Background: Defining molecular mechanisms that regulate AR activity is critical for understanding prostate cancer progression.
Results: CaMKK2 increases during disease progression, is transcriptionally regulated by the AR, promotes proliferation, and is required for optimal AR transcriptional activity.
Conclusion: CaMKK2 is in a feedback circuit to maintain AR activity.
Significance: The CaMKK2 pathway is a promising target for prostate cancer therapy.
Keywords: Androgen Receptor, Calcium, Calcium Calmodulin-dependent Protein Kinase (CaMK), Calmodulin, Prostate Cancer
Abstract
The androgen receptor (AR) plays a critical role in prostate cancer (PCa) progression, however, the molecular mechanisms by which the AR regulates cell proliferation in androgen-dependent and castration-resistant PCa are incompletely understood. We report that Ca2+/calmodulin-dependent kinase kinase 2 (CaMKK2) expression increases and becomes nuclear or perinuclear in advanced PCa. In the TRAMP (transgenic adenocarcinoma of mouse prostate) model of PCa, CaMKK2 expression increases with PCa progression with many cells exhibiting nuclear staining. CaMKK2 expression is higher in human castration-resistant tumor xenografts compared with androgen-responsive xenografts and is markedly higher in the AR-expressing, tumorigenic cell line LNCaP compared with cell lines that are AR-nonexpressing and/or nontumorigenic. In LNCaP cells, dihydrotestosterone induced CaMKK2 mRNA and protein expression and translocation of CaMKK2 to the nucleus. Conversely, androgen withdrawal suppressed CaMKK2 expression. Knockdown of CaMKK2 expression by RNAi reduced LNCaP cell proliferation and increased percentages of cells in G1 phase, whereas correspondingly reducing percentages in S phase, of the cell cycle. CaMKK2 knockdown reduced expression of the AR target gene prostate-specific antigen at both mRNA and protein levels, AR transcriptional activity driven by androgen responsive elements from the prostate-specific probasin gene promoter and levels of the AR-regulated cell cycle proteins, cyclin D1 and hyperphosphorylated Rb. Our results suggest that in PCa progression, CaMKK2 and the AR are in a feedback loop in which CaMKK2 is induced by the AR to maintain AR activity, AR-dependent cell cycle control, and continued cell proliferation.
Introduction
Work from multiple laboratories including our own, has delineated a signaling pathway termed the Ca2+/calmodulin (CaM)-dependent kinase cascade (1). At its top level, the CaM kinase cascade consists of Ca2+/CaM-dependent kinase kinases (CaMKKs) 1(α) and 2(β) (2–5). The downstream kinases phosphorylated and activated by CaMKK1 and/or CaMKK2 include the Ca2+/CaM-dependent protein kinases, CaMKI and CaMKIV, as “primary” targets and the non-Ca2+-requiring kinases, Akt, and 5′-AMP activated kinase as “secondary” targets. The latter two are so named because they are also activated by distinct upstream kinase kinases, PDK1 and LKB1, respectively (6–11). Upon activation, the downstream kinases regulate a remarkably broad spectrum of physiological processes including: gene transcription, mRNA translation, adaptation to cellular stress, tissue morphogenesis, cell cycle control, cell survival, and proliferation (1, 12, 13).
Early studies demonstrated that this signaling pathway can be triggered by elevations of intracellular Ca2+ in accord with the idea that the CaM kinase cascade is a cellular Ca2+-transducing mechanism (14, 15). The CaMKKs have been studied most intensively in relationship to the function and development of excitable cells such as neurons, however, certain characteristics of the pathway raise the possibility that the arm of the CaM kinase cascade triggered by CaMKK2 (referred to here as the CaMKK2 pathway) may have a unique role(s) in nonexcitable cells (13). First, although present at much lower levels than in the CNS, CaMKK2 is expressed in many tissues and cell types (1, 5). Second, whereas CaMKK1 is largely dependent for its activity on the presence of Ca2+/CaM, CaMKK2 shows considerable activity (∼70%) in the absence of Ca2+/CaM (5, 16). Recent studies have reported that the Ca2+/CaM autonomous activity of CaMKK2 is regulated by Ca2+/CaM-independent intramolecular autophosphorylation or by the non-Ca2+ requiring kinases, GSK3β and CDK5, respectively (16, 17). Although future studies will be required to fully elucidate the mechanism by which CaMKK2 activity is regulated, it may be reasonably concluded that CaMKK2 has robust constitutive activity and therefore is potentially capable of responding to stimuli of longer duration than predicted from the rapid kinetics of Ca2+ fluxes. Finally, CaMKK2 but not CaMKK1 is a regulator of the secondary targets, Akt and 5′-AMP activated kinase and therefore may have more diverse downstream targets than the latter (8–11, 18).
Based on their tissue distributions, involvement of either of the CaMKKs in the physiology or pathophysiology of the prostate gland was initially unappreciated (5). However, numerous large scale gene expression profiling, and proteomic studies have identified CaMKK2 as expressed at high levels in prostate cancer (PCa)2 (19–31). Individual studies reported CaMKK2 to be up-regulated in the transition from prostatic intraepithelial neoplasia (PIN) to PCa (22) or to be increased in metastatic samples relative to benign prostate (27). CaMKK2 mRNA expression in LNCaP PCa cells is increased after exposure to the synthetic androgen R1881, an effect shown to be mediated by a functional androgen-responsive element (ARE) in the CaMKK2 gene promoter (32, 33). CaMKK2 has been identified as a feature of an AR signature, a potential predictor of AR activity over the course of therapy for PCa, an observation of potential significance given the requirement of AR signaling activity for PCa progression (34, 35).
The evolution of PCa from early stages to advanced disease involves a complex series of events (35). Although suppression of AR activity by chemical or surgical castration (androgen deprivation therapy) is initially effective in producing disease remission, eventually tumors recur, a state termed castration resistant (CR) PCa (CRPC). In the latter state, AR activity has been restored to compensate for the reduction in systemic androgen and remains critical for tumor progression. Given the reported association of CaMKK2 with PCa and AR signaling, we undertook the work reported here with three aims: to provide insight into how the expression of CaMKK2 is related to PCa progression, to examine the regulation of CaMKK2 expression by the AR, and to place this mechanism in the context of AR-dependent control of PCa cell proliferation. We describe here a novel regulatory feedback loop in which the AR feeds forward to induce CaMKK2 expression and CaMKK2 in turn, feeds back to positively regulate the transcriptional activity of the AR. We propose that this positive feedback signaling circuit represents a means by which the AR regulates PCa cell cycling and growth in the progression to prostatic malignancy.
EXPERIMENTAL PROCEDURES
Chemicals
7H-Benzimidazol[2,1a]benz[de]isoquinoline-7-one-3-carboxylic acid (STO-609) was purchased from Tocris. Casodex (bicalutamide) and dimethyl sulfoxide were purchased from Sigma.
Cell Culture
Cell culture components were obtained from the following sources: RPMI 1640 and phenol-free RPMI 1640 medium (Invitrogen); F12-K and Eagle's minimum essential medium (ATCC); FBS (Atlanta Biologicals); l-glutamine, sodium pyruvate, penicillin/streptomycin, keratinocyte serum-free medium, human recombinant EGF, and bovine pituitary extract (Invitrogen); HEPES buffer, d-glucose (Fisher Scientific); and steroid-depleted (charcoal-stripped, CS) FBS (Gemini Bio-Products).
LNCaP cells were cultured in RPMI 1640 media supplemented with 10% FBS, 1% l-glutamine, 10 mm HEPES buffer, 1 mm sodium pyruvate, 1× penicillin/streptomycin, and 2.4 mg/ml of glucose. PC3 cells were routinely cultured in F12-K media supplemented with 10% FBS and 1× penicillin/streptomycin. DU145 cells were grown in Eagle's minimum essential medium supplemented with 10% FBS and 1× penicillin/streptomycin. RWPE-1 prostate epithelial cells were cultured in serum-free medium supplemented with 2.5 μg of human recombinant EGF, 25 mg of bovine pituitary extract, and 1× penicillin/streptomycin. All cultures were maintained in a humidified, 5% CO2, 95% air environment at 37 °C.
RPMI 1640 media supplemented with 10% FBS (referred to here as normal media) contains a low (“castrate”) level of testosterone. However, by concentration and metabolism, LNCaP cells maintain an intracellular concentration (10 nm) of dihydrotestosterone (DHT) sufficient to maintain normal rates of cell growth (36). Therefore to study the effects of AR signaling, steroid depletion conditions were achieved by replacing FBS with 5 or 10% CS-FBS in phenol-free RPMI 1640 media (referred to here as steroid-depleted media). Cells were grown for periods of time in this media and then treated with DHT or vehicle (EtOH) as indicated in the figure legends.
Tissue Preparation
Prostate tissue from nontransgenic wild type (C57BL/6 x FVB) mice, prostate and tumor tissue from TRAMP mice, and CWR22/CWR22R tumor xenografts were lysed and processed for either protein or RNA as previously described (37).
Western Blotting
Western blotting was performed as previously described (37). The antibodies used for Western blotting (apart from those used for subcellular distribution experiments, see “Nuclear-cytoplasmic distribution”) were: CaMKK2 (Abnova), PSA (Thermo Scientific), cyclin D1 (Cell Signaling), Rb (Cell Signaling), AR (Millipore), and GAPDH (Santa Cruz Biotechnology). Protein levels were quantified by densitometry using Quantity One software (Bio-Rad).
