Background: Defective ABCB11 causes severe progressive cholestatic liver disease from early infancy.
Results: Abcb11 knock-out C57BL/6J mice recapitulate human deficiency and, before cholestatic liver damage, exhibit impaired fatty acid β-oxidation.
Conclusion: Altered fatty acid oxidation may facilitate cholestatic liver damage.
Significance: Disrupted fatty acid β-oxidation may be essential to cholestatic liver injury in Abcb11 deficiency.
Keywords: Bile Acid, Fatty Acid Oxidation, Liver, Metabolomics, Transporters, Cholestasis
Abstract
The bile salt export pump (BSEP) is an ATP-binding cassette transporter that serves as the primary system for removing bile salts from the liver. In humans, deficiency of BSEP, which is encoded by the ABCB11 gene, causes severe progressive cholestatic liver disease from early infancy. In previous studies of Abcb11 deficiency in mice generated on a mixed genetic background, the animals did not recapitulate the human disease. We reasoned that ABCB11 deficiency may cause unique changes in hepatic metabolism that are predictive of liver injury. To test this possibility, we first determined that Abcb11 knock-out (KO) C57BL/6J mice recapitulate human deficiency. Before the onset of cholestasis, Abcb11 KO mice have altered hepatic lipid metabolism coupled with reduced expression of genes important in mitochondrial fatty acid oxidation. This was associated with increased serum free-fatty acids, reduced total white adipose, and marked impairment of long-chain fatty acid β-oxidation. Importantly, metabolomic analysis confirmed that Abcb11 KO mice have impaired mitochondrial fatty acid β-oxidation with the elevated fatty acid metabolites phenylpropionylglycine and phenylacetylglycine. These metabolic changes precede cholestasis but may be of relevance to cholestatic disease progression because altered fatty acid metabolism can enhance reactive oxygen species that might exacerbate cholestatic liver damage.
Introduction
The physiological concentration of hepatic bile acids is maintained through a complex feedback process (1). Impaired canalicular secretion and hepatic accumulation of BAs cause gross pathologic changes that may be preceded by molecular events that presage liver injury. The bile salt export pump (BSEP),3 encoded by ABCB11, is the primary efflux transporter of bile acids at the canalicular membrane of hepatocytes (2). When BSEP function is impaired (3–6), defective bile acid export leads to progressive cholestasis. ABCB11 deficiency is associated with progressive familial intrahepatic cholestasis type 2 (PFIC2), benign recurrent intrahepatic cholestasis type 2 (BRIC2), and several forms of acquired cholestasis (5). The extent of loss of BSEP function parallels the severity of cholestasis (6). Infants with defective ABCB11 experience a progressive cholestasis that requires liver transplant to avoid cirrhosis and liver failure (7). Uncorrected, ABCB11 loss of function is also associated with a high frequency of rarely seen hepatobiliary malignancies in children (8).
Importantly, Abcb11-null mice generated on a “mixed genetic” background as well as the FVB background (9), despite substantial accumulation of hepatic bile acids, show no evidence of progressive liver injury. The lack of progressive cholestasis was attributed to bile acid metabolism and composition differences between mice and humans as well as compensation by alternative bile acid exporters (9, 10). In contrast, we show herein that Abcb11 knock-out (KO) mice on a “pure” C57BL/6J background exhibit progressive cholestasis, much like human PFIC2. However, this progressive disease occurs despite the KO having altered bile acid metabolism and compensation of alternative bile acid exporters. We hypothesized that hepatocytes acquire metabolic changes before cholestatic liver injury is manifested.
Bile acids have long been considered essential for digestion but more recently have been characterized as important signaling molecules regulating liver metabolism (11, 12). For example, bile acids activate farnesoid X receptor (FXR/Nrlh4) to suppress multiple hepatic glucose metabolism genes (13), thereby modulating glucose metabolism. Bile acids also negatively regulate their biosynthesis via the intracellular FXR, repressing the key enzyme, cholesterol 7-α-hydroxylase (Cyp7α). In this context, we show that cholestasis and liver injury in Abcb11 deficiency is preceded by elevated hepatic bile acids and altered lipid metabolism; it is likely that these early changes are crucial to producing liver injury.
EXPERIMENTAL PROCEDURES
Animals
Wild type (WT) and Abcb11 knock-out (KO) mice generated on the C57BL/6 genetic background were purchased from the Jackson Laboratory; the KO mice were generated by backcrossing Abcb11-null mice on a mixed background with C57BL/6 mice for 10 generations. Animals were maintained in a temperature- and humidity-controlled room with free access to water and food with 12-h/12-h light/dark cycles. All procedures were reviewed and approved by the Animal Care and Use Committee of St. Jude Children's Research Hospital. Mice were monitored for more than 1 year. Blood was collected retro-orbitally. Alanine transferase, aspartate transferase, alkaline phosphatase activities, and triglyceride concentration were measured by standard assays. The body weight and liver weight of euthanized mice were recorded.
Microarray Analysis and Quantitative Real-time PCR (qPCR)
Total RNA was isolated from 1.5-month-old WT and KO mouse liver tissues by using the RNeasy kit (Qiagen), according to the manufacturer's protocol. The quality and integrity of the RNA was confirmed by analysis on an Agilent 2100 Bioanalyzer. Biotin-labeled targets, prepared from 100 ng of total RNA using the Affymetrix 3′IVT express protocol, were hybridized to mouse HT MG430 PM plate arrays and then processed automatically using the Affymetrix GeneTitan system. Signals from scanned arrays were summarized using the Robust Multi-array Average method (14, 15). Gene set enrichment analysis (GSEA) (14, 15) was performed using curated canonical pathways obtained from Ingenuity Systems. The same RNA was used in qPCR analysis for confirmation.
Metabolomic Analysis
Three days before sample collection, age-matched WT and KO mice were individually placed in and acclimated to Nalgene metabolic cages. Animals had free access to food and water. Urine was collected over a 24-h period; blood was obtained retro-orbitally for serum. Specimens were stored in −80 °C until use. Urine samples were prepared by mixing 20 μl of urine with 180 μl of 50% aqueous acetonitrile, and serum samples were prepared by mixing 10 μl of serum with 190 μl of 66% aqueous acetonitrile. Liver samples (100 mg) were minced in 1.0 ml of 50% aqueous acetonitrile and shaken for 15 min at room temperature. The mixture or homogenate was centrifuged at 14,000 × g for 20 min, and a 5-μl aliquot of supernatant was injected into an ultraperformance liquid chromatography-electrospray ionization-quadrupole time-of-flight mass spectrometry (UPLC-ESI-QTOFMS) system (Waters Corp.) with analysis in positive and negative ion selection modes. The data matrix of ions was analyzed by principal component analysis (PCA) and then by multivariate partial least squares discriminant analysis.
Chromatographic and spectral data of urine, serum, and liver samples were processed by MarkerLynx mass spectrometry software (Waters Corp.). PCA and partial least squares discriminant analysis were conducted to identify the potential biomarkers using SIMCA-P+12 (Umetrics). Metabolomics databases (Madison Metabolomics Consortium Database and METLIN) were searched to find potential candidates for ions with high contribution scores. Identities of the ions were further confirmed by comparing the fragmentation pattern and retention time with authentic compounds that were purchased from Sigma or synthesized, as previously reported (16). After potential biomarker candidates were identified in urine and serum, they were quantified by using an ACQUITY UPLC system coupled with a XEVO triple-quadrupole tandem mass spectrometer (Waters Corp.). Biomarkers were detected and quantified by multiple-reaction monitoring mass spectrometry. The concentrations of metabolites in urine and serum were determined by using calibration curves constructed with authentic standards. The concentrations of metabolites in urine were normalized to creatinine concentration. Chlorpropamide (0.5 μm) was used as the internal standard. Biomarkers in liver homogenate were quantified by using the Waters Corp. UPLC-ESI-QTOFMS system and QuanLynx software (Waters Corp.). The concentration of each analyte in liver homogenate was determined against calibration curves constructed with authentic standards. All correlation coefficients were greater than 0.99 for each calibration curve. Chlorpropamide at a final concentration of 5 μm was used as the internal standard.
