Abstract
Pregnane X Receptor (PXR), a master regulator of drug metabolism and inflammation, is abundantly expressed in the gastrointestinal tract. Baicalein and its O-glucuronide baicalin are potent anti-inflammatory and anti-cancer herbal flavonoids that undergo a complex cycle of interconversion in the liver and gut. We sought to investigate the role these flavonoids play in inhibiting gut inflammation by an axis involving PXR and other potential factors. The consequences of PXR regulation and activation by the herbal flavonoids, baicalein and baicalin were evaluated in vitro in human colon carcinoma cells and in vivo using wild-type, Pxr-null, and humanized (hPXR) PXR mice. Baicalein, but not its glucuronidated metabolite baicalin, activates PXR in a Cdx2-dependent manner in vitro, in human colon carcinoma LS174T cells, and in the murine colon in vivo. While both flavonoids abrogate dextran sodium sulfate (DSS)-mediated colon inflammation in vivo, oral delivery of a potent bacterial β-glucuronidase inhibitor eliminates baicalin’s effect on gastrointestinal inflammation by preventing the microbial conversion of baicalin to baicalien. Finally, reduction of gastrointestinal inflammation requires the binding of Cdx2 to a specific proximal site on the PXR promoter. Pharmacological targeting of intestinal PXR using natural metabolically labile ligands could serve as effective and potent therapeutics for gut inflammation that avert systemic drug interactions.
Introduction
For centuries, the roots of Scutellaria baicalensis Georgi (Labiatae) have been used for the treatment of allergic, inflammatory and cancer-related diseases in China and Japan [1], [2]. In these roots, the principal ingredients, in terms of both abundance and effectiveness as an anti-oxidant, are baicalein (5,6,7-trihydroxyflavone) and its 7-glucuronic acid conjugate, baicalin [2]. Both of these flavonoids have shown remarkable in vitro and in vivo pharmacodynamic activities related to their anti-oxidant, anti-inflammatory, anti-viral and anti-bacterial properties [2]. These constituents have a striking propensity to nonspecifically bind to proteins, thus masking their “true” pharmacodynamic targets [3]. Among several molecular targets described for baicalein/baicalin (e.g., transcription induction, enzyme inhibition/induction, reactive oxygen species) [4], [5], very few of these targets have emerged as important pharmacodynamic targets [6], [7]. Indeed, these properties of flavones have stimulated analog discovery efforts to optimize pharmacodynamic properties [3], [8].
Flavonoids, baicalin and baicalein abrogate inflammation in various organs, [9] as well as in inflammatory bowel disease (IBD) [10]. However, the role for baicalin in inflammatory bowel disease remains controversial [11]. Since, no mechanism for this effect has been reported, our efforts focused on whether the adopted orphan nuclear receptor, Pregnane X Receptor (PXR) [12], [13] could potentially mediate flavonoid effects in the gut. This receptor is abundantly expressed in the intestine and liver of mammals and rodents [14]. It is a master regulator of xenobiotic detoxification, and more recently, it has been shown to regulate (abrogate) inappropriate gut inflammation [15]. Furthermore, unlike any known receptor system described to date, PXR has the largest and most promiscuous ligand-binding pocket [16]. Indeed, ligand binding and the activation of PXR can be stereo (enantiomer)-specific with respect to the parent compound and metabolites [17], [18].
Using human colon cancer-derived cells, we found that baicalein, in contrast to baicalin, induces PXR through Cdx2. Baicalein also activated PXR in PXR-transactivation assays. Using animal models, we demonstrated that both baicalein and baicalin abrogate intestinal inflammation and reduce TNFα and IL-6 mRNA abundance. However, the beneficial effect of baicalein on intestinal inflammation was observed in wild-type (Pxr +/+) and hPXR mice but not in Pxr-null (Pxr −/−) mice. Baicalin, also abrogated inflammation, but this effect was abolished with oral gavage of Inh1 (Inhibitor 1), a novel microbe specific β-glucuronidase inhibitor [19] that prevents baicalin from converting to baicalein [20]. Further, we demonstrate that Cdx2 binds and activates RNA Polymerase (Pol) II recruitment on a specific site in the proximal PXR promoter (∼1.4 kb from the start site). In summary, we found that baicalein, but not its major metabolite (glucuronide) baicalin, protects from inappropriate intestinal inflammation in vivo through the induction of Cdx2 and PXR expression.
