Background: The role of iPLA2β as a regulator of inflammatory signaling and neointima formation is unknown.
Results: Smooth muscle-specific expression of iPLA2β exacerbates proinflammatory cytokine production, macrophage infiltration, and neointima formation.
Conclusion: Smooth muscle-specific iPLA2β participates in the initiation and early progression of vascular inflammation and neointima formation.
Significance: iPLA2β may represent a novel therapeutic target for attenuating vascular inflammation and restenosis.
Keywords: Angiotensin II, Cardiovascular Disease, Inflammation, Phospholipase A, Vascular Smooth Muscle Cells, 12/15-Lipoxygenase, Neointimal Formation, Restenosis, Vascular Inflammation, iPLA2
Abstract
Whether group VIA phospholipase A2 (iPLA2β) is involved in vascular inflammation and neointima formation is largely unknown. Here, we report that iPLA2β expression increases in the vascular tunica media upon carotid artery ligation and that neointima formation is suppressed by genetic deletion of iPLA2β or by inhibiting its activity or expression via perivascular delivery of bromoenol lactone or of antisense oligonucleotides, respectively. To investigate whether smooth muscle-specific iPLA2β is involved in neointima formation, we generated transgenic mice in which iPLA2β is expressed specifically in smooth muscle cells and demonstrate that smooth muscle-specific expression of iPLA2β exacerbates ligation-induced neointima formation and enhanced both production of proinflammatory cytokines and vascular infiltration by macrophages. With cultured vascular smooth muscle cell, angiotensin II, arachidonic acid, and TNF-α markedly induce increased expression of IL-6 and TNF-α mRNAs, all of which were suppressed by inhibiting iPLA2β activity or expression with bromoenol lactone, antisense oligonucleotides, and genetic deletion, respectively. Similar suppression also results from genetic deletion of 12/15-lipoxygenase or inhibiting its activity with nordihydroguaiaretic acid or luteolin. Expression of iPLA2β protein in cultured vascular smooth muscle cells was found to depend on the phenotypic state and to rise upon incubation with TNF-α. Our studies thus illustrate that smooth muscle cell-specific iPLA2β participates in the initiation and early progression of vascular inflammation and neointima formation and suggest that iPLA2β may represent a novel therapeutic target for preventing cardiovascular diseases.
Introduction
Neointima formation is a common feature of restenosis after balloon angioplasty, transplantation of vessels and organs, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, and atherosclerosis (1–5). Neointima formation has been extensively studied because of its multiple clinical implications. The persistently high rates of restenosis after vascular interventions indicate that the current understanding of the molecular mechanisms responsible for neointima formation is incomplete, however, and the clinical significance of neointima formation calls for identification of new therapeutic targets.
The vascular smooth muscle cell (VSMC)3 is a major cellular component of the blood vessel wall, and its primary physiological functions are to maintain homeostasis of blood flow and blood pressure within normal ranges. In healthy mature blood vessels, the VSMC exhibits a quiescent contractile phenotype and expresses a unique repertoire of smooth muscle contractile proteins. Upon various injurious stimuli, the VSMC dedifferentiates, rapidly switches from a contractile phenotype to a synthetic phenotype, and migrates from the medial to the intimal layer of the vessel wall where it proliferates to form neointima (1–6). In addition to migration and proliferation, VSMC with a synthetic phenotype can produce various proinflammatory cytokines in vitro and in vivo (7–9). Paradoxically, the initiation and early progression of vascular inflammation in restenosis has been attributed largely to interactions among macrophages, lymphocytes, and endothelial cells (1–5), despite the fact that the large number of VSMC in the vessel wall are capable of producing significant amounts of cytokines that could contribute to the evolution of the inflammatory process.
Phospholipases A2 (PLA2) comprise a family of enzymes that hydrolyze esterified fatty acid residues from the sn-2 position of glycerophospholipids to produce a free fatty acid (e.g. arachidonic acid (AA)) and a lysophospholipid (e.g. 1-radyl, 2-lyso-glycerophosphocholine (LPC)) (10). Based on their cellular location and the Ca2+ requirement for enzymatic activity, PLA2s are classified into three subfamilies as follows: secretory PLA2, cytosolic PLA2, and calcium-independent PLA2 (iPLA2). The iPLA2 enzymes recognized so far are located within cells, do not require Ca2+ for enzymatic activity, and are subject to irreversible inhibition by the suicide substrate bromoenol lactone (BEL) at concentrations that do not inhibit secretory PLA2 or cytosolic PLA2 enzymes (11).
The iPLA2 enzymes are also members of a larger family of lipases designated the patatin-like phospholipase domain-containing proteins (PNPLA), of which the human genome expresses nine members (PNPLA1–9) (12). PNPLA family members contain a protein domain discovered initially in patatin, which is a lipid hydrolase that is the most abundant protein of the potato tuber. Mammalian PNPLAs include lipid hydrolases with specificities for diverse substrates such as triacylglycerols, phospholipids, and retinol esters. PNPLA9 corresponds to group VIA PLA2 (iPLA2β), and its recognition predates that of the PNPLA family as a whole. Of the iPLA2 enzymes, iPLA2β was the first recognized, the most extensively studied, and the best characterized member. iPLA2β is ubiquitously expressed and is distributed mainly in cytoplasm under resting conditions, but upon cellular stimulation, it can translocate to membranous organelles where it hydrolyzes phospholipids to generate AA and LPC (13, 14), among other products. Both AA and LPC have intrinsic second messenger functions in some settings, can also be metabolized into diverse bioactive lipid mediators, and have been implicated in a variety of physiopathological processes (15).
We and others have shown that iPLA2β is expressed in cultured VSMC in vitro and in blood vessels in vivo (16–20), that iPLA2β enzymatic activity increases upon incubating VSMC with angiotensin II (Ang II), vasopressin, thrombin, and high concentrations of glucose in culture (16, 17, 20–22), and that agonist-induced release of free AA from VSMC is largely mediated by iPLA2β (21–23). Moreover, smooth muscle iPLA2β has been functionally implicated in Ca2+ influx (18), proliferation (17, 23), transcriptional regulation (16, 24), Ca2+ sensitization of smooth muscle contraction (19), and diabetes-associated vascular hypercontractility (20).
Whether iPLA2β plays a role in vascular inflammation and neointima formation has so far not been examined in any animal models of which we are aware. Here, we report that smooth muscle cell-specific iPLA2β responds to vascular injury and participates in the initiation and early progression of vascular inflammation and neointima formation in a murine carotid artery ligation model.
EXPERIMENTAL PROCEDURES
Materials and Animals
The antibody against iPLA2β was generated in our laboratory as described previously (16, 20). The antibodies against smooth muscle cell α-actin (SMαA) and FLAG were purchased from Sigma. The antibody against CD31 was purchased from BD Biosciences. The antibody against F4/80 was purchased from AbD Serotec (Raleigh, NC). The antibody against β-actin and PCNA was purchased from Cell Signaling (Danvers, MA). The antibody against cPLA2 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies against IL-6 and NFκB p65 were purchased from Abcam (Cambridge, MA). The antibody against TNF-α was purchased from IHC World (Woodstock, MD). Recombinant mouse TNF-α was purchased from R&D Systems (Minneapolis, MN). BEL, 17-octadecynoic acid, MK886, baicalein, and luteolin were purchased from Cayman (Ann Arbor, MI). Nordihydroguaiaretic acid and indomethacin were purchased from Biomol (Plymouth Meeting, PA). Other chemicals and materials were purchased from Sigma or Fisher unless indicated otherwise.
C57BL/6 and 12/15-lipoxygenase-null mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The iPLA2β-null mice were generated in the laboratory of Dr. John Turk, as described elsewhere (25). All animals used in this study were 8–10-week-old male mice. All animal studies were performed in accordance with the “Guidelines for the Care and Use of Experimental Animals,” American Association for Accreditation of Laboratory Animal Care, and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Cloning of Rabbit Smooth Muscle Myosin Heavy Chain Promoter and Mouse iPLA2β Promoter
Nested PCR was used for cloning of the rabbit smooth muscle myosin heavy chain (SMMHC) promoter. Briefly, the first pair of external primers (rabbit SMMHC-MluI-F1 and rabbit SMMHC-SpeI-R1, see supplemental Table 1) was used to amplify a 2,305-bp rabbit SMMHC promoter from the rabbit brain genomic DNA. The second pair of internal primers (rabbit SMMHC (−2251)-F2, rabbit SMMHC (−18)-R2, see supplemental Table 1) was used to amplify a 2,234-bp fragment (−2,251 to −18 bp relative to the transcription start site) using the first PCR product as a template. The 2,234-bp PCR product was sequenced and found to be almost identical to the published rabbit SMMHC promoter sequence (26). A 14-mer oligonucleotide corresponding to the −17 to −4 bp of the rabbit SMMHC promoter was added to the 3′-end of the 2,234-bp fragment by PCR to generate a 2,248-bp rabbit SMMHC promoter (−2251 to −4 bp).
A mouse bacterial artificial chromosome clone (RP23-300M4) containing iPLA2β gene was purchased from Invitrogen and used as PCR template. A 0.952-kb PCR fragment (−1,411 bp to −459 bp relative to the translational start site) containing a predicated iPLA2β promoter (−1,278 to −460 bp, analyzed by Genomatix MatInspector software) was amplified by PCR using a pair of primers (supplemental Table 1). After verification by DNA sequencing, this putative 0.952-kb iPLA2β promoter was subcloned into a pGL3 basic vector (Promega, Madison, WI) at KpnI and XhoI sites to generate iPLA2β promoter-Luc reporter.
Generation of Smooth Muscle-specific iPLA2β Transgenic Mice
Four sequential steps were taken to construct a smooth muscle- specific transgenic vector as described below. First, an additional 28-mer (−3 to +25 bp) oligonucleotide containing the rabbit SMMHC transcriptional start site was added to the 3′-end of 2,248-bp SMMHC promoter by PCR to generate a 2,276-bp SMMHC promoter (−2251 to +25 bp). Second, a NotI enzyme site in pCI vector (Promega, Madison, WI) was removed by NotI and SmaI enzyme digestion followed by large (Klenow) fragment of DNA polymerase and blunt ligation. The modified pCI vector was then cut by PstI and BamH1 to generate an ∼500-bp fragment containing a chimeric intron, a multiple cloning site, and a SV40 late poly(A). The ∼500-bp fragment was ligated into PCR-Blunt vector (Invitrogen) at PstI and BamH1 enzyme sites to generate an “intermediate vector 1.” Third, an ∼2,400-bp rat iPLA2β cDNA (19), containing a Kozak sequences at its 5′-end and a FLAG tag at its 3′-end, was amplified by PCR and then ligated into the intermediate vector 1 at NheI and SalI enzyme sites to generate an “intermediate vector 2.” Finally, the 2,276-bp rabbit SMMHC promoter was ligated into the “intermediate construct 2” at NotI and EcoRV enzyme sites to generate an iPLA2β smooth muscle-specific transgenic vector containing a rabbit SMMHC promoter, a chimeric intron derived from pCI vector, a rat iPLA2β-Flag cDNA, and SV40 late poly(A) derived from pCI vector (Fig. 2A).