Fatty Acid β-Oxidation (β-FAO) assay
β-FAO was assayed in liver extracts per a modified protocol (17). Briefly, fresh liver extracts were incubated at room temperature for 1 h with unlabeled palmitic acid or myristic acid (50, 100, 200, and 300 μm) mixed with radioactive tracers ([1-14C]palmitate (PerkinElmer Life Sciences) or [1-14C]myristic acid (American Radioactive Chemicals), respectively). CO2 was released by adding percholic acid and captured by hyamine hydroxide and short-chain metabolites (≤C6, acid-soluble metabolites) retained in perchloric acid. Fatty acid oxidation rates were represented as the production rates of acid-soluble metabolites and captured CO2. To test the effect of a Cd36-specific inhibitor, sulfo-N-succinimidyl oleate (SSO) (Toronto Research Chemicals), on fatty acid oxidation, liver extracts were pretreated with 50 μm SSO for 10 min at room temperature before assay. Peroxisomal β-FAO was determined by adding mitochondrial β-FAO inhibitors, malonyl-CoA (0.5 mm), glybenclamide (20 μm), and 3-mercaptoproprionic acid (0.8 mm), into the reaction mixture before the assay (18).
Statistics
All experimental values are presented as the means ± S.E. Statistical comparisons used the two-tailed Student's t test.
RESULTS
Abcb11 Deficiency Causes Liver Injury
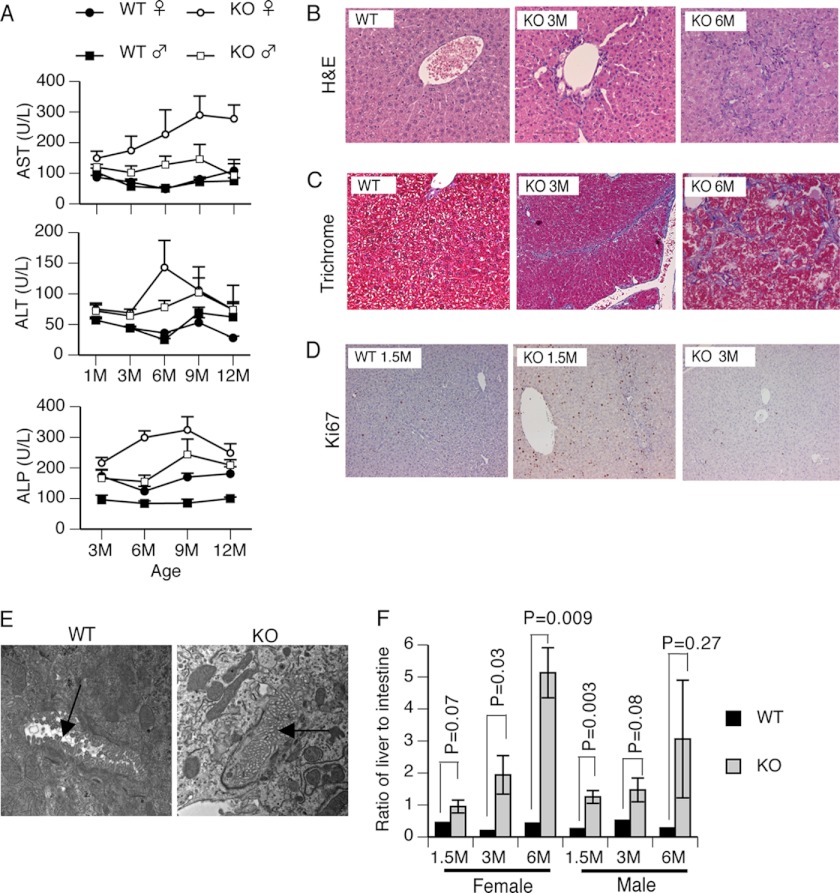
The Abcb11-null mice originally generated on the mixed genetic background showed no evidence of progressive liver injury (10). In contrast, Abcb11 KO mice generated on a “pure” C57BL/6J background (hereafter referred to as KO mice) exhibited a progressive increase in the activities of alanine transferase, aspartate transferase, and an intrahepatic cholestasis marker, alkaline phosphatase (Fig. 1A). These activities were strongly elevated in females. Histologic analysis was consistent with these findings, revealing age-dependent hepatocellular damage (Fig. 1B) in KO mice compared with WT mice. In 6-month-old KO mouse liver, trichrome staining indicated extensive fibrosis (Fig. 1C), whereas it was absent in the 1.5-month-old and minimal in the 3-month-old mouse liver. Although no change was seen in body weight between WT and KO (data not shown), the ratio of liver weight to body weight was greater than 8% in the KO mice (similar to that reported for Abcb11-null mice on the mixed background (10), compared with 4% in the WT mice (supplemental Fig. S1A). To determine whether hepatomegaly resulted from cellular proliferation or hypertrophy, we stained liver sections from KO and WT mice for Ki67 to assess DNA synthesis. Strong Ki67 staining was observed in KO liver (Fig. 1D). Specifically, for every 500 hepatocytes, 74 cells were Ki67-positive in KO versus 15 cells in WT (supplemental Fig. S1B) at 1.5 months. Although hepatocyte proliferation decreased with age to 11 and 16 in 3- and 6-month-old KO mice, respectively, it was still dramatically higher than that in 3-month-old WT mice (3 Ki67-positive cells per 500 hepatocytes) (supplemental Fig. S1B). Therefore, in KO hepatocytes, an early increase in hepatocyte proliferation accounts for the increase in liver/body weight ratio. Notably, after 1.5 months, KO animals showed increased necrosis and Kupffer cell hypertrophy. The KO also exhibited occluded canaliculi and apparent loss of microvilli as revealed by electron microscopy (EM) analysis (Fig. 1E). Cumulatively, the hepatomegaly, the progressive necrosis, fibrosis, and bile duct proliferation of the KO mice reflect those found in human deficiencies of ABCB11, PFIC2, and BRIC2 (19).
FIGURE 1.
Abcb11 deficiency induced progressive liver injury due to intrahepatic cholestasis. A, indicators of liver function, aspartate transferase (AST), alanine transferase (ALT), and alkaline phosphatase (ALP) activities were measured in serum collected retro-orbitally every month for up to 1 year. Herein, data at 1, 3, 6, 9, and 12 months are reported. n = 5–10. B, the progressive bile duct hyperplasia appeared in the 3- and 6-month-old KO mice. Liver sections were stained with H&E. C, fibrosis (blue staining) occurred in the livers of KO mice. Liver sections were stained with trichrome. D, massive proliferation of hepatocytes was shown in the KO mice. Positive Ki67 staining (brown) indicates proliferative hepatocytes in the liver sections. E, loss of canalicular microvilli in the KO mouse liver. Ultrastructure of liver was analyzed by electron microscopy. Canaliculi structure is indicated by an arrow (magnification, ×19,000). The pictures shown in B–E are representative of at least three mice in each group. F, bile acids accumulated progressively in the liver of KO mice. Bile pool sizes were separately measured by HPLC in the liver and in the small intestine, including contents and gall bladder. The bile pool size in the liver was normalized to that in the intestine. Values are mean ± S.E. (error bars) of ratios. p values are indicated in the graph. n = 3–7.
Abcb11 KO Mice Develop Intrahepatic Cholestasis
HPLC was used to determine bile acid pool size in liver, small intestine, and gall bladder. Although the total bile acid pool size relative to 100 g of body weight did not show changes between WT and KO (data not shown), its disposition in the KO differed from that in the WT. The relative bile acid pool size in the enterohepatic circulation was represented by the ratio of bile acid pool size of the liver to that of the small intestine. In both WT female and male mice, this ratio was consistently less than 0.5 across all ages from 1.5 to 6 months. But in the KO mice, the ratio increased progressively to 1 at 1.5 months to 5 in females and 3 in males at 6 months (Fig. 1F), suggesting a progressive intrahepatic cholestasis.
Additionally, metabolomic analysis by UPLC-ESI-QTOFMS of hepatic bile acid pool composition revealed differences between WT and KO. The PCA score plot showed bile acid metabolites that readily segregated the livers of WT and KO (supplemental Fig. S2A). Quantification followed identification of the bile acids by fragmentation pattern and retention time. The primary bile acid, β-muricholic acid (β-MCA), and its taurine conjugate (T-β-MCA) concentrations were dramatically increased in the KO liver (supplemental Fig. S2B). The hepatic concentration of T-β-MCA was over 5 times higher than that of unconjugated β-MCA. Moreover, the hepatic concentration of taurine was more than 6 times as high in the KO mice as in the WT mice. Taurine conjugation of bile acids increases their solubility and protects by reducing their inherent detergent-like properties (20). Moreover, taurine is itself protective against cholestasis (21, 22). Tetrahydroxylation of bile acids is also protective against intrahepatic cholestasis. The tetrahydroxylated bile acids 2β,3α,7α,12α-tetrol and 3α,6,7β,12α-tetrol and their taurine conjugates were undetectable in WT liver but were dramatically increased in KO (supplemental Fig. S2B).