Results
Baicalein, in Contrast to Baicalin, Induces PXR and Cdx2 in Colon Cancer Cells
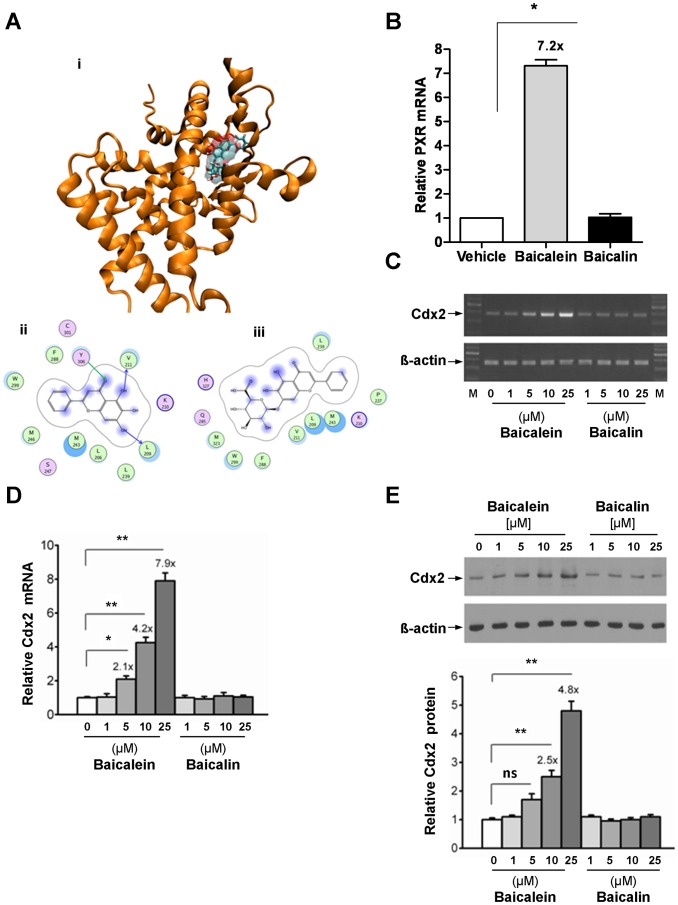
Although baicalein is known to activate PXR [21], the extent to which baicalin and baicalein affect PXR transcription and function is unknown. Molecular modeling of baicalein and baicalin showed that baicalein is predicted to have greater ligand binding residue interactions via H-bonds, which are absent for baicalin (Figure 1A). Notably, PXR transactivation studies in DPX2 cells showed that baicalein activates PXR, while baicalin had no PXR transactivation potential (Figure S1A) and minimal cellular toxicity (Figure S1B). Some herbal drug extracts (e.g., protocatechuic aldehyde) have been shown to activate and induce PXR [22]. Thus, we tested whether baicalein and its glucuronide metabolite, baicalin would induce PXR expression in LS174T colon cancer cells in vitro. Baicalein induced PXR (7.2 fold over control) while baicalin had no effect on PXR mRNA abundance (Figure 1B). Similar data were shown for another comparable Cdx2 expressing colon cell line, LoVo (Figure S1C).
Figure 1. Baicalein, in contrast to baicalin, docks within the PXR ligand binding domain and induces PXR and Cdx2 in LS174T colon carcinoma cells.
(A) Molecular docking of baicalein and baicalin to PXR ligand binding domain (LBD). Figure (i) represent molecular docking of baicalein to PXR LBD. The 2D schematic diagrams shown in (ii, baicalein) and (iii, baicalin) were generated using the LIGX module of MOE program. The binding site residues are colored by their nature, with hydrophobic residues in green, polar residues in purple and charged residues highlighted with bold contours. Blue spheres and contours indicate matching regions between ligand and receptors. Hydrogen bond interactions are shown by green and blue arrows for side chain and main chain interactions, respectively. (B) LS174T cells were exposed to 0.1% DMSO (vehicle) and baicalein (25 µM) or baicalin (25 µM) for 48 hours and total RNA was isolated for PXR mRNA expression analysis by real-time quantitative (RT-qPCR). (C & D) LS174T cells were exposed to different concentrations of baicalein or baicalin as illustrated, for 48 hours and total RNA isolated for Cdx2 mRNA expression analysis by (C) semi-quantitative polymerase chain reaction and by (D) RT-qPCR. β-actin was used as internal control. (E, top panel) Representative western blot of Cdx2 from the same experiment as in (C). (E, bottom panel) Absolute band intensity was quantified for each lanes of the western blot as in figure (E, top panel), using Image J software. Histogram, mean ± SEM. *P<.01; ** P<.001; ns, not significant.