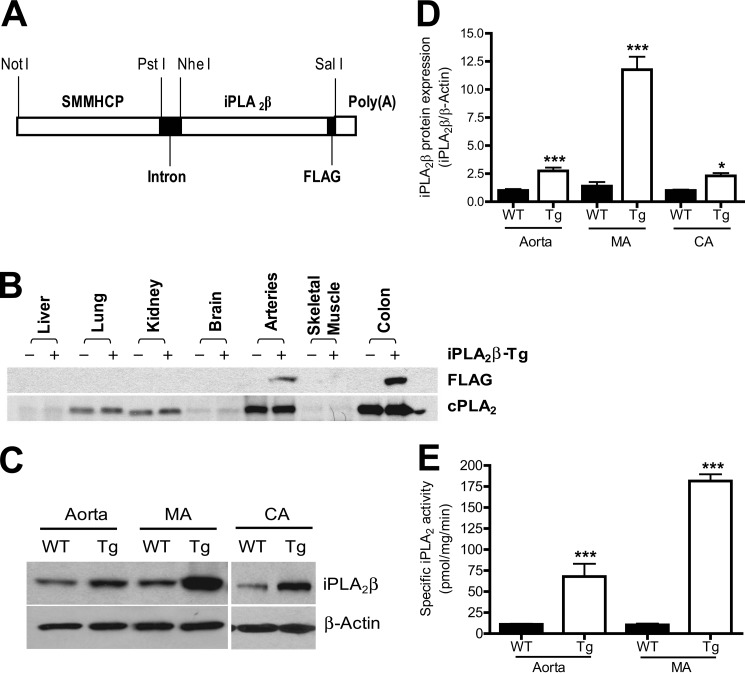
FIGURE 2.
iPLA2β is specifically expressed in smooth muscle cells in SM-iPLA2β-Tg mice. A, schematic diagram of the DNA construct used for generation of SM-iPLA2β-Tg mice. B, representative Western blots of various tissues isolated from SM-iPLA2β-Tg mice (+) and WT littermates (−). C, representative Western blots of aorta, mesentery artery (MA), and carotid artery (CA) isolated from SM-iPLA2β-Tg mice (Tg) and WT littermates (WT). D, summary of Western blots shown in C from 7 to 10 pairs of mice. E, summary of iPLA2 assay results (n = 3). *, p < 0.05; ***, p < 0.0001 versus WT.
The iPLA2β smooth muscle-specific transgenic vector was linearized by NsiI enzyme to remove the PCR-Blunt vector backbone. The linear DNA fragment was microinjected into zygotes from B6C3F1 mice (Harlan Laboratories, Indianapolis, IN) by the University of Kentucky Transgenic Mouse Facility. Pups derived from the microinjected embryos were screened for the presence of the iPLA2β transgene by mouse tail genotyping PCR using two sets of primers (supplemental Table 1) as follows: the first set of primers, Trans-iPLA2-up and Trans-iPLA2-down, was used to amplify a fragment from the 3′-end of iPLA2β to the 5′-end of the FLAG tag; the second set of primers, MHCP-Intron-F1 and iPLA2-R1, was used to amplify a fragment from 3′-end of a chimeric intron to 5′-end of iPLA2β. Seven independent founders were identified to be positive to both sets of PCR screenings. Pups derived from the seven founders were further subjected to Western blot using an anti-FLAG mAb. Three of seven founders were found expressing iPLA2β-FLAG tag protein in vascular smooth muscle tissues. Based upon levels of iPLA2β protein expression and iPLA2 enzymatic activity (data not shown), two independent founders with different levels of exogenous iPLA2β were retained in the laboratory and were backcrossed with C57BL/6J mice at least eight generations for the current studies.
Murine Carotid Artery Complete Ligation Model
Mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) in sterile saline. The carotid arteries were exposed through a small midline incision in the neck. The left common artery was ligated with a 5-0 suture just near its bifurcation to completely disrupt the blood flow (27). The right common carotid artery was used as a sham-operated control by passing the same suture below without ligation.
Local Administration of BEL or Antisense Oligonucleotide to Carotid Artery by Pluronic Gel
We used pluronic gel, an established local drug delivery method (28), to deliver BEL or antisense oligonucleotide to the carotid artery to inhibit iPLA2β and avoid potential systemic side effects. BEL or vehicle (Me2SO2) was mixed with 30% F-127 pluronic gel at 4 °C. The final concentration of BEL in pluronic gel was 91 μm. iPLA2β antisense or sense oligonucleotide was mixed with Lipofectamine 2000 reagent (Invitrogen) and then suspended in 30% F-127 pluronic gel at 4 °C. The final concentration of Lipofectamine 2000 reagent and oligonucleotides was 1% and 50 μg/ml, respectively. Immediately after left carotid artery ligation, 200 μl of F-127 pluronic gel containing BEL or vehicle or 100 μl of F-127 pluronic gel containing antisense or sense oligonucleotides were distally applied to the external surface of the carotid artery relative to the ligation site.
Morphometric Analysis
At 3 or 28 days after carotid artery ligation, mice were euthanized and perfused with PBS for 5 min followed by Formalde-Fresh solution (Fisher) for 30 min through the left ventricle under physiological pressure. The perfusion-fixed left carotid arteries were excised and embedded in paraffin or Tissue-Tek OCT compound. Serial 5-μm paraffin cross-sections or 10 μm cross-cryosections were obtained from each mouse, which covers 500–2,500 μm of carotid artery relative to the ligation site. Cross-sections were stained with the Elastic Stain kit (Fisher) or hematoxylin and eosin. All stained sections were photographed by an Olympus IX70 microscope equipped with Olympus DP70 digital camera. The circumference of the lumen, the internal elastic lamina, and the external elastic lamina were determined by Olympus MicroSuit™-B3 software. The areas surrounded by the luminal surface, internal elastic lamina, and external elastic lamina were then calculated. The neointimal area was calculated by subtracting the lumen area from the area inside the internal elastic lamina. The medial area was calculated by subtracting the area inside the internal elastic lamina from the area inside the external elastic lamina.
Immunocytochemistry
Paraffin cross-sections were deparaffinized with xylene and rehydrated in a graded ethanol series and unmasked by antigen unmasking solution (Vector Laboratories, Burlingame, CA) or proteinase K (Invitrogen). Endogenous peroxidases were quenched by 3% hydrogen peroxide, and nonspecific binding sites were blocked by using an avidin/biotin blocking kit (Vector Laboratories), followed by 10% normal goat serum. Slides were incubated with the following concentrations of primary antibodies overnight at 4 °C: anti-iPLA2β Ab (1:5,000 dilution), anti-FLAG Ab (1:100 dilution), anti-IL-6 Ab (1:800 dilution), anti-TNF-α Ab (no dilution), anti-NFκB P65 Ab (1:4,000 dilution), anti-F4/80 Ab (1:50 dilution), and anti-PCNA Ab (1:16,000 dilution). Slides were then subjected to the procedure of Vectastain Elite ABC system (Vector Laboratories). Immunoreactivity was visualized by 3,3′-diaminobenzidine (DAKO North America Inc., Carpinteria, CA) or 3-amino-9-ethyl carbazole (Biomeda Corp., Foster City, CA), followed by counterstaining with hematoxylin.
Western Blot Analysis
To obtain sufficient amount of proteins for immunoblotting, the ligated or nonligated carotid arteries from two mice were pooled for one sample preparation. Carotid arteries were frozen with liquid nitrogen and subjected to Western blot analysis as described previously (16, 19, 20, 24, 29–32).
Real Time PCR
Primer sequences used for quantification of mRNA levels from mouse carotid arteries by real time PCR are listed in supplemental Table 1 except for 18 S rRNA that has been described previously (16, 20, 24, 29, 33). The procedures of real time PCR were described previously (16, 20, 24, 29, 33).
iPLA2β Promoter Activity Analysis
2- or 9-passage rat aortic VSMC were grown in 12-well cell culture plates. When they achieved 70–80% confluence, cells were co-transfected with an iPLA2β-Luc reporter and pRL-TK vector (Promega) using Lipofectamine-Plus reagent. iPLA2β promoter activity was analyzed as described previously (24).
iPLA2 Assay
The iPLA2 activity was assayed using 14C-labeled 1-palmitoyl-2-[1-14C]palmitoyl-sn-glycero-3-phosphorylcholine (GE Healthcare), as described previously (19), or using arachidonoyl thio-PC as described previously (16, 20).
Primary VSMC Culture
The procedure for isolation and culture of primary aortic VSMC from male New Zealand White rabbits, Sprague-Dawley rats, iPLA2β-null mice, 12/15 lipoxygenase-null mice, and wild-type littermates was described previously (16, 19, 20, 24, 29–32). The usage of cultured rabbit, rat, and mouse VSMC was specifically indicated under the “Results” and in the figure legends.
Statistical Analysis
Each experiment was repeated independently at least three times. Data were expressed as mean ± S.E. Statistical analysis was performed by using unpaired t tests for two groups and one- or two-way analysis of variance with repeated measurement for multiple groups (GraphPad Prism 4).
RESULTS
iPLA2β Up-regulation in Response to Carotid Artery Ligation Precedes Neointima Formation
To determine whether iPLA2β participates in neointima formation, we first examined iPLA2β protein expression in a widely used model of vascular injury that involves ligation of the carotid artery (27). Immunoblotting analyses with our recently developed iPLA2β antibody (16) revealed a substantial increase in expression of iPLA2β protein in carotid arteries at 28 days after ligation compared with that in nonligated vessels from control mice (Fig. 1, A and B).
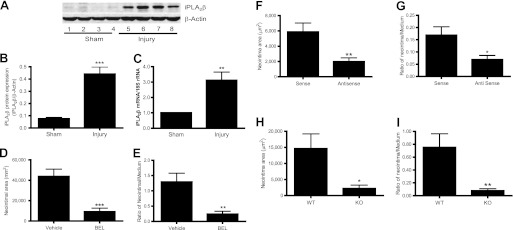
FIGURE 1.
iPLA2β is up-regulated in carotid artery by ligation and inhibition of iPLA2β with BEL, antisense oligonucleotide, and genetic deletion attenuates neointima formation. Ligated (injury) and nonligated (sham) carotid arteries were isolated from C57BL/6J mice at 3 days (C), 14 days (F and G), or 28 days (A and B, D and E, and H and I) after ligation and then subjected to Western blot analysis (A and B), real time PCR (C), or morphometric analysis (D–I). A, representative Western blots (each sample represents carotid arteries from two mice). B, summary of Western blot results shown in A (n = 5). C, summary of real time PCR results (n = 7). D and E, summary of neointimal area (D) and neointimal/medium ratio (E) from four pairs of mice that were perivascularly treated with BEL or vehicle (Me2SO2). F and G, summary of neointimal area (F) and neointimal/medium ratio (G) from nine pairs of mice that were perivascularly treated with iPLA2β antisense or sense oligonucleotides. H and I, summary of neointimal area (H) and neointimal/medium ratio (I) from 10 pairs of iPLA2β-null mice and WT littermates. Results are expressed as mean ± S.E. of four cross-sections that are 200 μm apart and cover 1,000–1,600 μm (D–G) and 1,000–1,400 μm (H and I) along the carotid artery from the ligation site. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To examine the temporal relationship of increased expression of iPLA2β protein and formation of neointima in response to carotid artery ligation, the arteries were isolated at 3 days after ligation and subjected to immunostaining analyses with our iPLA2β antibody. No neointima was observed 3 days after ligation, but increased expression of iPLA2β protein was clearly apparent in the tunica media of ligated vessels compared with that in nonligated vessels (supplemental Fig. 1A). To identify the cells responsible for increased expression of iPLA2β protein, immunostaining of carotid arteries 3 days after ligation was performed with our iPLA2β antibody and antibodies directed against markers for smooth muscle cells, for endothelial cells, and for macrophages. The supplemental Fig. 1B illustrates that iPLA2β largely co-localized with the smooth muscle marker α-actin (SMαA). Lesser amounts of iPLA2β were found to co-localize with the endothelial cell marker CD31 and the macrophage marker F4/80 (supplemental Fig. 1, C and D). These results indicate that increased expression of iPLA2β protein precedes neointima formation and may arise mainly from resident VSMC rather than from endothelial cells or macrophages.
