Abstract
During angiosperm evolution, innovations in vegetative and reproductive organs have resulted in tremendous morphological diversity, which has played a crucial role in the ecological success of flowering plants. Morindeae (Rubiaceae) display considerable diversity in growth form, inflorescence architecture, flower size, and fruit type. Lianescent habit, head inflorescence, small flower, and multiple fruit are the predominant states, but arborescent habit, non-headed inflorescence, large flower, and simple fruit states occur in various genera. This makes Morindeae an ideal model for exploring the evolutionary appearances and transitions between the states of these characters. We reconstructed ancestral states for these four traits using a Bayesian approach and combined nuclear/chloroplast data for 61 Morindeae species. The aim was to test three hypotheses: 1) self-supporting habit is generally ancestral in clades comprising both lianescent and arborescent species; 2) changes from lianescent to arborescent habit are uncommon due to “a high degree of specialization and developmental burden”; 3) head inflorescences and multiple fruits in Morindeae evolved from non-headed inflorescences and simple fruits, respectively. Lianescent habit, head inflorescence, large flower, and multiple fruit are inferred for Morindeae, making arborescent habit, non-headed inflorescence, small flower, and simple fruit derived within the tribe. The rate of change from lianescent to arborescent habit is much higher than the reverse change. Therefore, evolutionary changes between lianescent and arborescent forms can be reversible, and their frequency and trends vary between groups. Moreover, these changes are partly attributed to a scarcity of host trees for climbing plants in more open habitats. Changes from large to small flowers might have been driven by shifts to pollinators with progressively shorter proboscis, which are associated with shifts in breeding systems towards dioecy. A single origin of dioecy from hermaphroditism is supported. Finally, we report evolutionary changes from headed to non-headed inflorescences and multiple to simple fruits.
Introduction
During angiosperm evolution, changes in vegetative and reproductive organs have resulted in remarkable morphological diversity, which has played an important role in the ecological success of flowering plants [1]. The fusion of clustered fruits into multiple fruits (or syncarps) has occurred repeatedly in different lineages [1]–[2]. Some multiple fruits are important food sources for a wide range of animals, and the evolution of this type of compound fruit has been hypothesized as a result of selection by large animals [3]. This is based on the fact that multiple fruits are generally favored and their seeds are effectively dispersed by large frugivorous dispersers [4]. Flowering plants from different groups produce edible multiple fruits that are economically important. Examples include jackfruits and breadfruits (Moraceae), pineapples (Bromeliaceae), and noni fruits (Rubiaceae). Despite their crucial roles in different ecosystems and for the human society, little is known about the evolution of multiple fruits. This is partly due to the lack of robust phylogenies for the lineages that contain species producing multiple fruits and species bearing simple fruits. Molecular-based phylogenies are essential for placing patterns of any heritable trait in an evolutionary context [5].
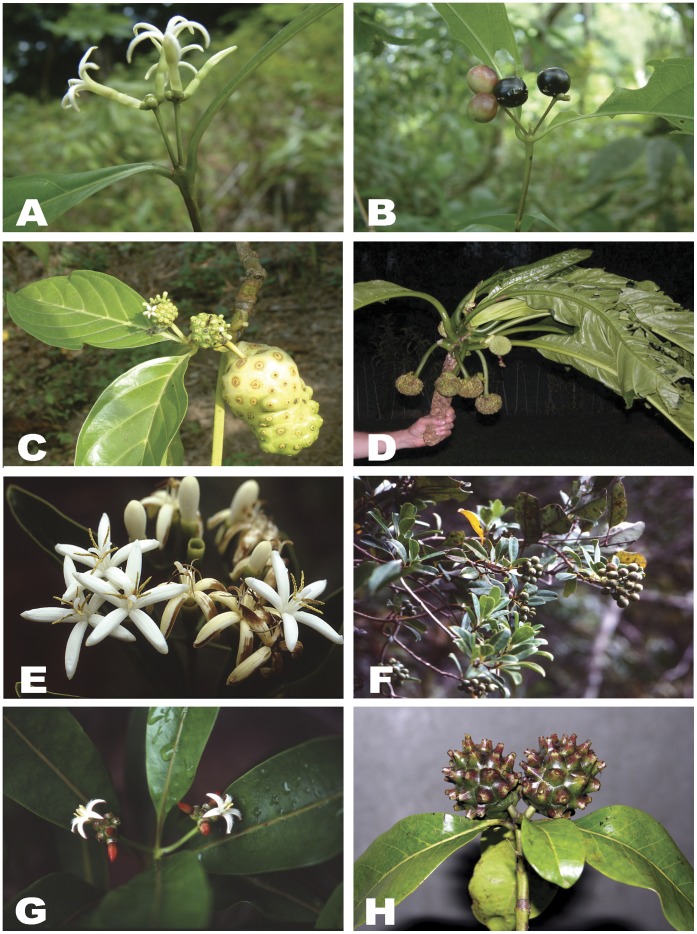
In the coffee family (Rubiaceae), most taxa with multiple fruits are members of the tribes Naucleeae [6]–[7] and Morindeae [8]–[10]. Fruits in Morindeae, belonging to the Psychotrieae alliance in the subfamily Rubioideae, are predominantly multiple fruits composed of two to many fully to basally fused drupaceous (fleshy) fruits (Fig. 1C–D, H), which are derived from ovaries of the adjacent flowers. This type of compound fruit is found in three (Coelospermum Blume, Gynochthodes Blume (Fig. 1H), and Morinda L. (Fig. 1C)) of the five genera currently recognized in the tribe, and is absent in the other two genera (Appunia Hook.f. and Siphonandrium K.Schum.) the infructescences (fruiting stage of inflorescences) of which are formed by clusters of simple, drupaceous fruits (Fig. 1B). A few members of Coelospermum are characterized by branched or headed infructescences bearing pedicellate (stalked), drupaceous fruits (Fig. 1F), while some Gynochthodes species have infructescences composed of pedicellate, drupaceous fruits grouped in umbels (flat-topped or rounded flower/fruit clusters with the pedicels arising from more or less the same point) or fascicles (tight bundles). It has been postulated by McClatchey [11] that multiple fruits of the broadly circumscribed Morinda (Morinda sensu lato), which included all lianescent and arborescent Morinda species with multiple fruits recently transferred to Gynochthodes [8], evolved from an ancestor with umbels and simple fruits by suppression of the pedicels and fusion of the ovaries of the adjacent flowers. This would imply that multiple fruits of Morinda and Gynochthodes (both sensu Razafimandimbison et al. [8]–[9]) are derived in Morindeae.
Figure 1. Characteristics and morphological variation of the tribe Morindeae (for details see text).
A–B: Appunia debilis; C: Morinda citrifolia; D: Morinda pacifica; E: Coelospermum fragrans; F: Coelospermum balansanum; G: Gynochthodes kanalensis; and H: Gynochthodes retusa (A–C by T. D. McDowell; D by F. Tronquet; E–G by J. T. Johansson; and H by K. Kainulainen).