Since flavonoids are known to regulate transcription factor (TF) activity directly (e.g., β-catenin/Tcf) [23] or indirectly (e.g., baicalein inhibition of NFκB) [24], and that PXR mRNA abundance is regulated at the level of transcription by intestine-specific and non-specific TFs [25], we chose to focus on the mechanism by which flavonoids regulate PXR transcription. PXR and the intestine specific homeobox protein caudal type homeobox 2 (Cdx2) are co-expressed in enterocytes [26], [27], with Cdx2 as a central regulator of intestinal differentiation [28], [29]. Furthermore, Cdx2 acts as a key regulator of intestinal homeostasis [30], [31], similar to our observations in Pxr −/− mice in vivo (unpublished observations). Given these possible similarities shared by Cdx2 and PXR, we sought to determine whether Cdx2 could transactivate PXR in 293T cells. We found that PXR promoter activity increased with Cdx2 dosage (Figure S1D). Baicalein was found to increase the expression of Cdx2 mRNA in LS174T cells (Figure 1C & D). A similar trend was also observed for Cdx2 protein expression (Figure 1E), albeit the expression abundance was lower than that observed for Cdx2 mRNA (4.8-fold versus 7.9-fold, respectively) (Figure 1E, bottom panel). Baicalin had no effect on Cdx2 mRNA or protein expression (Figure 1C–E). To further corroborate the apparent link between Cdx2 and PXR, several intestinal cells (HCT 116, SW 948, SW 403 and LoVo) expressing varying amounts of Cdx2 protein, were exposed to flavonoids. In contrast to baicalin, baicalein induced PXR expression in cell lines expressing Cdx2 protein (Figure S1C).
Baicalein, in Contrast to Baicalin, Induces PXR mRNA Expression through Cdx2
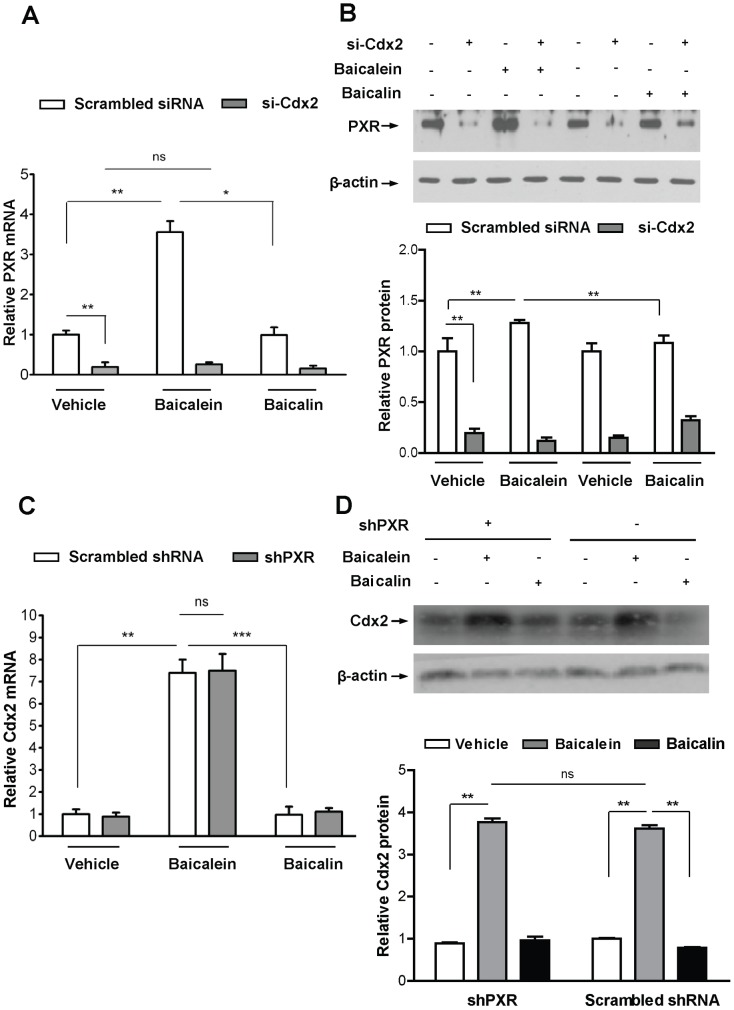
Next, we examined whether Cdx2 is necessary for baicalein-mediated induction of PXR in LS174T cells. Cdx2 silencing in LS174T cells (Figure S2) significantly reduced PXR mRNA (Figure 2A) and protein (Figure 2B) abundance. Baicalein induced PXR mRNA (Figure 2A) and protein (Figure 2B) in scrambled siRNA transfected but not in Cdx2 silenced LS174T cells. To further validate the relationship between Cdx2 function and PXR gene expression, HT-29-derived lines with tightly regulated Cdx2 activity [32], were used to confirm that PXR is a direct target of Cdx2 (Figure S3). As a corollary, DLD-1 derived lines with constitutively expressing Cdx2 shRNA were used to confirm reciprocal effects of Cdx2 and PXR (Figure S4). Conjointly, these results strongly argue for a direct transcriptional effect of Cdx2 on PXR transcription.
