Abstract
The infective juveniles (IJs) of entomopathogenic nematodes (EPNs) seek out host insects and release their symbiotic bacteria into their body cavity causing septicaemia, which eventually leads to host death. The interaction between EPNs and their hosts are only partially understood, in particular the host immune responses appears to involve pathways other than phagocytosis and the canonical transcriptional induction pathways. These pathways are genetically tractable and include for example clotting factors and lipid mediators. The aim of this study was to optimize the nematode infections in Drosophila melanogaster larvae, a well-studied and genetically tractable model organism. Here we show that two nematode species namely Steinernema feltiae and Heterorhabditis bacteriophora display different infectivity toward Drosophila larvae with the latter being less pathogenic. The effects of supporting media and IJ dosage on the mortality of the hosts were assessed and optimized. Using optimum conditions, a faster and efficient setup for nematode infections was developed. This newly established infection model in Drosophila larvae will be applicable in large scale screens aimed at identifying novel genes/pathways involved in innate immune responses.
Keywords: Drosophila melanogaster, Heterorhabditis bacteriophora, Galleria mellonella, Steinernema feltiae, entomopathogenic nematodes
Introduction
Entomopathogenic nematodes (EPNs) are soil-dwelling parasitic worms that infect and kill insects.1 Like many other nematode parasites, they exhibit non-feeding, free-living stages, also known as infective juveniles (IJs) that are tightly associated with their specific symbiotic bacteria. During an infection, IJs enter the insect host through their natural openings or by penetrating the cuticle, and release the symbiotic bacteria that kill and digest host tissues, thereby providing nutrients for the development of both nematodes and bacteria.2 To ensure a successful invasion and completion of their life cycle, EPNs and their symbionts employ various strategies to evade or suppress host immune responses, including the secretion of proteases,3 phenoloxidase inhibitors and toxins that interfere for example with phagocytosis.4,5 Potential insect hosts rely on both general immune mechanisms and mechanisms that are specific for EPNs.6 The first line of defense consists of physical structural barriers, such as the cuticle and the peritrophic membrane of the midgut and the respective epithelia. Once these barriers are breached, the insect defends itself against bacterial or parasitic infection using both cellular and humoral immune responses. Cellular immune reactions are mediated by different types of immune cells, which may contribute to phagocytosis, nodule formation and encapsulation. The nematode Heterorhabditis bacteriophora has been observed to be encapsulated by its insect host Manduca sexta.7 The humoral effectors recruited by host insects include the inducible antimicrobial peptides (AMPs), cell adhesion molecules, clotting components and the prophenoloxidase system.7-9 Those humoral factors are secreted into the hemolymph by both the fat body and by hemocytes. It has been shown that a set of mRNAs coding for specific pattern recognition proteins in M. sexta are upregulated upon the exposure of hosts to Heterorhabditis or its symbiont Photorhabdus luminescens alone.10
Besides their wide application as a biological control for insect pests,11 EPNs and their insect hosts are increasingly used as models for human parasitic nematode and vector-borne disease.6,12 Although a great deal of knowledge about the interaction between EPNs and their hosts, particularly on host immune responses has been gained from these studies, a more complete picture could be obtained by taking advantage of Drosophila melanogaster, a well-studied and genetically tractable model organism. The completion of the Drosophila genome sequencing project, microarray analysis and the use of genetic screens have led to a better understanding of host-pathogen interactions13 and hold the same promise for studies of EPN infections.
EPN infections of Drosophila were performed by Hallem et al.,14 who provided the first insights into the underlying effector mechanisms. The infection of Drosophila larvae with Heterorhabditis/Photorhabdus resulted in the temporary dynamic expression of a subset of antimicrobial peptide genes, a response that is induced specifically by the symbiotic bacterium Photorhabdus. However, Drosophila larvae with defects in the induction of AMPs show the same sensitivity toward EPNs as controls, indicating a dispensable role of AMPs and in fact of both the imd and Toll pathway in this infection model.14 In addition, Drosophila mutants for phagocytic receptors (Nimrod1 and Eater) survive the Heterorhabditis/Photorhabdus infection which is in line with the previously observed suppression of phagocytosis by Photorhabdus.4,9,15 In contrast, there are indications that several clotting factors help to combat nematode infection and that lipid mediators are involved.9,15 Nonetheless, the molecular basis of Drosophila-EPN interactions is far from being completely understood.