In parallel with the increase in hepatic bile acids, serum and urinary bile acids were also increased in the KO mice. The serum concentrations of β-MCA and its taurine conjugate were more than 35 times greater in the KO mice but progressively declined from 1.5 to 12 months (supplemental Fig. S1C). T-3α,6,7α,12α-tetrol and 3α,6,7β,12α-tetrol in the KO urine were more 50- and 10-fold higher than in the WT urine. The increase was progressive with age in the KO mice (supplemental Fig. S1D).
Regulation of Bile Acid Transporter and Biosynthetic Genes in the Absence of Abcb11
The increased serum bile acid concentration may reflect an increased expression of sinusoidal and basolateral bile acid efflux proteins. The up-regulation of these alternative bile acid transporters as well as canalicular bile acid transporters has been proposed by Ling and colleagues (9, 10) as an additional mechanism to account for the non-progressive liver damage in murine Abcb11 deficiency. Because of the elevated serum bile acids, we evaluated transporter expression in WT and KO mice using qPCR. Analysis of KO liver at 1.5 months showed strong up-regulation of the low affinity canalicular transporter Mdr1a (9), which was proposed as a compensatory transporter protecting murine liver (9). Importantly, mRNA encoding Oatp1a1, a bile acid uptake carrier, was reduced over 80-fold (supplemental Fig. S3A) in the KO liver. In contrast, expression of sinusoidal exporters, Mrp4, Ostα, and Ostβ, was strongly up-regulated (supplemental Fig. S3, B and C), which is similar to the previous report for Abcb11 deficiency (23).
To assess the potential contribution of bile acid biosynthetic genes to the bile acid elevation in KO mice, qPCR was conducted to detect their mRNA expression. The enzyme bile acid-CoA:amino acid N-acyltransferase (Baat) catalyzes glycine and taurine conjugation of primary bile acids (24). Baat was similarly expressed in WT and KO livers; therefore, it probably does not account for the 6–8-fold increase in hepatic taurine-conjugated β-MCA and chenodeoxycholate. Elevated taurine concentration in the KO liver most likely accounts for the increase in taurine-conjugated bile acids and is consistent with the time-dependent decrease in the primary bile acid, chenodeoxycholate. The rate-limiting enzymes of biosynthetic genes, Cyp7a1, Cyp7b1, Cyp8b1, and Cyp27a1, were strongly down-regulated in KO mice (supplemental Fig. S3D), indicating general down-regulation of bile acid synthesis. It is notable that most of these changes in gene expression have been reported in Abcb11 deficiency on either a mixed genetic background or FVB (9, 23).
Lipid Homeostasis Is Disrupted in the KO Mice
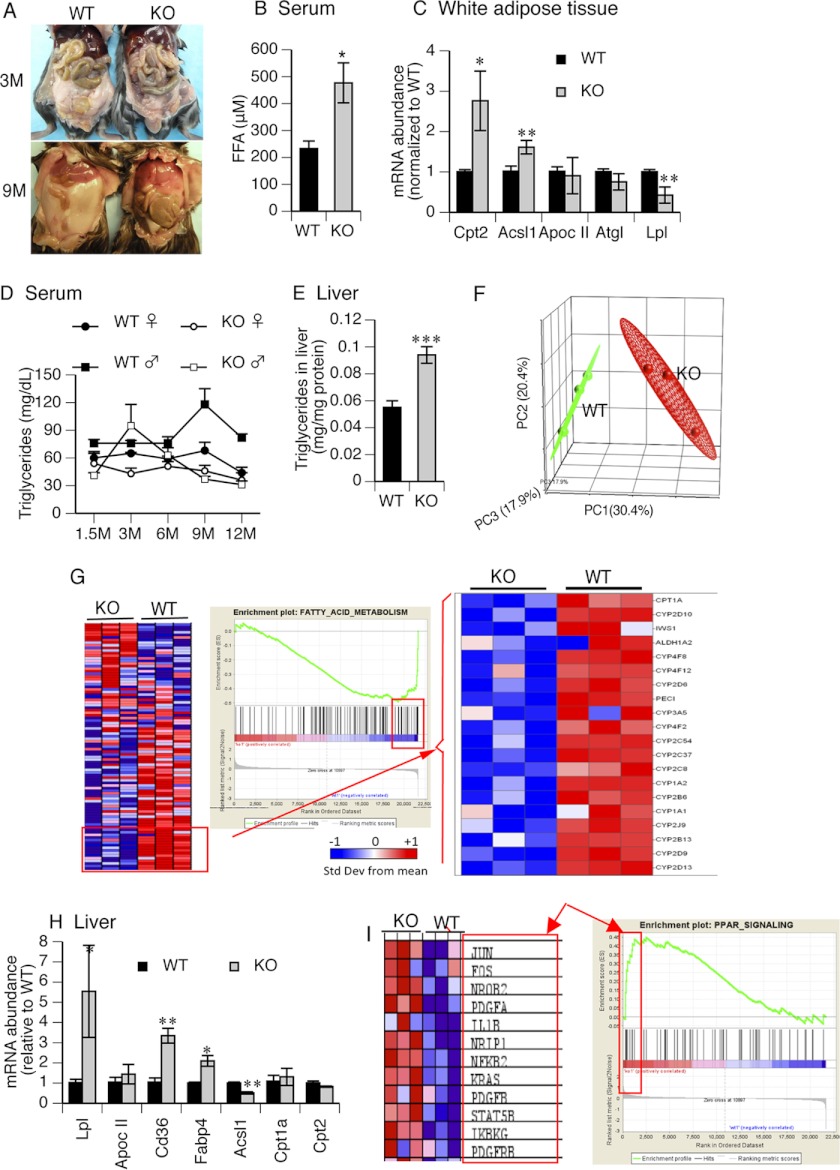
A surprising phenotype of the KO mice (not previously reported for murine Abcb11 deficiency) was a time-dependent reduction in mass of white adipose from 3 months to almost complete absence at 9 months as compared with the time-dependent accumulation in the WT mice (Fig. 2A). It is notable that at 1.5 months, there was no observable difference in white adipose between KO and WT mice (not shown). We hypothesized that this was due to decreased storage and/or increased lipolysis of white adipose. In animals with no visible difference in peripheral adipose (1.5 months) we determined that the serum concentration of free fatty acids (FFAs) increased over 2-fold (Fig. 2B). An analysis of adipose from comparably aged animals revealed a down-regulation in lipases (Lpl and Atgl). Moreover, in the KO, the fatty acid importer Cd36 was down-regulated (not shown); however, the key genes in β-FAO, Acsl1 and Cpt2, were both strongly increased in KO white adipose tissue, suggesting increased FA oxidation in the white adipose (Fig. 2C). Although serum triglycerides were generally lower in the KO mice (Fig. 2D), the hepatic triglyceride level significantly increased, which suggests that triglyceride secretion from liver is lower in the KO mice (Fig. 2E). This increase is unlikely to be due to increased FA uptake by either FATP2 (slc27a5) or FATP5 (slc27a5) because neither of these FA transporters are involved in FA uptake is increased in the KO (supplemental Fig. S4).
FIGURE 2.
KO mice exhibited alteration in fatty acid metabolism. A, white adipose tissue was gradually lost in the KO mice from 3 to 9 months. The pictures are representative of the WT and KO mice. B, free fatty acids were measured in serum collected by cardiac puncture. n = 3. C, expression of lipid metabolism genes was altered in the KO white adipose tissue. Expression of mRNA was measured by qPCR. Values in the graph are -fold changes after normalization to WT. n = 3–4. D, serum triglycerides were monitored in the WT and KO mice. n = 5–10. E, triglycerides were measured after being extracted from liver homogenates and normalized to total protein. n = 3. F, the WT gene expression profile was well segregated from KO mice analyzed by PCA. Liver tissues of the 1.5-month-old mice were used in the microarray. Green and red dots represent individual WT and KO mice, respectively. Ninety-five percent confidence boundaries are drawn around each group. n = 3. G, fatty acid metabolism was down-regulated in the KO liver. GSEA was performed on the microarray data using curated canonical pathways obtained from Ingenuity Systems. A heat map of all genes (left), an enrichment plot (middle), and a heat map of core genes (right) that contribute maximally to the enrichment score are displayed. H, expression of hepatic mitochondrial β-FAO genes was measured by qPCR. Values in the graph are -fold changes after normalization to WT. n = 3. I, up-regulation of PPAR signaling genes in the KO liver analyzed by GSEA. Relative expression of the PPAR “core” genes contributing to the maximum enrichment score (left) and the GSEA enrichment plot (right) are displayed. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. Error bars, S.E.