Besides its fruit diversity, Morindeae are also diverse in growth form, inflorescence architecture, and flower size. Lianescent (climbing) habit, headed inflorescence, and small flower are the predominant states, and occur in different genera of the tribe. This makes Morindeae an attractive model for exploring the evolutionary appearances and transitions between the major states of the four major characters (i.e., growth habit, inflorescence architecture, flower size, and fruit type) from a phylogenetic perspective. The recently published molecular phylogeny of Morindeae [9] provides a solid basis for such a study. These traits have been revealed to be evolutionarily labile in Morindeae [9], however a proper ancestral state reconstruction (hereafter called ASR) is essential in order to better understand their evolution. Moreover, a combination of these four characters has been used for circumscribing the five recognized genera of the tribe (Table 1).
Table 1. Morphological characteristics and other important information of the five recognized genera of the tribe Morindeae.
| Appunia Hook.f. | Coelospermum Blume | Gynochthodes sensulato Blume | Morinda sensustricto L.1 | SiphonandriumK.Schum.2 | |
| Geographic distribution | Neotropics | Tropical Asia andAustralasia | Tropical Asia, Australasia, andMadagascar | Pantropical | New Guinea |
| Number of species | Ca. 12 | Ca. 11 | Ca. 95 | Ca. 40 | 1 |
| Growth habit | Mostly frutescent | Mostly woody lianescent | Mostly woody lianescent, | Mostly arborescent and frutescent | Lianescent |
| Inflorescence architecture | Head inflorescences | Mostly non-headed inflorescences | Mostly head inflorescences | Head inflorescences | Head inflorescences |
| Flower size | Large (corolla tubes5–10 (23–24) mm long> corolla lobes 0.5–7 (15–17) mm) long | Small (corolla tubes3–7 (11) mm long <corolla lobes 4.5–16 mm) long | Small (corolla tubes0.7–5.5 mm long <corolla lobes 1.5–11 mm) long | Large (corolla tubes5–40 (80) mm long >corolla lobes 1–14 (22) mm) long | Small (corolla tubesca. 3 mm long >corolla lobes ca. 5 mm long) |
| Breeding systems | Hermaphroditic | Androdioecious or dioecious or functionally dioecious | Androdioecious or dioecious orfunctionally dioecious | Hermaphroditic | Dioecious |
| Fruit type | Simple, drupaceous fruits | Mostly simple, drupaceous fruits | Mostly multiple fruits | Multiple fruits | Simple, drupaceous fruits |
All lianescent, dioecious species of Morinda with small flowers have recently been transferred to Gynochthodes [8].
Filaments of Siphonandrium are tightly fused and its anthers are glued together, all forming a staminal tube. This feature is unique within Morindeae.
Morindeae comprise ca. 100 woody climbing species (ca. 62%), with only ca. 54 arborescent (tree or tree-like) and frutescent (shrubby) species (ca. 28%), and two suffrutescent (shrubby plants having woody stems only at the base) species. Frutescent plants are common in Appunia. Both arborescent and frutescent plants are predominant in Morinda but are extremely rare in Coelospermum (the arborescent C. reticulatum (F.Muell.) Benth.) and Gynochthodes (frutescent G. decipiens (Schltr.) Razafim. & B.Bremer and the arborescent G. trimera (Hillebr.) Razafim. & B.Bremer). Conversely, lianescent plants (woody vines or climbers) in the tribe are found mostly in Gynochthodes [8]–[9], with only one species in Appunia (A. megalantha C.M.Taylor & Lorence, [12]), two species in Morinda (M. longiflora G.Don and M. morindoides (Baker) Milne-Redh., [9]), and 10 species in Coelospermum [13]. There are at least two different (but not mutually exclusive) hypotheses regarding the evolutionary transitions between lianescent and arborescent growth forms. The first hypothesis considers self-supporting (arborescent or frutescent) habit to be the common ancestral condition for clades containing both lianescent and arborescent/frutescent species [14]–[15]. The second hypothesis states that the evolutionary change from lianescent to arborescent/frutescent habit is uncommon, because “the evolution of lianescence can carry a high degree of specialization and developmental burden that might limit evolution back to self-supporting growth forms” [16].
Head inflorescences (or heads) occur when two or more flowers are borne on a common receptacle (the end of the inflorescence stalk upon which the floral organs are borne). In Morindeae, these heads are composed of two to 50 flowers that are clustered tightly on the receptacles (Fig. 1A, C–D, G). These inflorescences may contain a single head or two to several heads, which are in turn arranged into various branching forms: umbels, corymbs (flat-topped or round-topped, racemose inflorescences with the lower pedicels longer than the upper), or panicles (branched clusters of flowers). Within a head the ovaries of the adjacent flowers may be fused or free. The majority of Morindeae species with heads have fused ovaries, but in Appunia (Fig. 1A) and Siphonandrium the ovaries are free. It is important to note that the degree of the fusion of ovaries prior to fruit development (pre-genital fusion) varies greatly among species, from only basally to completely fused. Only 27 Morindeae species (nine from Coelospermum and 18 from Gynochthodes) bear non-headed inflorescences, which are arranged in umbels, or compound umbels, or fascicles (in the latter genus) and panicles or corymbs (in the former genus). We postulated that heads evolved from non-headed inflorescences in Morindeae. In addition, inflorescences in Morindeae are mostly terminal on the shoot, sometimes leaf-opposed ("pseudo-terminal") and axillary. Axillary inflorescences are found in Siphonandrium, at least three species of Appunia, and ca. 18 species of Gynochthodes. Leaf-opposed inflorescences distinguish the mostly Asian, arborescent Morinda clade (including the Neotropical M. royoc L., the pantropical M. citrifolia L., and the African M. chrysorhiza (Thonn.) DC. and M. lucida Benth.) from the remaining Morinda [9]; this type of inflorescence is also known to occur in Appunia (e.g., A. surinamensis (Bremek.) Steyerm.).
In Morindeae, flowers in the same inflorescence or head appear to open successively over days or weeks [17] (Fig. 1A, C, E, G). The flowers vary greatly in size (Table 1); large flowers (corolla tube length/corolla lobe length >1) are found in Appunia and Morinda (Fig. 1A, C) and presumably pollinated by larger insects, such as long-proboscis moths [18]. Plants with small flowers (corolla tube length/corolla lobe length <1) are restricted to Coelospermum, Gynochthodes, and Siphonandrium (Fig. 1E, G), and are most likely to be pollinated by small insects (e.g., short-proboscis moths or small bees or flies) [19]–[20]. Overall, the species of Morinda have larger flowers than the species of Appunia, with the exception of the lianescent A. megalantha (corolla tubes of 23–24 mm long > corolla lobes of 15–17 mm long, [12]). Within Morinda, seven African species have much larger flowers than the remaining species of the genus. The flowers of Coelospermum are larger than those of the species of Gynochthodes [9]. Furthermore, Morindeae species vary in their breeding systems, and flowers are either bisexual or unisexual or functionally unisexual. Only the hermaphroditic condition has been reported in Appunia and Morinda [21], while the androdioecious (male and hermaphroditic individuals) [21]–[22], strict or functional dioecious [23]–[24], and hermaphroditic [21], [24] conditions are all known in Gynochthodes and Coelospermum [21]–[24]. The New Guinean Siphonandrium is dioecious (Table 1).
We reconstructed the evolution of fruit type, inflorescence architecture, flower size, and growth form across a phylogeny of the tribe Morindeae. We were particularly interested in testing the following hypotheses: 1) self-supporting habit is generally plesiomorphic in clades comprising both lianescent and arborescent species [14]; 2) evolutionary changes from lianescent to arborescent/frutescent habit are less frequent than the reverse change, from arborescent/frutescent to lianescent habit [16]; 3) and Head inflorescences and multiple fruits in Morindeae evolved from non-headed inflorescences and simple fruits, respectively. The ecological and evolutionary implications of the findings of this study are discussed.