To investigate the mechanism by which carotid artery ligation leads to increased iPLA2β protein expression, we examined iPLA2β mRNA levels by real time PCR, and we found them to be increased in carotid arteries 3 days after ligation (Fig. 1C), which may account in part for the increased expression of iPLA2β protein.
Inhibiting iPLA2β Activity or Expression by Perivascular Delivery of BEL or Antisense Oligonucleotides, Respectively, Suppresses Neointima Formation Induced by Carotid Artery Ligation, as Does Genetic Deletion of iPLA2β
To determine whether increased iPLA2β expression after carotid artery ligation plays a causal role in neointima formation, the iPLA2β inhibitor BEL (11) was delivered into the perivascular space in thermoreversible F127 pluronic gel (supplemental Fig. 1E) (28). The BEL concentration in the gel was 91 μm, but release of inhibitors from pluronic gel is a continuous and relatively slow process (28). The effective concentration of BEL that enters the carotid artery in vivo under these conditions is thus probably comparable with that used in vitro with cultured VSMC (16, 17, 20–22). Carotid arteries were isolated 28 days after ligation and sliced in serial sections to determine the effect of BEL on neointima formation (supplemental Fig. 1F). Representative images of Verhoeff-Van Gieson staining (supplemental Fig. 1G) and quantitative data indicate that perivascular delivery of BEL resulted in significant reduction of the neointimal area (Fig. 1D) and of the ratio of the neointimal area to the medial area (Fig. 1E).
BEL inhibits all iPLA2 isoforms (34), and any of them (e.g. iPLA2β versus iPLA2γ) might account for the effect of BEL to suppress neointima formation. BEL may also inhibit other unrecognized targets (35). We therefore examined the effects of an iPLA2β antisense oligonucleotide that we and others have previously demonstrated to selectively suppress iPLA2β protein expression and function effectively in cultured VSMC in a selective manner (16–18, 24). Antisense oligonucleotide is completely released from pluronic gel after 3 days (28), and we therefore harvested carotid arteries 14 days rather than 28 days after ligation to examine the effect of antisense oligonucleotide on neointima formation. Less neointima formation was observed 14 days after ligation compared with that at 28 days after ligation (e.g. Fig. 1, D versus F). Nonetheless, the iPLA2β antisense oligonucleotide inhibited neointima formation in a manner similar to BEL (Fig. 1, F and G; supplemental Fig. 1H). These results suggest that iPLA2β may play a causal role in neointima formation.
We also examined the effect of genetic deletion of iPLA2β on neointima formation 28 days after carotid artery ligation. The area of newly formed neointima in the wild-type (WT) littermates of iPLA2β-null mice was ∼3-fold lower than that in C57BL/6J mice (e.g. Fig. 1, D versus H), which probably reflects mouse strain differences because the iPLA2β-null mice were derived from 129/SvJ mouse embryonic stem cells (25). 129/SvJ mice are known to be more resistant to vascular injury responses to carotid artery ligation than are C57B/6J mice (36). Nonetheless, a significant decrease in neointimal area and in the ratio of the neointimal area to the medial area was observed for iPLA2β-null mice compared with their WT littermates (Fig. 1, H and I).
Development of a Novel Smooth Muscle-specific iPLA2β Transgenic Mouse Model (SM-iPLA2β-Tg)
Because iPLA2β is ubiquitously expressed (13), it is unclear what cell type (e.g. VSMC versus endothelial cell) expresses the pool of iPLA2β involved in neointima formation. To address this issue, we created transgenic mice that overexpress iPLA2β specifically in smooth muscle cells, which is similar to the increased iPLA2β expression that occurs in the media of the vascular wall in response to carotid artery ligation (Fig. 1, A–C, and supplemental Fig. 1, A–D).
To create these mice, we cloned a 2,276-bp SMMHC promoter from rabbit genomic DNA by nested PCR. Dual-Luciferase assay demonstrated that the cloned SMMHC promoter activity in cultured VSMC was 8–10-fold higher than that in cultured HeLa cells or GH3 cells (data not shown). As illustrated in Fig. 2A, the construct used to generate SM-iPLA2β-Tg mice is composed of a rabbit SMMHC promoter, a chimeric intron, a full-length rat iPLA2β cDNA coding sequence, a FLAG tag, and a poly(A) tail. Insertion of an intron between a smooth muscle-specific promoter and cDNA in transgenic vectors has been shown to increase transgene expression (37). Inclusion of a FLAG tag in the C terminus of iPLA2β allowed exogenous and endogenous iPLA2β to be distinguished without interfering with iPLA2β function (16, 19, 20).
Three independent founder lines of SM-iPLA2β-Tg mice were obtained, and the one that exhibited the highest level of iPLA2β expression was further characterized. First, to determine whether exogenous iPLA2β is expressed specifically in smooth muscle cells in SM-iPLA2β-Tg mice, transgene expression in various tissues was examined by immunoblotting with an anti-FLAG antibody. Fig. 2B illustrates that the FLAG-iPLA2β fusion protein product of the transgene was detectable only in smooth muscle cell-enriched organelles, such as arteries and colon. Interestingly, overexpression of exogenous iPLA2β in smooth muscle did not alter endogenous cPLA2α protein expression in these tissues.
Second, to verify that the FLAG-iPLA2β fusion protein arises only from smooth muscle cells, we performed immunostaining with an anti-FLAG antibody and observed that FLAG-expressing cells were readily apparent in the vascular smooth muscle cell layers of coronary arteries, renal arteries, carotid arteries, and aortas, in addition to the visceral smooth muscle cell layers of bladder, rectum, and ileum of the SM-iPLA2β-Tg mice but not control mice (supplemental Fig. 2).
Third, to examine the expression levels of iPLA2β from the endogenous gene and from the transgene, immunoblotting was performed with our iPLA2β antibody (16). Increased iPLA2β protein expression was observed in tissues of the transgenic mice of at least 2.5-fold in aorta, 10-fold in mesenteric arteries, and 2.3-fold in carotid arteries compared with WT littermates (Fig. 2, C and D).
Fourth, to determine whether iPLA2β protein arising from the transgene is enzymatically active, we measured iPLA2 activity using a radiolabeled phospholipid substrate and following release of the radiolabeled fatty acid product (19). The iPLA2-specific activities in aorta and mesenteric arteries of SM-iPLA2β-Tg mice were found to be significantly greater than those of WT littermates (Fig. 2E). These results are concordant with those from the immunoblotting studies (Fig. 2, C and D) and verify that iPLA2β that arises from the transgene is enzymatically active.
Smooth Muscle-specific Expression of iPLA2β Exacerbates Neointima Formation in Response to Carotid Artery Ligation
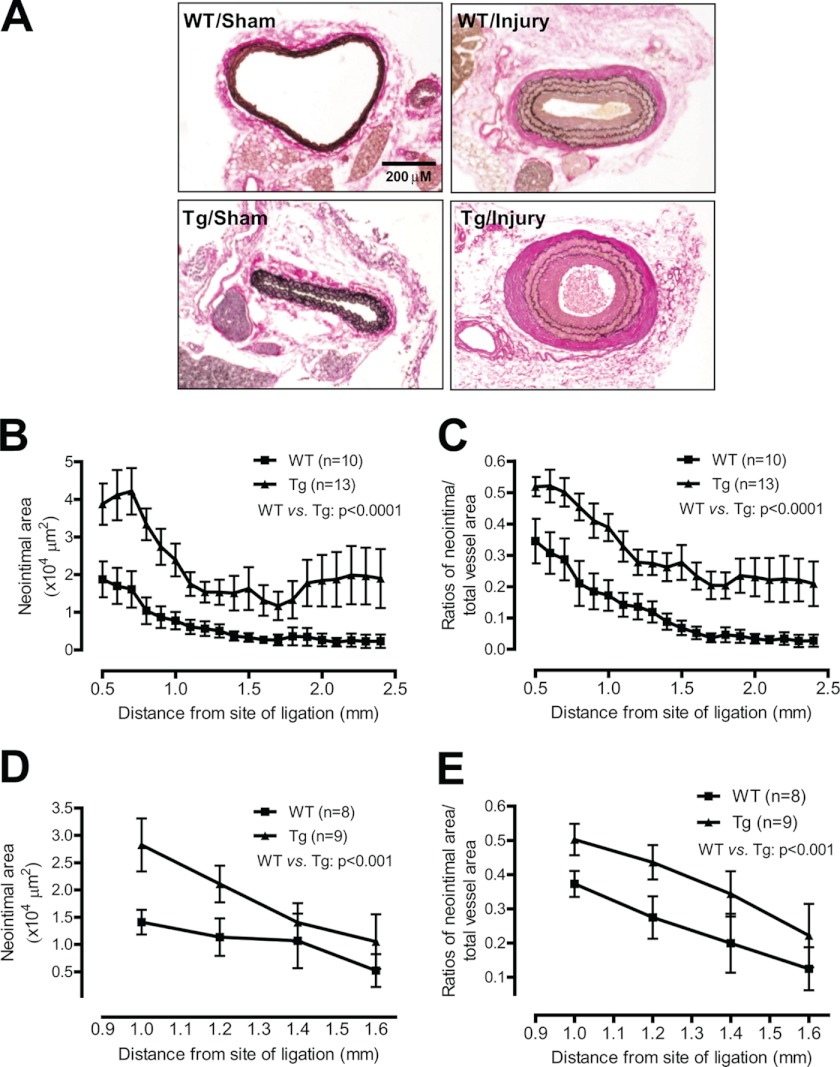
To determine whether smooth muscle-specific expression of iPLA2β affects neointima formation, we examined carotid arteries from SM-iPLA2β-Tg mice and WT littermates 28 days after ligation. No neointima was observed in either SM-iPLA2β-Tg mice or WT littermates in the absence of carotid ligation (Fig. 3A). This result suggests that smooth muscle-specific expression of iPLA2β is insufficient to induce neointima formation. Therefore, only ligated carotid arteries were subjected to quantitative analysis of neointima formation, which revealed that smooth muscle-specific expression of iPLA2β exacerbates ligation-induced increases in the neointimal area (Fig. 3B) and in the ratio of the neointimal and total area (Fig. 3C).
FIGURE 3.
Smooth muscle-specific expression of iPLA2β exacerbates carotid ligation-induced neointima formation. Ligated (injury) and nonligated (sham) carotid arteries were isolated from one line (A–C) or an independent line (D and E) of SM-iPLA2β-Tg mice (Tg) and WT littermates (WT) at 28 days after ligation and then subjected to morphometric analysis. A, representative photographs of Verhoef-Van Gieson staining. B–E, summary of neointimal area (B and D) and neointimal/total ratio (C and E). Results were analyzed by two-way analysis of variance.