Immunohistochemistry (IHC)
Sections of human PCa tissue from radical prostatectomies were obtained from de-identified paraffin blocks from the archives of the Department of Pathology, Roswell Park Cancer Institute (RPCI). Paraffin-embedded human PCa sections or mouse TRAMP tissue sections were de-paraffinized with xylene, rehydrated with alcohol, and placed in dH2O for 5 min before undergoing standard antigen retrieval conditions (citrate buffer, pH 6). Slides were equilibrated with Tris/phosphate buffer that was also used for the intermediate washing steps. Endogenous peroxidase activity was blocked by using 3% H2O2 and incubating for 15 min in the dark at room temperature. Nonspecific immunoglobulin binding sites were blocked with 10% normal goat serum (Invitrogen) for 30 min at room temperature. Additional blocking of avidin and biotin activities was performed by using the Avidin/Biotin blocking kit (Vector Labs). Slides were then incubated overnight with primary anti-CaMKK2 antibody (Prestige, Sigma) diluted 1:100 in 1% BSA in Tris/phosphate buffer at room temperature. Slides were washed, then incubated with biotinylated secondary antibody for 30 min at room temperature, followed by an additional 30-min incubation with ABC detection kit (Vector Labs). Endogenous peroxidase activity was revealed with 1 mg/ml of diaminobenzidine (DAB) (Sigma) and 1 μl/ml of H2O2 and incubating all slides simultaneously for 5 min in the dark at room temperature. Slides were then extensively washed with dH2O and stained with hematoxylin for ∼10 s. Slides incubated solely with goat serum and no primary antibody were considered negative IHC controls. Images were obtained on an Olympus BX45 microscope and processed using cellSens digital imaging software.
Nuclear-Cytoplasmic Distribution
Nuclear-cytoplasmic distribution was assessed by subcellular fractionation and indirect immunofluorescence microscopy. Subcellular fractionation of LNCaP cells was performed with the nuclear and cytoplasmic extraction kit according to the manufacturer's instructions (Thermo Scientific). Nuclear and cytosolic compartments were subjected to Western blotting for their respective markers, histone H3 (Abcam) and lactate dehydrogenase (LDH-A, U.S. Biologicals), to assess the effectiveness of the fractionation procedure. For comparability, CaMKK2 was detected with the same antibody (Prestige, Sigma) used for immunofluorescence microscopy.
Immunofluorescence microscopy was performed as follows. LNCaP cells were plated on coverslips in wells of a 6-well plate at a density of 160,000 cells/coverslip for 24 h and steroid depleted for an additional 24 h. Cells were then treated with 10 nm DHT or vehicle (EtOH) for 16 h. Following treatment, cells were washed twice with PBS then fixed with 4% paraformaldehyde solution (Affymetrix) for 15 min at room temperature. Following three washes in PBS, cells were permeabilized with 0.1% Triton X-100, 0.1% sodium deoxycholate in PBS for 10 min at room temperature. Cells were rinsed three times with PBS and then incubated in 5% BSA in PBS for 1 h at room temperature. Coverslips were incubated with primary AR (Abcam) and CaMKK2 (Prestige, Sigma) antibodies at dilutions of 1:200 and 1:500, respectively, overnight at 4 °C, rinsed with PBS and incubated with anti-mouse IgG-Alexa 647 and anti-rabbit IgG-Alexa 488 secondary antibodies (Molecular Probes, Invitrogen), respectively, at a dilution of 1:500 for 1 h at room temperature in the dark. Cells were then rinsed with PBS and mounted in Slowfade Gold reagent (Molecular Probes, Invitrogen) with DAPI (4 μg/ml). Cells were observed under an AxioImager fluorescence microscope (Zeiss) equipped with an Apotome device, which was utilized to acquire images of 30 optical sections and generate z-stacks of images. Sections were captured at a final magnification of ×630 and analyzed using AxioVision software.
RNA Interference and Transfection
The following siRNA duplexes (Ambion) were used: CaMKK2 siRNA#1, 5′-GGAUCUGAUCAAAGGCAUCtt-3′, 100 nm); CaMKK2 siRNA#2, 5′-GCAUCGAGUACUUACACUAtt-3′, 20–40 nm). Nonspecific (NS) control siRNAs were designed for, and used at the same concentrations as, their respective CaMKK2-targeting siRNAs. LNCaP cells were plated at a density of 1 × 106 cells per plate (100 mm) in antibiotic-free medium 24 h prior to transfection. Lipofectamine 2000 (Invitrogen) was used as the transfection agent. Following the addition of siRNA duplexes, cells were incubated in serum-free medium lacking antibiotics for 6 h. After the 6-h incubation, transfection components were discarded and replaced with complete antibiotic-free media.
Quantitative Reverse Transcription PCR (qRT-PCR)
RNA isolation and cDNA synthesis were performed as previously described (37). Primers for qRT-PCR were designed using the program PRIME (Genetics Computer Group). To assess specificity of primers, a preliminary end point PCR in the presence and absence of reverse transcriptase was performed as well as a melt curve analysis following qRT-PCR. In most cases primers spanned at least one exon/intron junction. The following primers are listed by target cDNA, forward primer and reverse primer, respectively: human CaMKK2, 5′-GCAGTGACGCGCTCCTCTCCAA-3′, 5′-TCCGCTCGTCCATGAATGGGCA-3′; human PSA, 5′-GGTTGTCTTCCTCACCCTGTCCG-3′, 5′-GCACCAGAACACCGCCGCA-3′; human GAPDH, 5′-TGGCATTGCCCTCAACGACCACTTTG-3′; 5′-TCCTCTTGTGCTCTTGCTGGGGCTG-3′; and human Cyclin D1, 5′-GCGAGGAACAGAAGTGCGAGGAGGAG-3′; 5′-CACGAACATGCAAGTGGCCCCCAG. qRT-PCR was performed on a Bio-Rad iCycler with SYBR Green (Bio-Rad) detection. Thermocycling parameters were: 2 min at 95 °C, 45 cycles at 95 °C for 15 s and 60 °C for 30 s, followed by a final extension of 30 min at 72 °C and a melt-curve analysis from 65 to 93.8 °C. The average starting quantity for each condition was calculated by the standard curve method using GAPDH as normalizer.
Semiquantitative RT-PCR
PCR was performed using Platinum Taq Polymerase under standard reaction conditions as recommended by the manufacturer (Invitrogen). The following primers are listed by target cDNA, forward primer and reverse primer, respectively; mouse CaMKK2, 5′-AAACCTGCTCACGAAGCAAG-3′, 5′-TGGTTTCCGTAGGACATGCTG-3′; and mouse GAPDH, 5′-AGCGAGACCCCACTAACATCAAAT-3′, 5′-ATCCACAGTCTTCTGGGTGGCA-3′.
Cell Proliferation and Viability
LNCaP cells were treated with CaMKK2-targeting or control siRNAs and grown for the time periods indicated in the legend to Fig. 4. At the respective time points, floating and attached cells were collected, and centrifuged at 1,500 × g for 5 min. Live and dead cells were distinguished and quantified by cell counting after trypan blue addition.
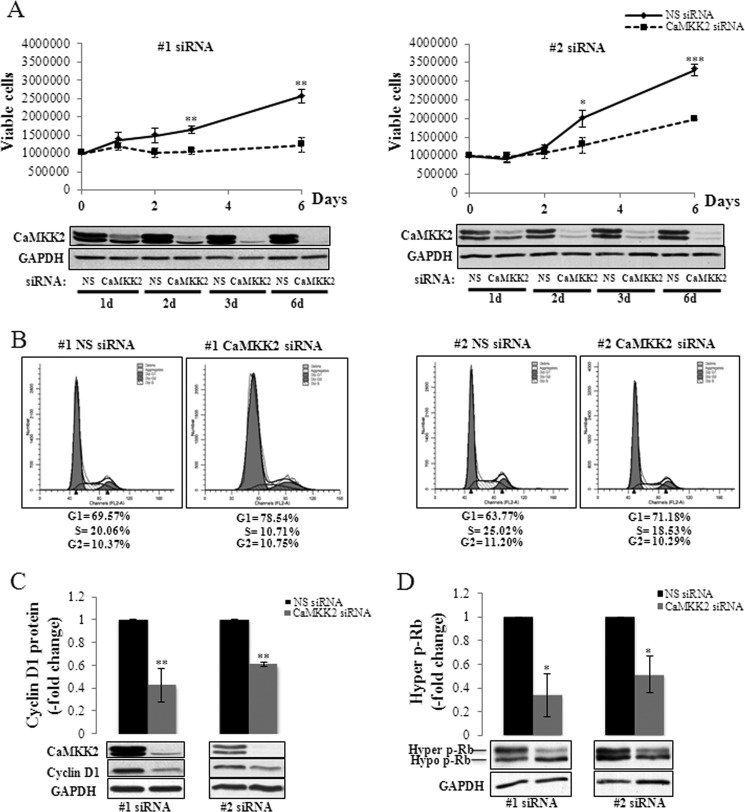
FIGURE 4.
