Summary
Mutational activation of KRas is the first and most frequently detected genetic lesion in pancreatic ductal adenocarcinoma (PDAC). However, the precise role of oncogenic KRas in the pathogenesis of PDAC is not fully understood. Here, we report that the endogenous expression of oncogenic KRas suppresses premature senescence in primary pancreatic duct epithelial cells (PDEC). Oncogenic KRas-mediated senescence bypass is conferred by the upregulation of the basic helix-loop-helix transcription factor Twist which in turn abrogates p16INK4A induction. Moreover, the KRas - Twist - p16INK4A senescence bypass pathway is employed in vivo to prevent inflammation-associated senescence of pancreatic ductal epithelium. Our findings indicate that oncogenic KRas could contribute to PDAC initiation by protecting cells from entering a state of permanent growth arrest.
Introduction
Ras proteins comprise a family of signal-transducing GTPases that are frequently mutated in human cancers. Oncogenic Ras mutations lock the protein in its GTP-bound form thus permitting its constitutive interaction with and activation of multiple effectors (Downward, 2003). The pathogenic role of oncogenic Ras has been attributed primarily to its promoting effects on cell proliferation and cell survival. In contrast, in normal primary cells oncogenic Ras can cause a permanent proliferative arrest known as premature senescence (Serrano et al., 1997). The induction of senescence by oncogenic Ras is largely mediated by the upregulation of inhibitors of cell proliferation including p16INK4A, p19ARF, p21CIP, and p53 and is thought to serve as a tumor suppressive process by preventing the expansion of cells bearing mutant Ras (Lowe et al., 2004). However, the capacity of oncogenic Ras to provoke senescence varies considerably depending on cellular context and biological setting. For example, the ectopic expression of oncogenic Ras in fibroblasts at supraphysiological levels can trigger senescence, whereas expression of oncogenic Ras at physiological levels fails to engage the senescence machinery (Serrano et al., 1997; Tuveson et al., 2004). In addition, while some studies utilizing mouse models of oncogenic KRas-driven tumorigenesis have documented the presence of senescent preneoplastic lesions in lung, colon, and pancreatic tissues (Bennecke et al., 2010; Collado et al., 2005; Morton et al., 2010), others have reported that senescence could not be detected in oncogenic KRas expressing tissues (Tuveson et al., 2004). Thus, it remains unclear to what extent the implementation of the senescence program is linked to the oncogenic potential of mutated Ras.
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death in the United States and carries a median survival of less than 6 months (Jemal et al., 2009; Warshaw and Fernandez-del Castillo, 1992). A distinguishing molecular feature of PDAC is the presence of activating KRas mutations in over 90% of tumors (Almoguera et al., 1988). Because of their unusually high prevalence and their detection at very early stages of disease, KRas mutations are considered a key genetic determinant in the initiation of PDAC. In support of this postulate, mice engineered to express mutated KRas specifically in the pancreas sustain a spectrum of neoplastic lesions that mirror histologically those observed in humans (Hingorani et al., 2003). Thus, understanding the mechanisms by which KRas mutations contribute to PDAC development is critical for the identification of effective strategies to detect and treat PDAC. To clarify the relationship between the mutational activation of KRas, induction of senescence and pancreatic tumorigenesis, we have examined the consequences of endogenous oncogenic KRas expression in primary pancreatic duct epithelial cells (PDEC), a potential cell of origin for PDAC.
Results
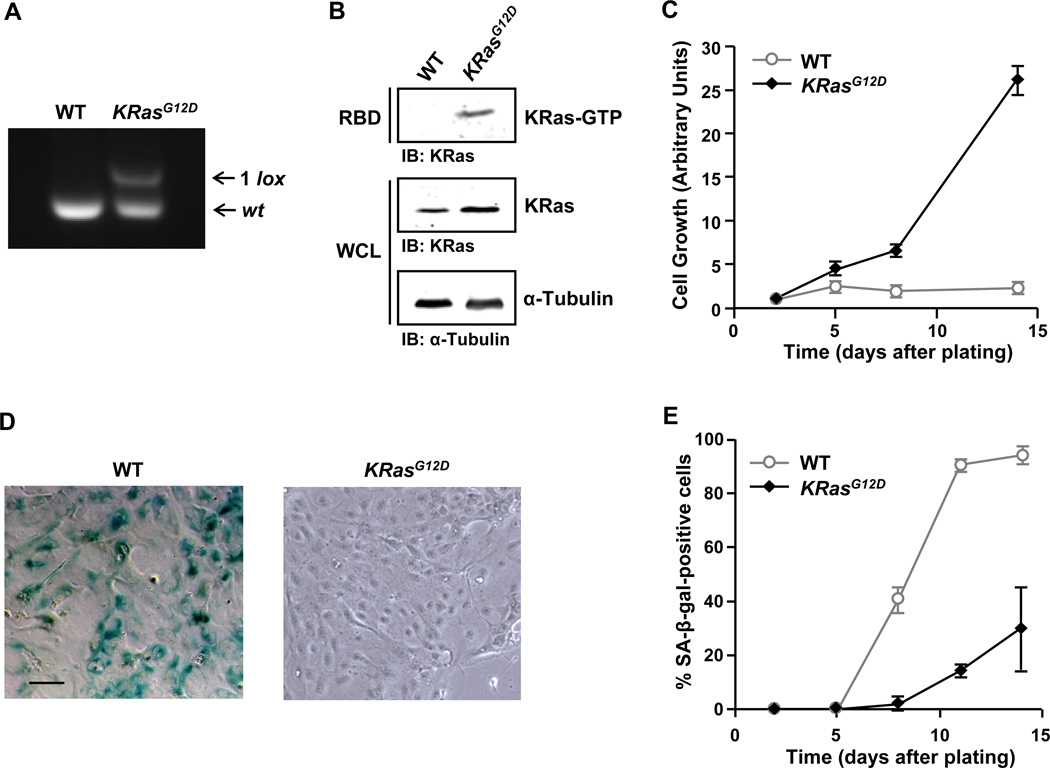
To assess the role of oncogenic KRas in pancreatic tumorigenesis, we have used a previously described cell culture system for primary mouse PDEC (Agbunag and Bar-Sagi, 2004; Agbunag et al., 2006). The endogenous expression of oncogenic KRas in these cells was achieved by their isolation from conditional oncogenic KRas (LSL-KRasG12D) knock-in mice (Jackson et al., 2001) followed by infection with recombinant adenoviruses encoding Cre recombinase. As a control, PDEC derived from the same mice were infected with recombinant adenoviruses encoding green fluorescent protein (GFP). The excision of the LSL cassette was verified by PCR (Figure 1A) and the expression of the KRasG12D allele was indicated by the pronounced increase in the levels of KRas-GTP (Figure 1B).
Figure 1. Oncogenic KRas protects PDEC from undergoing premature senescence.
(A) PCR analysis of genomic DNA prepared from LSL-KRasG12D PDEC infected with adenoviral-GFP (WT) or adenoviral-Cre (KRasG12D). The excision-recombination event of the LSL cassette leaves behind a single LoxP (1 lox) site.
(B) Measurement of Ras activation in WT and KRasG12D PDEC by GST-RBD pull-down assay. α-Tubulin serves as a loading control. IB, immunoblot; WCL, whole cell lysates.
(C) Growth analysis of WT and KRasG12D PDEC. The number of DAPI-stained nuclei was counted in 9 random fields of view (FOV) each containing at least 50 cells at day 2, 5, 8, and 14. The average number of nuclei present in the FOV at each time point was then normalized to the average number of nuclei per FOV at day 2. Error bars indicate standard deviation (SD). Data are representative of five independent experiments.