To determine whether smooth muscle-specific expression of iPLA2β affects vascular remodeling, we compared areas of the lumen, media, and total vessel in SM-iPLA2β-Tg mice and their WT littermates at 28 days after carotid artery ligation. Smooth muscle-specific expression of iPLA2β did not affect the luminal area (supplemental Fig. 3A) but was associated with significantly increased total vessel area and medial area (supplemental Fig. 3, B and C). This suggests that smooth muscle-specific expression of iPLA2β causes positive (expansive) vascular remodeling in which an increase in the neointimal area does not necessarily result in a decrease in the luminal area due to simultaneous vessel enlargement (27, 38).
To exclude the possibility that the exacerbated neointima formation in SM-iPLA2β-Tg mice is due to a nonspecific random insertion of the transgenic construct into chromosomes, we analyzed neointima formation in a second line of SM-iPLA2β-Tg mice derived from a different founder with a lower level of iPLA2β expression compared with that of the first SM-iPLA2β-Tg line studied. Similar results were obtained with the second transgenic line (Fig. 3, D and E).
Smooth Muscle-specific Expression of iPLA2β Promotes Inflammatory Cytokine Production, Macrophage Infiltration, and VSMC Proliferation in Response to Carotid Artery Ligation
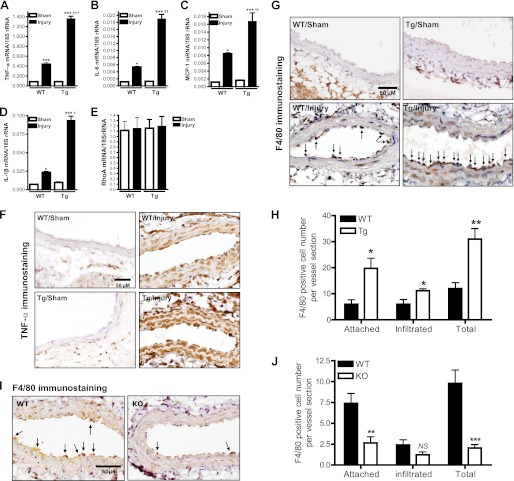
To gain insight into the mechanism by which smooth muscle iPLA2β mediates neointima formation in response to carotid artery ligation, we measured mRNA levels of proinflammatory cytokines in carotid arteries from SM-iPLA2β-Tg mice and WT littermates at 28 days after ligation. Fig. 4, A–D, illustrates that smooth muscle-specific expression of iPLA2β exacerbated the increase in mRNA levels for tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and monocyte chemotactic protein-1 (MCP-1) that occurred in response to carotid artery ligation, although basal expression was unaffected. Expression of RhoA (Fig. 4E) and ROCK2 (data not shown) mRNA was also unaffected.
FIGURE 4.
Effects of smooth muscle-specific expression of iPLA2β or genetic deletion of iPLA2β on carotid ligation-induced proinflammatory cytokine production and macrophage infiltration. A–E, summary of real time PCR results in ligated (Injury) and nonligated (Sham) carotid arteries from SM-iPLA2β-Tg mice (Tg) and WT littermates (WT) at 28 days after ligation (n = 3–6). F, representative TNF-α immunostaining of carotid artery cross-sections from Tg and WT mice at 3 days after ligation. G, representative F4/80 immunostaining of carotid artery cross-sections from Tg and WT mice at 3 days after ligation. Attached monocytes/macrophages were identified by F4/80-positive immunostaining, hematoxylin staining of distinct large nuclei, and tethered on the luminal surface of the vessel wall (vector arrow). Infiltrated monocytes/macrophages were F4/80-positive cells that localize in the media of the vessel wall. H, summary of F4/80-positive cells shown in G, n = 5. I, representative F4/80 immunostaining of carotid artery cross-sections from iPLA2β knock-out mice (KO) and WT littermates (WT) at 3 days after ligation. J, summary of F4/80-positive cells shown in I, n = 5. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus sham in A–E or WT in H and J. †, p < 0.05; ††, p < 0.01; †††, p < 0.001 versus WT/injury in A–E. NS, no significance.
To examine effects of iPLA2β on initiation and early progression of vascular inflammation, we determined TNF-α protein expression in carotid arteries from SM-iPLA2β-Tg mice and WT littermates at 3 days after ligation. This time point was selected because expression of iPLA2β increases at 3 days, but neointima formation has not yet begun (supplemental Fig. 1A). In nonligated vessels, little TNF-α immunostaining was detected for either SM-iPLA2β-Tg mice or their WT littermates (Fig. 4F). In contrast, a dramatic increase in TNF-α immunostaining was observed 3 days after carotid ligation in both genotypes, and smooth muscle-specific expression of iPLA2β amplified this increase. Similar effects were observed with immunostaining for IL-6 (supplemental Fig. 4A).
The fact that smooth muscle-specific expression of iPLA2β affects expression of multiple proinflammatory cytokines (Fig. 4, A–F) suggests the possibility that iPLA2β might affect a master regulator of inflammatory cytokine expression, such as NFκB. To test this possibility, we examined NFκB p65 immunostaining in carotid arteries from SM-iPLA2β-Tg mice and WT littermates at 3 days after ligation and found that the NFκB p65 immunostaining pattern (supplemental Fig. 4B) was similar to that of TNF-α (Fig. 4F) and IL-6 (supplemental Fig. 4A).
To investigate whether increased proinflammatory cytokine production by smooth muscle-specific expression of iPLA2β might result in elaboration of chemotactic signals that attract macrophage migration into the lesion site, carotid arteries were isolated from SM-iPLA2β-Tg mice and WT littermates at 3 days after ligation. Macrophages were identified by F4/80 immunostaining and hematoxylin staining of their distinctive large nuclei. In nonligated vessels, no macrophages were observed in either SM-iPLA2β-Tg mice or their WT littermates (Fig. 4G). In contrast, macrophages that had infiltrated the vascular wall were readily detectable after carotid ligation, and it was noteworthy that most of these macrophages were attached to the vessel wall, although some were observed in the media and adventitia of the vessels. This observation suggests that the 3-day time point represents an early stage in the process of macrophage infiltration in which attachment to the vessel has begun but penetration into the vessel wall has just begun. Nonetheless, substantially more macrophages were associated with ligated vessels of SM-iPLA2β-Tg mice compared with their WT littermates (Fig. 4H).
Macrophage vascular infiltration was also examined at 3 days after carotid artery ligation in iPLA2β-null mice and their WT littermates (Fig. 4, I and J), and the number of macrophages attached to the vessel wall was reduced in the former, although the number of macrophages that had infiltrated the vessel wall did not differ between those two genotypes.
Effects of smooth muscle-specific expression of iPLA2β on VSMC proliferation in vivo were examined by immunostaining for the proliferation marker PCNA in carotid arteries from SM-iPLA2β-Tg mice and their WT littermates at 3 days after ligation. The supplemental Fig. 5A illustrates that smooth muscle-specific expression of iPLA2β had no effect on PCNA immunostaining in nonligated vessels, but it was associated with a dramatic increase in PCNA immunostaining in ligated carotid arteries. VSMC migration and proliferation were also examined in aortic explants in fibrin gels. Representative micrographs and quantitative data illustrate that at least 10-fold more SMαA-positive cells migrated and/or proliferated from vessel explants from SM-iPLA2β-Tg mice compared with their WT littermates (supplemental Fig. 5, B and C).
12/15-Lipoxygenase Is Selectively Coupled to iPLA2β in Ang II- and AA-induced IL-6 mRNA Expression in Cultured VSMC
Results described so far demonstrate that smooth muscle-specific expression of iPLA2β is involved in the initiation and early progression of vascular inflammation and neointima formation in a murine carotid artery ligation model, but they do not identify the molecular mechanisms underlying these events.
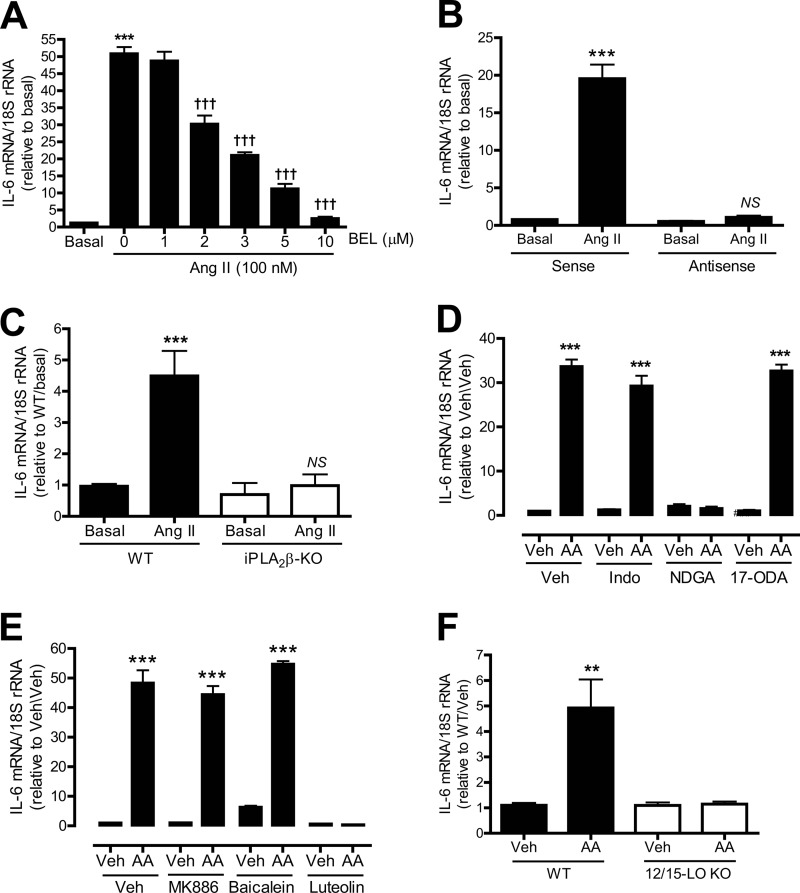
To address these issues, we examined whether iPLA2β is involved in Ang II-induced IL-6 mRNA expression in cultured rat aortic VSMC. A dramatic increase in IL-6 mRNA expression was observed in VSMC treated with Ang II compared with unstimulated cells (Fig. 5A). Pretreatment of VSMC with BEL potently inhibited Ang II-induced IL-6 mRNA up-regulation in a concentration-dependent manner.
FIGURE 5.