Figure 2. Baicalein, in contrast to baicalin, induces PXR and Cdx2 mRNA.
(A) Scrambled or Cdx2 siRNA (si-Cdx2) transfected LS174T colon cancer cells were exposed to 0.1% DMSO (vehicle), baicalein (25 µM) or baicalin (25 µM) for 48 hours. mRNA levels of PXR were quantified by RT-qPCR. (B, top panel) Representative western blot of PXR from the same experiment as in (A). (B, bottom panel) Absolute band intensity was quantified for each lanes of the western blot as in figure (B, top panel), using Image J software. (C) Scrambled or PXR shRNA (shPXR) transduced LS174T cells, were exposed to 0.1% DMSO (vehicle), baicalein (25 µM) or baicalin (25 µM) for 48 hours. Cdx2 mRNA levels were quantified by RT-qPCR. (D, top panel) Representative western blot of Cdx2 from the same experiment as in (C). (D, bottom panel) Absolute band intensity was quantified for each lanes of the western blot as in figure (D, top panel), using Image J software. Histogram, mean ± SEM. *P<.05; ** P<.01;*** P<.001; ns, not significant.
Baicalein, in Contrast to Baicalin, Induces Cdx2 Independent of PXR Expression
Next, we examined whether PXR is necessary for flavonoid-mediated induction of Cdx2. We found that PXR knockdown in LS174T cells (Figure S5) had no effect on the basal Cdx2 mRNA (Figure 2C), protein abundance (Figure 2D), and baicalein-mediated induction of Cdx2 mRNA (Figure 2C) or its protein abundance (Figure 2D). Similarly, PXR knockdown did not alter the effect of baicalin on Cdx2 mRNA expression levels (Figure 2C) nor its protein abundance (Figure 2D).
The Flavones Baicalein and Baicalin Abrogate Intestinal Inflammation in a Mouse Model of Colitis through a PXR-dependent Mechanism
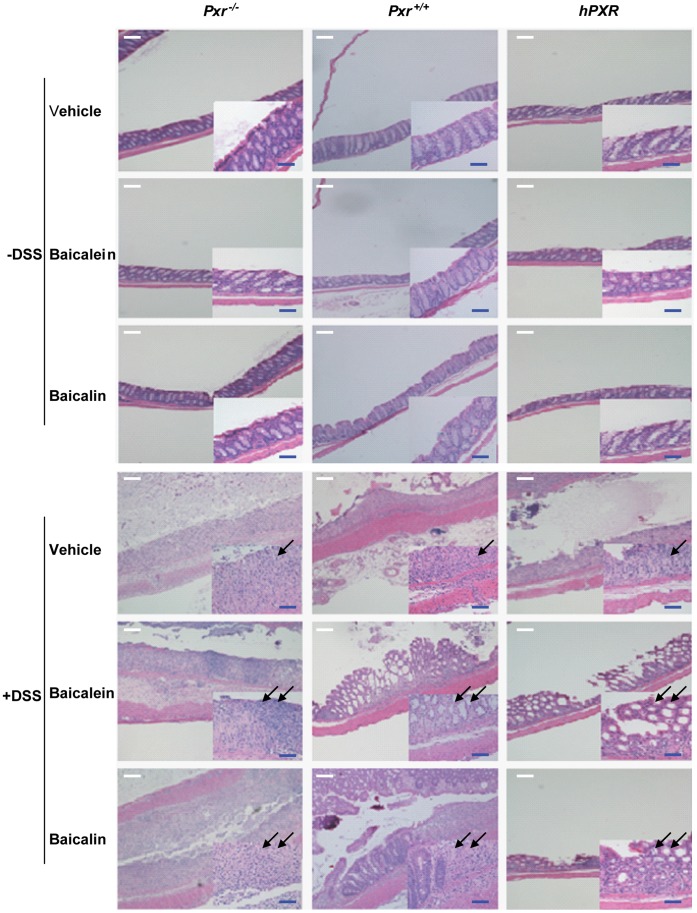
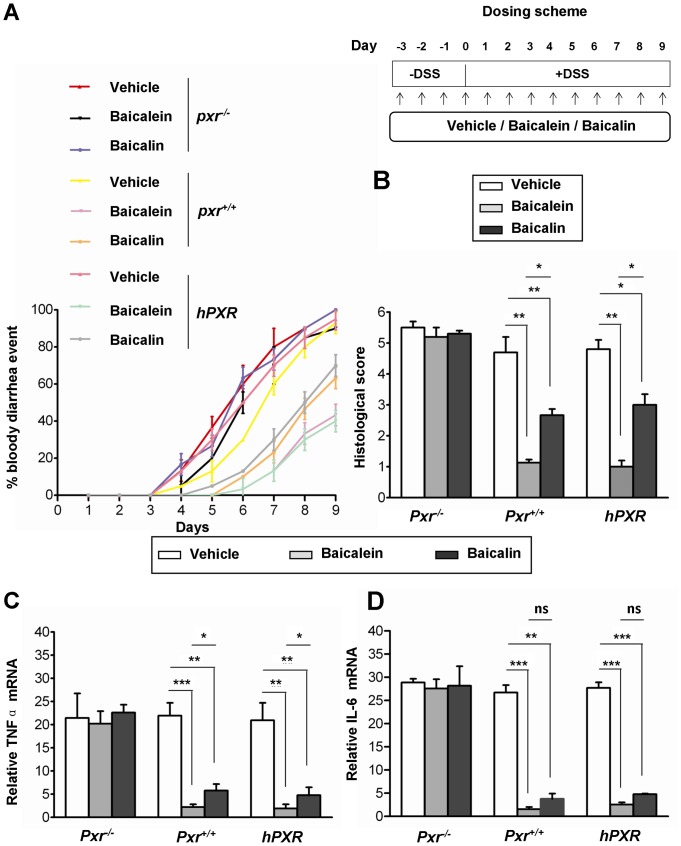
The dextran sodium sulfate (DSS) model of murine colitis closely simulates the human condition and is one commonly used model of human IBD. Baicalein has been previously shown to abrogate inflammation in such a model [10]. Similarly, PXR ligands also abrogate inflammation [15]. We investigated whether the anti-inflammatory actions of baicalein were dependent on Pxr in vivo. DSS exposed mice were treated with vehicle or flavonoids (baicalein, baicalin). The histopathology of the colon was examined by hematoxylin/eosin, as shown in Figure 3. The insets illustrate the effect of DSS (vehicle treated) on the intestinal mucosa where it induces significant inflammatory infiltrates (black arrow; Figure 3), regardless of the presence of Pxr. Indeed, both baicalein and baicalin protected against DSS-mediated intestinal inflammation and