(D) SA-β-gal staining of WT and KRasG12D PDEC cultures at day 8. Scale bar: 100 µm.
(E) Quantification of SA-β-gal staining in WT and KRasG12D PDEC cultures at day 2, 5, 8, 11, and 14. Cells were counterstained with Hoechst 33342 for β-gal quantification. Error bars indicate SD (n = 6 FOV). Data are representative of five independent experiments. See also Figure S1.
We have previously shown that primary PDEC undergo premature senescence in culture (Agbunag and Bar-Sagi, 2004). Consistent with these observations, control PDEC, hereafter referred to as wild type (WT), ceased growing within 5 days after plating, adopted an enlarged flattened morphology, and displayed senescence-associated-β-galactosidase (SA-β-gal) activity starting at day 8 and peaking at days 12–15 (Figures 1C, 1D, 1E, and S1). In contrast, the vast majority of KRasG12D-expressing PDEC, hereafter referred to as KRasG12D, continued proliferating and showed no SA-β-gal activity (Figures 1C, 1D, 1E, and S1). These findings indicate that oncogenic KRas can protect PDEC from premature senescence.
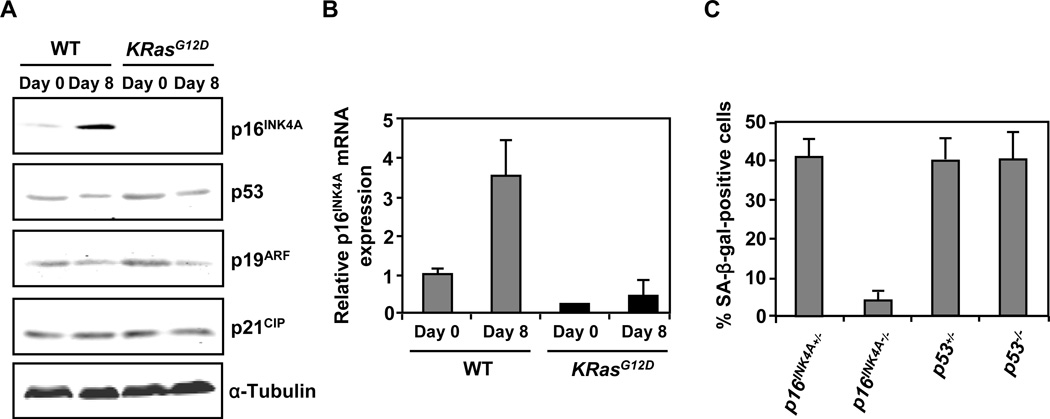
To investigate the mechanisms by which oncogenic KRas represses PDEC senescence, we first assessed the expression levels of the major senescence effectors p16INK4A, p19ARF, p21CIP, and p53 in WT and KRasG12D PDEC by Western blot analysis. As shown in Figure 2A, in WT PDEC the levels of p19ARF, p21CIP, and p53 remained essentially unchanged as the cells matured in culture. In contrast, p16INK4A protein and message levels increased markedly (Figures 2A and 2B) suggesting that the induction of premature senescence in PDEC might depend preferentially on p16INK4A upregulation. To test this idea directly, we examined the senescence phenotype of PDEC isolated from p16INK4A −/− mice and p53−/− mice (Jacks et al., 1994; Serrano et al., 1996) by SA-β-gal staining. Loss of p53 had no effect on the extent of PDEC senescence, while p16INK4A deficiency led to a significant reduction in PDEC senescence (Figure 2C). The senescence phenotype of the PDEC used as controls for these experiments (p16INK4A+/− and p53+/−) was indistinguishable from that observed in WT PDEC (data not shown). These observations suggest that premature senescence of PDEC requires the induction of p16INK4A but not p53. Noticeably, the induction of p16INK4A expression was abrogated in KRasG12D PDEC (Figures 2A and 2B) indicating that oncogenic KRas might confer senescence bypass via the suppression of p16INK4A induction.
Figure 2. Oncogenic KRas confers bypass of premature senescence in PDEC through the suppression of p16INK4A induction.
(A) Western blot analysis for senescence effectors in WT and KRasG12D PDEC cultures at day 0 and day 8. Equal loading was verified with anti-α-Tubulin.
(B) Quantitative RT-PCR analysis of p16INK4A in WT and KRasG12D PDEC cultures at day 0 and day 8. Error bars indicate SD (n = 3).
(C) Quantification of SA-β-gal staining in p16INK4A+/−, p16INK4A−/−, p53+/−, p53−/− PDEC cultures at day 8. Error bars indicate SD (n = 6 FOV). Data are representative of three independent experiments.
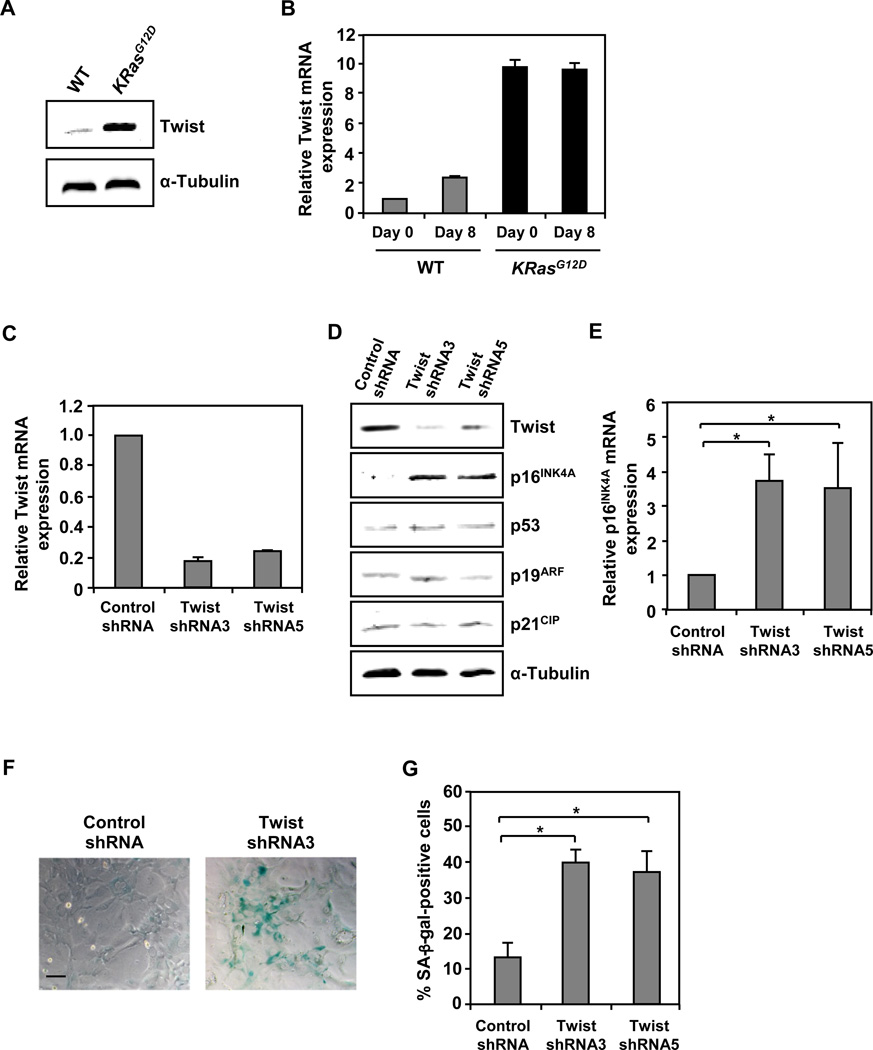
Next we sought to determine the mechanism by which oncogenic KRas prevents the upregulation of p16INK4A. We focused our attention on the basic helix-loop-helix transcription factor Twist (also known as Twist1) because of its documented ability to override premature senescence by abrogating p16INK4A expression (Ansieau et al., 2008) and its recently reported genetic interactions with Ras (Hurlbut et al., 2009). To examine the effect of oncogenic KRas on Twist expression, the levels of Twist mRNA and protein in WT and KRasG12D PDEC were compared. As illustrated in Figures 3A and 3B, Twist levels were markedly increased in KRasG12D PDEC indicating that Twist transcription might be regulated by oncogenic KRas signaling. Of note, oncogenic KRas did not induce expression of the Twist1 homologue, Twist2 (formerly known as Dermo-1) (Li et al., 1995) (data not shown). Initial analysis of Ras effector pathways that might target Twist transcription failed to implicate the Ras-ERK and Ras-PI3K signaling axes (data not shown). To establish whether Twist is essential for oncogenic KRas-mediated senescence bypass in PDEC, Twist expression was suppressed in KRasG12D PDEC using RNA interference. A significant attenuation of Twist mRNA and