Effects of inhibiting the iPLA2β/COX/LO/CYP pathway on Ang II- or AA-induced IL-6 mRNA expression in cultured VSMC. Rat aortic VSMC (A, B, D, and E) and mouse aortic VSMC (C and F) were pretreated with various inhibitors for 30 min and/or stimulated with Ang II (100 nm, 1 h) or AA (10 μm, 1 h). A, effects of inhibiting iPLA2 with various concentrations of BEL on Ang II-induced IL-6 mRNA expression. B, effects of iPLA2β antisense or sense oligonucleotides on Ang II-induced IL-6 mRNA expression. C, effects of genetic deletion of iPLA2β on Ang II-induced IL-6 mRNA expression. D, effects of COX2 inhibitor indomethacin (indo, 50 μm), LO inhibitor nordihydroguaiaretic acid (NDGA) (30 μm), and CYP inhibitor 17-octadecynoic acid (10 μm) on AA-induced IL-6 mRNA expression. E, effects of 5-LO inhibitor MK886 (1 μm), P-12-LO inhibitor baicalein (10 μm), and 12/15-LO inhibitor luteolin (50 μm) on AA-induced IL-6 mRNA expression. F, effects of genetic deletion of 12/15-LO on AA-induced IL-6 mRNA expression. Results are expressed as mean ± S.E. from at least three independent experiments. **, p < 0.01; ***, p < 0.001 versus basal in A–C, or vehicle (Veh, Me2SO2) in D–F. †††, p < 0.001 versus Ang II only in A. NS, no significance versus antisense/basal in B or iPLA2β-KO/basal in C.
To ensure that the effect of BEL on Ang II-induced IL-6 mRNA expression is attributable to inhibition of iPLA2β, rather than to another BEL-sensitive enzyme (34), rat aortic VSMC were preincubated with a well characterized iPLA2β antisense oligonucleotide that effectively suppresses iPLA2β expression (16, 17, 20–22). Fig. 5B illustrates that the iPLA2β antisense oligonucleotide, but not the corresponding sense oligonucleotide, abolished Ang II-induced IL-6 mRNA up-regulation.
The role of iPLA2β in Ang II-induced IL-6 mRNA up-regulation was also examined in aortic VSMC isolated from iPLA2β knock-out mice and WT littermates. Fig. 5C demonstrates that Ang II-induced IL-6 mRNA expression was also markedly suppressed in iPLA2β-deficient mouse VSMC compared with WT.
The products of iPLA2β action on phospholipids include a free fatty acid (e.g. AA) and a 2-lysophospholipid (e.g. LPC). AA can be further metabolized to a variety of biologically active eicosanoids via lipoxygenases (LO), cyclooxygenases (COX), and cytochrome P450-dependent epoxygenases (CYP) (15). To determine whether AA itself or an AA metabolite is involved in Ang II-induced and iPLA2β-mediated IL-6 mRNA expression, VSMC were pretreated with the LO inhibitor nordihydroguaiaretic acid, the COX inhibitor indomethacin, or the CYP inhibitor 17-octadecynoic acid, respectively, before addition of AA.
We used AA rather than Ang II as a stimulus because agonist-induced AA release is largely mediated by iPLA2β in VSMC (21–23), and Ang II simultaneously activates multiple intracellular signaling pathways in VSMC, which makes it difficult to evaluate whether the iPLA2β activation pathway is solely responsible for subsequent events. Fig. 5D shows that incubation of rat aortic VSMC with AA did induce a marked rise in IL-6 mRNA levels. Pretreating the cells with the LO inhibitor nordihydroguaiaretic acid completely prevented this response to AA, but the COX inhibitor indomethacin and the CYP inhibitor 17-octadecynoic acid had no effect. This suggests that AA metabolism by the LO pathway but not by the COX or CYP pathways is involved in Ang II-induced accumulation of IL-6 mRNA in cultured VSMC.
Of the LO isozymes, rat aortic VSMC express 5-LO, platelet-type 12-LO (P-12-LO), and leukocyte-type 12-LO (L-12-LO, which is highly homologous to human and rabbit 15-LO) (20). To determine which of these LO enzymes is involved in AA-induced IL-6 mRNA accumulation, rat aortic VSMC were pretreated with MK886 (a selective 5-LO inhibitor), baicalein (a selective P-12-LO inhibitor), or luteolin (a selective 12/15-LO inhibitor) (16, 20, 24), before addition of AA. Luteolin abolished AA-stimulated IL-6 mRNA accumulation, but baicalein and MK886 had no effect (Fig. 5E).
The potential role of 12/15-LO in AA-induced IL-6 gene transcription was also examined in aortic VSMC from 12/15-LO-null mice, which exhibited no rise in IL-6 mRNA levels upon incubation with AA, although basal levels were similar to WT. Thus, results from studies involving pharmacological inhibition and genetic ablation of 12/15-LO suggest that metabolites from that pathway produced from AA released by iPLA2β are involved in the signaling pathway through which Ang II stimulates IL-6 mRNA accumulation in VSMC.
Role of iPLA2β and 12/15-LO in TNF-α-induced TNF-α and IL-6 mRNA Expression in Cultured VSMC
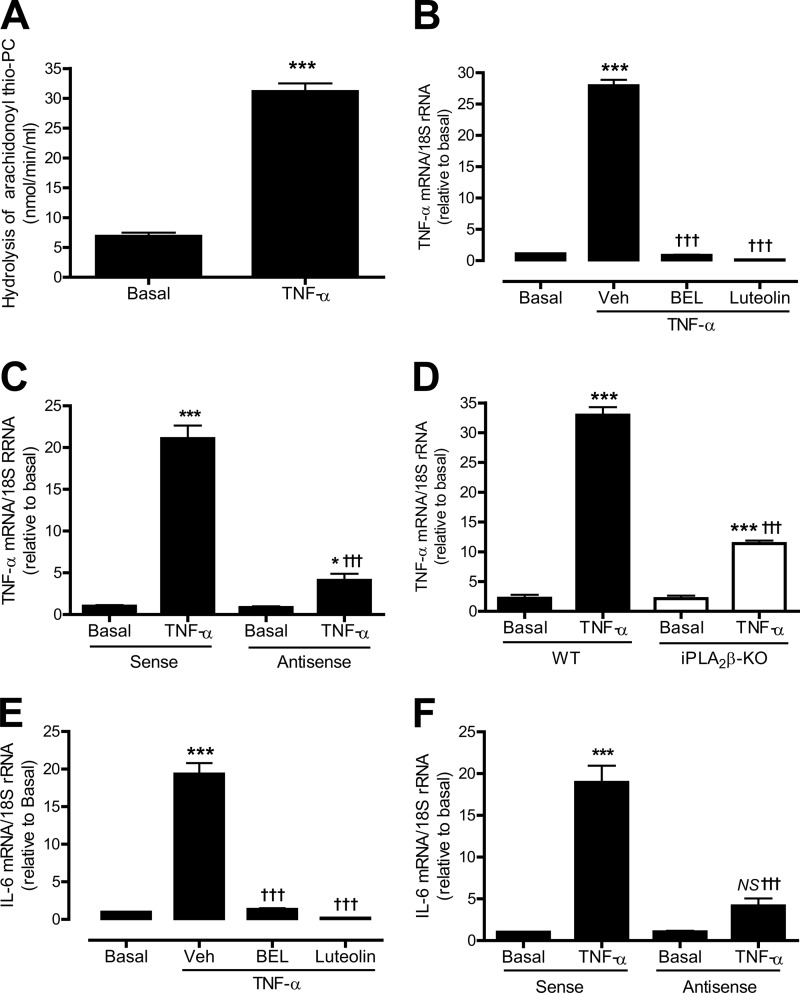
To determine whether iPLA2β and 12/15-LO are involved in proinflammatory cytokine production by VSMC in response to other agonists, rat aortic VSMC were incubated with TNF-α. TNF-α was selected because TNF-α is markedly up-regulated in carotid arteries 3 days after ligation (Fig. 4F) and is implicated in neointima formation in response to carotid artery ligation (39). An approximate 5-fold increase was found in iPLA2 specific activity in cells stimulated with TNF-α as compared with unstimulated cells (Fig. 6A).
FIGURE 6.
Effects of inhibiting iPLA2β or 12/15-LO on TNF-α-induced TNF-α or IL-6 mRNA expression in cultured VSMC. Rat aortic VSMC (A–C, E, and F) and mouse aortic VSMC (D) were pretreated with various inhibitors for 30 min and/or stimulated with TNF-α (10 ng/ml) for 5 min (A) or 1 h (B–F). A, effects of TNF-α stimulation on iPLA2-specific activity. B, effects of BEL (10 μm) or luteolin (50 μm) on TNF-α-induced TNF-α mRNA expression. C, effects of iPLA2β antisense or sense oligonucleotides on TNF-α-induced TNF-α mRNA expression. D, effects of genetic deletion of iPLA2β on TNF-α-induced TNF-α mRNA expression. E, effects of BEL (10 μm) or luteolin (50 μm) on TNF-α-induced IL-6 mRNA expression. F, effects of iPLA2β antisense or sense oligonucleotides on TNF-α-induced IL-6 mRNA expression. Results are expressed as mean ± S.E. from at least three independent experiments. *, p < 0.05; ***, p < 0.001 versus basal. †††, p < 0.001 versus vehicle (Veh, Me2SO2) in B and E or sense/TNF-α in C and F. NS, no significance.
TNF-α-induced iPLA2-specific activity (Fig. 6A) is associated with its dramatic effect on TNF-α mRNA accumulation (Fig. 6B). To determine whether iPLA2β and 12/15-LO are required for TNF-α-induced TNF-α mRNA accumulation, rat aortic VSMC were pretreated with the iPLA2 inhibitor BEL, the 12/15-LO inhibitor luteolin (Fig. 6B), and the iPLA2β antisense oligonucleotide (Fig. 6C), respectively. It was found that inhibiting iPLA2β with BEL or antisense oligonucleotide and inhibiting 12/15-LO with luteolin markedly suppressed TNF-α-induced TNF-α mRNA accumulation.
The effect of iPLA2β on TNF-α-induced TNF-α mRNA accumulation was also examined in mouse aortic VSMC isolated from iPLA2β-null mice and WT littermates. TNF-α could potently stimulate TNF-α mRNA expression in mouse aortic WT VSMC, but this response was also significantly suppressed in iPLA2β-deficient cells (Fig. 6D). These results indicate that the iPLA2β/12/15-LO pathway is involved in TNF-α-induced accumulation of TNF-α mRNA in VSMC.
TNF-α was also found to strongly stimulate IL-6 mRNA accumulation in rat aortic VSMC (Fig. 6E), and this response was also suppressed by the iPLA2β inhibitor BEL (Fig. 6E), by iPLA2β antisense oligonucleotide (Fig. 6F), by iPLA2β genetic deletion (data not shown), or by the 12/15-LO inhibitor luteolin (Fig. 6E). These results suggest that the iPLA2β/12/15-LO pathway may serve as a common component of a signaling network governing production of inflammatory cytokines by VSMC.