CaMKK2 regulation of PCa cell proliferation and cell cycle progression. A, CaMKK2 knockdown inhibits growth of LNCaP cells. LNCaP cells were transfected with either nonspecific (NS)- or CaMKK2-siRNAs and grown for the indicated time periods. Numbers of viable cells, determined by cell counting with trypan blue exclusion are shown with representative Western blots below. Results represent mean ± S.E. (n = 3 independent experiments). *, p < 0.05; **, p < 0.01; ***, p < 0.001, relative to NS siRNA time-matched controls. GAPDH is used as loading control. B, CaMKK2 knockdown arrests LNCaP cells in the G1 phase of the cell cycle. LNCaP cells were transfected with either NS- or CaMKK2-siRNAs, grown for 4 days, and analyzed by PI flow cytometry. Representative DNA content histograms (for each siRNA used) are shown. Results from this experiment and an additional independent experiment are averaged and shown in supplemental Fig. S5B. C and D, CaMKK2 knockdown reduces cyclin D1 and hyper p-Rb expression. LNCaP cells were transfected with either NS- or CaMKK2-siRNAs and grown for 72 h. Levels of cyclin D1 (C) and hyper p-Rb (D) proteins, quantified by Western blotting are shown with representative blots below. Results represent mean ± S.E. (n = 3 independent experiments). *, p < 0.05; **, p < 0.01, relative to NS-treated control cells. GAPDH is used as loading control.
Cell Cycle Analysis
LNCaP cells (1 × 106) were trypsinized, centrifuged, and resuspended in 1 ml of PBS buffer. Cells were fixed in 70% EtOH by dropwise addition of 2.8 ml of ice-cold 95% EtOH. Fixed cells were washed and resuspended twice in PBS, 1% BSA. Cell pellets were resuspended in Krishan buffer (0.1% sodium citrate, 0.05 mg/ml of propidium iodide (PI), 0.2% Nonidet P-40, 0.02 mg/ml of RNase A, 1 drop/100 ml of 1 n HCl) for 1 h. Cell suspensions were filtered and analyzed on a FACSCalibur flow cytometer. DNA content histograms were generated using ModFit LT 3.1 software.
Luciferase Reporter Assay
The pARE-luciferase reporter construct (pARE-Luc) containing three tandem AREs from the rat probasin gene promoter was kindly provided by K. Gurova. LNCaP cells (1 × 106) were plated in antibiotic-free medium 24 h prior to transfection. Cells were co-transfected with 40 nm CaMKK2 siRNA#2 or its respective negative control siRNA along with pARE-Luc (3 μg) and Renilla luciferase (pRL)-TK vector (0.5 μg) as an internal control. Lipofectamine 2000 (Invitrogen) was used as the transfection agent. Cells were steroid-depleted and then treated with DHT (10 nm) or vehicle (EtOH). Luciferase activity was measured using a dual luciferase reporter assay kit (Promega).
Statistical Analysis
All results are expressed as mean ± S.E. Statistical significance was evaluated using the unpaired Student's t test.
RESULTS
Although CaMKK2 has been identified as a gene expressed at high levels in malignant versus normal prostate tissue (19–31), we wanted to investigate whether CaMKK2 expression varied with the histological pattern of differentiation of prostate tumors and determine the subcellular localization of the expressed protein. For this purpose, we performed IHC for CaMKK2 on clinical samples of PCa procured from radical prostatectomies. Specimens from five patients were analyzed, all of which showed strong CaMKK2 immunoreactivity in malignant glands and four of which are illustrated in Fig. 1 (see supplemental Fig. S1). For this purpose we used an antibody of equivalent specificity to the CaMKK2 antibody used for Western blotting but which produced less background staining in IHC (supplemental Fig. S2).
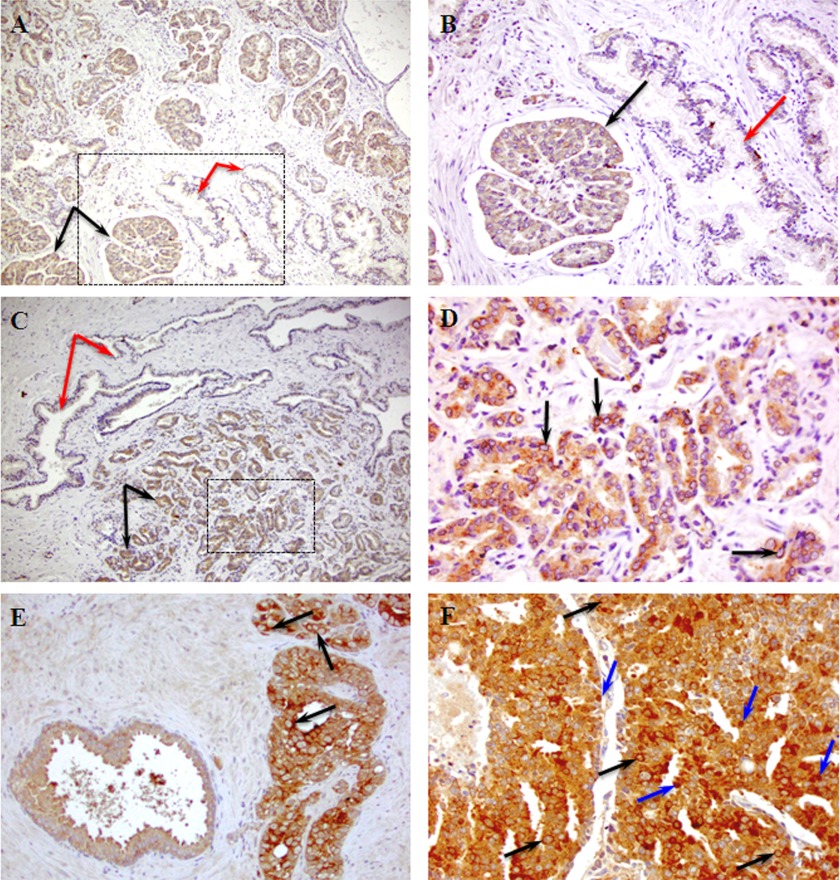
FIGURE 1.
CaMKK2 expression in clinical specimens of PCa. Shown are representative images of CaMKK2 IHC staining in patient samples with various histological Gleason scores3 representing disease advancement and predicting aggressiveness of prostate tumors. A, CaMKK2 shows stronger epithelial staining in malignant glands (black arrows) compared with adjacent benign glands (red arrows) in a patient specimen of GS 3 + 4. B, image A (boxed area) at higher magnification. C, CaMKK2 expression differences in a specimen of GS 3 + 4 from a second patient. D, image C (malignant area, boxed) at higher magnification. Note the perinuclear CaMKK2 staining (arrows). E, CaMKK2 expression is higher in a GS 4 + 4 tumor area (right) compared with an adjacent HGPIN area (left). Arrows indicate perinuclear CaMKK2 staining. F, CaMKK2 staining is uniformly intense in a GS 4 + 5 tumor specimen. Note the appearance of nuclear CaMKK2 staining (blue arrows) apart from the perinuclear staining (black arrows). Magnifications: A and C, ×10; B and E, ×20; D and F, ×40.
CaMKK2 immunostaining was more intense in malignant prostatic epithelium than in adjacent normal epithelium in two Gleason score (GS)3 3 + 4 specimens (Fig. 1, A–D and supplemental Fig. S1, A–D). CaMKK2 staining was mainly cytoplasmic although at a higher magnification, a subset of cells showed apparent perinuclear accumulation of immunoreactivity (Fig. 1D and supplemental Fig. S1D). In a patient specimen with both high grade PIN (HGPIN) and GS, 4 + 4 tumor, CaMKK2 showed strong cytoplasmic and prominent perinuclear staining in the tumor area (Fig. 1E and supplemental Fig. S1E). The highest GS specimen (4 + 5), revealed strong CaMKK2 expression, with perinuclear immunoreactivity and in many cells, nuclear localization of CaMKK2 (Fig. 1F and supplemental Fig. S1F). CaMKK2 has been reported to be cytoplasmically localized in a variety of tissues (1). The nuclear expression of CaMKK2 in advanced human PCa was unexpected and reminiscent of the increased nuclear expression of the AR in castration-resistant tumors (38).
We also investigated the expression of CaMKK2 during tumor progression in the transgenic adenocarcinoma of the mouse prostate (TRAMP) model (Fig. 2, A and B). TRAMP is an autochthonous4 model in which PCa progression recapitulates human disease (39). In this model, early PIN develops between 8 and 12 weeks of age, followed by carcinoma in situ at 12 weeks, moderately differentiated carcinoma by 18 weeks and poorly differentiated carcinomas from 24 to 30 weeks. In all cases, castration leads to the development of recurrent tumors. IHC was performed on normal prostate tissue and tumors representing various stages of progression. CaMKK2 showed moderate cytoplasmic and nuclear staining in nontransgenic mouse prostate tissue (Fig. 2A, panel a) and stronger staining in prostate tissue of a 15-week-old TRAMP mouse (Fig. 2A, panel b). Expression of CaMKK2 was further increased in both noncastrated and castrated advanced tumors (Fig. 2A, panels c and d, respectively) and nuclear staining intensity increased with progression. Western blotting and PCR of TRAMP tissue and tumor lysates confirmed that increased CaMKK2 protein and mRNA expression is associated with PCa progression (Fig. 2B and supplemental Fig. S3, respectively).
