolumns. Such microcolumns contain about 11 neurons and have a periodicity of about 80 microns, a periodicity that is disrupted in two examples of neurodegenerative disease. These observations raise two important questions: can microcolumns be identified in all cortical areas of all species, and are cellular microcolumns indicative of a modularity of intracortical connectivity that confines activity to domains of a size comparable to the cellular microcolumns?
Although the vertical chains of cells of the primate temporal cortex are far less conspicuous in other areas or in the cortex of nonprimates, there is sufficient interest in the idea of a mini- or microcolumnar organization of the cortex to make its generality worth considering. Minicolumns have turned up in various forms in recent and older literature. One compelling demonstration of vertical arrays of cortical cell bodies that may underlie a microcolumnar structure comes from the developing cortex in which postmitotic neurons, after leaving the proliferative neuroepithelium lining the walls of the lateral ventricles, ascend in linear arrays following a scaffold of neuroglial cell processes into the overlying cortical plate. Many of these radial chains of young neurons are clones of cells that originated from a common precursor, a potential basis for microcolumnar modularity in the definitive cortex (5). This remains a viable hypothesis, although it is now recognized that the progeny of some precursor cells can migrate tangentially in the intermediate zone before ascending to the cortex as widespread clones of cells, the radial and widespread clones possibly forming different cell types (6, 7). Moreover, this clonal modularity may apply only to the efferent pyramidal cells of the definitive cortex, the inhibitory neurons, or at least sizeable populations of them, migrating horizontally rather than radially into the cortex from the ganglionic eminence (8).
Physiologically speaking, if a microcolumn were to retain functional identity in the definitive cortex, it should be identifiable as a component of the larger and better known columnar arrays such as the barrels and ocular dominance columns, for these are much larger than the dimensions of the cellular modules visualized by Buldyrev et al. Barrels and ocular dominance columns contain the terminal ramifications of scores of thalamic afferents. Even if a column is defined on the basis of the extent of arborization of a single thalamic afferent fiber in the middle layers of the cortex, this is much more extensive than the dimensions of the microcolumns, measuring at least 600 μm in horizontal extent in monkeys and as much as 900 μm for some thalamic afferents in cats.
Evidence for a substructure in larger columnar arrays of the cortex comes from the work on the somatosensory cortex of cats and monkeys (9). Here, larger topographic zones (“segregates”) related to a body part (such as defined by a typical multiunit “mapping” experiment) contain narrow vertical “minicolumns” ≈50 μm in diameter in which all neurons have virtually identical receptive field locations, sizes, and shapes. There is no predictability about the receptive field shifts in moving from one minicolumn to the next, and receptive fields of neurons in neighboring minicolumns can differ significantly in location, size, and/or shape. Similar minicolumns are demonstrable as repeating high- and low-density periodicities within the larger segregates of radioactive 2-deoxy-d-glucose uptake produced by stimulation of part of the body (10). Can the cortex select these smaller domains from a more divergent thalamic input by surround inhibition alone, or is there an underlying anatomical microcolumn?
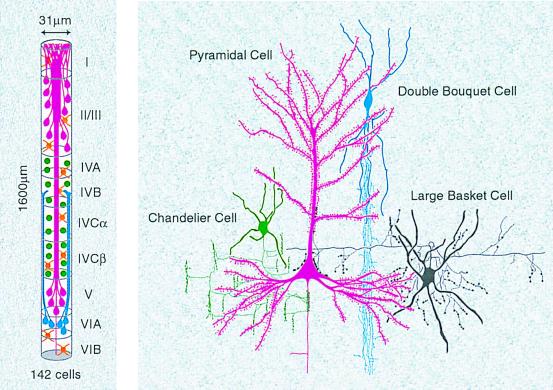
There are a number of examples of repeating microarrays of intracortical elements that could be interpreted as conforming to a microcolumnar pattern of vertical connections. These include the bundling of apical dendrites of pyramidal cells with somata located in layers II, III, and V (11). Each bundle ascends from layer V through the supervening layers, adding apical dendrites from pyramidal cells in more superficial layers as it does so. When fully formed, it consists of the dendrites of ≈142 pyramidal cells and has a diameter of ≈31 μm (Fig. 2). The pattern repeats in the horizontal dimension with a periodicity of ≈31 μm and a density of ≈1,270 per mm2 in the monkey visual cortex. (The apical dendrites of layer VI cells form their own independent ascending fasciculi, which end in layer IV.) It is likely that vertical modules visualized by imaging of voltage-sensitive dyes in developing cortex are made up of similar arrays of dendrites (12).