VSMC iPLA2β Expression Varies with Phenotypic State and Increases in Response to TNF-α
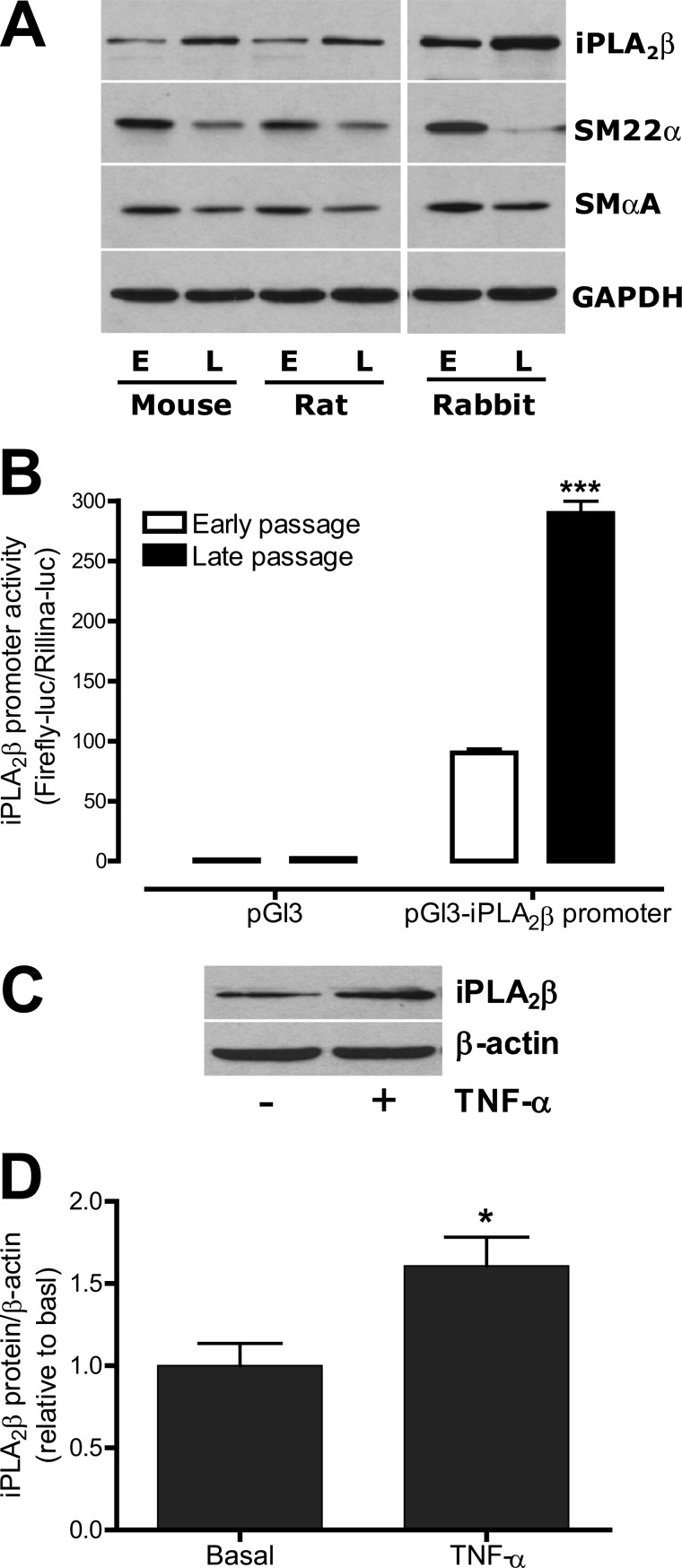
Immunohistological analyses of control nonligated carotid arteries in supplemental Fig. 1, A–D suggest that healthy VSMC with a contractile phenotype express only low levels of iPLA2β. Examination of iPLA2β protein expression levels in early and late passage VSMC isolated from mice, rats, and rabbits revealed that in each species iPLA2β protein expression levels were higher in late passage than in early passage VSMC (Fig. 7A). In contrast, expression levels of the contractile proteins SM22α and SMαA were lower in late passage than in early passage VSMC. These results suggest that the iPLA2β protein expression level of the VSMC varies with the phenotypic state of the cells.
FIGURE 7.
iPLA2β protein expression levels are higher in late passage than in early passage VSMC and can be further up-regulated by TNF-α in cultured VSMC. A, representative Western blots show expressions of iPLA2β, SM22α, SMαA, and GAPDH in cultured early and late passage aortic VSMC isolated from mice (5 passage versus 10 passages), rats (1 passage versus 9 passages), and rabbit (2 passage versus 9 passages). E, early passage; L, late passage. B, summary of a 0.952-kb mouse iPLA2β promoter activity in cultured early and later rat aortic VSMC (2 passage versus 9 passages). ***, p < 0.001 versus early passage VSMC. C, representative iPLA2β Western blots from rat aortic VSMC stimulated with or without TNF-α (10 ng/ml, 24 h). D, quantitative data shown in C. n = 8.
To investigate the mechanism that may underlie iPLA2β up-regulation in late passage VSMC, we cloned a 0.952-kb mouse iPLA2β promoter from a bacterial artificial chromosome clone and examined iPLA2β promoter activity in early and late passage rat aortic VSMC. We found that iPLA2β promoter activity in late passage VSMC was about 3-fold higher than that in early passage VSMC (Fig. 7B). This result suggests that transcriptional up-regulation of iPLA2β is, at least in part, responsible for the rise in iPLA2β protein levels in late passage VSMC.
Proinflammatory cytokines are known to be able to alter the VSMC phenotype (9). To explore the possibility that such effects include regulation of iPLA2β expression, rat aortic VSMC were incubated with and without TNF-α. As illustrated in Fig. 7, C and D, TNF-α did cause an increase in VSMC iPLA2β protein levels.
DISCUSSION
Although iPLA2β was once thought to serve as a housekeeping enzyme involved in phospholipid remodeling (40), subsequent evidence from our laboratory (16, 19, 20, 24), and many others (13, 14, 41), over the last 15 years has demonstrated that iPLA2β expression is regulated and that it can be activated in response to a variety of physiological stimuli in a number of cell types. Moreover, it is now clear that iPLA2β participates in signaling pathways that underlie processes that include insulin secretion, cell proliferation, apoptosis, gene expression, Ca2+ influx, and Ca2+ sensitization of vascular smooth muscle contraction (13, 14, 41). Importantly, alterations of iPLA2β expression or activity have been linked to many human diseases, including cancer, diabetes, neurodegenerative disorders, and Barth syndrome (an X-linked cardioskeletal myopathy) (13, 14, 41).
To examine the role of iPLA2β in vascular physiological and pathophysiological processes, we have created a transgenic mouse line in which iPLA2β is overexpressed in smooth muscle cells. Using these mice in conjunction with other approaches that include pharmacological inhibition of iPLA2β activity, suppression of iPLA2β expression with antisense oligonucleotides, and genetic deletion with iPLA2β-null mice, we have revealed a previously unrecognized role for iPLA2β in vascular inflammation and neointima formation in a carotid artery ligation model. We have found that vascular expression of iPLA2β protein increases markedly in response to carotid artery ligation and that this precedes the neointima formation (Fig. 1, A–C; supplemental Fig. 1, A–D).
Upon vascular injury, the concentrations of many molecules that promote neointima formation increase in the lesion site (1). Among them, TNF-α is of particular interest because in mice that lack functional TNF-α, the area of neointima formed in response to carotid artery ligation is 14-fold lower than that of WT controls (39). In addition, TNF-α stimulates iPLA2β activity in adult rat ventricular myocytes (42). Here, we demonstrate that expression of TNF-α and iPLA2β increases in a temporally and spatially related manner in carotid arteries 3 days after ligation (Fig. 4F and supplemental Fig. 1, A–D). Incubation of VSMC with TNF-α in culture induces increased expression of iPLA2β activity and protein (Figs. 6A and 7, C and D), which suggests that TNF-α might also increase iPLA2β expression in vivo. This would represent a novel mechanism by which proinflammatory cytokines influence neointima formation through a signaling pathway that involves iPLA2β.
Our studies also demonstrate expression of TNF-α itself is increased in response to TNF-α via a signaling pathway that involves iPLA2β (Figs. 4, A and F, and 6, B–D). This is a potentially important finding because it suggests the existence of a positive feedback loop that is initiated by proinflammatory cytokines such as TNF-α, perhaps derived from endothelial cells or infiltrating leukocytes, followed by activation of iPLA2β in VSMC, and then resulting in robust TNF-α production. Such a positive feedback loop could explain why TNF-α and iPLA2β are up-regulated coordinately, and these could be pivotal events in the initiation and early progression of vascular inflammation.
The mechanism by which iPLA2β protein expression is up-regulated in response to carotid artery ligation has not yet been elucidated. In particular, information regarding regulation of iPLA2β gene transcription levels in VSMC and vascular endothelial cells is limited. In cultured Chinese hamster ovary (CHO) cells, Seashols et al. (43) cloned a 1-kb human iPLA2β promoter and demonstrated that sterol regulator element-binding protein-2 (SREBP-2) binds to the iPLA2β promoter and is responsible for sterol depletion-induced stimulation of iPLA2β promoter activity. In cultured pancreatic islet β-cells, Lei et al. (44) reported that both basal and thapsigargin-induced iPLA2β expression is suppressed by a dominant negative SREBP-1 mutant. In cultured VSMC, we reported that iPLA2β mRNA is up-regulated in response to high concentrations of glucose in a time-dependent manner (20). A report by Zhou et al. (45) that SREBP-1 protein expression is enhanced in the injured vascular wall, especially within the neointima, and co-localizes with SMαA-positive cells raises interest in the possibility that SREBP is involved in increased iPLA2β expression in response to carotid artery ligation. It is tempting to speculate that carotid artery ligation may lead to increased SREBP-1 expression that in turn causes an increase in iPLA2β mRNA expression, and this possibility deserves further examination in the future.
It is of interest that smooth muscle-specific expression of iPLA2β alone is insufficient to induce neointima formation (Fig. 3A), proinflammatory cytokine production (Fig. 4, A–F), or macrophage infiltration (Fig. 4, G and H). These results suggest that iPLA2β remains inactive in the absence of vascular injury. Some cytosolic proteins, e.g. calmodulin, can interact with iPLA2β to maintain its inactive state, and some stimuli, e.g. thapsigargin, induce release of iPLA2β from the calmodulin-iPLA2β complex to activate iPLA2β (18, 46). It is therefore possible that increased TNF-α levels may result in iPLA2β activation through a mechanism that involves disassociation of an inhibitory complex. This may explain why both carotid artery ligation and increased expression of iPLA2β in smooth muscle cells are required for development of vascular inflammation and neointima formation in SM-iPLA2β-Tg mice.
Perhaps the most novel finding from this study is that we demonstrate that activation of iPLA2β in VSMC in response to carotid artery ligation is involved in the initiation and early progression of vascular inflammation. Several independent lines of evidence support this conclusion. First, in the absence of neointima formation, iPLA2β, TNF-α, and IL-6 protein levels increase in a temporally and spatially coordinated manner in response to carotid artery ligation (supplemental Figs. 1, A–D, and 4A and 4F). Second, smooth muscle-specific expression of iPLA2β results in increased levels of mRNA for several proinflammatory cytokines (Fig. 4, A–E). Third, smooth muscle-specific expression of iPLA2β elevates TNF-α and IL-6 proteins in the absence of neointima formation (Fig. 4F; supplemental Fig. 4A). Fourth, macrophage infiltration, which is a hallmark of early vascular inflammation, is enhanced by smooth muscle-specific expression of iPLA2β (Fig. 4, G and H) and is attenuated in vessels from iPLA2β-null mice (Fig. 4, I and J). Finally, inhibition of iPLA2β activity with BEL, suppression of iPLA2β expression with antisense oligonucleotide, and genetic deletion of iPLA2β each resulted in suppression of accumulation of mRNA for IL-6 and TNF-α in VSMC in response to incubation with Ang II or TNF-α (Figs. 5, A–C, and 6, B–F).