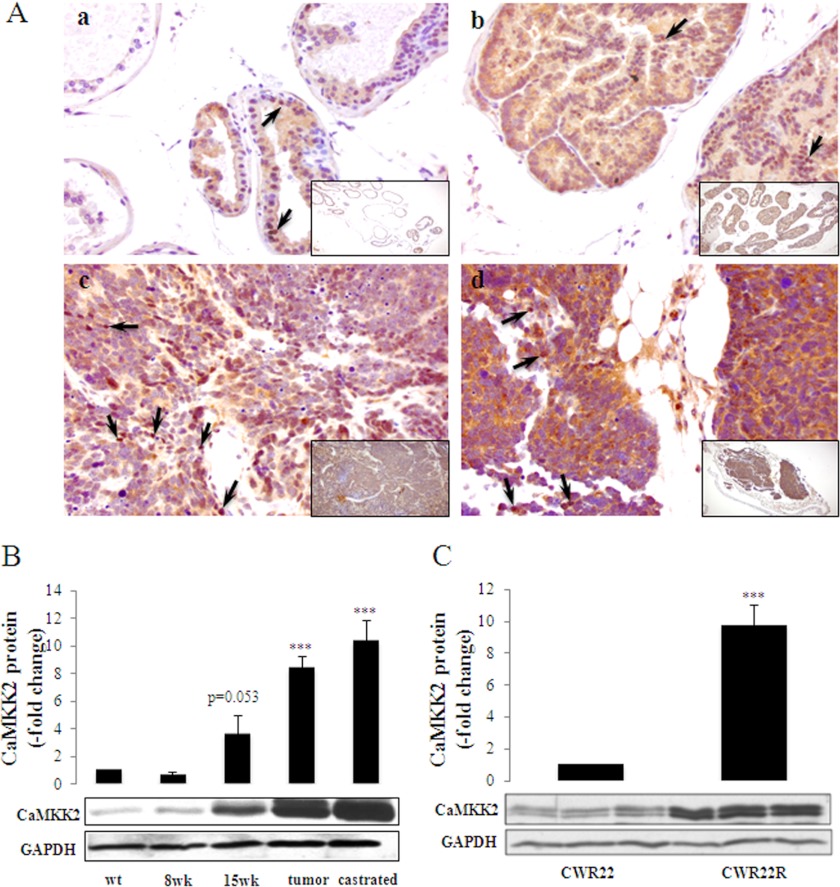
FIGURE 2.
Expression of CaMKK2 during tumor progression in transgenic and xenograft models of PCa. A, shown are representative images of CaMKK2 IHC staining in the TRAMP mouse over a course of PCa progression. a, nontransgenic (C57BL/6 x FVB) prostate tissue. b, prostate tissue of a 15-week-old TRAMP mouse. c, prostate tumor of a 27-week-old TRAMP mouse. d, prostate tumor of a 24-week-old castrated TRAMP mouse. Arrows indicate nuclear CaMKK2 staining. Magnifications: a-d, ×40; a-d, insets, ×10. B, samples were harvested at the indicated times of tumor progression (as in panel A with the addition of prostate tissue from an 8 weeks TRAMP mouse) and assessed by Western blotting for CaMKK2 protein expression. GAPDH was used as loading control. The graph represents mean ± S.E. (n = 5), ***, p < 0.001, relative to wt CaMKK2 levels. C, CaMKK2 protein expression in human CWR22 and CWR22R tumor xenografts. Tumors were harvested from three independent CWR22 and CWR22R tumor-bearing mice and lysates were subjected to Western blotting for CaMKK2 protein expression. GAPDH was used as loading control. The graph represents mean ± S.E. (n = 3) normalized to CWR22 CaMKK2 protein levels. ***, p < 0.001, relative to CWR22.
The association with advanced disease led us to investigate whether CaMKK2 is up-regulated in CRPC. In the serially transplantable CWR22 human xenograft, tumor growth is initially decreased by castration but over time, tumor eventually recurs, and is designated CWR22R (40). CaMKK2 protein expression is increased in the recurrent CWR22R xenograft compared with the androgen-dependent CWR22 xenograft (Fig. 2C). In summary, the data shown in Figs. 1 and 2 and supplemental Figs. S1 and S3, illustrate a strong association of CaMKK2 expression with advanced local, and recurrent, PCa suggesting that CaMKK2 may be required at elevated amounts in the nuclear compartment for a functional role(s) related to PCa progression. Consonant with these results, a tissue microarray analysis by IHC published during the course of our studies reported an up-regulation of CaMKK2 accompanying the development of CRPC (31).
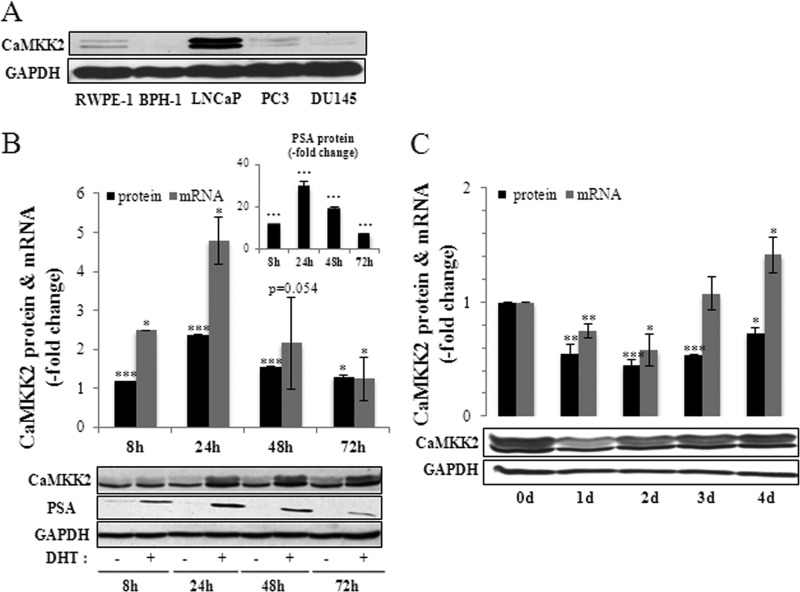
Because the AR is essential for development of organ-confined PCa and a driver of progression to CRPC (35, 38, 41), we compared CaMKK2 levels in various human prostate cell lines differing in their tumorigenicity and AR expression (Fig. 3A). CaMKK2 protein levels were considerably higher in the LNCaP cell line that is both tumorigenic and AR-positive, compared with nontumorigenic prostate cell lines (RWPE-1, BPH-1) or the tumorigenic cell lines PC3 and DU145 that do not express, or express low levels, of the AR (42). Because CaMKK2 levels are high in AR-positive LNCaP cells and the AR is the primary target of DHT action in LNCaP cells (43), we examined whether CaMKK2 expression is androgen-regulated. Corroborating previous reports (31–33), we found that CaMKK2 protein and mRNA levels were significantly up-regulated by DHT (maximally by 2.5- and 4.8-fold relative to vehicle controls, respectively; Fig. 3B). The time dependence of CaMKK2 up-regulation paralleled that of DHT induction of the endogenous AR target, PSA (Fig. 3B, inset).5 DHT induction of CaMKK2 expression was blocked by the AR antagonist Casodex (bicalutamide) indicating that it is mediated by the AR (supplemental Fig. S4). Conversely, steroid removal from the medium led to significant decreases in protein and mRNA levels of CaMKK2 (maximally to levels of 43 and 57% of 0 day of controls, respectively; Fig. 3C). Based on these results, we conclude that AR signaling can produce homeostatic adjustments in CaMKK2 expression levels.
FIGURE 3.
Androgen regulation of CaMKK2 expression. A, relative levels of CaMKK2 protein in prostatic cell lines as assessed by Western blotting. GAPDH is used as loading control. B, DHT stimulates CaMKK2 expression. LNCaP cells were cultured in steroid-depleted media for 24 h then treated with 10 nm DHT or vehicle (EtOH) for the indicated time periods. Levels of CaMKK2 protein and mRNA, quantified by Western blotting and qRT-PCR, respectively, are shown with a representative blot below. Values are normalized to time-matched vehicle controls and represent mean ± S.E. (n = 3 independent experiments). *, p < 0.05; **, p < 0.01; ***, p < 0.001, relative to time-matched vehicle controls. PSA protein expression in response to 10 nm DHT is shown in the inset. GAPDH is used as loading control. C, steroid depletion reduces CaMKK2 expression. LNCaP cells were cultured in normal media for 72 h then in steroid-depleted media for the indicated time periods. Levels of CaMKK2 protein and mRNA, quantified by Western blotting and qRT-PCR, respectively, are shown with a representative blot below. Results represent mean ± S.E. (n = 3 independent experiments). *, p < 0.05; **, p < 0.01; ***, p < 0.001, relative to 0 day controls. GAPDH is used as loading control.
The AR is a key regulator of PCa cellular proliferation and cell cycle transitions (45, 46). We therefore investigated whether CaMKK2 could be a mediator of AR-dependent PCa cell cycle control. Silencing of CaMKK2 expression by either of two independently targeting siRNAs led to significant decreases in the proliferation of LNCaP cells (Fig. 4A). Interestingly, CaMKK2 knockdown did not produce similar cytostatic effects in PC3 cells (supplemental Fig. S5A). This may be due either to a low concentration of CaMKK2 in these cells (Fig. 3A), or that the effect of CaMKK2 knockdown on PCa cell proliferation requires the presence of the AR and thus not observed in AR-negative PC3 cells.