To take full advantage of the combination of EPN infections and Drosophila genetics we wished to optimize the conditions for nematode infections in Drosophila larvae. This included the use of two EPN models (Heterorhabditis bacteriophora with its bacterium Photorhabdus luminescens and Steinernema feltiae with Xenorhabdus bovienii), different supporting media and different doses of IJs. Finally we developed a fast and efficient strategy for nematode infection, which allowed us to continuously monitor the infection process and will be suitable for large-scale screens to identify genes that modulate EPN infections.
Results
Entomopathogenic nematodes of the genera Steinernema and Heterorhabditis are able to infect a broad range of insect larvae, however, they often display specific host preferences.16 In Drosophila melanogaster, Hallem et al. have shown that Drosophila larvae were susceptible to IJs of Heterorhabditis bacteriophora,14 yet no study has been performed to compare the infectivity of the two different nematode species to Drosophila larvae. Here we tested the infectivity of H. bacteriophora and S. feltiae by infecting Drosophila larvae with high dose (1,000 IJs/larva). Figure 1 shows that most of the larvae were killed by S. feltiae already 24 h after infection, and all host larvae were dead after 48 h; whereas far fewer larvae succumbed to H. bacteriophora after both 24 h and 48 h. Clearly, both nematode species displayed the ability to infect and kill Drosophila larvae, but S. feltiae is more pathogenic than H. bacteriophora against Drosophila larvae.
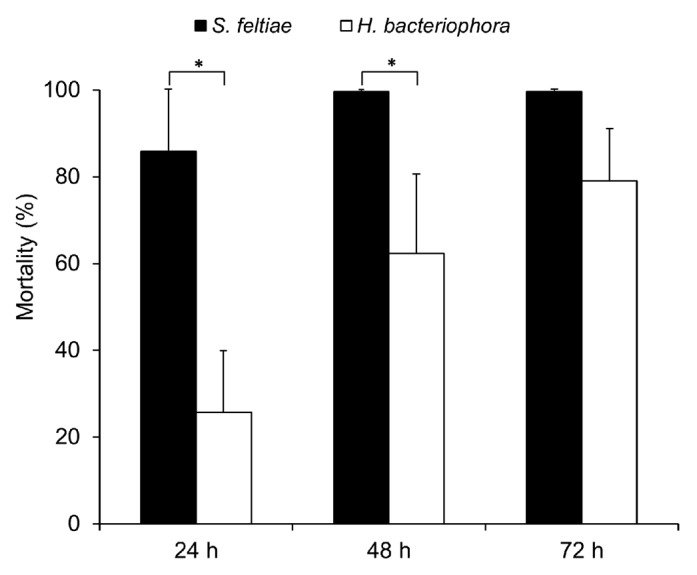
Figure 1. Infectivity of S. feltiae or H. bacteriophora on Drosophila larvae. Larvae were infected with nematodes at an infectious dose of 1000 IJs/larva in a 96-well assay with tissue paper as described in Material and Methods (mean ± S.D.; confidence levels are: *: < 0.05).
Since different supporting media applied in the nematode infection establishment may have different effect on infectivity, due to differences in host foraging strategies,17 we next assessed the effect of media (agarose and tissue paper) on nematode infection. Considering the high mortality rate caused by the high dose of nematodes, we conducted the infection with a lower dose IJs suspension (100 IJs/larva). For both nematode species, the infection involving tissue paper soaked with nematode suspension was more effective in killing host larvae than the one involving agarose (Fig. 2) but only with Steinernema, these effects were significant. For S. feltiae, host mortality increased significantly when tissue paper was employed, particularly at 48 h and 72 h post infection (p < 0.01 in Fig. 2). In addition, the replicates on tissue paper appeared to show somewhat less variation in comparison to that on agarose. Therefore, tissue paper was selected as the better support medium for nematode infection and was used to further studies.
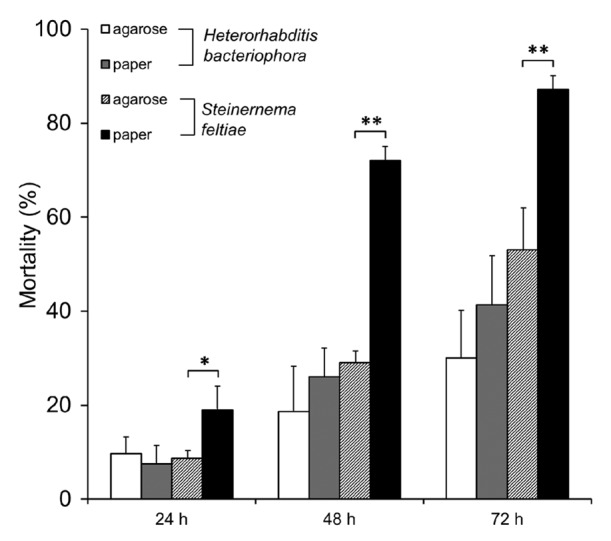
Figure 2. Effect of different supporting media on mortality rate of Drosophila larvae in nematode infection with S. feltiae or H. bacteriophora. Larvae were infected with nematodes at an infectious dose of 100 IJs/larva in a 96-well assay as described in Material and methods (mean ± S.D.; confidence levels are: *: < 0.05; **: < 0.01).