protein expression was attained using two independent targeting sequences (Figures 3C and 3D). The reduction in Twist expression coincided with a specific increase in p16INK4A expression (Figures 3D and 3E) and was accompanied by the induction of SA-β-gal activity (Figures 3F and 3G). In contrast, the knockdown of Twist in p16INK4A−/− PDEC was without an effect on SA-β-gal activity (Figure S2A). Together, these results indicate that Twist is a critical mediator of oncogenic KRas-dependent suppression of p16INK4A expression and senescence bypass in PDEC. Although Twist has been shown to cooperate with Ras in the induction of the epithelial-mensenchymal transition (EMT) (Ansieau et al., 2008), the expression of Twist in KRasG12D PDEC was not accompanied by the activation of the EMT program, as reflected by the persistence of expression of the epithelial marker E-cadherin, and absence of induction of the mesenchymal markers vimentin and α-smooth muscle actin (α-SMA) (Figures S2B, S2C, and S2D).
Figure 3. Oncogenic KRas-mediated bypass of premature senescence in PDEC is dependent on Twist.
(A) Western blot analysis for Twist in WT and KRasG12D PDEC cultures at day 8. α-Tubulin serves as a loading control.
(B) Quantitative RT-PCR analysis of Twist in WT and KRasG12D PDEC cultures at day 0 and day 8. Error bars indicate SD (n = 3).
(C) Quantitative RT-PCR analysis of Twist in KRasG12D PDEC cultures 8 days after infection with recombinant lentiviruses encoding shRNA targeted against GFP (control shRNA) or Twist (Twist shRNA3 or Twist shRNA5). Error bars indicate SD (n = 3).
(D) Western blot analysis for Twist and senescence effectors in KRasG12D PDEC cultures 8 days after infection with recombinant lentiviruses encoding control shRNA, Twist shRNA3, or Twist shRNA5. Equal loading was verified with anti-α-Tubulin.
(E) Quantitative RT-PCR analysis of p16INK4A in KRasG12D PDEC cultures 8 days after infection with recombinant lentiviruses encoding control shRNA, Twist shRNA3, or Twist shRNA5. Error bars indicate SD (n = 3). *, p-value < 0.05.
(F) SA-β-gal staining of KRasG12D PDEC cultures 8 days after infection with recombinant lentiviruses encoding control shRNA or Twist shRNA3. Scale bar: 100 µm.
(G) Quantification of SA-β-gal staining in KRasG12D PDEC cultures 8 days after infection with recombinant lentiviruses encoding control shRNA, Twist shRNA3, or Twist shRNA5. Error bars indicate SD (n = 6 FOV). *, p-value < 0.05. Data are representative of three independent experiments. See also Figure S2.
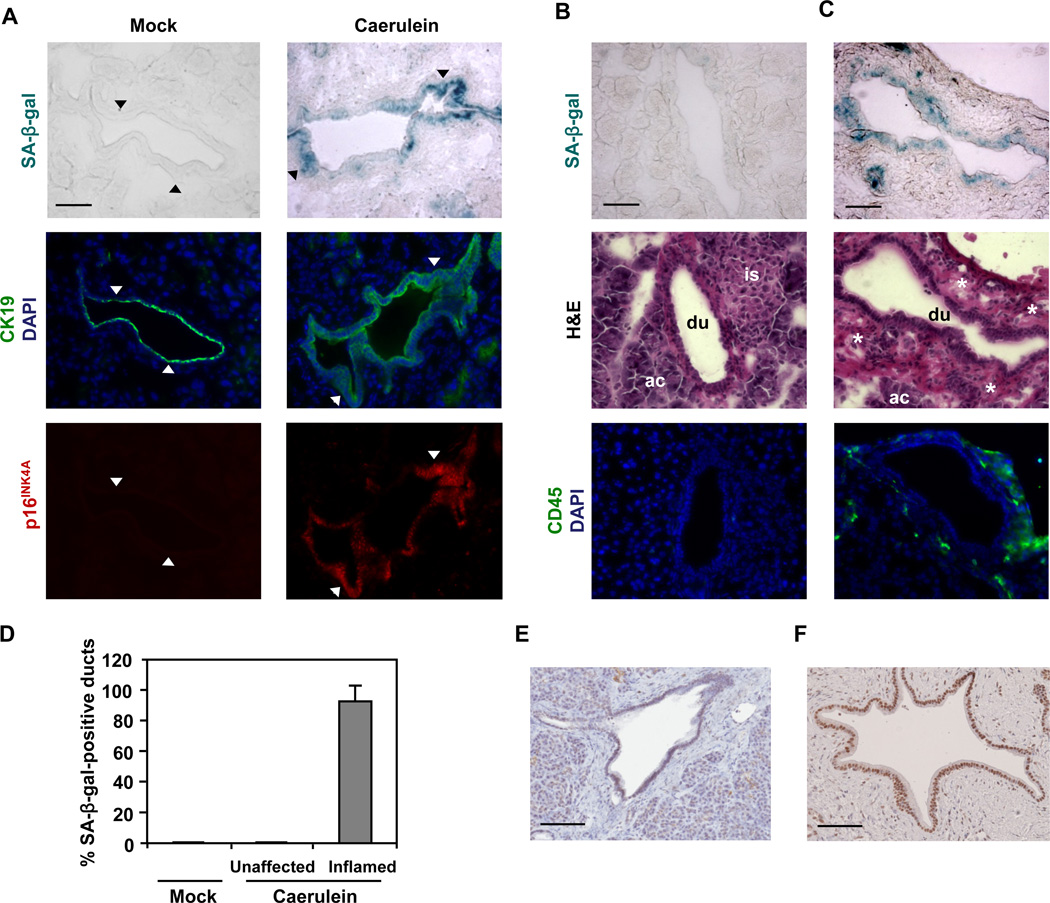
To investigate the physiological relevance of senescence abrogation by oncogenic KRas, we set out to identify an in vivo context in which premature senescence occurs in a p16INK4A-dependent manner. Because inflammation is a well-established risk factor for pancreatic cancer (Lowenfels et al., 1993; Malka et al., 2002) and pro-inflammatory signals have been implicated in senescence induction (Acosta et al., 2008; Kuilman et al., 2008), we examined whether inflammatory conditions may trigger senescence in pancreatic ductal epithelium in vivo. To elicit pancreatic inflammation, mice were subjected to a series of eight hourly intraperitoneal injections of supraphysiological levels of caerulein over 2 consecutive days. This protocol has been shown to induce exocrine pancreatic injury followed by a mild inflammatory response (Carriere et al., 2009; Jensen et al., 2005; Willemer et al., 1992). Three days after the last caerulein injection, pancreata were harvested and subjected to SA-β-gal assays. As illustrated in Figure 4A, mock-injected pancreata had no detectable SA-β-gal activity, while pancreatic ducts in caerulein-treated pancreata displayed pronounced SA-β-gal activity. Consistent with the growth arrest state attributed to senescent cells, the SA-β-gal-positive ductal cells were negative for the proliferation marker Ki67 (Figure S3). It should be noted that the inflammatory response following caerulein-induced injury is focal. Significantly, only pancreatic ducts that were adjacent to inflamed areas as evident by loss of acinar cells and presence of immune infiltrates stained positively for SA-β-gal activity (Figures 4B, 4C, and 4D). This spatial correlation indicates that the pancreatic ductal epithelium can be induced to undergo premature senescence in vivo in response to inflammatory insults.