To determine whether the slowing of proliferation of LNCaP cells by CaMKK2 knockdown could be explained by losses of cell viability, we quantified viable and dead cells at each time point for both siRNAs, in at least three independent experiments per siRNA. Neither siRNA produced changes in percent viability ((live/live + dead) × 100) significantly different from their respective control siRNAs (#1, 80.1 versus 90.8, respectively, p = 0.10; #2, 77.4 versus 82.1, respectively, p = 0.11). Therefore, cell loss appears to be incapable of explaining the decreased number of LNCaP cells as a result of CaMKK2 silencing. This suggested that effects on cell number are due instead to alterations of cell cycle kinetics. To evaluate this hypothesis, we performed PI-flow cytometry on LNCaP cells transfected with either of the two CaMKK2 targeting siRNAs. The results were that both CaMKK2 siRNAs, relative to their respective NS control siRNAs, produced significant increases in numbers of cells in G1 phase (#1, 80.92 ± 2.48 versus 70.35 ± 0.78, p < 0.05; #2, 71.81 ± 0.63 versus 64.11 ± 0.34, p < 0.01, respectively) and to significant decreases in number of cells in S phase (#1, 7.34 ± 3.37 versus 19.56 ± 0.49, p < 0.05; #2, 17.86 ± 0.66 versus 23.85 ± 1.17, p < 0.05, respectively) (n = 2 independent experiments for each siRNA used, supplemental Fig. S5B). Representative DNA content histograms are shown in Fig. 4B. These results indicate that depletion of CaMKK2 leads to cell cycle arrest at the G1/S interface.
Control of G1 to S phase progression involves, among other mechanisms, up-regulation of cyclin D1 leading to increased formation of D1-cdk4/6 complexes, multisite (“hyper”) phosphorylation and inactivation of the retinoblastoma (Rb) tumor suppressor, and consequent E2F-dependent expression of genes required for S phase entry. In PCa cells, androgen deprivation results in G1 arrest, loss of cyclin D1 expression, and reduced Rb phosphorylation (45, 46). Conversely, androgen stimulation of LNCaP cells up-regulates D-type cyclin expression and Rb phosphorylation. Androgen-dependent induction of cyclin D1 is reported to occur by AR stimulation of the expression of multiple genes leading to the activation of mTOR, which then mediates an increase in cyclin D1 protein by a translational mechanism (47). We examined the effect of CaMKK2 depletion on the levels of cyclin D1 and hyperphosphorylated Rb (p-Rb) in LNCaP cells. CaMKK2 knockdown caused significant decreases in levels of cyclin D1 (to 43–61% of NS siRNA control) and p-Rb (to 34–52% of NS siRNA control) consistent with G1 arrest (Fig. 4, C and D). The decrease in cyclin D1 protein was not accompanied by a change in cyclin D1 mRNA upon CaMKK2 knockdown indicating that this effect is due to a post-transcriptional mechanism (supplemental Fig. S5C).
The similarity of these phenotypes between CaMKK2 and AR pathways is consistent with two possible explanations. CaMKK2 could act as an independent mediator of AR action. In this mechanism, androgen stimulation of CaMKK2 expression leads to the activation of a kinase(s) downstream of CaMKK2, which in turn up-regulates cell cycle proteins. This could occur by direct phosphorylation of the cell cycle regulators, activation of their transcription or more indirectly, by regulation of anabolic metabolism (31, 48–50). However, because the AR is known to be phosphorylated at multiple sites (51) and also to bind calmodulin (52), we considered here the alternate possibility that the functional consequence of up-regulation of CaMKK2 by androgen is to increase the cell cycle modulatory activity of the AR itself, perhaps as a mechanism for AR activation/re-activation in PCa progression. Such a feedback mechanism would be consistent with up-regulation and nuclear accumulation of CaMKK2 in late, and possibly in recurrent, disease (Figs. 1 and 2, and supplemental Fig. S3) and with the slowing of the proliferation of AR-positive LNCaP, but not AR-negative PC3, cells by CaMKK2 silencing (Fig. 4A and supplemental Fig. S5A).
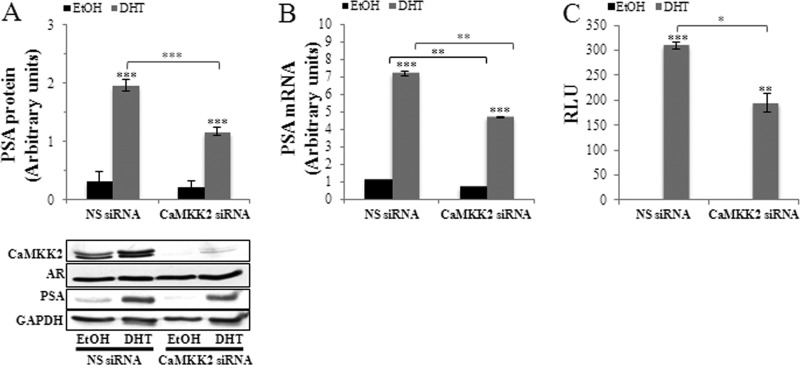
To test this hypothesis, we silenced CaMKK2 expression and quantified expression of the AR-target gene, PSA. CaMKK2 knockdown6 produced a significant reduction of DHT-stimulated PSA expression at both its protein and mRNA levels, (to 59 and 65% of NS siRNA controls, respectively) (Fig. 5, A and B, see also supplemental Fig. S6A). These results indicate that the CaMKK2 pathway exerts a modulatory role in maintaining AR activity. AR protein levels remained unaltered, suggesting that the observed changes in PSA expression are the result of acute regulation of AR transcriptional activity and not AR expression or stability (Fig. 5A and supplemental Fig. S6C). To further test this hypothesis, we used a reporter assay in which luciferase expression is driven by tandem AREs from the prostate-specific probasin gene promoter (43). Similarly to the effect of CaMKK2 knockdown on PSA mRNA expression, which is a measure of AR transcriptional activity at the PSA promoter, probasin ARE activity was significantly less in DHT-stimulated, CaMKK2-depleted cells (63% of NS siRNA control; Fig. 5C). We also examined whether the effect of CaMKK2 silencing on the AR required androgen binding. Although probasin ARE transcriptional activity was very low in the absence of androgen precluding accurate assessment by luciferase assay, CaMKK2 silencing did produce a significant decrease in PSA mRNA under steroid depletion conditions, suggesting that the effect of the CaMKK2 pathway on the AR does not require its ligand-bound state and thus could be a functional mechanism during the development of castration-resistant PCa (Fig. 5B).7
FIGURE 5.
CaMKK2 regulates transcriptional activity of the AR. A, CaMKK2 knockdown inhibits androgen-induced PSA protein expression. LNCaP cells were transfected with NS- or CaMKK2- siRNA (#2) in normal media for 48 h, switched to steroid-depleted media for 24 h, and then treated with 10 nm DHT or vehicle (EtOH) for an additional 24 h. Levels of PSA protein were quantified by Western blotting and normalized to GAPDH. A representative blot is shown below. GAPDH is used as loading control. Results represent mean ± S.E. in arbitrary units (n = 3 independent experiments). Note that AR protein levels are unaltered under these conditions. B, CaMKK2 knockdown inhibits androgen-induced PSA mRNA expression. LNCaP cells were transfected with NS- or CaMKK2-siRNA (#2) in normal media for 24 h, switched to steroid-depleted media for 72 h, and then treated with 10 nm DHT or vehicle (EtOH) for an additional 16 h. Levels of PSA mRNA were quantified by qRT-PCR and normalized to GAPDH. Results represent mean ± S.E. in arbitrary units (n = 3 independent experiments). C, CaMKK2 knockdown inhibits AR transcriptional activity. LNCaP cells were transfected with the probasin ARE luciferase reporter along with NS- or CaMKK2-siRNAs for 6 h, after which cells were switched to steroid-depleted media for 24 h then stimulated with either DHT (10 nm) or EtOH for an additional 16 h. Firefly luciferase activity normalized to Renilla luciferase activity is shown. Results represent mean relative light units (RLU) ± S.E. (n = 2 independent experiments). A-C, *, p < 0.05; **, p < 0.01; ***, p < 0.001, either of DHT-treated cells relative to EtOH-treated cells (over bars) or of NS relative to CaMKK2-siRNA-treated cells (over brackets).