To establish nematode infections for use in genetic screens in Drosophila, it is important to identify an optimal dosage, which results in low-to-intermediate mortality rate of the wild-type Drosophila, allowing detection of increased mortality caused by of loss-of-function or RNAi-mediated knockdowns. Conversely high mortality rates may decrease in gain-of-function backgrounds. Therefore different doses of IJs from Heterorhabditis (25, 100 and 1,000 IJs/larva) were assayed in W1118 larvae. The mortality of Drosophila larvae showed dose dependence (Fig. 3). With the minimum dose of H. bacteriophora tested here, only 30% of the larvae were killed at 72 h post infection; a moderate mortality rate was obtained with a dose of 100 IJs/larva, while with 1,000 IJs/ larva, around 80% were dead after 72 h (Fig. 3). We conclude that to identify negative effects on the immune response against EPNs, 100 IJs/larva is the optimal dose for a single host challenged at RT, while the ten times higher concentration may be suitable to identify dominant activators of the immune response.
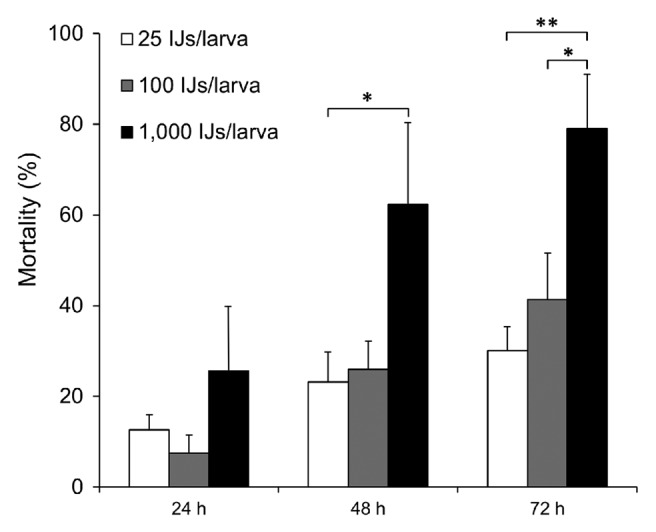
Figure 3. Effect of different doses on mortality rate of Drosophila larvae in nematode infection with H. bacteriophora. Larvae were infected with nematodes at the indicated infectious doses in a 96-well assay as described in Material and Methods (mean ± S.D; confidence levels are: *: < 0.05; **: < 0.01).
In addition to allowing detection of modifiers of the immune response, the feasibility of genetic screens also depends on the time and effort required for the phenotypic analysis. Since the 96-well plate assay we have been using so far requires individual inspection of the larvae in each well, we wished to establish an easier and faster assay. Therefore we developed a ‘plastic bag assay’ in which 50 host larvae were mixed with IJs in a transparent re-sealable plastic bag (Fig. S1). To score the survival easily we used the H. bacteriophora/GFP expressing P. luminescens complex (Heterorhabditis bacteriophora TT01), since both infected and dead hosts could be easily scored as GFP-positive (Figs. 4B and S1). To test the plastic bag assay, we used a previously described line (black cells/imd double mutant, Bc/imd), for which increased susceptibility toward Heterorhabditis/Photorhabdus had been described indicating a more subtle role for both crystal cells and the imd pathway during EPN infections.15 Using the novel assay, we could fully confirm the increase in susceptibility. Titration of the nematodes may even indicate that the assay is more robust and less sensitive to variation in nematode concentration (Fig. 4A) since all nematode densities caused significantly higher mortality in the positive control, Bc/imd. Both the GFP-expressing bacteria in the hemolymph and the nematodes were visible in infected larva that had been retrieved from the plastic bags and inspected under UV light. Septicaemia with GFP-Photorhabdus led to a signal throughout the hemolymph while Heterorhabditis nematodes were visible as dark shades in the GFP signal (Fig. 4B).