Because these lipid changes occur in young animals, prior to frank liver damage, and abnormal lipid metabolism is associated with liver damage (25, 26), we analyzed gene expression in the livers of 1.5-month-old mice that showed no evidence of liver damage. PCA of all hepatic microarray gene expression patterns allowed a clear distinction between 1.5-month WT and KO mice (Fig. 2F). GSEA, a computational method that identifies concordant differences between groups on the basis of enrichment of genes, revealed a strong reduction in the expression of core genes in FA metabolism (Fig. 2G) and reduced expression of β-FAO genes. We quantified selected genes in FA metabolism by qPCR. We found strong down-regulation of Acsl1, a crucial initial step in β-FAO (Fig. 2H). Surprisingly, we found an increase in expression of genes important to uptake and a chaperone of FFA (Cd36 and Fabp4, respectively) but also a 5.5-fold increase in Lpl.
Free fatty acids are endogenous ligands of PPARs (27, 28). GSEA indicated that the PPAR signaling pathway was activated in livers of KO mice (Fig. 2I). Although our GSEA data support PPAR activation of Cd36, we cannot rule out the possibility that it is also up-regulated by PXR (29, 30) because PXR expression is increased in KO, and bile acids activate PXR (supplemental Fig. S5).
Metabolomic Analysis Indicates Impaired β-FAO in KO Mice
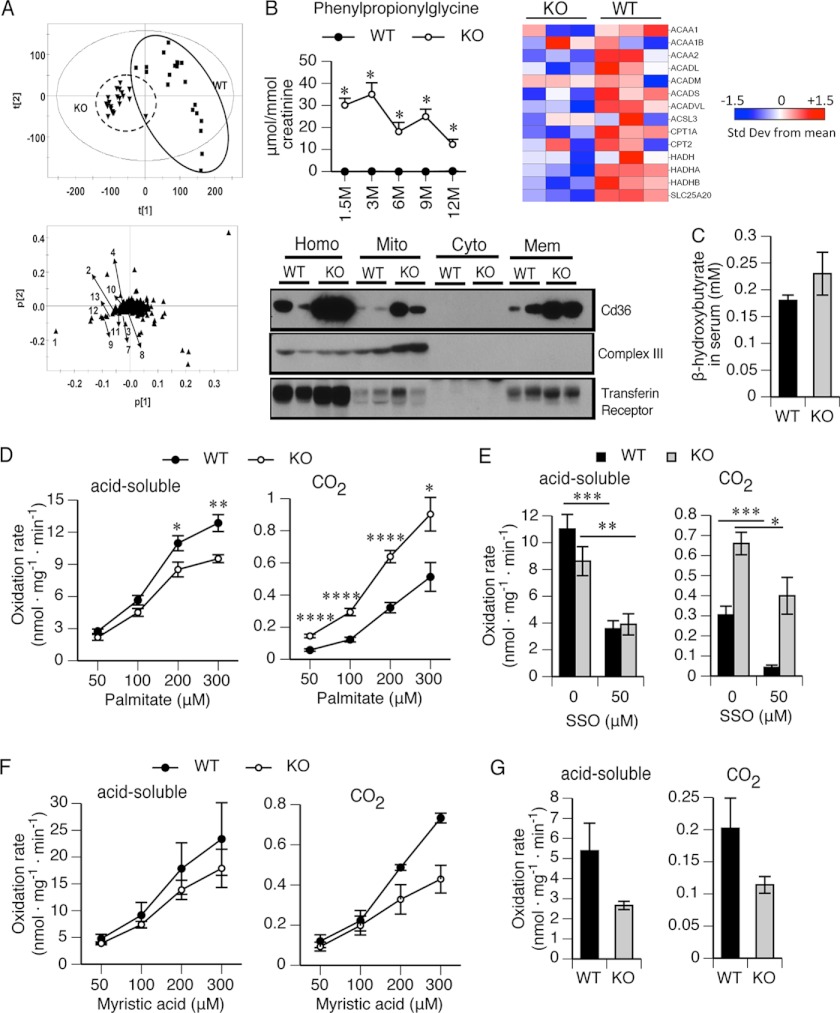
Based upon PPAR activation and elevated FFA levels, we hypothesized that fatty acid metabolism would be different in KO mice. To determine this, serum and urine samples (24 h) were collected from young mice (1.5 months) to old mice (12 months) housed in metabolic cages, and UPLC-ESI-QTOFMS was performed. Unsupervised PCA yielded good separation of the data sets from KO and WT mice (Fig. 3A, top), and specific metabolites readily distinguished WT and KO mice (supplemental Table S1). The loading plot (Fig. 3A, bottom) identified ions in the urine that provided the greatest separation between KO and WT mice. Although acylglycines are usually minor metabolites, large amounts of several fatty acid-derived acylglycine metabolites were readily detected in the urine of 1.5-month-old and older KO mice (Fig. 3B). Phenylpropionylglycine was mostly undetectable from WT mice but increased to more than 35 μmol/mmol creatinine in KO mice. Other acylglycine conjugates of β-FAO metabolites (hippuric acid, cinnamoylglycine, and phenylacetylglycine) were also elevated in KO mice (supplemental Fig. S6). Defective mitochondrial β-FAO is characterized by urinary excretion of phenylpropionylglycine, a β-FAO metabolite that defines medium-chain acyl-CoA dehydrogenase deficiency (31). The accumulation of these intermediate metabolites of β-FAO is consistent with down-regulation of mitochondrial β-FAO in the KO liver and supported by the GSEA analysis (Fig. 3B, right).
FIGURE 3.
Mitochondrial β-FAO in KO mice was impaired. A, identification of biomarkers of impaired fatty acid metabolism in urine using UPLC-ESI-QTOFMS-based metabolomics. Shown are a score scatter plot of a PCA model and PCA loading scatter plots of ions in urine of KO and WT mice at ages of 1.5–12 months. ▾, KO; ■, WT; ▴, an ion characterized by unique mass and retention time. The ions that contribute to the interclass difference were selected as candidate biomarkers. Some representative metabolites are labeled (arrows) in the loading scatter plots. B, highly elevated phenylpropionylglycine (left) was detected in urine of the KO mice at 1.5, 3, 6, 9, and 12 months. The concentration was normalized to creatinine concentration. Chlorpropamide (0.5 μm) was used as the internal standard. n = 3–6. Relative expression of hepatic mitochondrial β-FAO (right) genes is displayed. The liver fractions (bottom) were prepared by a series of centrifugations. Cd36 protein expression was determined by immunoblotting. Complex III and transferrin receptor served as markers of mitochondria and plasma membranes, respectively. Homo, whole homogenate; Mito, mitochondria; Cyto, cytosol; Mem; crude plasma membrane. C, serum was collected by cardiac puncture and used for β-hydroxybutyrate measurement. n = 6–7. D, fatty acid oxidation was measured by incubating liver homogenates with cold palmitate (50, 100, 200, and 300 μm) using 14C-labeled palmitate as tracer. The oxidation rates normalized to total hepatic protein were represented as acid-soluble metabolites and CO2. n = 6. E, palmitic acid (300 μm) oxidation rates were measured in liver homogenate with or without SSO (50 μm). The oxidation rates normalized to total hepatic protein were represented as acid-soluble metabolites and CO2. n = 4. F, oxidation of myristic acid (50, 100, 200, and 300 μm) was measured in liver homogenates using 14C-labeled myristic acid as tracer. The oxidation rates normalized to total hepatic protein were represented as acid-soluble metabolites and CO2. n = 3. G, peroxisomal oxidation of palmitate was measured in liver homogenates using inhibitors of mitochondrial β-FAO, malonyl-CoA (0.5 mm), glybenclamide (20 μm), and 3-mercaptoproprionic acid (0.8 mm). n = 3; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001. Error bars, S.E.
Cd36 is expressed at the plasma membrane and outer mitochondrial membrane, where it acts as a FA transporter (32–34), and Cd36 knock-out studies reveal its role in mitochondrial FA oxidation independent of Cpt1 activity (35). We determined Cd36 protein expression and localization by subcellular fractionation. In WT animals, Cd36 was primarily found in the plasma membranes (Fig. 3B, bottom). However, in the KO, a majority of Cd36 was in the mitochondria (supplemental Fig. S7). In contrast, although the overall level of Cd36 was increased (10-fold) in the KO (as revealed by the amount in liver homogenate (labeled Homo in Fig. 3), the proportion in the KO mitochondria was substantially elevated. We therefore reasoned that mitochondrial Cd36 might enhance FA uptake in the KO.
We hypothesized that the increased FA uptake in the liver would lead to an increased formation of acetyl-CoA and enhanced ketogenesis, producing an increase of β-hydroxybutyrate. Analysis of serum levels revealed increased concentrations of β-hydroxybutyrate in the KO (Fig. 3C).