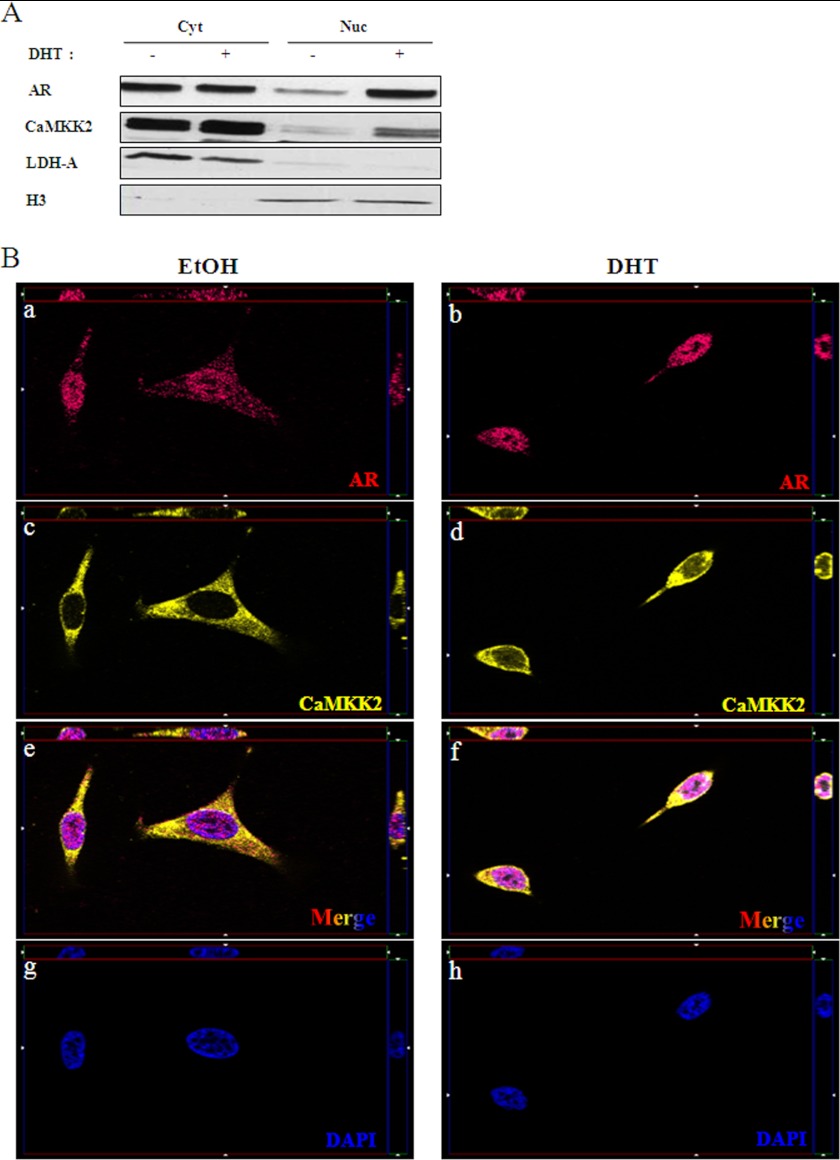
The observation of some high grade malignant cells exhibiting CaMKK2 nuclear staining (Fig. 1F and supplemental Fig. S1F) raised the possibility that the AR not only promotes synthesis of new CaMKK2 molecules but also their nuclear translocation. To test this hypothesis, we treated LNCaP cells with DHT or vehicle and determined CaMKK2 localization by subcellular fractionation and immunofluorescence microscopy (Fig. 6). In the absence of androgen, CaMKK2 is almost exclusively cytoplasmic as has been reported (1). However, addition of DHT induced a portion of the CaMKK2 to exhibit nuclear localization (Fig. 6, A and B, panels c–f). The cytoplasm to nucleus translocation of CaMKK2 is similar to that of AR under these conditions (Fig. 6, A and B, panels a and b). These results suggest that the nuclear translocation of CaMKK2 is AR-dependent. CaMKK2 may therefore be capable of regulating AR activity either in the cytoplasm or at genomic sites and function as a mechanism by which the AR maintains activity in advanced PCa.
FIGURE 6.
DHT induces CaMKK2 nuclear translocation. A, LNCaP cells were cultured in steroid-depleted media for 24 h, then treated with 10 nm DHT or vehicle (EtOH) for an additional 16 h. Cells were then fractionated into cytoplasmic and nuclear compartments and assessed for CaMKK2 and AR expression by Western blotting. Lactate dehydrogenase (LDH-A) and histone H3 were used for assessing purity of cytoplasmic and nuclear fractions, respectively. Shown is a representative blot of two independent experiments. B, representative images of immunofluorescent staining for AR (a, b, red), CaMKK2 (c, d, yellow), DAPI (g, h, blue), and merged images (e, f, tricolor) and in LNCaP cells treated with 10 nm DHT or EtOH for 16 h. Single plane apotome (x-y plane) and z-sections (bordering the x-y planes) confirm CaMKK2 presence in the nucleus. Total magnification, ×630. Images are representative of two independent experiments.
Finally, we examined the effect of the CaMKK inhibitor STO-609 (53) on LNCaP proliferation in the presence and absence of androgen (supplemental Fig. S7). STO-609 inhibited proliferation, with an apparent increase in its potency under steroid depletion conditions. Due to toxic effects of STO-609 at high doses we were precluded from testing concentrations of >100 μm and thus could not precisely determine maximal efficacy in both conditions. The potentiation of the inhibitory action of STO-609 by androgen depletion is also supportive of a CaMKK2-AR interaction of potential relevance for PCa cell growth.
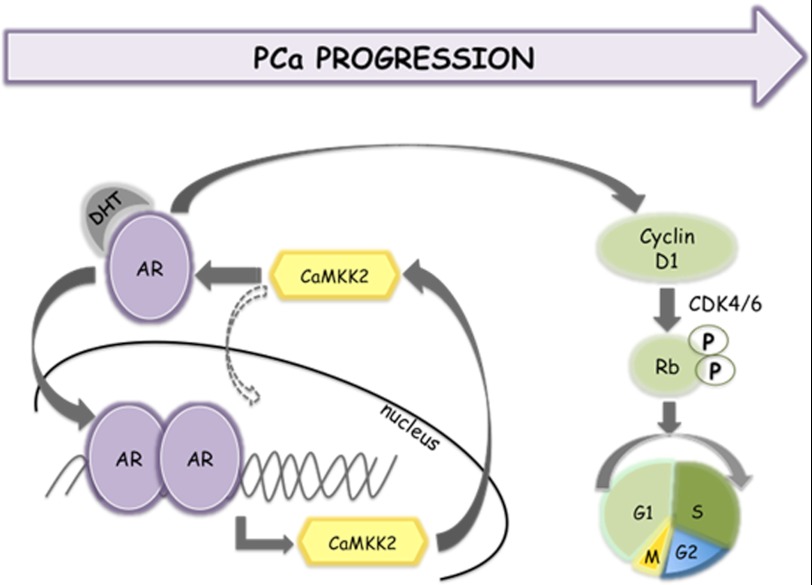
The results of this study suggest a hypothetical model schematically illustrated in Fig. 7. In this model, CaMKK2 and the AR interact in a positive feedback loop by which increases in activity of the AR during PCa progression result in higher levels of CaMKK2 that in turn promote AR transcriptional activity and cell proliferation.
FIGURE 7.
Schematic model for a CaMKK2-AR feedback loop in PCa progression. See text for details.
DISCUSSION
During the growth of an androgen-responsive tumor, AR activity is critical for control of the cycling and proliferation of malignant cells. Following decreases as a consequence of androgen deprivation therapy, AR activity is eventually restored due to mutational alterations in its structure, expression, and localization (35). Post-translational modifications of the AR such as phosphorylation can provide additional avenues by which the activity of the AR is sustained during androgen-dependent and castration-resistant PCa progression. However, the multiplicity of signaling pathways and their respective AR phosphorylation sites (at least 16 of which have been reported to date) present a complex and poorly understood picture (51). It is currently thought that there are two ways in which PCa progression results in increased phosphorylation of the AR. Androgen binding or mutational alteration of the structure of the AR leads to solvent exposure of potential phosphorylation sites and increased kinase accessibility. Alternatively, reduction in interaction of the AR with phosphatase(s) results in net increased phosphorylation of the AR (54). Both mechanisms imply that the constitutive activities of the kinases are sufficient for AR phosphorylation.
We present here data in support of a third model illustrated in Fig. 7. This model envisages a relatively simple, positive feedback loop in which increased AR signaling, as a consequence of locally advanced PCa or CRPC, transcriptionally induces CaMKK2 mRNA and protein, increasing the activity of the CaMKK2 pathway that in turn feeds back to stimulate AR-dependent transcriptional activity and cell cycle progression.
At present we do not know whether the overall activity or setpoint of this loop is modulated by other stimuli, such as Ca2+-elevating signals. Compared with non-neoplastic cells, LNCaP and other malignant cells require much lower concentrations of extracellular Ca2+ to proliferate (55, 56). And, as noted above, CaMKK2 exhibits considerable (∼70% of total) constitutive, Ca2+ -independent activity in vitro. It is therefore possible that induction of CaMKK2 protein by the AR could yield an increase in active CaMKK2 sufficient for feedback regulation of the AR, either in the absence of Ca2+ elevation, or perhaps with the additional intracellular Ca2+ needed for optimal CaMKK2 activity supplied by androgen-induced increases in Ca2+ influx through LNCaP plasma membrane L-type Ca2+ channels (57).
It is also unclear whether in LNCaP cells, a CaMKK2 driven cascade impinges upon cell cycle regulators in an AR-independent fashion (48–50). Activity of the AR is critical for G1-S phase progression in PCa cells (45, 46). Therefore our data that CaMKK2 knockdown reduces AR transactivation driven by two promoters (PSA and probasin) and phenotypically replicates the reductions in levels of phospho-RB and cyclin D1 protein but not cyclin D1 mRNA predicted from an action via the AR, strongly suggest that the CaMKK2-cell cycle regulation described here is AR-mediated to at least some degree.
Taken together, these observations suggest that an AR-CaMKK2 regulatory loop is capable of operating as a contained, or relatively contained, two-component system. This raises an intriguing possibility that any event that occurs during PCa progression and/or development of CRPC that augments AR activity, such as gene amplification, mutation, alternate splicing etc. would be reinforced and strengthened by this mechanism. In other words, for the CaM kinase cascade to promote AR-mediated pathogenesis it is not necessary for CaMKK2 to be oncogenic per se (although it is not precluded), but simply to be utilized by the AR in concert with mechanisms triggering (or triggered by) recurrence to maintain AR activity during tumor progression.
Current conceptual models of signaling pathways active in PCa do not incorporate the CaMKK2 pathway (35, 45, 46, 51). It will be of interest to explore this pathway both from the standpoint of delineating the downstream effectors responsible for transmitting enhanced CaMKK2 activity to the AR, the mechanism by which the activity of the AR is modulated, and how this is translated into altered cell cycle progression. Our data suggest that this is an acute regulation rather than a change in AR concentration, as has been observed with some protein kinases capable of AR phosphorylation (51), although this conclusion will require longer term stability studies. It will be of particular interest to probe the relationship between nuclear accumulation of the AR and that of CaMKK2 in different models of PCa progression.