crypt loss; however, this effect was lost in the absence of Pxr (double black arrow; Figure 3). The humanized mice showed effects and responses similar to those of wild-type mice (Pxr+/+). Clinically, DSS-exposed mice treated with baicalein and baicalin, showed a reduced cumulative incidence of “bloody diarrhea” as compared to the vehicle-exposed mice, that was more pronounced in Pxr +/+ and hPXR as compared to the Pxr −/− mice (Figure 4A). Using the same mice on day 9, the colon histological (inflammation) score was determined. Baicalein significantly reduced the histological score in DSS-exposed Pxr+/+ and hPXR mice but not in Pxr −/− mice (Figure 4B). Surprisingly, baicalin significantly reduced the histological score in DSS-exposed Pxr+/+ and hPXR mice but not in Pxr −/− mice (Figure 4B). However, the effects of baicalein were significantly greater than those of baicalin (Figure 4B). Messenger RNA expression levels of the mediators of inflammation, TNFα and IL-6, were also assessed in the intestinal mucosa of the same mice. Baicalein and baicalin significantly reduced both TNFα (Figure 4C) and IL-6 (Figure 4D) mRNA expression levels in DSS-treated Pxr+/+ and hPXR mice but not in Pxr −/− mice. Once again, the effects of baicalein were significantly greater than those of baicalin (Figure 4D).
Figure 3. Protective effects of flavonoids in DSS-induced colitis in mice.
Histopathological examination of the colon of mice exposed to DSS and/or flavones (Baicalein, Baicalin) is illustrated. The histology (inset shows higher magnification views) represents the prototypical injury to the colon induced by DSS. Pxr+/+: wild-type mice; Pxr−/−: Pxr-null mice; hPXR: humanized PXR mice. –DSS: DSS untreated mice; +DSS: DSS treated mice; single black arrow: vehicle exposed mucosa; double black arrow: flavonoids exposed mucosa. Scale bar, 50 µM.
Figure 4. Flavonoids protect against DSS-induced bloody diarrhea, colitis score and inflammatory cytokine expressions.
(A) Mice exposed to DSS, as represented in Figure 3 according to the dosing scheme illustrated in upper right panel of (A), were evaluated for the presence of bloody diarrhea after flavonoids treatment. Data plotted as percentage (%) of total mice that had bloody diarrhea on different days of DSS treatment. (B) The histologic score was determined on day 9, when mice were sacrificed. (C & D) Total RNA was isolated from colons of DSS treated mice as in figure (A & B) and analyzed for (C) TNFα and (D) IL-6 mRNA expressions by RT-qPCR. Histogram and data points, mean ± SEM. *P<.05; **P<.01; ***P<.001; ns, not significant.