Figure 4. Inflammatory insult triggers premature senescence in pancreatic ductal epithelium in vivo.
(A–D) Caerulein was administered to 1-month-old mice as 8 hourly intraperitoneal injections (50 ng/g of body weight/injection) for two days. Three days after the last caerulein injection, pancreata were harvested. At least 3 sections were analyzed per animal (n = 5 per genotype per treatment).
(A) SA-β-gal staining and immunofluorescence staining for p16INK4A and CK19 on consecutive sections of pancreata from WT mice treated with caerulein or saline (mock). CK19 was used to identify ductal cells. Nuclei were counterstained with DAPI. Arrowheads mark corresponding areas.
(B and C) SA-β-gal staining, hematoxylin and eosin (H&E) staining, and immunofluorescence staining for CD45 on consecutive sections of pancreata from WT mice treated with caerulein. Pancreatic ducts in unaffected (B) or inflamed (C) areas are from the same tissue section. CD45 was used to identify leukocytes. Nuclei were counterstained with DAPI. ac, acinus; du, duct; is, islet; asterisk, immune infiltrate.
(D) Quantification of SA-β-gal staining in pancreata from WT mice treated with caerulein or saline (mock). Pancreatic ducts bearing 10% or more β-gal-positive cells in each duct were scored as positive. At least four pancreatic ducts were scored in each mouse. Error bars indicate SD (n = 5).
(E and F) Immunohistochemical analysis of p16INK4A in human chronic pancreatitis tissue samples. Representative areas of normal pancreatic (E) and pancreatitis (F) tissues are shown. Scale bars: 50 µm (A, B, and C), 100 µm (E and F). See also Figure S3.
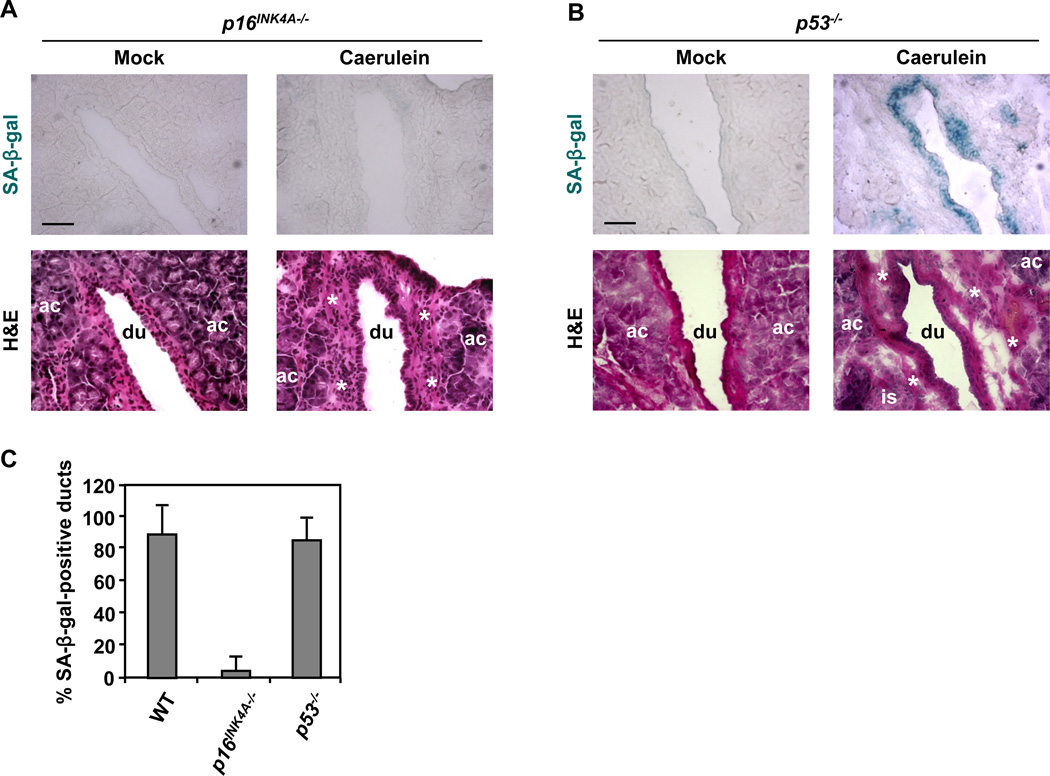
To explore the relationship between inflammation-induced premature senescence in pancreatic ducts and p16INK4A expression, the levels of p16INK4A were assessed in pancreata from mock-injected and caerulein-injected mice by immunofluorescence staining. In agreement with published data documenting the lack of p16INK4A expression in the mouse pancreas (Krishnamurthy et al., 2004), pancreatic ducts from mock-injected mice were negative for p16INK4A staining (Figure 4A). In contrast, p16INK4A staining was robust in pancreatic ducts from caerulein-injected animals (Figure 4A). Significantly, human chronic pancreatitis was found to be also associated with the ductal upregulation of p16INK4A (Figures 4E and 4F). These results along with the observation that strong p16INK4A immunostaining coincided with high SA-β-gal activity (Figure 4A) raised the possibility that inflammation-induced premature senescence in pancreatic ducts is conferred by the upregulation of p16INK4A. To test this idea, we treated p16INK4A−/− mice with caerulein and analyzed the pancreata for SA-β-gal activity. As shown in Figures 5A and 5C, inflammation-induced premature senescence of the ductal epithelium was rescued by p16INK4A-deficiency. By comparison, pancreatic ducts from caerulein-injected p53−/− mice retained the capacity to undergo premature senescence (Figures 5B and 5C). The inflammatory response per se did not appear to be altered by p16INK4A deficiency as determined by gross inspection of tissue sections and the abundance of CD45-positive cells (Figure S4). These observations indicate that p16INK4A is required for inflammation-associated premature senescence in pancreatic ducts and p53 is dispensable for this process.
Figure 5. Inflammation-induced premature senescence in pancreatic ductal epithelium depends on p16INK4A upregulation.
Caerulein was administered as described in Figure 4. At least 3 sections were analyzed per animal (n = 5 per genotype per treatment).