From a clinical perspective, CaMKK2 could potentially perform a regulatory role in both androgen-sensitive PCa and CRPC because the AR is a driver of progression in both cases, however, our data that CaMKK2 is up-regulated in recurrent CWR22R versus androgen-sensitive, CWR22 tumors suggest that this signaling pathway may be one of the escape routes by which the AR maintains activity in the face of decreases in testicular androgen production. Hence, a therapeutic agent targeting CaMKK2 may be relatively inactive in monotherapy but potentially effective in combination with androgen ablation to suppress a phosphorylation-based mechanism triggered in relapsed tumors by which the AR maintains optimal control of malignant cell growth.
Supplementary Material
Acknowledgments
We thank Katerina Gurova for providing the pARE-Luciferase plasmid. We also thank Elaine Goldstein for general technical assistance, Ellen Karasik for histological services, Michalis Mastri for assistance with immunofluorescence experiments, Michael Moser for discussions and technical advice, and Brian Gillard for animal studies support. We gratefully acknowledge Thomas Pretlow (Case Western Reserve University) for providing the CWR22 xenograft.
This work was supported, in whole or in part, by National Institutes of Health Grants NS50385 (to A. M. E.) and CA095367 (to B. A. F.) and University at Buffalo Foundation and RPCI Cancer Center Support Grant CA016056.

This article contains supplemental Figs. S1–S7.
The Gleason score (GS) is a number based on a histopathological grading system for predicting the aggressiveness of prostate tumors. The GS is the sum of two numbers. Each number is a grade from 1 to 5 representing tumor differentiation where a grade of 1 is well differentiated and 5, poorly differentiated. The first number is the most common tumor pattern and the second number is the second most common tumor pattern.
Autochthonous refers to a tumor found in a location (in this case the prostate) from where it arose. It is distinguished from the term orthotopic, which typically refers to a xenograft of heterologous cells into the prostate of a recipient host.
As reflected by both CaMKK2 and PSA expression, the time course of AR activity in LNCaP cells during continuous exposure to DHT is biphasic (Fig. 3B). Wang et al. (44) also reported that PSA mRNA peaks at 16–24 h of exposure of LNCaP cells to DHT and then steadily declines over time. This phenomenon was attributed to transient occupancy of AR coactivator complexes at PSA regulatory regions (44). The biphasic, but inverted, time course of CaMKK2 expression in response to androgen deprivation may also be due to this phenomenon (Fig. 3C).
As shown in Fig. 5A and supplemental Fig. S6B, DHT had little effect on CaMKK2 levels after RNA interference indicating that the extent of CaMKK2 knockdown was not blunted by a compensatory AR-dependent up-regulation of CaMKK2.
PSA protein levels in the absence of androgen were below the limit of accurate quantification and thus we were unable to compare differences between siRNA-treated cells at the protein level under these conditions (Fig. 5A).
- PCa
- prostate cancer
- AR
- androgen receptor
- PIN
- prostatic intraepithelial neoplasia
- ARE
- androgen-responsive element
- CRPC
- castration resistant PCa
- DHT
- dihydrotestosterone
- IHC
- immunohistochemistry
- NS
- nonspecific
- qRT
- quantitative reverse transcription
- TRAMP
- transgenic adenocarcinoma of mouse prostate
- Rb
- retinoblastoma.
REFERENCES
- 1. Colomer J., Means A. R. (2007) Physiological roles of the Ca2+/CaM-dependent protein kinase cascade in health and disease. Subcell. Biochem. 45, 169–214 [DOI] [PubMed] [Google Scholar]
- 2. Tokumitsu H., Enslen H., Soderling T. R. (1995) Characterization of a Ca2+/calmodulin-dependent protein kinase cascade. J. Biol. Chem. 270, 19320–19324 [DOI] [PubMed] [Google Scholar]
- 3. Edelman A. M., Mitchelhill K. I., Selbert M. A., Anderson K. A., Hook S. S., Stapleton D., Goldstein E. G., Means A. R., Kemp B. E. (1996) Multiple Ca2+/calmodulin-dependent protein kinase kinases from rat brain. J. Biol. Chem. 271, 10806–10910 [DOI] [PubMed] [Google Scholar]
- 4. Kitani T., Okuno S., Fujisawa H. (1997) Molecular cloning of Ca2+/calmodulin-dependent protein kinase kinase β. J. Biochem. 122, 243–250 [DOI] [PubMed] [Google Scholar]
- 5. Anderson K. A., Means R. L., Huang Q. H., Kemp B. E., Goldstein E. G., Selbert M. A., Edelman A. M., Fremeau R. T., Means A. R. (1998) Components of a calmodulin-dependent protein kinase cascade, molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase β. J. Biol. Chem. 273, 31880–31889 [DOI] [PubMed] [Google Scholar]
- 6. Haribabu B., Hook S. S., Selbert M. A., Goldstein E. G., Tomhave E. D., Edelman A. M., Snyderman R., Means A. R. (1995) Human calcium-calmodulin-dependent protein kinase I, cDNA cloning, domain structure and activation by phosphorylation at threonine 177 by calcium-calmodulin-dependent protein kinase I kinase. EMBO J. 14, 3679–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Selbert M. A., Anderson K. A., Huang Q. H., Goldstein E. G., Means A. R., Edelman A. M. (1995) Phosphorylation and activation of Ca2+-calmodulin-dependent protein kinase IV by Ca2+-calmodulin-dependent protein kinase Ia kinase. Phosphorylation of threonine 196 is essential for activation. J. Biol. Chem. 270, 17616–17621 [DOI] [PubMed] [Google Scholar]
- 8. Yano S., Tokumitsu H., Soderling T. R. (1998) Calcium promotes cell survival through CaM-K kinase activation of the protein kinase-B pathway. Nature 396, 584–587 [DOI] [PubMed] [Google Scholar]
- 9. Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. (2005) Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2, 9–19 [DOI] [PubMed] [Google Scholar]
- 10. Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., Witters L. A. (2005) The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280, 29060–29066 [DOI] [PubMed] [Google Scholar]
- 11. Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., Carling D. (2005) Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2, 21–33 [DOI] [PubMed] [Google Scholar]
- 12. Hardie D. G. (2007) AMP-activated/SNF1 protein kinases, conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8, 774–785 [DOI] [PubMed] [Google Scholar]
- 13. Wayman G. A., Lee Y. S., Tokumitsu H., Silva A. J., Soderling T. R. (2008) Calmodulin kinases, modulators of neuronal development and plasticity. Neuron 59, 914–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park I. K., Soderling T. R. (1995) Activation of Ca/calmodulin-dependent protein kinase (CaM-kinase) IV by CaM-kinase kinase in Jurkat T lymphocytes. J. Biol. Chem. 270, 30464–30469 [DOI] [PubMed] [Google Scholar]
- 15. Aletta J. M., Selbert M. A., Nairn A. C., Edelman A. M. (1996) Activation of a calcium-calmodulin-dependent protein kinase I cascade in PC12 cells. J. Biol. Chem. 271, 20930–20934 [DOI] [PubMed] [Google Scholar]
- 16. Tokumitsu H., Hatano N., Fujimoto T., Yurimoto S., Kobayashi R. (2011) Generation of autonomous activity of Ca2+/calmodulin-dependent protein kinase kinase β by autophosphorylation. Biochemistry 50, 8193–8201 [DOI] [PubMed] [Google Scholar]
- 17. Green M. F., Scott J. W., Steel R., Oakhill J. S., Kemp B. E., Means A. R. (2011) Ca2+/calmodulin-dependent protein kinase kinase β is regulated by multisite phosphorylation. J. Biol. Chem. 286, 28066–28079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Green M. F., Anderson K. A., Means A. R. (2011) Characterization of the CaMKKβ-AMPK signaling complex. Cell. Signal. 23, 2005–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dhanasekaran S. M., Barrette T. R., Ghosh D., Shah R., Varambally S., Kurachi K., Pienta K. J., Rubin M. A., Chinnaiyan A. M. (2001) Delineation of prognostic biomarkers in prostate cancer. Nature 412, 822–826 [DOI] [PubMed] [Google Scholar]
- 20. Welsh J. B., Sapinoso L. M., Su A. I., Kern S. G., Wang-Rodriguez J., Moskaluk C. A., Frierson H. F., Jr., Hampton G. M. (2001) Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 61,5974–5978 [PubMed] [Google Scholar]
- 21. Ernst T., Hergenhahn M., Kenzelmann M., Cohen C. D., Bonrouhi M., Weninger A., Klären R., Gröne E. F., Wiesel M., Güdemann C., Küster J., Schott W., Staehler G., Kretzler M., Hollstein M., Gröne H. J. (2002) Decrease and gain of gene expression are equally disciminatory markers for prostate carcinoma. Am. J. Pathol. 160, 2169–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ashida S., Nakagawa H., Katagiri T., Furihata M., Iiizumi M., Anazawa Y., Tsunoda T., Takata R., Kasahara K., Miki T., Fujioka T., Shuin T., Nakamura Y. (2004) Molecular features of the transition from prostatic intraepithelial neoplasia (PIN) to prostate cancer, genome-wide gene expression profiles of prostate cancers and PINs. Cancer Res. 64, 5963–5972 [DOI] [PubMed] [Google Scholar]