Impaired Fatty Acid Metabolism in KO Mice Is Confirmed by in Vitro FAO Assays
Cd36-mediated FAO can be discriminated from Cpt1 by using palmitate (C16) and the Cd36-specific competitive inhibitor SSO. Mitochondrial oxidation of long-chain fatty acid was evaluated by using palmitate (C16) as a substrate in FAO assays. The rate of acid-soluble metabolite formation by β-FAO was significantly lower in KO mitochondria than in WT at all concentrations of palmitate (Fig. 3D, left). Although more than 90% of palmitate was oxidized to acid-soluble metabolites, ∼10% underwent complete oxidation to CO2 (Fig. 3D, right). Unexpectedly, palmitate oxidation to CO2 was significantly greater in the KO mice (Fig. 3D, right). We hypothesized that this discrepancy was caused by greater Cd36-mediated mitochondrial import of fatty acids in the KO mice (36). To test this, SSO was used in the same assays. The generation of acid-soluble metabolites was inhibited to a similar extent by SSO in WT and KO mice (Fig. 3E), consistent with the role of Cd36 in mitochondrial β-FAO. In contrast, inhibition of CO2 production from palmitate was significantly less in KO than in WT mice, with SSO showing an inhibition of 85% in WT mice but less than 40% in the KO mice (Fig. 3E). This differential effect of SSO in the KO liver suggests that elevated Cd36 initially facilitates FA uptake and oxidation in the KO. However, although FAO is generally reduced in the KO, mitochondrial fatty acid uptake strongly increases in the KO mitochondria as palmitate concentration increases from 50 to 200 μm (not shown). In total, our findings indicate that FAO is impaired in the KO. However, impaired β-FAO does not reflect a general disruption of mitochondrial function in the KO because the activity of components of the electron transport chain (complexes I, III, and IV) is not reduced (supplemental Fig. S8).
To further elucidate the basis for the defective FAO, myristic acid was used as a FAO substrate because previous studies successfully used it to interrogate mitochondrial medium-chain FAO (18). The overall FA oxidation rate of myristic acid was lower in KO, both in terms of production of acid-soluble metabolites and CO2, compared with that in WT (Fig. 3F). In total, these findings, coupled with the FA gene expression data and the accumulation of FA metabolites, indicate that for the liver, the primary defect is medium-chain acyl-CoA dehydrogenase oxidation deficiency.
In addition to the mitochondria, peroxisomes also metabolize fatty acids. Peroxisomal FAO was tested in liver extracts in the presence of Cpt1 inhibitors: malonyl-CoA, glibenclamide, and 3-mercaptoproprionic acid (Fig. 3G). Peroxisomal FAO was lower in the KO liver homogenate when using a mitochondrial β-FAO inhibitor mixture in in vitro assays.
ω-FAO in the Endoplasmic Reticulum in KO Mice Is Up-regulated
In the normal basal state, 90% of short- and medium-chain FAs are oxidized in the mitochondria, and the remaining 10% are oxidized in the peroxisomes and microsomes. When mitochondrial β-FAO is impaired, alternative metabolism of FAs, such as by ω-oxidation (ω-FAO), occurs in the endoplasmic reticulum by Cyp4a family members (37) This produces intermediate chain length FA metabolites (medium-chain (C7–14) dicarboxylic acids) (38–40), which were identified by a scatterplot of metabolite ions and a loading plot of sera from KO and WT mice (Fig. 4A). Quantification revealed strong accumulation of suberic acid (C8), azelaic acid (C9), pimelic acid (C7), sebacic acid (C10), and undecanediocic acid (C11) in the knockout (Fig. 4B). However, a notable feature of the kinetics of these metabolites is the absence of a difference between WT and KO at 1.5 months. Because Cyp4a12 is elevated at 1.5 months at the mRNA (Fig. 4C) and protein (Fig. 4D) level, this finding supports the idea that ω-FAO only occurs after the accumulation of fatty acid intermediates that are substrates for Cyp4a12.
FIGURE 4.
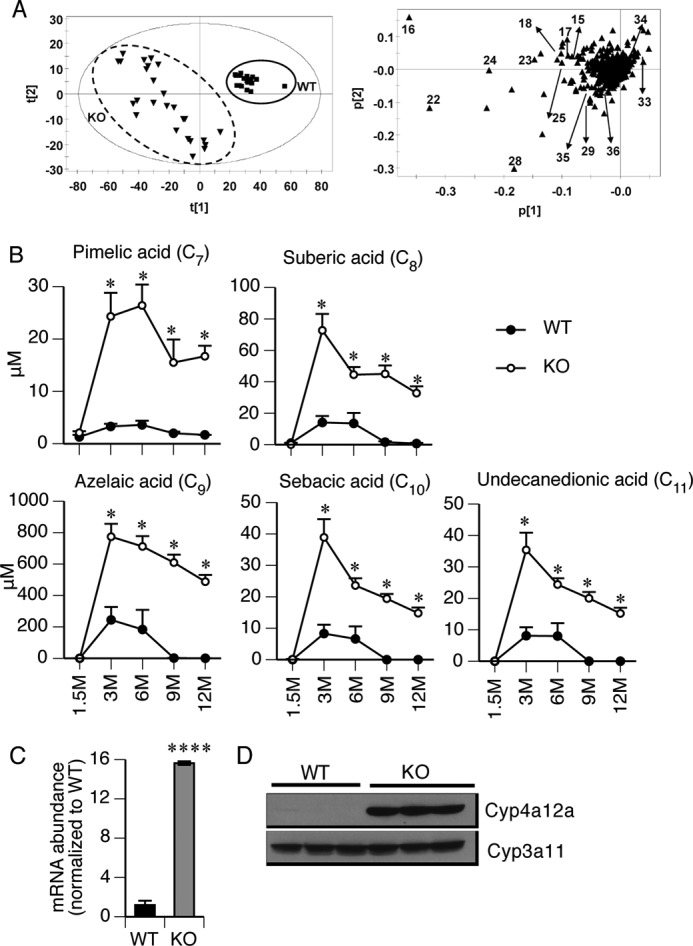
Microsomal ω-oxidation of fatty acid was activated in KO mice. A, identification of biomarkers in serum using UPLC-ESI-QTOFMS-based metabolomics. Shown are a score scatter plot of a PCA model and PCA loading scatter plots of ions in serum of WT and KO mice at 1.5–12 months of age. ▾, KO; ■, WT; ▴, an ion characterized by unique mass and retention time. The ions that contribute to the interclass difference were selected as candidate biomarkers. Some representative metabolites are labeled (arrows) in the loading scatter plots. B, metabolomic analysis detected highly elevated concentrations of five medium-chain dicarboxylic acids in the KO serum at 3–12 months of age. n = 3–6. C and D, expression of Cyp4a12a mRNA and protein in the liver tissues was measured by qPCR and immunoblotting, respectively. Cyp3a11 served as a loading control in the immunoblotting assay. *, p ≤ 0.05; ****, p ≤ 0.0001. Error bars, S.E.
Glycolysis Is Down-regulated in the KO Mice
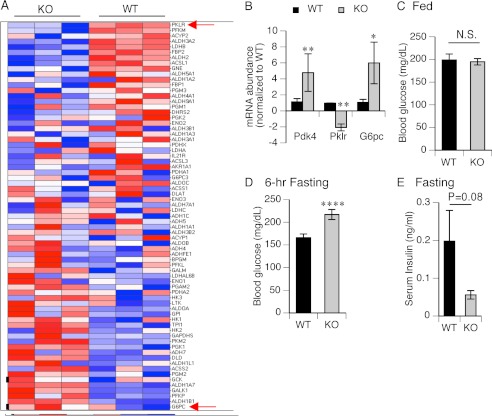
We hypothesized that the early switch in FFA oxidation in KO might be due to a glycolytic shift because BA can alter glyconeogenic and glycolytic regulatory genes (13). The heat map revealed a markedly different pattern of glycolytic/gluconeogenic gene expression in WT and KO mice (Fig. 5A). Among the genes, a rate-limiting enzyme of glycolysis, Pklr, was decreased, whereas a rate-limiting enzyme of gluconeogenesis, G6pc, increased. This was confirmed by qPCR and also demonstrated that pyruvate dehydrogenase kinase, Pdk4, a gene that phosphorylates PDH and reduces oxidative glucose metabolism, was up-regulated 4-fold in KO mice and is a reported FXR target (41) (Fig. 5B). These gene changes might be expected to preserve glucose levels and support a switch in hepatic glucose metabolism.
FIGURE 5.