We have previously demonstrated that, in cultured VSMC, 12/15-LO is downstream of iPLA2β in the signaling pathways underlying Ang II-induced RGS2 transcriptional activation (16) and cAMP-response element-binding protein phosphorylation (24) and RhoA/Rho-kinase/CPI-17 phosphorylation induced by incubation with high concentrations of glucose (20). Consistent with those reports, our current studies illustrate that 12/15-LO is also a component downstream of iPLA2β in a signaling pathway that underlies increases in levels of mRNA for IL-6 and TNF-α in VSMC incubated with AA or TNF-α (Figs. 5, D–F, and 6, B and E).
Consistent with a role for 12/15-LO in production of proinflammatory cytokines, Natarajan et al. (47) reported that the 12/15-LO product hydroperoxyoctadecadienoic acid is a potent stimulator of NFκB activity in primary cultured porcine VSMC. Dwarakanath et al. (48) reported that hydroperoxyoctadecadienoic acid causes increased transcription of the MCP-1 and TNF-α genes in cultured human VSMC in an NFκB p65-dependent manner. Similarly, Chava et al. (49) reported that the 12/15-LO product hydroxyeicosatetraenoic acid stimulates IL-6 mRNA accumulation in primary cultured rat VSMC in a cAMP-response element-binding protein-dependent manner. Concordant with those reports, our current studies demonstrate that smooth muscle-specific expression of iPLA2β results in a marked increase in NFκB p65 protein immunostaining in response to carotid artery ligation (supplemental Fig. 4B), which suggests that NFκB p65 may serve as a common transcriptional factor that links the iPLA2β/12/15-LO signaling pathway to production of multiple proinflammatory cytokines, although the detailed mechanism by which this pathway leads to NFκB p65 is the subject of ongoing inquiry.
A role for iPLA2β in vascular inflammation has not been well recognized previously. An early study by Walev et al. (50) indicated that the iPLA2β inhibitor BEL diminishes LPS-induced IL-1β secretion by inhibiting the inflammasome in mononuclear cells, but a more recent study by Franchi et al. (51) demonstrated that this is an off-target effect of BEL that does not result from iPLA2β inhibition. Whether iPLA2β is involved in transcriptional regulation of IL-1β or other proinflammatory cytokines is a question that has been largely unexplored in any cell type.
Our findings that iPLA2β is involved in proinflammatory cytokine production in VSMC provides new insight into interactions among cellular participants in vascular inflammation, which has long been thought to involve mainly monocytes/macrophages, other leukocytes, and endothelial cells, despite the fact that VSMC can also produce proinflammatory cytokines both in vitro and in vivo (7–9) and that this could be a significant source of cytokines in view of the large number of VSMC in the vascular wall. Evidence from our current studies indicates that VSMC are not merely passive responders to signals from macrophages or endothelial cells but rather actively interact with other cells in the vascular wall and participate in proinflammatory cytokine production in a coordinated manner that leads to the initiation and early progression of vascular inflammation.
Involvement of iPLA2β in cell proliferation has been demonstrated in several types of cultured cells (41), including VSMC (17, 23), but there has been little attention to this issue in animal models that are more relevant to physiological and pathological processes in vivo. Our current studies are the first of which we are aware to address these issues with four independent lines of investigation that include the following: 1) pharmacological inhibition of iPLA2β with BEL (Fig. 1, D and E); 2) suppression of iPLA2β expression with antisense oligonucleotides (Fig. 1, F and G); 3) genetic deletion of iPLA2β by homologous recombination in iPLA2β-null mice (Fig. 1, H and I); and 4) smooth muscle-specific expression of iPLA2β (Fig. 3, A–E; supplemental Fig. 5, A and B). Findings from each approach are complementary and consistently indicate that iPLA2β plays a critical role in neointima formation.
The pharmacological iPLA2β inhibitor BEL used in our in vitro and in vivo experiments has off-target effects that include inhibition of other serine lipases (34), serine proteases (51), and a number of other enzymes (35). Conclusions based on experiments involving BEL thus require confirmation by experiments from independent lines of investigation. Although iPLA2β global knock-out mice and iPLA2β antisense oligonucleotides are generally thought to be more specific than BEL, such reagents do not discriminate among cell types. Smooth muscle cell-specific iPLA2β transgenic mice that are described here are useful in that regard, but there is always the concern of whether overexpressed iPLA2β is same as endogenous iPLA2β. Conditional iPLA2β knock-out mice that selectively fail to express iPLA2β only in smooth muscle cells are required to clarify these issues.
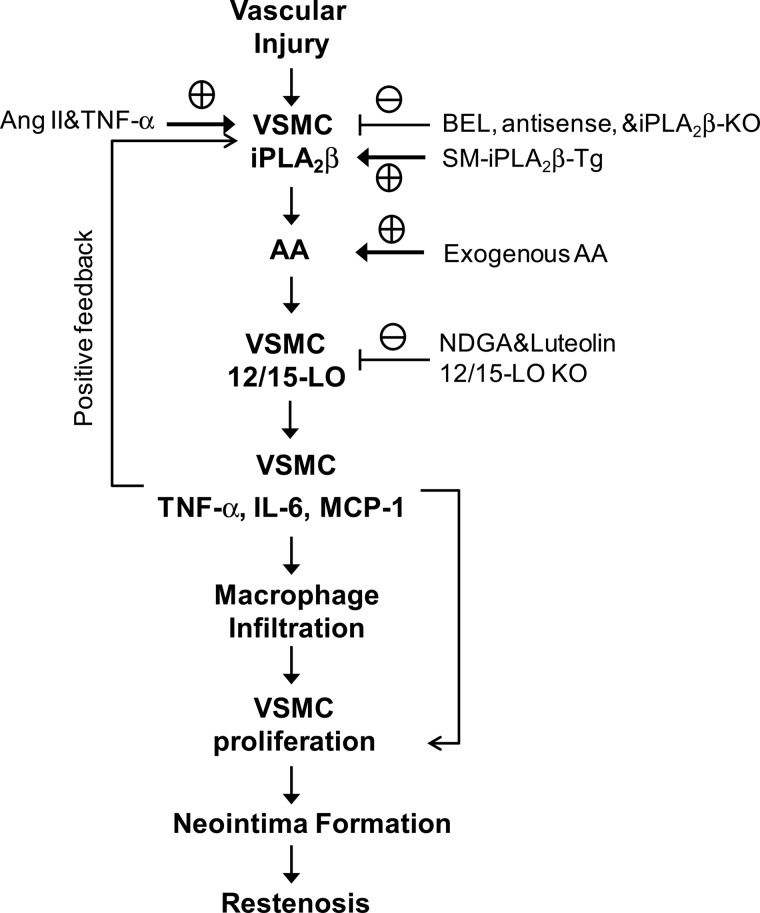
In summary, our results demonstrate that activation of iPLA2β in VSMC is involved in the initiation and early progression of vascular inflammation and neointima formation in a mouse carotid artery ligation model. Fig. 8 summarizes a model that integrates our major findings and experimental approaches and proposes a sequence of biochemical events in VSMC signaling pathways. Activation of iPLA2β by carotid artery ligation is proposed to liberate AA that is metabolized by the 12/15-LO enzyme to produce eicosanoid mediators that elicit a train of events that lead to production of inflammatory cytokines, infiltration of macrophages into the vascular wall, proliferation of VSMC, formation of neointima, and stenosis or restenosis of the vessel to produce luminal compromise or occlusion. Our results indicate that smooth muscle iPLA2β may represent a novel therapeutic target for development of new therapeutic agents to attenuate or prevent such vaso-occlusive events in human cardiovascular diseases.
FIGURE 8.
Model for vascular injury-induced and iPLA2β-mediated vascular inflammation and neointima formation. See text for details.
Acknowledgment
We thank Ming Zhang for excellent technical assistance in breeding of iPLA2β-Tg mice and WT littermates.
This work was supported, in whole or in part, by National Institutes of Health HL088389 and HL088389-02S1 (to Z. G.), HL082791 (to M. G.), P20 RR021954 from NCRR and P20 GM103527 from NIGMS, and USPHS Grants R37-DK34388, P41-RR00954, P60-DK20579, and P30-DK56341 (to J. T.). This work was also supported by the Commonwealth of Kentucky Diabetes Research Trust Fund (to Z. G.) and a postdoctoral fellowship from the American Heart Association (to S. L.).

This article contains supplemental Experimental Procedures, Figs. 1–5, Table 1, and additional references.
- VSMC
- vascular smooth muscle cell
- iPLA2
- calcium-independent phospholipase A2
- AA
- arachidonic acid
- LPC
- 1-radyl, 2-lyso-glycerophosphocholine
- BEL
- bromoenol lactone
- Ang II
- angiotensin II
- SM-iPLA2β-Tg
- iPLA2β smooth muscle-specific transgenic mice
- SMαA
- smooth muscle cell α-actin
- SMMHC
- smooth muscle heavy chain
- MCP-1
- monocyte chemotactic protein-1
- NFκB
- nuclear factor κ-light-chain-enhancer of activated B cell
- PCNA
- proliferating cell nuclear antigen
- LO
- lipoxygenase
- COX
- cyclooxygenase
- CYP
- cytochrome P450-dependent epoxygenase
- PNPLA
- phospholipase domain-containing protein.