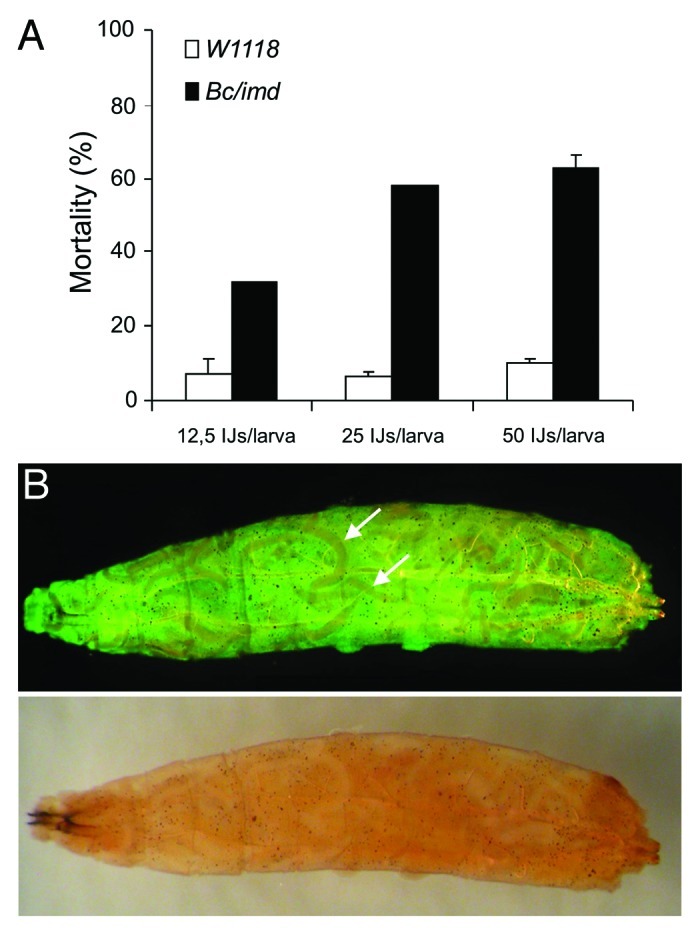
Figure 4. (A) The plastic bag assay recapitulates the results of a 96-well assay in a comparison between wild-type and Bc/imd larvae. Mortality of D. melanogaster was documented 48h after the infection with different doses of H. bacteriophora in plastic bags at 29°C, (B) a Bc/imd larva infected with H. bacteriophora harbouring GFP-expressing P. luminescens, visualized under UV light (upper part, arrows – note the nematodes which are visible as black shades in an green fluorescent background due to systemic infection with GFP-expressing Photorhabdus) and normal light (lower part).
Discussion
The aim of this study was to optimize the conditions to use Drosophila melanogaster as a model host for infection with entomopathogenic nematodes. Our goal was to identify the optimal conditions for a previously described assay that involves 96-well plates and to develop a faster assay that facilitates continuous monitoring of the infected larvae and is suitable for larger screens. A key result is the observation that when Drosophila larvae are used as hosts, Steinernema appears to be the more effective parasite and therefore parasitisation strategies may differ between the two species. This should be taken into account when modifier screens are designed. For example in order to identify dominant modifiers, based on the work presented here it is possible to use either a high infectious dose of Heterorhabditis or a lower concentration of Steinernema. Such screens may partially identify the same effector mechanisms but also responses that are specific and may correlate with the level of specialization.
One aspect of this specialization is even apparent in our simple infection setup, where we observe differences in the support medium (paper vs. agarose) with the Steinernema and not when we use Heterorhabditis. This finding is consistent with previously described differences in the ways the two nematodes seek out their prospective hosts. In case of agarose it is possible for larvae to move to the top of wells and partly avoid the contact with nematodes. Wrinkled tissue paper fills the whole well and the distribution of nematodes is expected to be more equal. The difference in mortality between media is not apparent during H. bacteriophora infections. We propose that the explanation may lie in the different ecology and behavior of the two nematode species. H. bacteriophora cruise to find hosts and are not dependent on the medium whereas S. feltiae can benefit more from tissue paper because it is intermediary in its search behavior sharing some characteristics of both ambush and cruise foragers.17,18
To identify positive regulators and effectors involved in the host immune response, we have established conditions for both the 96-well and the plastic bag assay that lead to moderate mortality of Drosophila larvae allowing us to monitor an increase in mortality in hypomorphic or null-mutants. The lower pathogenicity of the H. bacteriophora made it a suitable infectious agent for screens (Figs. 1 and 3). To allow larger screens for host immune responses in a systemic and time-efficient manner, we developed the plastic bag assay, by setting up an infection of a group of host larvae sharing the same space, rather than being infected singly. Additionally, the application of the H. bacteriophora harbouring GFP expressing P. luminescens made it feasible to monitor the infection from a very early time point after infection and in real-time (see Supplemental Material). A drawback of this method is that a small fraction of the larvae are injured and may be infected due to crowding and injuring from other larvae rather than the infection with EPNs (i.e., they were not GFP positive). We anticipate that the method in its present version will be suitable for targeted screens with a throughput of dozens of genes/month.