Results
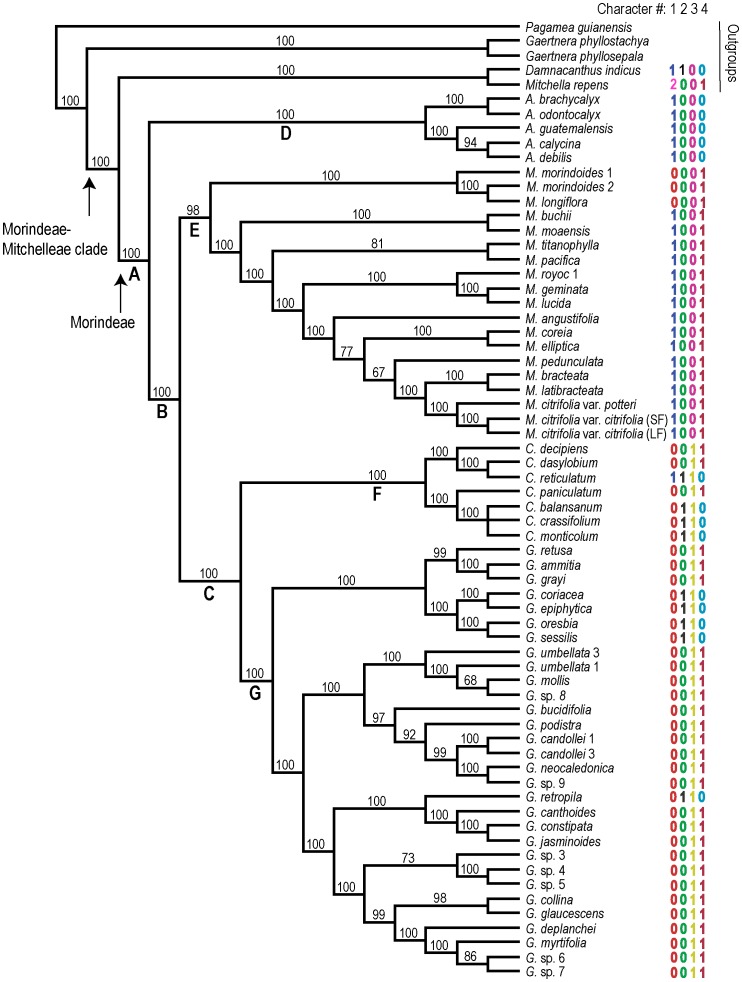
The Bayesian majority rule consensus tree generated from the combined nrETS/nrITS/trnT-F data and shown in Figure 2 was fully resolved. Its overall topology is almost identical to that of the Bayesian majority rule consensus tree published in Razafimandimbison et al. [9].
Figure 2. Bayesian majority rule consensus tree from the combined nrETS/nrITS/trnT-F data of 61 Morindeae taxa.
Values above nodes are the posterior probabilities. Capital letters A–G denote selected nodes whose state probabilities were estimated for the states of the four characters (1–4). Data shown across the tips are growth habit (character 1: 0 = lianescent, 1 = arborescent, 2 = herbaceous), inflorescence architecture (character 2: 0 = headed inflorescences (heads); 1 = non-headed inflorescences), flower size (character 3: 0 = large, 1 = small), and fruit type (character 4: 0 = simple fruits, 1 = fused or multiple fruits). SF and LF stand for small and large fruits, respectively.
Ancestral State Reconstructions of Growth form, Inflorescence Architecture, Flower Size, and Fruit Type in Morindeae
Two types of ASRs were performed (one with the outgroup Damnacanthus indicus C.F.Gaertn. and Mitchella repens L. (tribe Mitchelleae), hereafter called ASR with outgroup, and the other without the outgroup, hereafter called ASR without outgroup), to infer the ancestral states of growth form, inflorescence architecture, flower size, and fruit type (characters 1–4, respectively) at seven important nodes of Morindeae (nodes A–G, Fig. 2). The results of these ASRs are summarized in Tables 2,3. For growth form (character 1) and flower size (character 3) the ratios q01/q10 from the ASRs with outgroup, respectively, were 4.820 and 2.691 for Morindeae (Table 2). This indicates that the rates of changes from lianescent to arborescent habit and from large to small flower were higher than the rates of the reverse directions, from arborescent to lianescent habit and from small to large flower. In contrast, for inflorescence architecture (character 2) and fruit type (character 4), the ratios q01/q10, respectively, were 0.676 and 0.677 for Morindeae (Table 2); this means that the rates of changes from non-headed to headed inflorescence and from multiple to simple fruit are higher than the rates of the reverse changes, from headed to non-headed inflorescence and from simple to multiple fruit.
Table 2. Bayesian reconstruction of ancestral states in the four characters (1–4) at seven nodes (A–G) across a posterior sample of trees including Morindeae but no outgroup.
| Character* | ||||
| 1 (growth form) | 2 (inflorescence architecture) | 3 (flower size) | 4 (fruit type) | |
| Coded character states | lianescent = 0 and arborescent = 1 | headed = 0 andnon-headed = 1 | large = 0 andsmall = 1 | simple = 0 andfused = 1 |
| q01/q10 | 4.820 | 0.676 | 2.691 | 0.677 |
| κ | 0.961 (0.363–1.508) | 0.711 (0.111–1.315) | 1.124 (0.239–1.950) | 0.787 (0.142–1.332) |
| Node A (Morindeae) | 0.861 | 0.584 | 0.980 | 0.370 |
| Node B (the Morinda-Coelospermum-Gynochthodes clade) | 0.992 | 0.586 | 0.878 | 0.181 |
| Node C (the Coelospermum-Gynochthodes) | 0.998 | 0.584 | 0.000 | 0.185 |
| Node D (Appunia) | 0.007 | 0.909 | 1.000 | 0.989 |
| Node E (Morinda) | 0.860 | 0.910 | 1.000 | 0.025 |
| Node F (Coelospermum) | 0.998 | 0.565 | 0.000 | 0.214 |
| Node G (Gynochthodes) | 1.000 | 0.583 | 0.000 | 0.186 |
For each character 1–4, the following information is provided: the ratio of the average rate q01 to the average rate q10, the average and 95% highest posterior density (HPD) of κ, and the marginal posterior probabilities of having state 0 in each of the seven nodes (A–G). As all characters are binary, the marginal posterior probability of having state 1 is one minus the probability of state 0. The 95% HPD of κ excludes 0 in all cases, which is a strong indication that branch lengths carry information about the amount of change in the morphological characters.