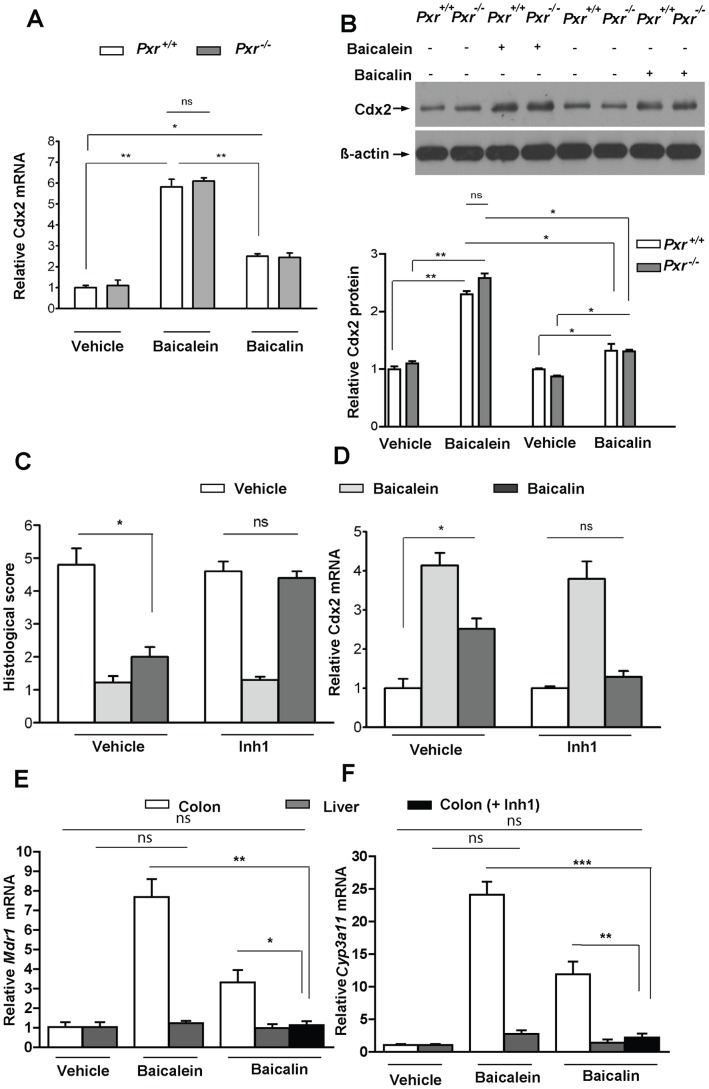
The Flavones Baicalein and Baicalin Induce Cdx2 in the Intestinal Mucosa through a PXR-Independent Mechanism in vivo
Both baicalein and baicalin induced Cdx2 mRNA expression (Figure 5A) and protein abundance (Figure 5B), which was independent of Pxr content in the intestinal mucosa (Figure 5B, bottom panel). Moreover, the effects of baicalein were significantly greater than those of baicalin (Figure 5A & B).
Figure 5. Flavonoids induce Cdx2 mRNA and protein regardless of Pxr genotype in murine intestinal mucosa.
(A & B) Pxr +/+ and Pxr −/− mice (DSS untreated) exposed to flavones (baicalein, baicalin), as represented in Figure 3, were evaluated for (A) Cdx2 mRNA expression by RT-qPCR and (B, top panel) Cdx2 protein abundance by western blot. (B, bottom panel) Absolute band intensity was quantified for each lanes of the western blot as in figure (B, top panel), using Image J software. (C & D) Pxr +/+ mice (DSS treated) pre-exposed to Inh1 and subsequently treated with flavones (baicalein, baicalin), as in Figure 3, were evaluated for (C) histological score and (D) Cdx2 mRNA expression (analyzed by RT-qPCR) in colon isolated on day 9 of DSS treatment. (E & F) Pxr +/+ mice were treated with flavonoids (baicalein, baicalin) and mRNA expressions of Mdr1 and Cyp3a11 were analyzed in colon and liver. Flavonoids (baicalein, baicalin) have no effect on induction of (E) Mdr1 and (F) Cyp3a11 mRNA in Pxr +/+ mice liver; however, they robustly induce gene expression in the colon. Inh1 abolishes baicalin’s effect on induction of Mdr1 and Cyp3a11 mRNA in colon, as assessed by RT-qPCR. Histogram, mean ± SEM. * P<.05; ** P<.01; ns, not significant.