The tripartite model: nematode, bacteria and Drosophila larva, has been recently employed in several studies to identify factors involved the host immune response.9,14,15 However, the full picture of the host-pathogen interaction is far from being understood. We expect that screens based on the methods developed here will help in the further characterization of this natural infection model.
Material and Methods
Culture of nematodes
Entomopathogenic nematodes Steinernema feltiae (isolated from Prosenice) and Heterorhabditis bacteriophora (H222 strain, isolated from Pouzdrany) were kindly provided by Dr. Z. Mracek (Institute of Entomology, Ceske Budejovice, Czech Republic). Both nematodes were cultured in vivo using larvae of the great wax moth, Galleria mellonella at room temperature (RT). Drosophila larvae are not suitable hosts for laboratory culture of EPNs as the yields of IJs are too low for routine use in nematode infection assays. Released IJs were collected and stored in tap water with 0.075% formaldehyde at RT.
Culture of Drosophila melanogaster
Fly strains were kept under standard condition. Infections of Drosophila larvae were conducted with wild-type W1118 from Bloomington stock center (stock 6326) and Bc/imd double mutant generated by us, as positive control due to its high susceptibility to nematode infection.15
Infection of D. melanogaster larvae
Infection of D. melanogaster larvae with IJs was modified after Hallem et al.14 Second and third instar D. melanogaster wild-type larvae (W1118) were rinsed briefly in water and placed in wells of a microtiter plate (96-well, flat bottom, Costar) containing 10 μl of 1.25% agarose (Lonza) or approx. One cm2 of tissue paper. IJs were washed with tap water, diluted to the final density and 10 μl of required nematode suspension was added to each well containing one Drosophila larva. For uninfected controls, 10 ul of tap water was added to each well containing Drosophila larva. The assay plate was covered with Parafilm®.
In the infection assay in plastic bags (Fig. S1), 50 larvae were placed in a re-sealable transparent plastic bag (6 x 10 cm), in which one layer of tissue paper (with stapled edges preventing larvae from drowning) was soaked with different dose of nematode suspension (12.5, 25, 50 IJs/Drosophila larva).
Infections were conducted at RT in the dark. Survival was quantified under a dissecting microscope after 24, 48 and 72 h. Survival rates were determined based on movement, either spontaneous or in response to gentle prodding with forceps and typical reddish coloration of cadavers caused by Photorhabdus19 in case of H. bacteriophora or yellow coloration in case of S. feltiae. GFP-expressing Photorhabdus (a kind gift from Todd Ciche) was used to monitor the infection in the plastic bag assay, see Fig. S1 and Movie S1.
All experiments were run in triplicates using 48–100 larvae of Drosophila larvae per group. The results are expressed as the mean ± SD; the level of significance was analyzed by Student’s t-test.
Supplementary Material
Acknowledgments
Our research is supported by research grants from the Swedish Research Council (VR-NT 2010-5118), Cancerfonden (CAN 2010/553), the Swedish Foundation for International Cooperation in Research and Higher Education (STINT), the Carl-Tryggers Foundation (P.H. and U.T.) and the Grant Agency of the Czech Republic (P.H., GA206/09/P470).