Figure 2.
(Left) Organization of a columnar array of apical dendrites of layers II/II and V pyramidal cells in the primary visual cortex of macaque monkeys. Numbers to right represent layers of cortex. Redrawn from A. Peters (11). (Right) Typical layer III pyramidal cell and three named types of inhibitory interneuron, each with its specific type of axonal arborization terminating on different parts of the pyramidal cell. Primate cerebral cortex. Redrawn from J. DeFelipe and I. Fariñas (18).
Another set of microcolumnar arrays, descending rather than ascending in the cortex and made up of axons rather than dendrites, is especially evident in the cortex of primates (13). The arrays are formed by tightly packed bundles of vertical axon branches derived from double bouquet cells with somata located in layer II and upper layer III (Figs. 2 and 3). The bundles of axons derived from these GABA/peptide cells are ≈10 μm in diameter and descend to layer V, where they disperse in horizontal branches. They have a periodicity of 15–30 μm and a density of ≈1,000/mm2. The bundles are not coextensive with the apical dendritic bundles of pyramidal cells.
Figure 3.
(Left) Laser confocal scanning micrograph from a vertical section through layer III of the cerebral cortex of a monkey, showing double bouquet cells and their descending axon bundles, stained immunocytochemically for the calcium binding protein, calbindin. (Right) Photomicrograph of a tangential section through layer III of the same cortex showing the repeating pattern of the bundles of calbindin-immunoreactive axons. From material described in ref. 13 (×250).
It would be easy to regard one or both of these repeating vertical units as components of a more fundamental microcolumn. However, unlike the microcolumns of cell bodies (4), their tight columnarity belies an underlying dispersion of connectivity: the branches of the apical and basal dendrites of pyramidal cells extend horizontally for hundreds of microns beyond the narrow apical bundles, and the narrow columns of double bouquet cell axons, in terminating on these branches rather than on the apical dendrites (13), can be seen as dispersing connectivity across the dendritic fields of pyramidal cells with apical dendrites located in many apical dendritic bundles (unless there is some remarkable specificity in the manner in which the double bouquet cell axons synaptically target the pyramidal cells).
Connectional specificity might provide the final clues in the quest for the cortical microcolumn if it could be demonstrated that there is a repeating pattern of intrinsic connectivity that conforms to the microcolumnar arrays of cell somata. This has not yet been done. As the cell types of the cerebral cortex become better characterized morphologically, chemically, and physiologically (14), the details of the types of connections that they establish with one another within the cortex are becoming established. There have been attempts to describe a “canonical” circuit showing the contributions of individual cell types at the heart of the terminations of thalamic afferents to the circuitry leading from input to output (15). The incorporation of the wealth of new data into a scheme relevant to a pattern of repeating microcolumns is still some distance away. Indeed, the richness of the new data only serves to make the task an increasingly onerous one. For a time it seemed as if the rather stereotyped axonal ramifications of the various classes of cortical interneurons (Fig. 2) and the patterns of intracortical collateralization of the efferent axons of the pyramidal cells would eventually yield a basic circuit diagram. Recording from connected pairs of cortical neurons in vitro, followed by morphological recovery of the neurons and often their synapses as well, has been a very powerful tool in this regard (16, 17). It has, however, continued to present new challenges to building a canonical circuit diagram. Who would have imagined, for example, that the dynamic properties of synapses formed by branches of the same axon of a GABAergic interneuron on neighboring pyramidal cells would differ from cell to cell (17)?
The rodent cerebral cortex has served as the preparation of choice for in vitro studies, but it is difficult to know whether it is a prototypical cortex. One has the impression that cell morphology, certainly at the axonal level of organization, is far more stereotyped in the primate cortex and best seen in the parietal, temporal, and frontal association areas. This is definitely true of the double bouquet cells, which are not visible in their full primate form in the cortex of rodents, leading Cajal, who never studied primates other than the human, to consider them a uniquely evolved feature of the human brain (1).