(A and B) SA-β-gal staining and H&E staining on consecutive sections of pancreata from p16INK4A−/− (A) and p53−/− mice (B) treated with caerulein or saline (mock). Ac, acinus; du, duct; is, islet; asterisk, immune infiltrate. Scale bars: 50 µm.
(C) Quantification of SA-β-gal staining in pancreata from WT, p16INK4A−/−, and p53−/− mice treated with caerulein. Only pancreatic ducts in inflamed areas were counted. At least four pancreatic ducts were scored in each mouse. Error bars indicate SD (n = 5). See also Figure S4.
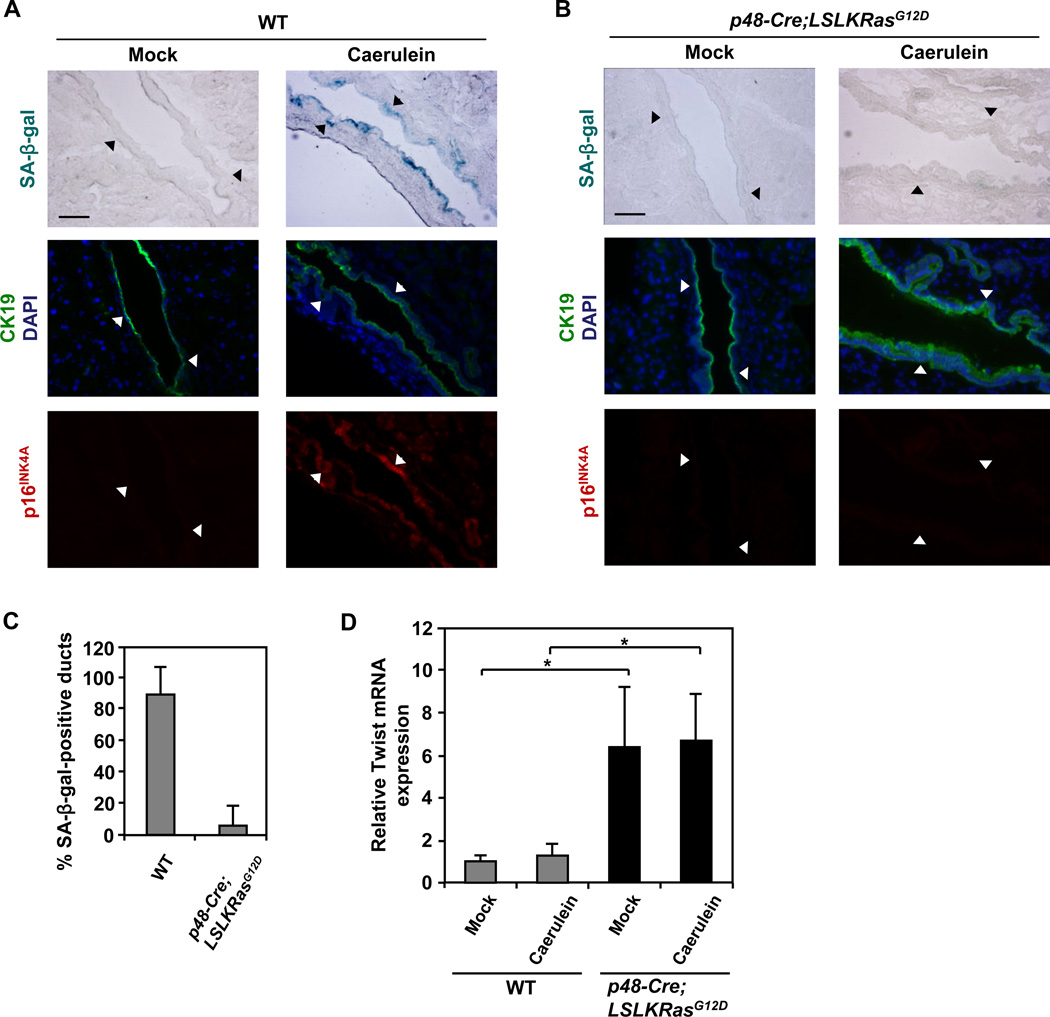
Given our in vitro findings that oncogenic KRas inhibits premature senescence in PDEC via the suppression of p16INK4A, we next asked whether oncogenic KRas can circumvent inflammation-induced premature senescence in pancreatic ductal epithelium in vivo. The LSL-KRasG12D allele was conditionally activated in the pancreas by interbreeding LSL-KRasG12D mice with p48-Cre mice (Kawaguchi et al., 2002). Lineage tracing studies previously demonstrated uniform expression of Cre recombinase throughout the pancreas in p48-Cre mice (Kawaguchi et al., 2002) and PCR analysis confirmed the presence of the recombined allele in pancreatic ducts of p48-Cre;LSL-KRasG12D mice used for the experiments (Figure S5A). As illustrated in Figures 6A, 6B, and 6C, pancreatic ducts in p48-Cre;LSL-KRasG12D mice treated with caerulein failed to senesce, and the abrogation of senescence was associated with suppression of p16INK4A expression. These results indicate that endogenous expression of oncogenic KRas in vivo protects the pancreatic ductal epithelium from undergoing premature senescence by suppressing the induction of p16INK4A in response to inflammation. Significantly, Twist levels were increased in pancreata from p48-Cre;LSL-KRasG12D mice (Figure 6D) implicating Twist in the protective effects of oncogenic KRas on inflammation-associated senescence. Consistent with our in vitro findings, the expression of Twist in pancreata from p48-Cre;LSL-KRasG12D mice was not associated with EMT induction as demonstrated by persistent expression of E-cadherin and absence of α-SMA induction in pancreatic ducts (Figures S5B and S5C).
Figure 6. Inflammation-associated premature senescence of pancreatic ductal epithelium in vivo can be blocked by oncogenic KRas through the suppression of p16INK4A.
Caerulein was administered as described in Figure 4. At least 3 sections were analyzed per animal (n = 5 per genotype per treatment).
(A and B) SA-β-gal staining and immunofluorescence staining for p16INK4A and CK19 on consecutive sections of pancreata from WT (A) and p48-Cre;LSL-KRasG12D (B) mice treated with caerulein or saline (mock). CK19 was used to identify ductal cells. Nuclei were counterstained with DAPI. Arrowheads mark corresponding areas. Scale bars: 50 µm.
(C) Quantification of SA-β-gal staining in pancreata from WT and p48-Cre;LSL-KRasG12D mice treated with caerulein. Only pancreatic ducts in inflamed areas were counted. At least four pancreatic ducts were scored in each mouse. Error bars indicate SD (n = 5).
(D) Quantitative RT-PCR analysis of Twist in pancreata from WT and p48-Cre;LSL-KRasG12D mice treated with caerulein or saline (mock). Error bars indicate SD (n = 5 per genotype). *, p-value < 0.05. See also Figure S5.
Discussion
In the present study, we describe a previously unrecognized functional facet of oncogenic KRas -- the capacity to suppress premature senescence of pancreatic ductal epithelium. Because in the setting of evolving pancreatic neoplasms, activating KRas mutations are the first genetic alteration to be detected (Hruban et al., 2000), senescence bypass could serve as a mechanism to impart selective advantage to the cellular precursors of pancreatic cancer. Moreover, the enhanced fitness that oncogenic KRas confers on pancreatic ductal epithelium may explain why KRas mutations can be tolerated by these cells.