Glossary
Abbreviations:
- AMPs
antimicrobial peptides
- EPNs
entomopathogenic nematodes
- IJs
infective juveniles
- RT
room temperature
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental material can be found at: www.landesbioscience.com/journals/fly/article/19553
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/19553
References
- 1.Bednarek A, Gaugler R. Compatibility of soil amendments with entomopathogenic nematodes. J Nematol. 1997;29:220–7. [PMC free article] [PubMed] [Google Scholar]
- 2.ffrench-Constant R, Waterfield N, Daborn P, Joyce S, Bennett H, Au C, et al. Photorhabdus: towards a functional genomic analysis of a symbiont and pathogen. FEMS Microbiol Rev. 2003;26:433–56. doi: 10.1111/j.1574-6976.2003.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 3.Cabral CM, Cherqui A, Pereira A, Simões N. Purification and characterization of two distinct metalloproteases secreted by the entomopathogenic bacterium Photorhabdus sp. strain Az29. Appl Environ Microbiol. 2004;70:3831–8. doi: 10.1128/AEM.70.7.3831-3838.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva CP, Waterfield NR, Daborn PJ, Dean P, Chilver T, Au CP, et al. Bacterial infection of a model insect: Photorhabdus luminescens and Manduca sexta. Cell Microbiol. 2002;4:329–39. doi: 10.1046/j.1462-5822.2002.00194.x. [DOI] [PubMed] [Google Scholar]
- 5.Waterfield NR, Ciche T, Clarke D. Photorhabdus and a host of hosts. Annu Rev Microbiol. 2009;63:557–74. doi: 10.1146/annurev.micro.091208.073507. [DOI] [PubMed] [Google Scholar]
- 6.Castillo JC, Reynolds SE, Eleftherianos I. Insect immune responses to nematode parasites. Trends Parasitol. 2011;27:537–47. doi: 10.1016/j.pt.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Li XY, Cowles RS, Cowles EA, Gaugler R, Cox-Foster DL. Relationship between the successful infection by entomopathogenic nematodes and the host immune response. Int J Parasitol. 2007;37:365–74. doi: 10.1016/j.ijpara.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Wilhelmsson C, Hyrsl P, Loof TG, Dobes P, Klupp M, et al. Pathogen entrapment by transglutaminase--a conserved early innate immune mechanism. PLoS Pathog. 2010;6:e1000763. doi: 10.1371/journal.ppat.1000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eleftherianos I, Joyce S, Ffrench-Constant RH, Clarke DJ, Reynolds SE. Probing the tri-trophic interaction between insects, nematodes and Photorhabdus. Parasitology. 2010;137:1695–706. doi: 10.1017/S0031182010000508. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers RU. Mass production of entomopathogenic nematodes for plant protection. Appl Microbiol Biotechnol. 2001;56:623–33. doi: 10.1007/s002530100711. [DOI] [PubMed] [Google Scholar]
- 12.Stock SP. Insect-parasitic nematodes: from lab curiosities to model organisms. J Invertebr Pathol. 2005;89:57–66. doi: 10.1016/j.jip.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Tzou P, De Gregorio E, Lemaitre B. How Drosophila combats microbial infection: a model to study innate immunity and host-pathogen interactions. Curr Opin Microbiol. 2002;5:102–10. doi: 10.1016/s1369-5274(02)00294-1. [DOI] [PubMed] [Google Scholar]
- 14.Hallem EA, Rengarajan M, Ciche TA, Sternberg PW. Nematodes, bacteria, and flies: a tripartite model for nematode parasitism. Curr Biol. 2007;17:898–904. doi: 10.1016/j.cub.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Hyrsl P, Dobes P, Wang Z, Hauling T, Wilhelmsson C, Theopold U. Clotting factors and eicosanoids protect against nematode infections. J Innate Immun. 2011;3:65–70. doi: 10.1159/000320634. [DOI] [PubMed] [Google Scholar]
- 16.de Doucet MM, Bertolotti MA, Giayetto AL, Miranda MB. Host range, specificity, and virulence of Steinernema feltiae, Steinernema rarum, and Heterorhabditis bacteriophora (Steinernematidae and Heterorhabditidae) from Argentina. J Invertebr Pathol. 1999;73:237–42. doi: 10.1006/jipa.1998.4831. [DOI] [PubMed] [Google Scholar]
- 17.Grewal PS, Lewis EE, Gaugler R, Campbell JF. Host finding behaviour as a predictor of foraging strategy in entomopathogenic nematodes. Parasitology. 1994;108:207–15. doi: 10.1017/S003118200006830X. [DOI] [Google Scholar]
- 18.Simões N, Rosa JS. Pathogenicity and host specificity of entomopathogenic nematodes. Biocontrol Sci Technol. 1996;6:403–12. doi: 10.1080/09583159631370. [DOI] [Google Scholar]
- 19.Poinar GO, Jr., Hess RT, Cole A. Replication of an Iridovirus in a nematode (Mermithidae) Intervirology. 1980;14:316–20. doi: 10.1159/000149202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