Table 3. Bayesian reconstruction of ancestral states in the four characters (1–4) at seven nodes (A–G) across a posterior sample of trees including Morindeae as well as the outgroup taxa Damnacanthus indicus and Mitchella repens (Mitchelleae).
| Character | ||||
| 1 (growth form) | 2 (inflorescence architecture) | 3 (flower size) | 4 (fruit type) | |
| Coded character states | lianescent (0), arborescent (1),and herbaceous (2) | headed (0) and non-headed (1) | large (0) and small (1) | simple (0) and fused (1) |
| κ | 0.981 (0.394–1.516) | 0.671 (0.061–1.259) | 1.144 (0.221–1.996) | 0.784 (0.128–1.312) |
| Node of Morindeae-Mitchelleae clade | 0.258, 0.216, 0.524 | 0.513 | 0.996 | 0.359 |
| Node A (Morindeae) | 0.733, 0.199, 0.067 | 0.566 | 0.994 | 0.371 |
| Node B (the Morinda-Coelospermum- Gynochthodes clade) | 0.970, 0.011, 0.019 | 0.568 | 0.886 | 0.166 |
| Node C (the Coelospermum-Gynochthodes) | 0.991, 0.001, 0.008 | 0.565 | 0.000 | 0.171 |
| Node D (Appunia) | 0.007, 0.980, 0.013 | 0.916 | 1.000 | 0.987 |
| Node E (Morinda) | 0.733, 0.202, 0.065 | 0.917 | 1.000 | 0.023 |
| Node F (Coelospermum) | 0.990, 0.001, 0.009 | 0.540 | 0.000 | 0.207 |
| Node G (Gynochthodes) | 0.998, 0.000, 0.002 | 0.564 | 0.000 | 0.172 |
For each character 1–4, the average and 95% highest posterior density of κ is provided. For character 1, we also provide the marginal posterior probabilities of having state 0, 1, and 2, respectively, in each of the eight selected nodes (the Mitchelleae-Morindeae root node and nodes A–G). For character 2–4, we provide the marginal posterior probabilities of having state 0 for the same eight nodes. The 95% HPD of κ excludes 0 in all cases, which is a strong indication that branch lengths carry information about the amount of change in the morphological characters.
The node of the Morindeae-Mitchelleae clade ( = the Mitchelleae-Morindeae common ancestor) (Fig. 2) was inferred with strong and moderate support, respectively, as large flower and multiple fruit in the ASR with outgroup; however, the results of this analysis were inconclusive for growth habit and inflorescence architecture (Table 3). The outcomes of the ASRs with and without outgroup were very similar for the seven important nodes of Morindeae (nodes A–G, Fig.2) (Tables 2,3). At node A (the Morindeae common ancestor) the lianescent habit, head inflorescence, large flower, and multiple fruit states were inferred; however the support was weak for head inflorescence, moderate for lianescent habit and multiple fruit, and strong for large flower. Within Morindeae lianescent habit was strongly inferred at node B (the Morinda-Coelospermum-Gynochthodes clade), node C (Coelospermum-Gynochthodes clade), node E (Morinda), node F (Coelospermum), and node G (Gynochthodes), while arborescent habit was resolved at node D (Appunia). For inflorescence architecture (character 2) head inflorescence was inferred at nodes B-G; the support was moderate for nodes B–C and F–G but strong for nodes D–E. For flower size (character 3) large flower was strongly inferred at nodes B and D–E, whereas nodes C and F–G were unambiguously resolved as small flower. Finally, for fruit type (character 4) fused fruit (multiple fruit) was strongly inferred at nodes B–C and nodes E–G, however simple fruit was highly resolved at node D (Tables 2,3).
Discussion
We performed ASRs with and without outgroup in order to assess the influence of the outgroup taxa on the outcomes. The fact that the results of these two ASRs are very similar for the seven nodes of Morindeae (nodes A–G, Fig. 2, Tables 2,3) suggests that the inclusion of the outgroup taxa (Damnacanthus indicus and Mitchella repens) has almost no effect on the analyses. The ASR with outgroup infers large flower with strong support and multiple fruit with moderate support at the node of the Morindeae-Mitchelleae clade. Lianescent habit, head inflorescence, large flower, and multiple fruit are inferred at node A (Morindeae) (Tables 2,3). If these inferences are correct, these states are interpreted as plesiomorphic for Morindeae and are plesiomorphic within Morindeae, with respect to the later changes (i.e., arborescent habit, non-headed inflorescence, small flower, and simple fruit) in the group.
Evolution of Growth form in Morindeae and its Ecological and Evolutionary Implications
Two-thirds of the species in Morindeae are represented by the lianescent species of Gynochthodes, while only two Morinda and one Appunia species are lianas. Conversely, a single species of Coelospermum (C. reticulatum) and two Gynochthodes (G. decipiens and G. trimera, not investigated in this study) species are arborescent. The fact that lianescent habit is inferred at node A (Morindeae) means that this state is plesiomorphic at node B (the Morinda-Coelospermum-Gynochthodes clade), node C (the Coelospermum-Gynochthodes clade), and nodes E–G (Morinda, Coelospermum, and Gynochthodes, respectively). Arborescent habit is inferred as apomorphic at node D (Appunia), and seems to have arisen at least three times within Morindeae: Appunia, Morinda (node E: M. butchii Urb. to M. citrifolia L., Fig. 2), and Coelospermum (node F: C. reticulatum, Fig. 2). Our findings provide no support for the reported prevalence of a plesiomorphic arborescent habit in lineages containing both lianescent and arborescent plants [14]. In fact, a plesiomorphic lianescent habit and multiple independent origins of arborescent from lianescent habit have recently been inferred for the primarily lianescent subfamily Secamonoideae in the family Apocynaceae [16] and the family Menispermaceae [25]. Moreover, the rate of change from lianescent to arborescent habit in Morindeae is significantly higher than the reverse change, from arborescent to lianescent habit (q01/q10 = 4.820>1, Table 2); this is inconsistent with Lahaye et al.’s [16] claim that “the evolution of lianescence can carry a high degree of specialization and developmental burden that might limit evolution back to self-supporting growth forms”. Based on the evidence presented above we argue that evolutionary changes between arborescent and lianescent habits can be reversible, and that their frequency and trends seem to vary between groups. In addition, the weak-stem condition of shrubs and treelets in Appunia (observations by T. D. McDowell) and the scandent- or vining-branch condition of shrubs or treelets in the Neotropical Morinda royoc [26] may be viewed as a reflection of their origins from lianescent forms. We find no evidence of any reversal from arborescent to lianescent habit in the 61 Morindeae species included in this study. On the other hand, the sole lianescent species (A. megalantha) in the otherwise arborescent Appunia, not investigated in this study due to lack of material for sequencing, may represent a unique case of an evolutionary reversal from arborescent to lianescent habit in the tribe.
Furthermore, the acquisition of arborescent habit in Appunia seems to have coincided with the diversification of the genus in the Neotropics. Consequently, the evolutionary changes from lianescent to arborescent habit within Morindeae may in part be attributed to reduced competition for open ground and a scarcity of host trees for climbing plants in more open habitats [15]–[16]. This could explain the abundance of some species of the Asian, arborescent Morinda in sparse forests on hill slopes or open disturbed forests and the common occurrence of many Asian, lianescent Gynochthodes in forests or thickets on mountains [27]. The pantropical, arborescent Morinda citrifolia L. is also commonly found on seashores and sparse forests throughout its geographic ranges [26]–[27]. Similarly, the Neotropical M. royoc is common in pine savannas and coastal strands [26]. Finally, five Appunia species of the Neotropical Guianas region are shrubs, which frequently occur at forest edges, in clearings along riverbanks, and in disturbed, opened sites (observations by T. D. McDowell).