The propensity of cultured primary cells to undergo premature senescence has been described before and has been attributed to the induction of p16INK4A expression in response to stress conditions imposed by an inappropriate growth environment (Ben-Porath and Weinberg, 2004; Sherr and DePinho, 2000). The senescence displayed by cultured PDEC likely reflects a similar mechanism as indicated by the increase in p16INK4A levels observed when the cells are maintained in culture. In vivo however, the link between p16INK4A upregulation and senescence induction has not been firmly established. Although p16INK4A levels are elevated in aging or stressed senescent tissues in vivo, so are p53 levels, and studies utilizing genetically engineered mice have implicated both p16INK4A and p53 in affecting senescence under these conditions (Campisi and d'Adda di Fagagna, 2007; Collado et al., 2007). Thus, it has been postulated that the relative contribution of the p16INK4A and p53 pathways to senescence might depend on the cellular context and the type of stress signals (Campisi and d'Adda di Fagagna, 2007). Our present findings implicate selectively p16INK4A in a heretofore unreported senescence response invoked in vivo by inflammatory stress in the absence of oncogene activation. The precise nature of the signals elicited by the inflammatory milieu to promote the p16INK4A-dependent induction of PDEC senescence remains to be established. However, experimental acute pancreatitis triggered by caerulein administration has been shown to induce an increase in the levels of proinflammatory cytokines such as TNF-alpha, IL-6, IL-8, and IL-10 (Fu et al., 1997; Norman, 1998; Van Laethem et al., 1998). IL-6 and IL-8 have both been implicated in the senescence response to oncogenic stress (Acosta et al., 2008; Kuilman et al., 2008), thus raising the possibility that they could play a similar role in the setting of pancreatic inflammation.
The in vivo and in vitro abrogation of senescence by oncogenic KRas reported here represents a departure from the more frequently described senescence-triggering effect of oncogenic Ras. The latter has been linked predominantly to hyperproliferative signals that result from a high Ras gene dosage (DeNicola and Tuveson, 2009; Ji et al., 2009; Sarkisian et al., 2007). Our findings demonstrate that PDEC harboring an endogenous allele of oncogenic KRas not only fail to activate the senescence program but are wired to suppress senescence through the upregulation of Twist. This observation is consistent with an earlier finding that Twist suppresses the senescence of immortalized human prostate cell lines (Kwok et al., 2007). Moreover, a connection between Twist expression and senescence bypass has been documented recently in the setting of oncogenic stress and has been attributed to the activity of Twist as a transcriptional repressor of the two senescence inducers p16INK4A and p21CIP (Ansieau et al., 2008). PDEC expressing oncogenic KRas rely on the same mechanism, namely Twist-dependent transcriptional repression of p16INK4A, to override premature senescence caused by environmental stress. As such, the coincident expression of oncogenic KRas and Twist in PDEC may facilitate the initiation of the tumorigenic state through the inactivation of a proliferative barrier imposed by p16INK4A expression. The increased expression of Twist displayed by human pancreatic cancer cells lends further support to this idea (Satoh et al., 2008). It is noteworthy that in studies utilizing mouse models that are different from the one employed in this study, the endogenous expression of oncogenic KRas in the pancreas has been reported to trigger senescence in preneoplastic lesions (Collado et al., 2005; Morton et al., 2010). The factors that might contribute to this apparent discrepancy remain to be delineated but could in principle include both cell autonomous and non-cell autonomous determinants related to strain-dependent variations in the expression levels of oncogenic KRas, the types of KRas activating mutation, and the Cre-driving promoters.
The loss of p16INK4A function occurs in approximately 90% of pancreatic cancers and is brought about by mutation, deletion and epigenetic silencing. However, the inactivation of p16INK4A is generally seen at a later stage of neoplastic progression subsequent to the acquisition of oncogenic KRas mutations (Moskaluk et al., 1997; Wilentz et al., 1998). Therefore, the expression of Twist may allow oncogenic KRas-expressing PDEC to escape senescence at an earlier stage when the p16INK4A locus has not been disrupted yet. The pressure to lose p16INK4A function as the disease advances might reflect alterations in Twist transcriptional repressive activity possibly resulting from changes in the abundance and/or post-translational modifications of Twist binding partners. The finding that the oncogenic KRas-Twist axis is exploited in the context of inflammation-associated senescence is of particular interest given the compelling evidence both from human studies and experimental models for a strong link between pancreatitis and an increased risk of pancreatic cancer (Carriere et al., 2009; Gidekel Friedlander et al., 2009; Guerra et al., 2007; Lowenfels et al., 1993; Malka et al., 2002; Morris et al., 2010). Accordingly, Twist expression could critically affect the impact of inflammatory conditions on the tumorigenic potential of pancreatic ductal cells harboring oncogenic KRas.
The induction of Twist by Ras is not unprecedented (Liu et al., 2009) and studies in Drosophila have reported genetic interactions between Twist and Ras (Hurlbut et al., 2009). However, the precise signaling events by which Ras stimulates Twist expression have not been defined. Indeed, it appears that multiple Ras effector pathways can contribute to an increase in Twist transcripts. These include the PI3K and ERK pathways as well as the NF-κB, JAK/STAT, and GSK-3β/β-catenin pathways (Dupont et al., 2001; Hong et al., 2009; Howe et al., 2003; Pham et al., 2007; Sosic et al., 2003; Zhu and Tan, 2005). The relative engagement of these pathways would vary depending on cell type and environmental conditions. Thus, the contribution of the Ras-Twist-p16INK4A axis to senescence bypass is likely to be context-dependent. Furthermore, although Twist has been implicated in the induction of EMT programs in epithelial cells (Yang et al., 2004), our findings indicate this aspect of Twist function is not essential for its senescence suppressing activity.
In addition to being locked in a state of permanent growth arrest, senescent cells have been noted to secrete an increased amount of pro-inflammatory mediators including cytokines, chemokines, growth factors and extracellular proteases (Coppe et al., 2010; Kuilman and Peeper, 2009). Hence the induction of ductal cell senescence under conditions of pancreatic injury and inflammation may constitute a mechanism to maintain and amplify the inflammatory microenvironment. Given the pro-tumorigenic impact of inflammatory conditions, senescent lesions within the pancreas may play a direct role in fueling the neoplastic process by promoting the unscheduled growth of precursor cells harboring KRas mutations.
Experimental Procedures
Mice and caerulein treatment
The LSL-KRasG12D, p16INK4A−/−, p53−/− and, p48-Cre strains have been previously described (Jacks et al., 1994; Jackson et al., 2001; Kawaguchi et al., 2002; Sharpless et al., 2001). 1-month-old mice were subjected to 8 hourly intraperitoneal injections of caerulein (50 ng/g of body weight/injection) on 2 consecutive days. Pancreata were harvested 3 days after the last injection. All animal care and procedures followed National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at NYU School of Medicine.
Isolation, culture, and infection of PDEC
Isolation and culture of PDEC were performed as previously described (Agbunag et al., 2006). PDEC were isolated from 2–3 month-old mice and propagated in Matrigel (Becton Dickinson). GFP pAdEasy-1 and Cre pAdEasy-1 adenoviral vectors were gifts from Gustavo Leone. Purified adenovirus particles were obtained by CsCl equilibrium centrifugation. 600 adenovirus particles/cell were added to the PDEC suspension. In a microfuge tube, cell/virus mixtures were rocked every 15 min for 1 h at 37°C, and then embedded in Matrigel. Two days later, the infection was repeated. PDEC were propagated in Matrigel, and then transferred to a 1% Matrigel-coated plate at the time of the experiment. Lentiviral vectors containing shRNAs directed against the Twist gene (shRNA Twist3 and shRNA Twist5) and control shRNA directed against the GFP gene were kind gifts from the Robert A. Weinberg laboratory (Yang et al., 2004). KRasG12D PDEC or p16INK4A−/− PDEC were infected with lentivirus (MOI = 20) using 10 µg/ml polybrene (Chemicon), cultivated on Matrigel with medium containing 2% Matrigel for 3 days, and transferred to a 1% Matrigel-coated plate.