Primate temporal cortex with its pronounced cellular microcolumnarity might present a better model in determining features generalizable to all cortical areas. In a sense, the barrel cortex of rodents and the higher primate visual cortex, the one with its isomorphic view of the body surface and the other with its infinitely precise laminar and columnar cellular specification for stimulus feature extraction, may represent endpoints in the evolution of the two orders, one of which noses and whisks its way around its environment and the other of which extracts an extraordinary richness of visual detail from its environment. The experimental paradigms provided by the barrel and the ocular dominance column tend to influence the way we look at the cerebral cortex as a whole, but neither is clearly built up from microcolumnar units of cells or connections. The temporal cortex and the association cortex of which it forms a part may prove to be better models for corticality, justifying Brodmann's calling them homotypical. Their stereotypy may indeed have been promoted in primate evolution, leading to that explosive growth of the association areas that commenced in the australopthecines some two million years ago.
The jury is still out on whether anatomical microcolumns are fundamental units of organization in all cortical areas of all species and, until we have more comprehensive data on the intricacies of intracortical connectivity, it may be premature to view anatomical microcolumns as indicative of a fine-grain functional modularity of cortex, although many of us find this to be an attractive hypothesis.
Footnotes
See companion article on page 5039.
References
- 1.DeFelipe J, Jones E G. Cajal on the Cerebral Cortex. An Annotated Translation of the Complete Writings. New York: Oxford Univ. Press; 1988. [Google Scholar]
- 2.Von Bonin G, Mehler W R. Brain Res. 1971;27:1–10. doi: 10.1016/0006-8993(71)90367-2. [DOI] [PubMed] [Google Scholar]
- 3.Mountcastle V B. J Neurophysiol. 1957;20:408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- 4.Buldyrev S V, Cruz L, Gomez-Isla T, Gomez-Tortosa E, Havlin S, Le R, Stanley H E, Urbanc B, Hyman B T. Proc Natl Acad Sci USA. 2000;97:5039–5043. doi: 10.1073/pnas.060009897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kornack D R, Rakic P. Neuron. 1995;15:311–321. doi: 10.1016/0896-6273(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 6.Tan S S, Kallioniatis M, Sturm P P, Reese B E, Faulkner-Jones B. Neuron. 1998;21:295–304. doi: 10.1016/s0896-6273(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 7.Ware M L, Tavazoie S F, Reid C B, Walsh C A. Cereb Cortex. 1999;9:636–645. doi: 10.1093/cercor/9.6.636. [DOI] [PubMed] [Google Scholar]
- 8.Andersen S A, Eisenstadt D D, Shi L, Rubenstein J L R. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- 9.Favorov O V, Diamond M D, Whitsel B L. Proc Natl Acad Sci USA. 1987;84:6606–6610. doi: 10.1073/pnas.84.18.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tommerdahl M, Favorov O, Whitsel B L, Nakhle B, Gonchar Y A. Cereb Cortex. 1993;3:399–411. doi: 10.1093/cercor/3.5.399. [DOI] [PubMed] [Google Scholar]
- 11.Peters A. In: Cerebral Cortex, Vol. 10, Primary Visual Cortex of Primates. Peters A, Rockland K S, editors. New York: Plenum; 1994. pp. 1–36. [Google Scholar]
- 12.Yuste R, Peinado A, Katz L C. Science. 1992;257:665–686. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]
- 13.DeFelipe J, Hendry S H C, Hashikawa T, Molinari M, Jones E G. Neuroscience. 1990;37:655–673. doi: 10.1016/0306-4522(90)90097-n. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi Y, Kubota Y. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 15.Douglas R, Martin K A C. J Physiol (London) 1991;440:735–769. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomson A M, Deuchars J. Cereb Cortex. 1997;7:510–522. doi: 10.1093/cercor/7.6.510. [DOI] [PubMed] [Google Scholar]
- 17.Markram H. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 18.DeFelipe J, Fariñas I. Prog Neurobiol. 1992;39:563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]