Evolution of Inflorescence Architecture and its Evolutionary Implications
The majority of species in Morindeae with head inflorescence belong to Gynochthodes, although they occur in all five recognized genera (Table 1). Conversely, nine of the 11 Coelospermum species and 18 Gynochthodes species have non-headed inflorescences. If the weakly inferred head inflorescence at node A (Morindeae) is correct, this state is interpreted as plesiomorphic within Morindeae (for nodes B–G); this is inconsistent with our hypothesis of a derived head inflorescence within the tribe. The inferred plesiomorphic head inflorescence for nodes B–G, although weakly supported for nodes B–C and F–G, is consistent with highly to moderately supported plesiomorphic multiple fruits in nodes B–C and F–G. Multiple fruits can only be produced by taxa with head inflorescences, although plants with headed inflorescences can also produce simple fruits (e.g., Appunia (node D), Fig. 2). The evolutionary changes from headed to non-headed inflorescence occurred at least four times within Morindeae: twice each in Coelospermum (the Australian C. reticulatum and the New Caledonian C. balansanum group, Fig. 2) and Gynochthodes (the G. coriacea group and the Australian G. retropila (Halford & A.J.Ford) Razafim. & B.Bremer, Fig. 2). This is, to our current knowledge, the first report of evolutionary changes from headed to non-headed inflorescences in Rubiaceae.
The findings of this study raise new interesting questions. We do not know if the formation of non-headed from head inflorescences passes through the development of pedicels (umbels) followed by the formation of inflorescence branches in the umbellate forms to produce elongated, branched inflorescences. Alternatively, the non-headed inflorescence could be derived from a branched inflorescence of many heads if flower number was reduced to leave only one flower per receptacle. Unfortunately, discrete state ASR cannot tell us anything about the intermediate evolutionary changes leading to the formation of non-headed inflorescences in Coelospermum and Gynochthodes. Detailed comparative morphological and developmental studies combined with phylogeny are essential in order to elucidate the underlying developmental basis between the states of inflorescence architecture in Morindeae [28]–[29].
Evolution of Flower Size in Morindeae and its Ecological and Evolutionary Implications
Almost all species of Morindeae with large flowers belong to the arborescent Appunia and Morinda, with the exception of the sole lianescent Appunia species, A. megalantha [12], and the two lianescent Morinda species, M. longiflora and M. morindoides. Conversely, Morindeae plants with small flowers are mostly the lianescent species of Coelospermum, Gynochthodes, and Siphonandrium, except the two arborescent Gynochthodes species (G. decipiens and G. trimera) and the lianescent G. sublanceolata Miq. Our ASRs strongly infer large flowers at the Morindeae-Mitchellae root node as well as node A (Morindeae), meaning that this state is plesiomorphic for the tribe Morindeae, the Morinda-Coelospermum-Gynochthodes clade (node B), Appunia (node D), and Morinda (node E). Small flowers are derived for the Coelospemum-Gynochthodes clade (node C), Coelospermum (node F), and Gynochthodes (node G). In other words, small flowers seem to have evolved only once from the large flowers within Morindeae (Fig. 2). It is worth noting that G. sublanceolata and G. decipiens, with large flowers but not included in this study, may represent one or two cases of reversals from small to large flowers.
The Coelospemum-Gynochthodes clade (node C) contains over 60% of the species in the tribe, and produce small flowers with inconspicuous colors that are most likely to be pollinated by small insects (e.g., short-proboscis moths or small bees or flies). Pollinators with progressively shorter proboscis may have been driving the transition from large to small flowers and an accompanying increase in speciation rate. Furthermore, change from large to small flowers in the Coelospemum-Gynochthodes clade appears to have been associated with a gender dimorphism transition. Androgynoecious (male and hermarphroditic) and dioecious conditions are only known from the lianescent species of Gynochthodes and Coelospermum [19]–[24] (Table 1). Thus, the high incidence of dioecy in the Coelospermum-Gynochthodes clade is correlated with woody, climbing growth habit, small flowers pollinated probably by unspecialized pollinators, and fleshy fruits. This pattern is consistent with those that have been reported from island habitats and various tropical forests [30]–[37]. Therefore, this study presents further support for the importance of these traits in the evolution of dioecy. On the other hand, it is important to note that all hermaphroditic members of Appunia and Morinda with large flowers also have the woody (but arborescent or frutescent) habit and fleshy fruits. This suggests that woodiness and fruit fleshiness alone cannot fully predict dioecy in the tribe Morindeae. In sum, the members of the Coelospemum-Gynochthodes clade display island syndrome characteristics, which are consistent with the fact that many of their species are indeed island endemics [13], [24].
In contrast to the Coelospermum-Gynochthodes clade, the large, mostly white flowers of Appunia and Morinda may be pollinated by larger insects, such as long-proboscis moths. This is consistent with the report on the Asian, arborescent Morinda coreia Buch.-Ham. being pollinated by hawkmoths in India [18]. The fact that the species of Appunia and Morinda are hermarphroditic suggests a single origin of dioecy in the Coelospermum-Gynochthodes clade from hermaphroditism. Members of Appunia and Morinda are predominantly distributed in continental areas (Africa mainland, continental Asia, and South and Central America), and show characteristics of the mainland pollinations and floral traits [30], [33]–[34], [36]–[38].
Evolution of Multiple Fruits in Morindeae and its Ecological and Evolutionary Implications
Most Morindeae, about 90% of the species, bear multiple fruits. The majority of these species belong to Gynochthodes and Morinda, with only three species (two investigated in this study, Fig. 2) in Coelospermum. The infructescences of Appunia and Siphonandrium are composed of simple, drupaceous fruits. Our ASRs with moderate certainty infer multiple fruits at node Morindeae-Mitchelleae and node A (Morindeae). If correct, this state is plesiomorphic for Morindeae, the Coelospermum-Gynochthodes clade (node C), Morinda (node E), Coelospermum (node F), and Gynochthodes (node G) (Tables 2,3). This is inconsistent with the hypothesis of a derived multiple fruit for the broadly delimited Morinda (including the lianescent Morinda species transferred to Gynochthodes sensu Razafimandimbison et al. [8]–[9]), as postulated by McClatchey [11]. Simple, drupaceous fruits are derived for Appunia (node D) and seem to have arisen at least five times within Morindeae: once in Appunia, twice each in Coelospermum (the Australian C. reticulatum and the New Caledonian C. balansanum group), and Gynochthodes (the G. coriacea group and the Australian G. retrophila) (Fig. 2). This is, to our knowledge, the first report of an evolutionary transition from multiple to simple fruits in Rubiaceae. Within the Coelospermum-Gynochthodes clade (node C) the evolutionary change from multiple to simple fruits coincides with that of from headed to non-headed inflorescences. However, it is interesting that Appunia (node D) seems to have retained the plesiomorphic headed inflorescences but acquire simple fruits.
Like the acquisition of arborescent habit, the derivation of simple fruits in Appunia seems to have coincided with the divergence of the Appunia lineage in the Neotropics. The change from multiple to simple fruits in this genus is in part attributed to shifts in seed dispersal vectors. Seeds of the simple, drupaceous fruits of Appunia species are presumably dispersed by birds, whereas seeds of the larger multiple fruits are dispersed effectively by large frugivorous animals [3]. The same mechanism seems to underlie the evolutionary change from multiple to simple fruits within the Coelospemum-Gynochthodes clade.