Baicalin’s Effect on Intestinal Inflammation and Cdx2 Expression is Mediated through the Formation of Baicalein in vivo
The in vivo data suggested that baicalin, which is not an efficient inducer or activator of PXR, was surprisingly dependent on PXR for its anti-inflammatory actions in the gut. However, baicalin, is converted to baicalein by microbial β-glucuronidase in vivo [2]. Baicalin is a good substrate for microbial β-glucuronidase (Km ∼0.038–0.01), and it is paradoxically a weak inhibitor of the enzyme (Brenda, www. Brenda-enzymes.org) [33]. To test whether baicalin inhibited microbial β-glucuronidase in vivo, protein (enzyme source) was extracted from mouse fecal pellets and incubated with 7-Ethyl-10-hydroxycamptothecin glucuronide (SN-38-G) (substrate) at various time intervals [19]. The resulting product, SN-38 (aglycone), was measured by spectrophotometry (Emission wavelength ∼425 nm) (Figure S6A). β-glucuronidase activity was minimally inhibited by baicalin in murine fecal extracts. However, a recently described inhibitor of microbial β-glucuronidase, Inh1 [19], was significantly more efficient at inhibiting β-glucuronidase (Figure S6B). To determine whether the anti-inflammatory actions of baicalin were indeed due to its in situ conversion to baicalein, mice were treated with Inh1, before being treated with baicalin. Inh1 dramatically normalized the baicalin-mediated reduction in histological scores in DSS-exposed mice (Figure 5C) and Cdx2 mRNA expression in untreated mice (Figure 5D).
Flavone Concentrations that Inhibit Intestinal Inflammation in vivo do not Activate PXR in the Liver
The flavonoids baicalein and baicalin are thought to exert systemic drug interaction effects through enzyme induction/inhibition [2]. To determine whether baicalein and baicalin administered at a dose and schedule of 20 mg/kg daily for 13 days led to the systemic activation of PXR, non-DSS exposed mice were exposed to vehicle or flavonoids. The colon and liver were excised after 13 days, and total RNA was extracted for real-time quantitative PCR of the classical PXR target genes, Mdr1 and Cyp3a11. Both baicalein and baicalin induced Mdr1 (Figure 5E) and Cyp3a11 (Figure 5F) mRNA expression in the colon but not in the liver. Given that there was significant conversion of baicalin to baicalein in the gut (Mdr1 and Cyp3a11 mRNA were not induced in the colon when Inh1 was present, Figure 5E & F, respectively) and that this conversion was responsible for the in vivo effects of baicalin on intestinal inflammation and Cdx2 expression, we conclude that the effect of baicalin on Mdr1 and Cyp3a11 in the colon was due to its conversion to baicalein.
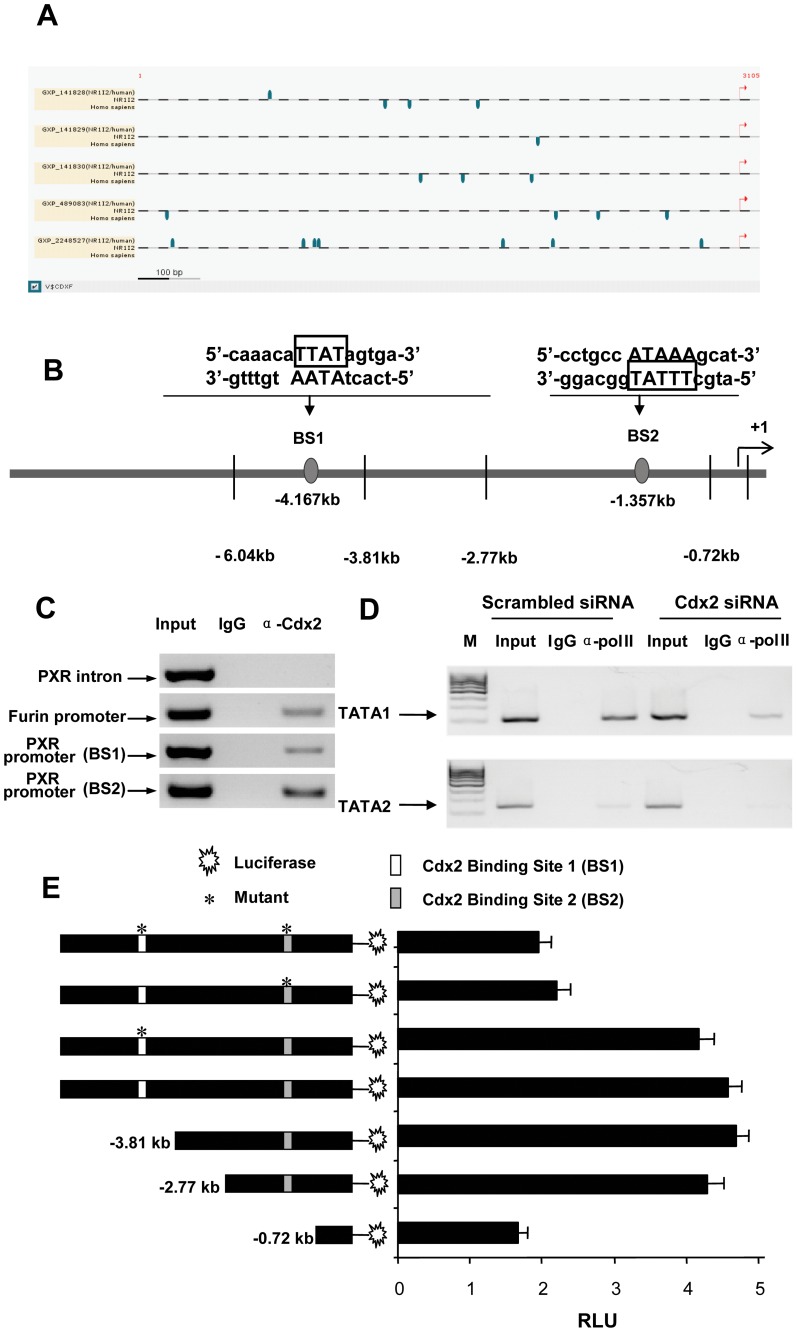
Cdx2 Binds to Specific PXR Proximal Promoter Element and Recruits Pol II
A bioinformatics analysis revealed several putative Cdx2 binding sites on the 3 kb proximal promoter of the PXR (-) strand (Figure 6A). A ChIP analysis revealed that only sites 1 and 2 (denoted BS1 and BS2 in Figure 6B, respectively) bind to endogenous Cdx2 (Figure 6C). The EMSA results show that both BS1 (Figure S7) and BS2 (Figure S8) bind Cdx2. The EMSA showed several non-specific bands, which have been sequentially excluded as they were not specific to Cdx2 (Figure S7 and S8).