KRasG12D allele recombination assay
For verification of Cre-mediated recombination, DNA was prepared from LSL-KRasG12D PDEC infected with adenoviral-GFP (WT) or adenoviral-Cre (KRasG12D), or from pancreatic ducts or pancreata from 1-month-old WT or p48-Cre;LSL-KRasG12D mice by using DNeasy Blood & Tissue kit (Qiagen). Genomic DNA was amplified by PCR to demonstrate 265 bp and 305 bp products that are specific for the WT and recombined alleles, respectively (Jackson et al., 2001).
Ras activation assay
The levels of Ras–GTP were determined by the GST–RBD pull-down assay, as described previously (Boykevisch et al., 2006). The following antibodies were used: mouse anti-KRas (Santa-Cruz) and mouse anti-α-Tubulin (Sigma).
Senescence-associated-β-galactosidase (SA-β-gal) assay
Adherent cells or frozen sections of pancreatic tissue were fixed with 2% formaldehyde/0.2% glutaraldehyde in PBS for 3–5 min, washed with PBS, stained at 37°C for 12–16 h in X-Gal solution (1 mg/ml X-Gal, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, and 1 mM MgCl2 in PBS at pH6.0), and counterstained with Hoechst 33342 (Invitrogen) for β-gal quantification. Slides were examined on a Zeiss Axiovert 200M microscope. For quantification of SA-β-gal staining in PDEC cultures, at least 150 cells/FOV were counted. In the pancreatic tissue, pancreatic ducts bearing 10% or more β-gal-positive cells in each duct were scored as positive.
Immunoblot analysis
Cells were lysed with 25 mM Tris at pH7.5, 120 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 1% NP-40, 10% Glycerol, 10 µg/ml aprotinin, 1 mM phenylmethanesulfonyl fluoride (PMSF), 1 mM Na2VO4, 0.25% sodium deoxycholate, 10 mM NaF, 10 µg/ml pepstatin, 10 µg/ml leupeptin, 10 µg/ml trypsin inhibitor, and 10 mM benzamidine. Cell lysates were separated by electrophoresis in SDS polyacrylamide gels and transferred to nitrocellulose membranes. After blocking, the membranes were incubated with primary antibodies. Subsequently, membranes were incubated with IRDye 800-conjugated goat anti-rabbit (Rockland) or Alexa Fluor 680 goat anti-mouse (Molecular Probes) and visualized with the Odyssey Infrared Imaging System (LiCor). The following antibodies were used: rabbit anti-p16INK4A (Santa-Cruz), rabbit anti-p19ARF (gift from Charles J. Sherr), mouse anti-p21CIP (Becton Dickinson), mouse anti-p53 (Santa-Cruz), rabbit anti-Twist (Santa-Cruz), and mouse anti-α-Tubulin (Sigma).
Quantitative RT-PCR
Total RNA was extracted from PDEC or pancreata by using RNeasy mini kit (Qiagen) and reverse transcribed with QuantiTect reverse transcription kit (Qiagen). PCR reactions were performed using the SYBR Green PCR Master Mix (USB). Expression levels were normalized by cyclophilin A.
Human pancreas specimens
The use of human tissue for this study was reviewed and approved by the Institutional Review Board of NYU School of Medicine and samples were obtained after informed consent. 5 µm sections were cut from formalin-fixed paraffin-embedded samples for the purpose of immunohistochemistry.
Histology and immunofluorescence
Pancreata were removed and snap frozen in OCT compound (Tissue-Tek). Sections of 8 µm were air-dried and hydrated in 70% ethanol. H&E staining was performed by incubation in hematoxylin (Sigma) followed by eosin (Sigma). Sections were dehydrated (70, 95, 100% ethanol) and mounted with Permount (Fisher). For immunofluorescence of pancreata, frozen sections were fixed in 4% paraformaldehyde for 10 min, permeabilized with 0.25% Triton X-100 for 10 min, and blocked with 10% serum/0.1% Tween-20 for 1 h. Slides were incubated with primary antibodies diluted in 1% BSA/0.5% Tween-20 overnight at 4°C. Slides were then incubated with Alexa Fluor-labeled secondary antibodies (Invitrogen) diluted in 1% BSA for 1 h and mounted using Vectashield mounting medium with DAPI (Vector Laboratories). For immunofluorescence of PDEC, cells grown on 1% Matrigel-coated coverslips were fixed in 3% paraformaldehyde for 30 min, permeabilized with 0.2% Triton X-100 for 10 min, and blocked with 2% BSA for 1 h. PDEC were incubated with primary antibodies diluted in 2% BSA for 1h followed by Alexa Fluor-labeled secondary antibodies (Invitrogen) diluted in 2% BSA for 1 h. PDEC were then incubated with DAPI for 15 min and mounted with Immuno-mount (Shandon) containing 0.04% paranitrodiphenylene (Sigma). Slides were examined on a Zeiss Axiovert 200M microscope. The following antibodies were used: rat anti-CD45 (Becton Dickinson), rat anti-CK19 (TromaIII, developed by Rolf Kemler and obtained from Developmental Studies Hybridoma Bank), mouse anti-E-cadherin (Becton Dickinson), rabbit anti-Ki67 (Novocastra), rabbit anti-p16INK4A (Santa-Cruz), and mouse anti-α-SMA (Sigma).
Immunohistochemistry
Pancreata were fixed in 10% formalin overnight and embedded in paraffin. For immunohistochemistry, slides (5 µm) were deparaffinized, rehydrated, were quenched in 0.6% hydrogen peroxide/methanol for 15 min, and antigens were boiled for 15 min in a microwave oven in 10 mM sodium citrate (pH6.0) for antigen retrieval. Sections were blocked with 5% serum/1% BSA/0.5% Tween-20 for 1 h. Slides were incubated with primary antibodies diluted in blocking buffer overnight at room temperature. Following the primary antibody, slides were incubated with biotinylated secondary antibodies (Vector Laboratories) followed by ABC solution (Vector Laboratories) and developed with 3,3′-diaminobenzidine tetrahydrochloride for 15 min. Slides were counterstained with hematoxylin, dehydrated, and mounted with Permount (Fisher). Slides were examined on a Zeiss Axiovert 200M microscope. The following antibodies were used: mouse anti-p16INK4A (Santa-Cruz) and rat anti-CD45 (Becton Dickinson).
Statistical analyses
Data were analyzed by Student's t test (paired, two-tailed) and results were considered significant at p-value < 0.05. Results are presented as mean and standard deviation (±SD).
Supplementary Material
Significance.
Cellular senescence, a state of stable proliferative arrest, is a potent tumor suppressor mechanism and senescence bypass represents an important step in tumor development. In this study we demonstrate that inflammatory stress can provoke a p16INK4A-dependent senescence of pancreatic ductal cells, and that this response is abrogated by oncogenic KRas through the upregulation of Twist and the consequent suppression of p16INK4A expression. These findings identify a mechanism by which oncogenic KRas may contribute to pancreatic tumorigenesis and warrant the evaluation of oncogenic KRas-mediated senescence escape as a potential target for therapeutic intervention.