The degree of ovary fusion prior to fruit development in head inflorescences varies greatly from only a basal, partial fusion to completely fused ovaries among Morinda and Gynochthodes. This variation, which is rarely mentioned by Rubiaceae systematists [26], merits consideration for its ecological implications. Clusters of simple fruits of Appunia are likely to be dispersed individually by frugivorous birds. Multiple fruits composed of partly to fully fused ovaries are presumably dispersed as single units, while those formed by basally fused ovaries could well be dispersed individually by frugivorous birds or as single units by larger frugivorous dispersers. Furthermore, we suspect that in many members of Gynochthodes and Morinda ovaries of the adjacent flowers are basally fused prior to and during maturation of the anthers, and that ovary fusion extends midway during fructification. This type of ovary fusion was reported for Breonia richardsonii Razafim. in the tribe Naucleeae of the subfamily Cinchonoideae (Rubiaceae) by Razafimandimbison [39].
Future Perspectives
The Bayesian phylogenetic approach used here provides a sound framework for examining the evolution of distinctive vegetative (growth habit) and reproductive traits (flower, inflorescence, and fruit structures), which have broad ecological importance and potential impact on our understanding of speciation and diversity. Methods, which rely upon mapping discrete character states across a phylogeny, inevitably reduce the complexity of character variation among a diverse group of species. Thus, the arborescent habit includes all non-liana woody shrubs and trees (large trunked trees (e.g., noni, Morinda citrifolia), suffrutescent plants (e.g., M. buchii Urb.), shrubs or treelets with scandent- or vining-branches (e.g., Morinda royoc), and weakly branching treelets (e.g., Appunia debilis Sandwith)). Similarly, the character states "large flower" and "small flower" and their diagnosis based upon corolla tube/lobe ratio summarize diverse flower sizes. The presence or absence of head inflorescences involves the complication of comparing much-branched inflorescences with unbranched inflorescences: either may have flowers in heads or not. Fruit fusion, though variable in degree, is summarized in the character states as simple or multiple. Despite the simplification of diverse characteristics into discrete character states, the essential outcomes of these analyses are clearly evident across the phylogenetic span of this inquiry: repeated shifts have occurred in the evolution of the growth habit, inflorescence architecture, flower size, and fruit across the species of the Morindeae. Moreover, the direction of these evolutionary changes has often been unexpected and at odds with currently accepted hypotheses. Finally, the findings of this study provide a new context for viewing patterns of character evolution and examining their ecological and developmental basis.
Materials and Methods
Taxon Sampling and Data Collection
The sampling used for this study coincided with the molecular phylogenetic study of Morindeae by Razafimandimbison et al. [9], on the basis of which new generic limits of the tribe were established. This latter study resulted in the transfer of all lianescent, dioecious Morinda species to Gynochthodes and all species of Sarcopygme Setch & Christoph. to Morinda [8]–[9]. Accordingly, the newly combined names of Morinda and Gynochthodes, respectively, were utilized in this study to replace the names of the sampled Sarcopygme and lianescent Morinda used in Razafimandimbison et al. [9]. Five Morindeae taxa (Appunia tenuiflora (Benth.) Jacks & Hook.f., Morinda royoc L. 2, and Gynochthodes candollei Montrouz. 2, 4, and 5) with incomplete sequences were excluded from this study to decrease the percentage of missing information in the combined nrETS/nrITS/trnT-F matrix and obtain a well-resolved phylogeny of Morindeae for basing our ASRs. We investigated a total of 66 taxa, and all information about the voucher specimens and sequences used in the study is published in Razafimandimbison et al. [9].
All morphological characteristics of the five genera of Morindeae summarized in Table 1 were based on data from field notes made by SGR (for Coelospermum, Gynochthodes, and the paleotropical Morinda) and by TDM (for Appunia and the Neotropical Morinda). This was coupled with data compiled by SGR from herbarium specimens on loan from many herbaria (BR, K, L, MO, P, S, TAN, TEF, UPS, [40]) and the literature [8]–[9], [12]–[13], [21]–[22], [24], [26]–[27].
Laboratory Work and Phylogenetic Analyses
The protocols used for DNA extraction, amplification, and sequencing are outlined in Razafimandimbison et al. [9]. The alignment of the combined nrETS/nrITS/trnT-F data was re-adjusted after the removal of A. tenuiflora, M. royoc 2, and G. candollei 2, 4, and 5. We treated each of the three gene regions as a separate partition and selected likelihood models following Razafimandimbison et al. [9]. As a consequence, we applied separately parameterized GTR+ G models to the trnT-F and nrITS partitions and a separately parameterized HKY+ G model to the nrETS partition. The gamma distributed rate heterogeneity across sites was approximated with four discrete categories. Flat Dirichlet priors were applied to the state frequencies and to the substitution rates of the GTR model, whereas a flat beta distribution was used as prior for the transition-to-transversion rate. A uniform prior on the interval (0.1, 50) was applied to the gamma curve shape parameter α. The prior on branch lengths was an exponential distribution with mean 0.1. Rate heterogeneity across partitions was modeled according to a proportional model with a flat Dirichlet prior. Tree topologies were treated as a priori equally likely. Three runs of Metropolis-coupled MCMC was run for 25×106 generations, each run starting from a random tree with initial branch lengths set to 0.1. Each run included four chains, three of which were incrementally heated to a temperature of 0.15 to ensure swap rates between adjacent chains between 10 and 70%. Every 1000th generation of the cold MCMC chain was sampled. Stationarity and convergence of runs, as well as the correlation of split frequencies between the runs were checked using the program AWTY [41]. We checked the effective sample size (ESS) of parameters using the program Tracer v.1.5.0 [42]. Trees sampled from the first 12.5×106 generations were discarded as burn-in. All saved trees (after excluding burn-ins) from the three independent runs were pooled for a consensus tree.
Reconstruction of Ancestral States
A variety of comparative phylogenetic methods have been used for reconstructing ancestral states of characters and mapping character changes across lineages: maximum parsimony [43]–[44], maximum likelihood [45], Bayesian inference [46]–[48], and stochastic character mapping [49]. The influences of method choice in reconstructing ancestral states of characters are well documented [2], [50]; it has recently been demonstrated that homoplasious characters are sensitive to choice of method [2].