Alteration of glycolysis/gluconeogenesis in the KO liver. A, heat map display of 71 genes within the glycolysis/gluconeogenesis pathway. Genes are ordered by average differential expression between KO and WT samples. The positions of Pklr and G6pc are indicated by arrows and represent the most down-regulated and up-regulated genes within the pathway, respectively. B, expression of mRNAs of Pdk4, Pklr, and G6pc, was detected by qPCR. Values are -fold changes after normalization to WT. Positive values represent increase, and negative represent decrease. n = 3. C and D, blood glucose levels were measured at the fed condition and after 6 h of fasting. n = 5–7. E, serum insulin was measured after overnight fasting. n = 5–7. N.S, no significance; ****, p ≤ 0.0001. Error bars, S.E.
To test this possibility, blood glucose levels were measured between fed and fasting state. Blood glucose levels were no different between WT and KO mice under fed conditions (Fig. 5C). However, in KO mice, after 6 h of fasting, blood glucose was substantially higher than that in WT mice (Fig. 5D). The reduction in serum insulin levels under fasting conditions in the KO is consistent with this finding.
Greater Oxidative Stress in the KO Mouse Liver
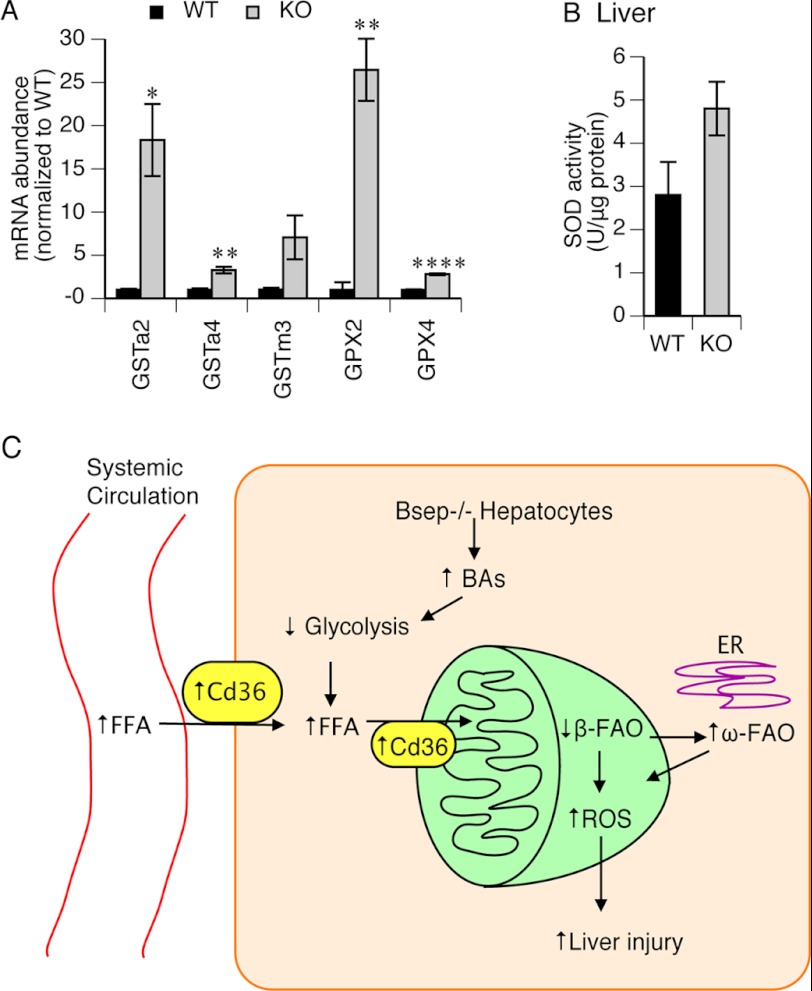
Recent studies indicate that mitochondrial long chain FA metabolites generate reactive oxygen species (42). Because of the high level of FA metabolites formed in Abcb11 deficiency, we hypothesized that the transcription factor, Nrf2 a sensor of intracellular reactive oxygen species, would be activated. Nrf2 activation can be revealed by up-regulation of a series of anti-oxidative stress genes, including glutathione pathway genes (43–45). Expression of Gst isoforms (Gsta2, Gsta4, and Gstm3) and Gpx isoforms (Gpx2 and Gpx4) was increased dramatically in the KO liver (Fig. 6A). Hepatic SOD activity was also increased in the KO mice (Fig. 6B). These findings indicate greater oxidative stress in the KO mouse liver.
FIGURE 6.
Oxidative stress responses were increased in KO liver. A, expression of hepatic isoforms of Gst and Gpx were detected by qPCR. Values represent -fold change after normalization to WT. n = 3. B, SOD activity was measured with the liver homogenate. n = 3. *, p ≤ 0.05; **, p ≤ 0.01; ****, p ≤ 0.0001. C, schematic model of changes leading to liver injury in the KO mice. In the C57BL/6 mice, BSEP deficiency induced progressive intrahepatic cholestasis and accumulation of hepatic bile acid, which leads to a metabolic change, a decrease in glycolysis. Increased expression of Cd36 on cytoplasmic and mitochondrial membranes results in accumulation of free fatty acids in mitochondria, which impairs mitochondrial β-fatty acid oxidation. Microsomal ω-fatty acid oxidation is highly activated following impaired mitochondrial β-fatty acid oxidation. As a result, intracellular ROS is accumulated and causes liver injury and eventually leads to liver inflammation, necrosis, and fibrosis. Error bars, S.E.
DISCUSSION
In humans, ABCB11 deficiency disrupts bile salt homeostasis, producing pathophysiologic changes that have been primarily attributed to hepatic bile acids (46). Here we describe for the first time an Abcb11-deficient mouse model that recapitulates PFIC2 in humans (i.e. KO (Abcb11 KO C57BL/6) mice showed progressive accumulation of hepatic bile acids and progressive liver injury under normal dietary conditions). In contrast, the Abcb11-null mice generated on the mixed background only exhibited mild, nonprogressive liver injury (10), which was explained by elevation of tetrahydroxylated bile acids (47) and up-regulation of compensatory bile acid efflux transporters (9). However, in this Abcb11 KO C57BL/6 mouse model, the increased formation of tetrahydroxylated bile acids and up-regulation of efflux transporters are not sufficient to alleviate the progressive hepatic damage induced by bile acid overload. To elucidate the mechanism of liver damage in KO mice, hepatic gene expression and metabolomic analyses were conducted in mice at 1.5 months, an age when the KO mice showed no histologic evidence of hepatic damage but accumulated bile acids.
In this study, hepatic accumulation of BAs was associated with activation of the FXR-signaling pathway. For example, Shp, a direct target of FXR (48), was transcriptionally up-regulated in 1.5-month-old KO mice (supplemental Fig. S9), consistent with the down-regulation of BA biosynthetic genes Cyp7a and Cyp7b. Although the BA exporters Ostα and Ostβ were up-regulated, consistent with FXR activation (49), they did not prevent BA accumulation or counteract hepatic BA overaccumulation in the absence of Abcb11.
Prominent changes in hepatic gene expression suggested that KO mice undergo a metabolic switch before the onset of liver damage. The reduced Pklr and increased Pdk4 (a FXR target (41)) and G6pc support this proposition and suggest deregulation of the glycolysis/gluconeogenesis pathway in the KO, which is further supported by elevated blood glucose under fasting conditions in the KO mice. Notably, a genome-wide association study found that altered fasting glucose levels were related to an SNP between ABCB11 and G6P catalytic subunit 2 (50, 51). These findings suggest that human deficiencies in ABCB11 contribute to impaired glucose regulation.
The Randle hypothesis (52) proposed that the competition between free fatty acids and glucose for mitochondrial oxidation reduces glucose utilization. We speculate that glucose utilization in the KO liver is further deregulated by the increased uptake and metabolism of free fatty acids. This hypothesis is supported by the reduction of white adipose mass, increased serum free fatty acids, and increased formation of fatty acid intermediary metabolites in the KO mice. This could occur because KO hepatocytes have an enhanced capacity to import free fatty acids secondary to increased expression of the fatty acid transporter protein, Cd36, and the intracellular fatty acid chaperone, Fabp4. Together with up-regulated hepatic lipolysis due to increased expression of Lpl and its activator Apoc II, the KO hepatocytes become overloaded with fatty acids. Likewise, in nonalcoholic fatty liver disease, fatty acids are excessive in the liver, leading to dramatic alteration of lipid metabolism by altering fatty acid oxidation (25).
Free fatty acids induce lipoapoptosis in hepatocytes and may impair FAO and cause hepatic damage (53, 54). Nonesterified saturated free fatty acids, such as palmitate, are toxic to hepatocytes; thus, in Abcb11 deficiency, elevation of FFAs may produce liver damage secondary to elevation in reactive oxygen species (ROS). Nrf2 has been recognized as a sensor of ROS (43, 44). Activation of Nrf2 by ROS induces a series of antioxidant genes, such as glutathione S-transferases and superoxide dismutases. Consistent with an increase in ROS, livers from KO mice show up-regulation in a suite of Nrf2 target genes (Fig. 6). The source of the ROS is possibly an enhanced flux of fatty acids through the mitochondria, but it may also be due to activation of microsomal ω-FAO in the KO liver because microsomal ω-FAO is a potent source of ROS (55).