Acknowledgements
We are grateful to T. Jacks, D.A. Tuveson, R.A. DePinho, C.V. Wright, R.A. Weinberg, G. Leone, H.C. Crawford, C.J. Sherr, R. Kemler for mice and reagents. We thank C. H. Hajdu for support and advice with human pancreatic tissue collection, J. Mallen-St Clair for advice on the animal experiments, L.J. Taylor for help with data analysis and manuscript preparation, E. Hernando for helpful suggestions, J.L. Zhou for technical assistance, and all members of the Bar-Sagi laboratory for comments and discussions. This work was supported by National Institutes of Health Grant CA055360 (D.B.-S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Agbunag C, Bar-Sagi D. Oncogenic K-ras drives cell cycle progression and phenotypic conversion of primary pancreatic duct epithelial cells. Cancer Res. 2004;64:5659–5663. doi: 10.1158/0008-5472.CAN-04-0807. [DOI] [PubMed] [Google Scholar]
- Agbunag C, Lee KE, Buontempo S, Bar-Sagi D. Pancreatic duct epithelial cell isolation and cultivation in two-dimensional and three-dimensional culture systems. Methods Enzymol. 2006;407:703–710. doi: 10.1016/S0076-6879(05)07055-2. [DOI] [PubMed] [Google Scholar]
- Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Weinberg RA. When cells get stressed: an integrative view of cellular senescence. J Clin Invest. 2004;113:8–13. doi: 10.1172/JCI200420663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennecke M, Kriegl L, Bajbouj M, Retzlaff K, Robine S, Jung A, Arkan MC, Kirchner T, Greten FR. Ink4a/Arf and oncogene-induced senescence prevent tumor progression during alternative colorectal tumorigenesis. Cancer Cell. 2010;18:135–146. doi: 10.1016/j.ccr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Boykevisch S, Zhao C, Sondermann H, Philippidou P, Halegoua S, Kuriyan J, Bar-Sagi D. Regulation of ras signaling dynamics by Sos-mediated positive feedback. Curr Biol. 2006;16:2173–2179. doi: 10.1016/j.cub.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Carriere C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem Biophys Res Commun. 2009;382:561–565. doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola GM, Tuveson DA. RAS in cellular transformation and senescence. Eur J Cancer. 2009;45(Suppl 1):211–216. doi: 10.1016/S0959-8049(09)70036-X. [DOI] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Dupont J, Fernandez AM, Glackin CA, Helman L, LeRoith D. Insulin-like growth factor 1 (IGF-1)-induced twist expression is involved in the anti-apoptotic effects of the IGF-1 receptor. J Biol Chem. 2001;276:26699–26707. doi: 10.1074/jbc.M102664200. [DOI] [PubMed] [Google Scholar]
- Fu K, Sarras MP, Jr, De Lisle RC, Andrews GK. Expression of oxidative stress-responsive genes and cytokine genes during caerulein-induced acute pancreatitis. Am J Physiol. 1997;273:G696–G705. doi: 10.1152/ajpgi.1997.273.3.G696. [DOI] [PubMed] [Google Scholar]
- Gidekel Friedlander SY, Chu GC, Snyder EL, Girnius N, Dibelius G, Crowley D, Vasile E, DePinho RA, Jacks T. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell. 2009;16:379–389. doi: 10.1016/j.ccr.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Hong KO, Kim JH, Hong JS, Yoon HJ, Lee JI, Hong SP, Hong SD. Inhibition of Akt activity induces the mesenchymal-to-epithelial reverting transition with restoring E-cadherin expression in KB and KOSCC-25B oral squamous cell carcinoma cells. J Exp Clin Cancer Res. 2009;28:28. doi: 10.1186/1756-9966-28-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe LR, Watanabe O, Leonard J, Brown AM. Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Res. 2003;63:1906–1913. [PubMed] [Google Scholar]
- Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- Hurlbut GD, Kankel MW, Artavanis-Tsakonas S. Nodal points and complexity of Notch-Ras signal integration. Proc Natl Acad Sci U S A. 2009;106:2218–2223. doi: 10.1073/pnas.0812024106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Ji B, Tsou L, Wang H, Gaiser S, Chang DZ, Daniluk J, Bi Y, Grote T, Longnecker DS, Logsdon CD. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–1082. doi: 10.1053/j.gastro.2009.05.052. 1082 e1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- Kwok WK, Ling MT, Yuen HF, Wong YC, Wang X. Role of p14ARF in TWIST-mediated senescence in prostate epithelial cells. Carcinogenesis. 2007;28:2467–2475. doi: 10.1093/carcin/bgm185. [DOI] [PubMed] [Google Scholar]
- Li L, Cserjesi P, Olson EN. Dermo-1: a novel twist-related bHLH protein expressed in the developing dermis. Dev Biol. 1995;172:280–292. doi: 10.1006/dbio.1995.0023. [DOI] [PubMed] [Google Scholar]
- Liu M, Casimiro MC, Wang C, Shirley LA, Jiao X, Katiyar S, Ju X, Li Z, Yu Z, Zhou J, et al. p21CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc Natl Acad Sci U S A. 2009;106:19035–19039. doi: 10.1073/pnas.0910009106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andren-Sandberg A, Domellof L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, Levy P, Ruszniewski P. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849–852. doi: 10.1136/gut.51.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JPt, Cano DA, Sekine S, Wang SC, Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508–520. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B, Jamieson NB, Oien KA, Lowy AM, Brunton VG, et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107:246–251. doi: 10.1073/pnas.0908428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997;57:2140–2143. [PubMed] [Google Scholar]
- Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76–83. doi: 10.1016/s0002-9610(97)00240-7. [DOI] [PubMed] [Google Scholar]
- Pham CG, Bubici C, Zazzeroni F, Knabb JR, Papa S, Kuntzen C, Franzoso G. Upregulation of Twist-1 by NF-kappaB blocks cytotoxicity induced by chemotherapeutic drugs. Mol Cell Biol. 2007;27:3920–3935. doi: 10.1128/MCB.01219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- Satoh K, Hamada S, Kimura K, Kanno A, Hirota M, Umino J, Fujibuchi W, Masamune A, Tanaka N, Miura K, et al. Up-regulation of MSX2 enhances the malignant phenotype and is associated with twist 1 expression in human pancreatic cancer cells. Am J Pathol. 2008;172:926–939. doi: 10.2353/ajpath.2008.070346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, Wu EA, Horner JW, DePinho RA. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, DePinho RA. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- Sosic D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169–180. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- Van Laethem JL, Eskinazi R, Louis H, Rickaert F, Robberecht P, Deviere J. Multisystemic production of interleukin 10 limits the severity of acute pancreatitis in mice. Gut. 1998;43:408–413. doi: 10.1136/gut.43.3.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- Wilentz RE, Geradts J, Maynard R, Offerhaus GJ, Kang M, Goggins M, Yeo CJ, Kern SE, Hruban RH. Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res. 1998;58:4740–4744. [PubMed] [Google Scholar]
- Willemer S, Elsasser HP, Adler G. Hormone-induced pancreatitis. Eur Surg Res. 1992;24(Suppl 1):29–39. doi: 10.1159/000129237. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Zhu YQ, Tan XD. TFF3 modulates NF-{kappa}B and a novel negative regulatory molecule of NF-{kappa}B in intestinal epithelial cells via a mechanism distinct from TNF-{alpha} Am J Physiol Cell Physiol. 2005;289:C1085–C1093. doi: 10.1152/ajpcell.00185.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.