The Bayesian approach implemented in the computer program BayesTraits v. 1.0 [48] appears to preserve the highest amount of uncertainty in ASR of discrete characters [2], [50]. It takes into account both phylogenetic uncertainty and branch length, and also permits one to explore a variety of models for character transition and to investigate nodes of interest [2], [50]. We performed ASRs of the four characters of the tribe Morindeae (growth habit, inflorescence architecture, flower size, and fruit type) using the software BayesTraits as described by [48] and on two posterior tree samples, one in which all outgroup taxa (Fig. 2) had been pruned and one in which we kept the outgroup taxa Mitchella repens and Damnacanthus indicus of the tribe Mitchelleae, known from previous studies to be the closest relatives of the Morindeae [51]. Pagamea guianensis Aubl. and Gaertnera phyllostachya Baker were pruned from the analyses, because they represent the poorly sampled tribe Gaertnereae and appear on long branches in the phylogeny. Before proceeding with the ASR, we checked trees for the node-density artifact [52] using the on-line implementation at http://www.evolution.reading.ac.uk/pe/index.html. The following four discrete characters were reconstructed for the ingroup taxa: growth form lianescent (0), arborescent (including frutescent and suffrutescent plants of Morinda, the weak-stemmed shrubs or treelets of Appunia, and the scandent- or vining branched shrubs or treelets of Morinda royoc L., i.e., all non-liana woody shrubs and trees) (1), and herbaceous (only relevant for the outgroup taxon M. repens) (2); inflorescence headed (0) or non-headed (1); flowers large (corolla tube length/corolla lobe length >1) (0) or small (corolla tube length/corolla lobe length <1) (1); and fruits simple (0) or fused (1). State probabilities were estimated for the following seven selected nodes in the Bayesian majority rule consensus tree (Fig. 2): Morindeae (node A), the Morinda-Coelospermum-Gynochthodes clade (node B), the Coelospermum-Gynochthodes clade (node C), Appunia (node D), Morinda (node E), Coelospermum (node F), and Gynochthodes (node G). Node A corresponds to the root of the tree when the outgroup taxa had been excluded. In addition, we reconstructed the root node (joining the Mitchelleae and Morindeae) in the analyses involving the two outgroup taxa of Mitchelleae. Reversible-jump MCMC was used to integrate over models. For single binary characters, there are four possible models, one two-rate model in which forward (q01) and backward (q10) rates are free, one single-rate model in which q01 and q10 are constrained to be equal, two single-rate models in which either q01 or q10 is estimated, and the reverse rate is fixed to zero. Ratios of q01 to q10 deviating from 1 indicate that the rate of change in one direction is higher than in the opposite direction.
We used a uniform prior on the models and an exponential prior on rates, the mean of which was seeded by a uniform hyperprior on the interval (0, 10). By applying an exponential prior on rates we say that moderate rates are a priori more likely than high rates and that strong evidence from the data is required to accept high-rate estimates. We also included the branch-length transformation parameter κ in the model [53]. This parameter raises original branch lengths to the κ power. If κ = 0, all branches are equally long, i.e., change is independent of branch lengths. If κ = 1, branch lengths are not modified and change is perfectly proportional to the original branch lengths. κ >1 indicates that change accelerates with increasing branch length and 0< κ <1 indicates that change decelerates with decreasing branch lengths. The prior on κ is a uniform distribution on the interval (0, 5) (A. Meade, pers. com.). The MCMC was run for 220×106 generations, the first 20×106 of which were discarded as burnin. A sample was saved from the posterior every 1000th generation. The rate deviation of the normal distribution was set to obtain an MCMC acceptance rate between 20% and 40%. Each analysis was conducted three times to check that similar harmonic mean likelihoods were obtained across runs.
Acknowledgments
We thank the following herbaria for allowing access to their collections: BR, CAY, K, L, MO, P, S, TAN, TEF, UPS, and US; the DGF (Direction Générale des Forêts) and MNP (Madagascar National Parks) in Madagascar for issuing collecting permits for SGR; Missouri Botanical Garden, Madagascar Program for logistical support; Parc Botanique et Zoologique de Tsimbazaza and Missouri Botanical Garden, Madagascar Program (Lalao Andriamahefarivo and Faranirina Lantoarisoa) for arranging collecting permits for SGR; Kent Kainulainen for technical assistance with Figure 1; and Andrew J. Ford, Johan T. Johansson, Frédéric Tronquet, and Kent Kainulainen for kindly providing photos of Morindeae taxa.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by grants from the Swedish Research Council and the Knut and Alice Wallenberg Foundation to Birgitta Bremer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: University Press. 1994. 511p
- 2.Xiang QY, Thomas DT. Tracking character evolution and biogeographic history through time in Cornaceae - Does choice of methods matter? J Syst Evol. 2008;46:349–374. [Google Scholar]
- 3.Eyde RH. The case for monkey-mediated evolution in big-bracted dogwoods. Arnodia. 1985;45:2–9. [Google Scholar]
- 4.Corlett RT. Frugivory and seed dispersal by vertebrates in the Oriental (Indomalayan) Region. Biol Rev Cambridge Phil Soc. 1998;73:413–448. doi: 10.1017/s0006323198005234. [DOI] [PubMed] [Google Scholar]
- 5.Rowe N, Speck T. Plant growth forms: an ecological and evolutionary perspective. New Phytol. 2005;166:61–72. doi: 10.1111/j.1469-8137.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- 6.Razafimandimbison SG, Bremer B. Tribal delimitation of Naucleeae (Rubiaceae): inference from molecular and morphological data. Syst Geogr Pl. 2001;71:515–538. [Google Scholar]
- 7.Razafimandimbison SG, Bremer B. Phylogeny and classification of Naucleeae (Rubiaceae) inferred from molecular (nrITS, rbcL, and trnT-F) and morphological data. Am J Bot. 2002;89:1027–1041. doi: 10.3732/ajb.89.7.1027. [DOI] [PubMed] [Google Scholar]
- 8.Razafimandimbison SG, Bremer B. Nomenclatural changes and taxonomic notes in the tribe Morindeae (Rubiaceae). Adansonia. 2011;33:281–307. [Google Scholar]
- 9.Razafimandimbison SG, McDowell TD, Halford DA, Bremer B. Molecular phylogenetics and generic assessment in the tribe Morindeae (Rubiaceae-Rubioideae): how to circumscribe Morinda L. to be monophyletic? Mol Phylogenet Evol. 2009;52:879–886. doi: 10.1016/j.ympev.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Razafimandimbison SG, McDowell TD, Halford DA, Bremer B. Origin of the pantropical and nutriceutical Morinda citrifolia L. (Rubiaceae): comments on its distribution range and circumscription. J. Biogeogr. 2010;37:520–529. [Google Scholar]
- 11.McClatchey WC. Nelson SC, editor. Diversity of growth forms, and uses in the Morinda citrifolia L. complex. 2003. pp. 5–10. editor. Proceeding of the 2002 Hawai’i Noni Conference. Honolulu: University of Hawaii at Manoa.
- 12.Taylor CM, Lorence D. Rubiacearum Americanum Magna Hama Pars XXII: Notable new species of South American Coutarea, Morinda, Patima, and Rosenbergiodebndron. Novon 95–105. 2010.
- 13.Johansson JT. Revision of Caelospermum Blume (Rubiaceae, Rubioideae, Morindeae). Blumea. 1988;33:265–297. [Google Scholar]
- 14.Speck T, Rowe NP, Civeyrel L, Classen-Bockhoff R, Neinhuis C, et al. Stuessy TF, Mayer V, Hörandl E, editors. The potential of plant biomechanics in functional biology and systematics. 2003. pp. 241–271. Deep morphology: toward a renaissance of morphology in plant systematics. Lichtenstein: ARG Ganter Verlag.