We propose that in Abcb11 deficiency, elevated bile acids produce a change in metabolic state (Fig. 6C) by disrupting glycolysis and gluconeogenesis. A consequence is an increase in reliance upon fatty acids. However, defects in fatty acid oxidation lead to cellular changes that cooperate with the detergent characteristics of BAs to induce liver damage. Importantly, this paper provides not just new metabolic biomarkers that precede cholestatic liver injury but a mechanism that could be extrapolated to other cholestatic conditions (e.g. drug-induced) that arise secondary to inhibition or loss of ABCB11 function.
Acknowledgments
We thank Sharon Naron and Angela McArthur for excellent editorial assistance and the Schuetz laboratory for critical review of the manuscript. We thank Sharon Frase in the St. Jude Imaging Core for the liver EM.
This work was supported, in whole or in part, by National Institutes of Health Grants 2R01-GM60904, R21HL114066, P30 CA21745, and CA21865. This work was also supported by the American Lebanese Syrian Associated Charities.

This article contains supplemental Materials and Methods, Table S1, and Figs. S1–S9.
- BSEP
- bile salt export pump
- PFIC2
- progressive familial intrahepatic cholestasis type 2
- BRIC2
- benign recurrent intrahepatic cholestasis type 2
- GSEA
- gene set enrichment analysis
- PCA
- principal component analysis
- FXR
- farnesoid X receptor
- FAO
- fatty acid oxidation
- SSO
- sulfo-N-succinimidyl oleate
- UPLC
- ultraperformance liquid chromatography
- ESI
- electrospray ionization
- QTOFMS
- quadropole time-of-flight mass spectrometry
- β-MCA
- β-muricholic acid
- qPCR
- quantitative real-time PCR
- FA
- fatty acid
- FFA
- free fatty acid
- ROS
- reactive oxygen species.
REFERENCES
- 1. Chiang J. Y. (2009) Bile acids. Regulation of synthesis. J. Lipid Res. 50, 1955–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Trauner M., Boyer J. L. (2003) Bile salt transporters. Molecular characterization, function, and regulation. Physiol. Rev. 83, 633–671 [DOI] [PubMed] [Google Scholar]
- 3. Hayashi H., Takada T., Suzuki H., Akita H., Sugiyama Y. (2005) Two common PFIC2 mutations are associated with the impaired membrane trafficking of BSEP/ABCB11. Hepatology 41, 916–924 [DOI] [PubMed] [Google Scholar]
- 4. Noe J., Kullak-Ublick G. A., Jochum W., Stieger B., Kerb R., Haberl M., Müllhaupt B., Meier P. J., Pauli-Magnus C. (2005) Impaired expression and function of the bile salt export pump due to three novel ABCB11 mutations in intrahepatic cholestasis. J. Hepatol. 43, 536–543 [DOI] [PubMed] [Google Scholar]
- 5. Stieger B., Meier Y., Meier P. J. (2007) The bile salt export pump. Pflugers Arch. 453, 611–620 [DOI] [PubMed] [Google Scholar]
- 6. Lam P., Pearson C. L., Soroka C. J., Xu S., Mennone A., Boyer J. L. (2007) Levels of plasma membrane expression in progressive and benign mutations of the bile salt export pump (Bsep/Abcb11) correlate with severity of cholestatic diseases. Am. J. Physiol. Cell Physiol. 293, C1709–C1716 [DOI] [PubMed] [Google Scholar]
- 7. Jansen P. L., Strautnieks S. S., Jacquemin E., Hadchouel M., Sokal E. M., Hooiveld G. J., Koning J. H., De Jager-Krikken A., Kuipers F., Stellaard F., Bijleveld C. M., Gouw A., Van Goor H., Thompson R. J., Müller M. (1999) Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology 117, 1370–1379 [DOI] [PubMed] [Google Scholar]
- 8. Knisely A. S., Strautnieks S. S., Meier Y., Stieger B., Byrne J. A., Portmann B. C., Bull L. N., Pawlikowska L., Bilezikçi B., Ozçay F., László A., Tiszlavicz L., Moore L., Raftos J., Arnell H., Fischler B., Németh A., Papadogiannakis N., Cielecka-Kuszyk J., Jankowska I., Pawłowska J., Melín-Aldana H., Emerick K. M., Whitington P. F., Mieli-Vergani G., Thompson R. J. (2006) Hepatocellular carcinoma in 10 children under 5 years of age with bile salt export pump deficiency. Hepatology 44, 478–486 [DOI] [PubMed] [Google Scholar]
- 9. Lam P., Wang R., Ling V. (2005) Bile acid transport in sister of P-glycoprotein (ABCB11) knockout mice. Biochemistry 44, 12598–12605 [DOI] [PubMed] [Google Scholar]
- 10. Wang R., Salem M., Yousef I. M., Tuchweber B., Lam P., Childs S. J., Helgason C. D., Ackerley C., Phillips M. J., Ling V. (2001) Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc. Natl. Acad. Sci. U.S.A. 98, 2011–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang W., Ma K., Zhang J., Qatanani M., Cuvillier J., Liu J., Dong B., Huang X., Moore D. D. (2006) Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312, 233–236 [DOI] [PubMed] [Google Scholar]
- 12. Houten S. M., Watanabe M., Auwerx J. (2006) Endocrine functions of bile acids. EMBO J. 25, 1419–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma K., Saha P. K., Chan L., Moore D. D. (2006) Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Invest. 116, 1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S., Mesirov J. P. (2005) Gene set enrichment analysis. A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Irizarry R. A., Hobbs B., Collin F., Beazer-Barclay Y. D., Antonellis K. J., Scherf U., Speed T. P. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 [DOI] [PubMed] [Google Scholar]
- 16. Cho J. Y., Matsubara T., Kang D. W., Ahn S. H., Krausz K. W., Idle J. R., Luecke H., Gonzalez F. J. (2010) Urinary metabolomics in Fxr-null mice reveals activated adaptive metabolic pathways upon bile acid challenge. J. Lipid Res. 51, 1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hirschey M. D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D. B., Grueter C. A., Harris C., Biddinger S., Ilkayeva O. R., Stevens R. D., Li Y., Saha A. K., Ruderman N. B., Bain J. R., Newgard C. B., Farese R. V., Jr., Alt F. W., Kahn C. R., Verdin E. (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Narayan S. B., Boriack R. L., Messmer B., Bennett M. J. (2005) Establishing a reference interval for measurement of flux through the mitochondrial fatty acid oxidation pathway in cultured skin fibroblasts. Clin. Chem. 51, 644–646 [DOI] [PubMed] [Google Scholar]
- 19. Evason K., Bove K. E., Finegold M. J., Knisely A. S., Rhee S., Rosenthal P., Miethke A. G., Karpen S. J., Ferrell L. D., Kim G. E. (2011) Morphologic findings in progressive familial intrahepatic cholestasis 2 (PFIC2). Correlation with genetic and immunohistochemical studies. Am. J. Surg Pathol. 35, 687–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hofmann A. F., Small D. M. (1967) Detergent properties of bile salts. Correlation with physiological function. Annu. Rev. Med. 18, 333–376 [DOI] [PubMed] [Google Scholar]
- 21. Mühlfeld A., Kubitz R., Dransfeld O., Häussinger D., Wettstein M. (2003) Taurine supplementation induces multidrug resistance protein 2 and bile salt export pump expression in rats and prevents endotoxin-induced cholestasis. Arch. Biochem. Biophys. 413, 32–40 [DOI] [PubMed] [Google Scholar]
- 22. Timbrell J. A., Seabra V., Waterfield C. J. (1995) The in vivo and in vitro protective properties of taurine. Gen. Pharmacol. 26, 453–462 [DOI] [PubMed] [Google Scholar]
- 23. Wang R., Lam P., Liu L., Forrest D., Yousef I. M., Mignault D., Phillips M. J., Ling V. (2003) Severe cholestasis induced by cholic acid feeding in knockout mice of sister of P-glycoprotein. Hepatology 38, 1489–1499 [DOI] [PubMed] [Google Scholar]
- 24. Falany C. N., Johnson M. R., Barnes S., Diasio R. B. (1994) Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J. Biol. Chem. 269, 19375–19379 [PubMed] [Google Scholar]