REFERENCES
- 1. Schwartz S. M., deBlois D., O'Brien E. R. (1995) The intima. Soil for atherosclerosis and restenosis. Circ. Res. 77, 445–465 [DOI] [PubMed] [Google Scholar]
- 2. Ross R. (1999) Atherosclerosis is an inflammatory disease. Am. Heart J. 138, S419–S420 [DOI] [PubMed] [Google Scholar]
- 3. Libby P. (2002) Inflammation in atherosclerosis. Nature 420, 868–874 [DOI] [PubMed] [Google Scholar]
- 4. Costa M. A., Simon D. I. (2005) Molecular basis of restenosis and drug-eluting stents. Circulation 111, 2257–2273 [DOI] [PubMed] [Google Scholar]
- 5. Glass C. K., Witztum J. L. (2001) Atherosclerosis. The road ahead. Cell 104, 503–516 [DOI] [PubMed] [Google Scholar]
- 6. Owens G. K., Kumar M. S., Wamhoff B. R. (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84, 767–801 [DOI] [PubMed] [Google Scholar]
- 7. Schober A., Weber C. (2005) Mechanisms of monocyte recruitment in vascular repair after injury. Antioxid. Redox. Signal. 7, 1249–1257 [DOI] [PubMed] [Google Scholar]
- 8. Doran A. C., Meller N., McNamara C. A. (2008) Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 28, 812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raines E. W., Ferri N. (2005) Thematic review series. The immune system and atherogenesis. Cytokines affecting endothelial and smooth muscle cells in vascular disease. J. Lipid Res. 46, 1081–1092 [DOI] [PubMed] [Google Scholar]
- 10. Dennis E. A. (1994) Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 269, 13057–13060 [PubMed] [Google Scholar]
- 11. Hazen S. L., Zupan L. A., Weiss R. H., Getman D. P., Gross R. W. (1991) Suicide inhibition of canine myocardial cytosolic calcium-independent phospholipase A2. Mechanism-based discrimination between calcium-dependent and -independent phospholipases A2. J. Biol. Chem. 266, 7227–7232 [PubMed] [Google Scholar]
- 12. Kienesberger P. C., Oberer M., Lass A., Zechner R. (2009) Mammalian patatin domain containing proteins. A family with diverse lipolytic activities involved in multiple biological functions. J. Lipid Res. 50, S63–S68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma Z., Turk J. (2001) The molecular biology of the group VIA Ca2+-independent phospholipase A2. Prog. Nucleic Acids Res. Mol. Biol. 67, 1–33 [DOI] [PubMed] [Google Scholar]
- 14. Murakami M., Taketomi Y., Miki Y., Sato H., Hirabayashi T., Yamamoto K. (2011) Recent progress in phospholipase A2 research. From cells to animals to humans. Prog. Lipid Res. 50, 152–192 [DOI] [PubMed] [Google Scholar]
- 15. Natarajan R., Nadler J. L. (2004) Lipid inflammatory mediators in diabetic vascular disease. Arterioscler. Thromb. Vasc. Biol. 24, 1542–1548 [DOI] [PubMed] [Google Scholar]
- 16. Xie Z., Gong M. C., Su W., Turk J., Guo Z. (2007) Group VIA phospholipase A2 (iPLA2β) participates in angiotensin II-induced transcriptional up-regulation of regulator of G-protein signaling-2 in vascular smooth muscle cells. J. Biol. Chem. 282, 25278–25289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yellaturu C. R., Rao G. N. (2003) A requirement for calcium-independent phospholipase A2 in thrombin-induced arachidonic acid release and growth in vascular smooth muscle cells. J. Biol. Chem. 278, 43831–43837 [DOI] [PubMed] [Google Scholar]
- 18. Smani T., Zakharov S. I., Csutora P., Leno E., Trepakova E. S., Bolotina V. M. (2004) A novel mechanism for the store-operated calcium influx pathway. Nat. Cell Biol. 6, 113–120 [DOI] [PubMed] [Google Scholar]
- 19. Guo Z., Su W., Ma Z., Smith G. M., Gong M. C. (2003) Ca2+-independent phospholipase A2 is required for agonist-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 278, 1856–1863 [DOI] [PubMed] [Google Scholar]
- 20. Xie Z., Gong M. C., Su W., Xie D., Turk J., Guo Z. (2010) Role of calcium-independent phospholipase A2β in high glucose-induced activation of RhoA, Rho kinase, and CPI-17 in cultured vascular smooth muscle cells and vascular smooth muscle hypercontractility in diabetic animals. J. Biol. Chem. 285, 8628–8638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jenkins C. M., Han X., Mancuso D. J., Gross R. W. (2002) Identification of calcium-independent phospholipase A2 (iPLA2) β, and not iPLA2γ, as the mediator of arginine vasopressin-induced arachidonic acid release in A-10 smooth muscle cells. Enantioselective mechanism-based discrimination of mammalian iPLA2s. J. Biol. Chem. 277, 32807–32814 [DOI] [PubMed] [Google Scholar]
- 22. Lehman J. J., Brown K. A., Ramanadham S., Turk J., Gross R. W. (1993) Arachidonic acid release from aortic smooth muscle cells induced by [Arg8]vasopressin is largely mediated by calcium-independent phospholipase A2. J. Biol. Chem. 268, 20713–20716 [PubMed] [Google Scholar]
- 23. Moon S. H., Jenkins C. M., Mancuso D. J., Turk J., Gross R. W. (2008) Smooth muscle cell arachidonic acid release, migration, and proliferation are markedly attenuated in mice null for calcium-independent phospholipase A2β. J. Biol. Chem. 283, 33975–33987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie Z., Liu D., Liu S., Calderon L., Zhao G., Turk J., Guo Z. (2011) Identification of a cAMP-response element in the regulator of G-protein signaling-2 (RGS2) promoter as a key cis-regulatory element for RGS2 transcriptional regulation by angiotensin II in cultured vascular smooth muscles. J. Biol. Chem. 286, 44646–44658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bao S., Miller D. J., Ma Z., Wohltmann M., Eng G., Ramanadham S., Moley K., Turk J. (2004) Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J. Biol. Chem. 279, 38194–38200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kallmeier R. C., Somasundaram C., Babij P. (1995) A novel smooth muscle-specific enhancer regulates transcription of the smooth muscle myosin heavy chain gene in vascular smooth muscle cells. J. Biol. Chem. 270, 30949–30957 [DOI] [PubMed] [Google Scholar]
- 27. Kumar A., Lindner V. (1997) Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler. Thromb. Vasc. Biol. 17, 2238–2244 [DOI] [PubMed] [Google Scholar]
- 28. Villa A. E., Guzman L. A., Poptic E. J., Labhasetwar V., D'Souza S., Farrell C. L., Plow E. F., Levy R. J., DiCorleto P. E., Topol E. J. (1995) Effects of antisense c-Myb oligonucleotides on vascular smooth muscle cell proliferation and response to vessel wall injury. Circ. Res. 76, 505–513 [DOI] [PubMed] [Google Scholar]
- 29. Guo Z., Su W., Allen S., Pang H., Daugherty A., Smart E., Gong M. C. (2005) COX-2 up-regulation and vascular smooth muscle contractile hyper-reactivity in spontaneous diabetic db/db mice. Cardiovasc. Res. 67, 723–735 [DOI] [PubMed] [Google Scholar]
- 30. Pang H., Guo Z., Su W., Xie Z., Eto M., Gong M. C. (2005) RhoA-Rho kinase pathway mediates thrombin- and U-46619-induced phosphorylation of a myosin phosphatase inhibitor, CPI-17, in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 289, C352–C360 [DOI] [PubMed] [Google Scholar]
- 31. Pang H., Guo Z., Xie Z., Su W., Gong M. C. (2006) Divergent kinase signaling mediates agonist-induced phosphorylation of phosphatase inhibitory proteins PHI-1 and CPI-17 in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol 290, C892–C899 [DOI] [PubMed] [Google Scholar]
- 32. Xie Z., Su W., Guo Z., Pang H., Post S. R., Gong M. C. (2006) Up-regulation of CPI-17 phosphorylation in diabetic vasculature and high glucose cultured vascular smooth muscle cells. Cardiovasc. Res. 69, 491–501 [DOI] [PubMed] [Google Scholar]
- 33. Su W., Guo Z., Randall D. C., Cassis L., Brown D. R., Gong M. C. (2008) Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am. J. Physiol. Heart Circ. Physiol. 295, H1634–H1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jenkins C. M., Mancuso D. J., Yan W., Sims H. F., Gibson B., Gross R. W. (2004) Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 279, 48968–48975 [DOI] [PubMed] [Google Scholar]
- 35. Song H., Ramanadham S., Bao S., Hsu F. F., Turk J. (2006) A bromoenol lactone suicide substrate inactivates group VIA phospholipase A2 by generating a diffusible bromomethyl keto acid that alkylates cysteine thiols. Biochemistry 45, 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harmon K. J., Couper L. L., Lindner V. (2000) Strain-dependent vascular remodeling phenotypes in inbred mice. Am. J. Pathol. 156, 1741–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stec D. E., Morimoto S., Sigmund C. D. (2001) Vectors for high level expression of cDNAs controlled by tissue-specific promoters in transgenic mice. BioTechniques 31, 256–260 [DOI] [PubMed] [Google Scholar]
- 38. Kumar A., Hoover J. L., Simmons C. A., Lindner V., Shebuski R. J. (1997) Remodeling and neointimal formation in the carotid artery of normal and P-selectin-deficient mice. Circulation 96, 4333–4342 [DOI] [PubMed] [Google Scholar]
- 39. Rectenwald J. E., Moldawer L. L., Huber T. S., Seeger J. M., Ozaki C. K. (2000) Direct evidence for cytokine involvement in neointimal hyperplasia. Circulation 102, 1697–1702 [DOI] [PubMed] [Google Scholar]
- 40. Balsinde J., Dennis E. A. (1997) Function and inhibition of intracellular calcium-independent phospholipase A2. J. Biol. Chem. 272, 16069–16072 [DOI] [PubMed] [Google Scholar]
- 41. Hooks S. B., Cummings B. S. (2008) Role of Ca2+-independent phospholipase A2 in cell growth and signaling. Biochem. Pharmacol. 76, 1059–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu S. J., McHowat J. (1998) Stimulation of different phospholipase A2 isoforms by TNF-α and IL-1β in adult rat ventricular myocytes. Am. J. Physiol. 275, H1462–H1472 [DOI] [PubMed] [Google Scholar]
- 43. Seashols S. J., del Castillo Olivares A., Gil G., Barbour S. E. (2004) Regulation of group VIA phospholipase A2 expression by sterol availability. Biochim. Biophys. Acta 1684, 29–37 [DOI] [PubMed] [Google Scholar]
- 44. Lei X., Zhang S., Barbour S. E., Bohrer A., Ford E. L., Koizumi A., Papa F. R., Ramanadham S. (2010) Spontaneous development of endoplasmic reticulum stress that can lead to diabetes mellitus is associated with higher calcium-independent phospholipase A2 expression. A role for regulation by SREBP-1. J. Biol. Chem. 285, 6693–6705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou R. H., Pesant S., Cohn H. I., Eckhart A. D. (2008) Enhanced sterol response element-binding protein in postintervention restenotic blood vessels plays an important role in vascular smooth muscle proliferation. Life Sci. 82, 174–181 [DOI] [PubMed] [Google Scholar]
- 46. Wolf M. J., Wang J., Turk J., Gross R. W. (1997) Depletion of intracellular calcium stores activates smooth muscle cell calcium-independent phospholipase A2. A novel mechanism underlying arachidonic acid mobilization. J. Biol. Chem. 272, 1522–1526 [DOI] [PubMed] [Google Scholar]
- 47. Natarajan R., Reddy M. A., Malik K. U., Fatima S., Khan B. V. (2001) Signaling mechanisms of nuclear factor-κB-mediated activation of inflammatory genes by 13-hydroperoxyoctadecadienoic acid in cultured vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 21, 1408–1413 [DOI] [PubMed] [Google Scholar]
- 48. Dwarakanath R. S., Sahar S., Reddy M. A., Castanotto D., Rossi J. J., Natarajan R. (2004) Regulation of monocyte chemoattractant protein-1 by the oxidized lipid, 13-hydroperoxyoctadecadienoic acid, in vascular smooth muscle cells via nuclear factor-κB (NF-κB). J. Mol. Cell. Cardiol. 36, 585–595 [DOI] [PubMed] [Google Scholar]
- 49. Chava K. R., Karpurapu M., Wang D., Bhanoori M., Kundumani-Sridharan V., Zhang Q., Ichiki T., Glasgow W. C., Rao G. N. (2009) CREB-mediated IL-6 expression is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. Arterioscler. Thromb. Vasc. Biol. 29, 809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Walev I., Klein J., Husmann M., Valeva A., Strauch S., Wirtz H., Weichel O., Bhakdi S. (2000) Potassium regulates IL-1β processing via calcium-independent phospholipase A2. J. Immunol. 164, 5120–5124 [DOI] [PubMed] [Google Scholar]
- 51. Franchi L., Chen G., Marina-Garcia N., Abe A., Qu Y., Bao S., Shayman J. A., Turk J., Dubyak G. R., Núñez G. (2009) Calcium-independent phospholipase A2β is dispensable in inflammasome activation and its inhibition by bromoenol lactone. J. Innate Immun. 1, 607–617 [DOI] [PMC free article] [PubMed] [Google Scholar]