- 15.Whitlock BA, Hale AM. The phylogeny of Ayenia, Byttneria, and Rayleya (Malvaceae s.l.) and its implication for the evolution of growth forms. Syst Bot. 2011;36:129–136. [Google Scholar]
- 16.Lahaye R, Giveyrel L, Speck T, Rowe NP. Evolution of shrub-like growth forms in the lianoid subfamily Secamonoideae (Apocynaceae s.l.) of Madagascar: phylogeny, biomechanics, and development. Amer J Bot. 2005;92:1381–1396. doi: 10.3732/ajb.92.8.1381. [DOI] [PubMed] [Google Scholar]
- 17.Robbrecht E. Tropical woody Rubiaceae. Opera Bot Belg. 1988;1:1–271. [Google Scholar]
- 18.Raju AJS, Rao SP, Ezradaman V, Zafar R, Kalpana PR, et al. The hawkmoth Macroglossum gyrans and its interaction with some plant species at Visakhapatnam. Zoos’ Print J. 2004;19:1595–1598. [Google Scholar]
- 19.Halford DA, Ford AJ. Two species of Morinda L. (Rubiaceae) from north-east Queensland. Austrobaileya. 2009;8:81–90. [Google Scholar]
- 20.Halford DA, Ford AJ. Coelospermum purpureum Halford & A.J. Ford (Rubiaceae), a new species from north-east Queensland. Austrobaileya. 2009;8:69–76. [Google Scholar]
- 21.Burck MW. Sur l’organisation florale chez quelques Rubiacées. Suite. Ann Jard Bot Buitenzorg. 1883;3:109. [Google Scholar]
- 22.Johansson JT. The genus Morinda (Morindeae, Rubioideae, Rubiaceae) in New Caledonia. Taxonomy and phylogeny. Opera Bot. 1994;122:5–67. [Google Scholar]
- 23.Liu Y, Luo Z, Wu X, Bai X, Zhang D. Pollinators with progressively shorter proboscis may have been driving the transition from large to small flowers and an accompanying increase in speciation rate. Pl Syst Evol. 2012;298:775–785. [Google Scholar]
- 24.Wong KM. A synopsis of Morinda (Rubiaceae) in the Malay Peninsula, with two new species. Malayan Nat J. 1984;38:89–98. [Google Scholar]
- 25.Ortiz RD, Kellogg CEA, Werff HVD. Molecular phylogeny of the mooseed family (Menispermaceae): implications for morphological diversification. Am J Bot. 2007;94:1425–1438. doi: 10.3732/ajb.94.8.1425. [DOI] [PubMed] [Google Scholar]
- 26.Burger W, Taylor CM. Family # 202 Rubiaceae. Burger W, editor. 1993;33:1–333. editor. Flora Costaricensis. Fieldiana Bot. [Google Scholar]
- 27.Tao C, Taylor CM. Rubiaceae. Fl China. 2011;19:220–229. [Google Scholar]
- 28.Endress PK. Disentangling confusions in inflorescence morphology: patterns and diversity of reproductive shoot ramification in angiosperms. J Syst Evol. 2010;48:225–239. [Google Scholar]
- 29.Feng CM, Xiang QY, Franks RG. Phylogeny-based developmental analyses illuminate evolution of inflorescence architectures in dogwoods (Cornus s.l., Cornaceae). New Phytol. 2011;191:850–869. doi: 10.1111/j.1469-8137.2011.03716.x. [DOI] [PubMed] [Google Scholar]
- 30.Bawa KS. Evolution of dioecy in flowering plants. Ann Rev Ecol Syst. 1980;11:15–39. [Google Scholar]
- 31.Bawa KS. Outcrossing and the incidence of dioecism in island floras. Am Nat. 1982;119:866–871. [Google Scholar]
- 32.Bawa KS. Pollination of tropical dioecious angiosperms: a reassessment? No, not yet. Am J Bot. 1994;81:456–460. [Google Scholar]
- 33.Bawa KS, Bullock SH, Perry DR, Coville RE, Grayum MH. Reproductive biology of tropical lowland rain forest trees. II. Pollination systems. Am J Bot. 1985;72:346–356. [Google Scholar]
- 34.Bawa KS, Opler PA. Dioecism in tropical forest trees. Evolution. 1975;29:167–179. doi: 10.1111/j.1558-5646.1975.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 35.Muenchov GE. Is dioecy associated with fleshy fruit? Am J Bot. 1987;74:287–293. [Google Scholar]
- 36.Renner S, Ricklefs RE. Dioecy and its correlates in the flowering plants. Am J Bot. 1995;82:596–606. [Google Scholar]
- 37.Weller SG, Sakai AK. Using phylogenetic approaches for the analysis of plant breeding system evolution. Ann Rev Ecol Syst. 1999;30:167–199. [Google Scholar]
- 38.Tetsuto A. Threatened pollination systems in native floras of the Ogasawara (Bonin) Islands. Ann Bot. 2006;98:317–334. doi: 10.1093/aob/mcl117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Razafimandimbison SG. A systematic revision of Breonia (Rubiaceae-Naucleeae). Ann Missouri Bot Gard. 2002;89:1–37. [Google Scholar]
- 40.Holmgren PK, Holmgren NH, Barnett LC. Index herbarium. Part I: the herbaria of the world, 8th edition. New York: New York Botanical Garden. 693 p. 1990.
- 41.Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL. AWTY (Are We There Yet?): A system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics. 2008;24:581–583. doi: 10.1093/bioinformatics/btm388. [DOI] [PubMed] [Google Scholar]
- 42.Rambaut A, Drummond AJ. Tracer version 1.5. Edinburgh: University of Edinburgh. Available from. 2009. http://tree.bio.ed.ac.uk/software/tracer/
- 43.Maddison DR, Maddison WP. MacClade: Analysis of phylogeny and character evolution. Version 3.0. Sunderland: Sinauer Associates. 1992.
- 44.Maddison D, Maddison WP. Mesquite: a modular system for evolutionary analysis (online). Version 2.01. Available from. 2007. http://mesquiteproject.org.
- 45.Pagel M. The maximum likelihood approach to reconstructing ancestral character states of discrete on phylogenies. Syst Biol. 1999;48:612–622. [Google Scholar]
- 46.Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP. Bayesian inference of phylogeny and its impact on evolutionary biology. Science. 2001;294:2310–2314. doi: 10.1126/science.1065889. [DOI] [PubMed] [Google Scholar]
- 47.Ronquist F. Bayesian inference of character evolution. Trends Ecol Evol. 2004;9:475–481. doi: 10.1016/j.tree.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Pagel M, Meade A, Barker D. Bayesian estimation of ancestral states on phylogenies. Syst Biol. 2004;53:673–684. doi: 10.1080/10635150490522232. [DOI] [PubMed] [Google Scholar]
- 49.Huelsenbeck J, Bollback JP. Empirical and hierarchical Bayesian estimation of ancestral states. Syst Biol. 2001;50:351–366. [PubMed] [Google Scholar]
- 50.Ekman S, Andersen HL Wedin M. The limitations of ancestral state reconstruction and the evolution of the ascus in the Lecanorales (Lichenized Ascomycota). Syst Biol. 2008;57:141–156. doi: 10.1080/10635150801910451. [DOI] [PubMed] [Google Scholar]
- 51.Razafimandimbison SG, Rydin C, Bremer B. Evolution and trends in the Psychotrieae alliance (Rubiaceae): A rarely reported evolutionary change from one-seeded carpels to many-seeded carpels. Mol Phylogenet Evol. 2008;48:207–223. doi: 10.1016/j.ympev.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 52.Venditti C, Meade A, Pagel M. Detecting the node-density artifact in phylogeny reconstruction. Syst Biol. 2006;55:637–643. doi: 10.1080/10635150600865567. [DOI] [PubMed] [Google Scholar]
- 53.Pagel M. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc Royal Soc London, ser B. 1994;255:37–45. [Google Scholar]