- 25. Malhi H., Gores G. J. (2008) Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin. Liver Dis. 28, 360–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malhi H., Gores G. J. (2008) Cellular and molecular mechanisms of liver injury. Gastroenterology 134, 1641–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Desvergne B., Michalik L., Wahli W. (2006) Transcriptional regulation of metabolism. Physiol. Rev. 86, 465–514 [DOI] [PubMed] [Google Scholar]
- 28. Desvergne B. (2007) PPARs special issue. Anchoring the present to explore the future. Biochim. Biophys. Acta 1771, 913–914 [DOI] [PubMed] [Google Scholar]
- 29. Zhou J., Febbraio M., Wada T., Zhai Y., Kuruba R., He J., Lee J. H., Khadem S., Ren S., Li S., Silverstein R. L., Xie W. (2008) Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARγ in promoting steatosis. Gastroenterology 134, 556–567 [DOI] [PubMed] [Google Scholar]
- 30. Zhou J., Zhai Y., Mu Y., Gong H., Uppal H., Toma D., Ren S., Evans R. M., Xie W. (2006) A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J. Biol. Chem. 281, 15013–15020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rinaldo P., O'Shea J. J., Coates P. M., Hale D. E., Stanley C. A., Tanaka K. (1988) Medium-chain acyl-CoA dehydrogenase deficiency. Diagnosis by stable-isotope dilution measurement of urinary n-hexanoylglycine and 3-phenylpropionylglycine. N. Engl. J. Med. 319, 1308–1313 [DOI] [PubMed] [Google Scholar]
- 32. Smith B. K., Jain S. S., Rimbaud S., Dam A., Quadrilatero J., Ventura-Clapier R., Bonen A., Holloway G. P. (2011) FAT/CD36 is located on the outer mitochondrial membrane, upstream of long-chain acyl-CoA synthetase, and regulates palmitate oxidation. Biochem. J. 437, 125–134 [DOI] [PubMed] [Google Scholar]
- 33. Talanian J. L., Holloway G. P., Snook L. A., Heigenhauser G. J., Bonen A., Spriet L. L. (2010) Exercise training increases sarcolemmal and mitochondrial fatty acid transport proteins in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 299, E180–E188 [DOI] [PubMed] [Google Scholar]
- 34. Abumrad N. A., el-Maghrabi M. R., Amri E. Z., Lopez E., Grimaldi P. A. (1993) Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 268, 17665–17668 [PubMed] [Google Scholar]
- 35. Holloway G. P., Jain S. S., Bezaire V., Han X. X., Glatz J. F., Luiken J. J., Harper M. E., Bonen A. (2009) FAT/CD36-null mice reveal that mitochondrial FAT/CD36 is required to up-regulate mitochondrial fatty acid oxidation in contracting muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R960–R967 [DOI] [PubMed] [Google Scholar]
- 36. Holloway G. P., Snook L. A., Harris R. J., Glatz J. F., Luiken J. J., Bonen A. (2011) In obese Zucker rats, lipids accumulate in the heart despite normal mitochondrial content, morphology, and long-chain fatty acid oxidation. J. Physiol. 589, 169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hardwick J. P., Osei-Hyiaman D., Wiland H., Abdelmegeed M. A., Song B. J. (2009) PPAR/RXR regulation of fatty acid metabolism and fatty acid ω-hydroxylase (CYP4) isozymes. Implications for prevention of lipotoxicity in fatty liver disease. PPAR Res. 2009, 952734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Preiss B., Bloch K. (1964) ω-Oxidation of long chain fatty acids in rat liver. J. Biol. Chem. 239, 85–88 [PubMed] [Google Scholar]
- 39. Pettersen J. E., Jellum E., Eldjarn L. (1972) The occurrence of adipic and suberic acid in urine from ketotic patients. Clin. Chim. Acta 38, 17–24 [DOI] [PubMed] [Google Scholar]
- 40. Grego A. V., Mingrone G. (1995) Dicarboxylic acids, an alternate fuel substrate in parenteral nutrition. An update. Clin. Nutr. 14, 143–148 [DOI] [PubMed] [Google Scholar]
- 41. Savkur R. S., Bramlett K. S., Michael L. F., Burris T. P. (2005) Regulation of pyruvate dehydrogenase kinase expression by the farnesoid X receptor. Biochem. Biophys. Res. Commun. 329, 391–396 [DOI] [PubMed] [Google Scholar]
- 42. Seifert E. L., Estey C., Xuan J. Y., Harper M. E. (2010) Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J. Biol. Chem. 285, 5748–5758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cho H. Y., Reddy S. P., Kleeberger S. R. (2006) Nrf2 defends the lung from oxidative stress. Antioxid. Redox Signal. 8, 76–87 [DOI] [PubMed] [Google Scholar]
- 44. Cho H. Y., Reddy S. P., Yamamoto M., Kleeberger S. R. (2004) The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 18, 1258–1260 [DOI] [PubMed] [Google Scholar]
- 45. Venugopal R., Jaiswal A. K. (1998) Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene 17, 3145–3156 [DOI] [PubMed] [Google Scholar]
- 46. Kagawa T., Watanabe N., Mochizuki K., Numari A., Ikeno Y., Itoh J., Tanaka H., Arias I. M., Mine T. (2008) Phenotypic differences in PFIC2 and BRIC2 correlate with protein stability of mutant Bsep and impaired taurocholate secretion in MDCK II cells. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G58–G67 [DOI] [PubMed] [Google Scholar]
- 47. Perwaiz S., Forrest D., Mignault D., Tuchweber B., Phillip M. J., Wang R., Ling V., Yousef I. M. (2003) Appearance of atypical 3α,6β,7β,12α-tetrahydroxy-5β-cholan-24-oic acid in spgp knockout mice. J. Lipid Res. 44, 494–502 [DOI] [PubMed] [Google Scholar]
- 48. Lu T. T., Makishima M., Repa J. J., Schoonjans K., Kerr T. A., Auwerx J., Mangelsdorf D. J. (2000) Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 6, 507–515 [DOI] [PubMed] [Google Scholar]
- 49. Lee F. Y., Lee H., Hubbert M. L., Edwards P. A., Zhang Y. (2006) FXR, a multipurpose nuclear receptor. Trends Biochem. Sci. 31, 572–580 [DOI] [PubMed] [Google Scholar]
- 50. Takeuchi F., Katsuya T., Chakrewarthy S., Yamamoto K., Fujioka A., Serizawa M., Fujisawa T., Nakashima E., Ohnaka K., Ikegami H., Sugiyama T., Nabika T., Kasturiratne A., Yamaguchi S., Kono S., Takayanagi R., Yamori Y., Kobayashi S., Ogihara T., de Silva A., Wickremasinghe R., Kato N. (2010) Common variants at the GCK, GCKR, G6PC2-ABCB11, and MTNR1B loci are associated with fasting glucose in two Asian populations. Diabetologia 53, 299–308 [DOI] [PubMed] [Google Scholar]
- 51. Chen W. M., Erdos M. R., Jackson A. U., Saxena R., Sanna S., Silver K. D., Timpson N. J., Hansen T., Orrù M., Grazia Piras M., Bonnycastle L. L., Willer C. J., Lyssenko V., Shen H., Kuusisto J., Ebrahim S., Sestu N., Duren W. L., Spada M. C., Stringham H. M., Scott L. J., Olla N., Swift A. J., Najjar S., Mitchell B. D., Lawlor D. A., Smith G. D., Ben-Shlomo Y., Andersen G., Borch-Johnsen K., Jørgensen T., Saramies J., Valle T. T., Buchanan T. A., Shuldiner A. R., Lakatta E., Bergman R. N., Uda M., Tuomilehto J., Pedersen O., Cao A., Groop L., Mohlke K. L., Laakso M., Schlessinger D., Collins F. S., Altshuler D., Abecasis G. R., Boehnke M., Scuteri A., Watanabe R. M. (2008) Variations in the G6PC2/ABCB11 genomic region are associated with fasting glucose levels. J. Clin. Invest. 118, 2620–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Randle P. J. (1998) Regulatory interactions between lipids and carbohydrates. The glucose fatty acid cycle after 35 years. Diabetes Metab. Rev. 14, 263–283 [DOI] [PubMed] [Google Scholar]
- 53. Fromenty B., Berson A., Pessayre D. (1997) Microvesicular steatosis and steatohepatitis. Role of mitochondrial dysfunction and lipid peroxidation. J. Hepatol. 26, Suppl. 1, 13–22 [DOI] [PubMed] [Google Scholar]
- 54. Schönfeld P., Wojtczak L. (2008) Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic. Biol. Med. 45, 231–241 [DOI] [PubMed] [Google Scholar]
- 55. Lewis D. F., Sheridan G. (2001) Cytochromes P450, oxygen, and evolution. ScientificWorldJournal 1, 151–167 [DOI] [PMC free article] [PubMed] [Google Scholar]