- 23. Lapointe J., Li C., Higgins J. P., van de Rijn M., Bair E., Montgomery K., Ferrari M., Egevad L., Rayford W., Bergerheim U., Ekman P., DeMarzo A. M., Tibshirani R., Botstein D., Brown P. O., Brooks J. D., Pollack J. R. (2004) Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc. Natl. Acad. Sci. U.S.A. 101, 811–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu Y. P., Landsittel D., Jing L., Nelson J., Ren B., Liu L., McDonald C., Thomas R., Dhir R., Finkelstein S., Michalopoulos G., Becich M., Luo J. H. (2004) Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J. Clin. Oncol. 22, 2790–2799 [DOI] [PubMed] [Google Scholar]
- 25. Kristiansen G., Pilarsky C., Wissmann C., Kaiser S., Bruemmendorf T., Roepcke S., Dahl E., Hinzmann B., Specht T., Pervan J., Stephan C., Loening S., Dietel M., Rosenthal A. (2005) Expression profiling of microdissected matched prostate cancer samples reveals CD166/MEMD and CD24 as new prognostic markers for patient survival. J. Pathol. 205, 359–376 [DOI] [PubMed] [Google Scholar]
- 26. Lozano J. J., Soler M., Bermudo R., Abia D., Fernandez P. L., Thomson T. M., Ortiz A. R. (2005) Dual activation of pathways regulated by steroid receptors and peptide growth factors in primary prostate cancer revealed by factor analysis of microarray data. BMC Genomics 2005, 6, 109, doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varambally S., Yu J., Laxman B., Rhodes D. R., Mehra R., Tomlins S. A., Shah R. B., Chandran U., Monzon F. A., Becich M. J., Wei J. T., Pienta K. J., Ghosh D., Rubin M. A., Chinnaiyan A. M. (2005) Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell 8, 393–406 [DOI] [PubMed] [Google Scholar]
- 28. Hendriksen P. J., Dits N. F., Kokame K., Veldhoven A., van Weerden W. M., Bangma C. H., Trapman J., Jenster G. (2006) Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res. 66, 5012–5020 [DOI] [PubMed] [Google Scholar]
- 29. True L., Coleman I., Hawley S., Huang C. Y., Gifford D., Coleman R., Beer T. M., Gelmann E., Datta M., Mostaghel E., Knudsen B., Lange P., Vessella R., Lin D., Hood L., Nelson P. S. (2006) A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc. Natl. Acad. Sci. U.S.A. 103, 10991–10996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tomlins S. A., Chinnaiyan A. M. (2007) in Prostate Cancer (Chung L. W. K., Isaacs W. B., Simons J. W., eds) pp. 247–267, Humana Press, Clifton, NJ [Google Scholar]
- 31. Massie C. E., Lynch A., Ramos-Montoya A., Boren J., Stark R., Fazli L., Warren A., Scott H., Madhu B., Sharma N., Bon H., Zecchini V., Smith D. M., Denicola G. M., Mathews N., Osborne M., Hadfield J., Macarthur S., Adryan B., Lyons S. K., Brindle K. M., Griffiths J., Gleave M. E., Rennie P. S., Neal D. E., Mills I. G. (2011) The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 30, 2719–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nelson P. S., Clegg N., Arnold H., Ferguson C., Bonham M., White J., Hood L., Lin B. (2002) The program of androgen-responsive genes in neoplastic prostatic epithelium. Proc. Natl. Acad. Sci. U.S.A. 99, 11890–11895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frigo D. E., Howe M. K., Wittmann B. M., Brunner A. M., Cushman I., Wang Q., Brown M., Means A. R., McDonnell D. P. (2011) CaM kinase kinase β-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Res. 71,528–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mendiratta P., Mostaghel E., Guinney J., Tewari A. K., Porrello A., Barry W. T., Nelson P. S., Febbo P.G. (2009) Genomic strategy for targeting therapy in castration-resistant prostate cancer. J. Clin. Oncol. 27, 2022–2029 [DOI] [PubMed] [Google Scholar]
- 35. Bluemn E. G., Nelson P. S. (2012) The androgen/androgen receptor axis in prostate cancer. Curr. Opin. Oncol. 24, 251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sedelaar J. P., Isaacs J. T. (2009) Tissue culture media supplemented with 10% fetal calf serum contains a castrate level of testosterone. The Prostate 69, 1724–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feliciano D. M., Edelman A. M. (2009) Repression of Ca2+/calmodulin-dependent protein kinase IV signaling accelerates retinoic acid-induced dfferentiation of human neuroblastoma cells. J. Biol. Chem. 284, 26466–26481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gregory C. W., Johnson R. T., Jr., Mohler J. L., French F. S., Wilson E. M. (2001) Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 61, 2892–2898 [PubMed] [Google Scholar]
- 39. Gingrich J. R., Barrios R. J., Foster B. A., Greenberg N. M. (1999) Pathologic progression of autochthonous prostate cancer in the TRAMP model. Prostate Cancer Prostatic Dis. 2, 70–75 [DOI] [PubMed] [Google Scholar]
- 40. Nagabhushan M., Miller C. M., Pretlow T. P., Giaconia J. M., Edgehouse N. L., Schwartz S., Kung H. J., de Vere White R. W., Gumerlock P. H., Resnick M. I., Amini S. B., Pretlow T. G. (1996) CWR22, the first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res. 56, 3042–3046 [PubMed] [Google Scholar]
- 41. Snoek R., Cheng H., Margiotti K., Wafa L. A., Wong C. A., Wong E. C., Fazli L., Nelson C. C., Gleave M. E., Rennie P. S. (2009) In vivo knockdown of the androgen receptor results in growth inhibition and regression of well established, castration-resistant prostate tumors. Clin. Cancer Res. 15, 39–47 [DOI] [PubMed] [Google Scholar]
- 42. Tilley W. D., Wilson C. M., Marcelli M., McPhaul M. J. (1990) Androgen receptor gene expression in human prostate carcinoma cell lines. Cancer Res. 50, 5382–5386 [PubMed] [Google Scholar]
- 43. Tararova N. D., Narizhneva N., Krivokrisenko V., Gudkov A. V., Gurova K. V. (2007) Prostate cancer cells tolerate a narrow range of androgen receptor expression and activity. Prostate 67, 1801–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Q., Carroll J. S., Brown M. (2005) Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol. Cell 19, 631–642 [DOI] [PubMed] [Google Scholar]
- 45. Balk S. P., Knudson K. E. (2008) AR, the cell cycle, and prostate cancer. Nucl. Recept. Signal. 6, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schiewer M. J., Augello M. A., Knudsen K. E. (2012) The AR-dependent cell cycle, mechanisms and cancer relevance. Mol. Cell Endocrinol. 352, 34–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu Y., Chen S. Y., Ross K. N., Balk S. P. (2006) Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 66, 7783–7792 [DOI] [PubMed] [Google Scholar]
- 48. Kahl C. R., Means A. R. (2004) Regulation of cyclin D1/CDK4 complexes by calcium/calmodulin-dependent protein kinase I. J. Biol. Chem. 279, 15411–15419 [DOI] [PubMed] [Google Scholar]
- 49. Rodriguez-Mora O. G., LaHair M. M., McCubrey J. A., Franklin R. A. (2005) Calcium/calmodulin-dependent kinase I and calcium/calmodulin-dependent kinase kinase participate in the control of cell cycle progression in MCF-7 human breast cancer cells. Cancer Res. 65, 5408–5416 [DOI] [PubMed] [Google Scholar]
- 50. Schmitt J. M., Abell E., Wagner A., Davare M. A. (2010) ERK activation and cell growth require CaM kinases in MCF-7 breast cancer cells. Mol. Cell. Biochem. 335, 155–171 [DOI] [PubMed] [Google Scholar]
- 51. Gioeli D., Paschal B. M. (2012) Post-translational modification of the androgen receptor. Mol. Cell. Endocrinol. 352, 70–78 [DOI] [PubMed] [Google Scholar]
- 52. Cifuentes E., Mataraza J. M., Yoshida B. A., Menon M., Sacks D. B., Barrack E. R., Reddy G. P. (2004) Physical and functional interaction of androgen receptor with calmodulin in prostate cancer cells. Proc. Natl. Acad. Sci. U.S.A. 101, 464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tokumitsu H., Inuzuka H., Ishikawa Y., Ikeda M., Saji I., Kobayashi R. (2002) STO-609, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 277, 15813–15818 [DOI] [PubMed] [Google Scholar]
- 54. Yang C. S., Xin H. W., Kelley J. B., Spencer A., Brautigan D. L., Paschal B. M. (2007) Ligand binding to the androgen receptor induces conformational changes that regulate phosphatase interactions. Mol. Cell. Biol. 27, 3390–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boynton A. L., Whitfield J. F., Isaacs R. J., Tremblay R. G. (1977) Different extracellular calcium requirements for proliferation of nonneoplastic, preneoplastic, and neoplastic mouse cells. Cancer Res. 37, 2657–2661 [PubMed] [Google Scholar]
- 56. Liao J., Schneider A., Datta N. S., McCauley L. K. (2006) Extracellular calcium as a candidate mediator of prostate cancer skeletal metastasis. Cancer Res. 66, 9065–9073 [DOI] [PubMed] [Google Scholar]
- 57. Steinsapir J., Socci R., Reinach P. (1991) Effects of androgen on intracellular calcium of LNCaP cells. Biochem. Biophys. Res. Commun. 179, 90–96 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.