Figure 6. Cdx2 binds to two specific endogenous binding sites on the proximal PXR promoter.
(A) Schematic details of the sites (blue marker) that were identified with a high match [matrix scores (≥0.85)], represent potential functional Cdx2 binding sites on PXR promoter. (Note: three potential Cdx1 binding sites, which are also identified on analysis, were excluded from consideration). The in silico analysis identified a total of 19 potential Cdx1/2 binding sites, of which, we verified endogenous binding for two sites, BS1 and BS2 by (C) Chromatin Immunoprecipitation (ChIP) assays. BS, Cdx2 binding site. ChIP assays were performed with Cdx2 and non-specific IgG antibodies and Cdx2 binding was assessed on PXR promoter (BS1 and BS2), PXR intron (negative control) and Furin promoter (positive control) regions. (D) Pol II ChIP assays were performed, using chromatin extracted from LS174T cells that were exposed to scrambled or Cdx2 siRNA (si-Cdx2). Pol II occupancy was assessed on PXR proximal promoter (TATA box) region, using Pol II and non-specific IgG antibodies as a control. (E) Cdx2 transactivation assay was performed in 293T cells, transfected with ∼6 kb PXR promoter reporter constructs (with or without deletion and mutations, as shown in the figure) and Cdx2 expression plasmid. Data expressed as RLU. RLU, relative light unit. Histogram, mean ± SD.
To determine whether Cdx2 is a functional component of PXR transcription (i.e., whether it binds to the PXR promoter but does not initiate transcription through Pol II recruitment), Pol II ChIP assays were performed using nuclear extracts from LS174T cells that were silenced for Cdx2 expression. The results showed that Pol II was efficiently recruited to the PXR proximal promoter (TATA box) in scrambled siRNA transfected cells; however, qualitatively Pol II was reduced in cells in which Cdx2 had been silenced (Figure 6D). To further validate the functional consequence of Cdx2 binding to BS1 and BS2 of the ∼6 kb PXR promoter, we constructed several PXR deletion mutants and performed PXR transactivation assays with the Cdx2 expression plasmid in 293T cells. The ∼6 kb promoter activity was nearly identical to the ∼2.8 kb deletion construct; however, the ∼0.7 kb promoter had a clearly reduced (by ∼60%) transactivation potential compared to the ∼2.8 kb promoter, suggesting that BS2 is likely the functionally active binding site for Cdx2 on the PXR promoter (Figure 6E). To validate this finding, we serially mutated BS1 and/or BS2. The BS1 mutation had no effect on PXR promoter activity in the presence of Cdx2; however, mutation of BS2 and/or BS1 plus BS2 significantly reduced the ∼6.0 kb PXR promoter activity (by ∼60%), which was identical to that observed with the ∼0.8 kb deletion construct (Figure 6E).
Discussion
Using genetic mouse models of drug metabolism and inflammation, we identified PXR as a critical target of baicalein in abrogating IBD. Of note, PXR has been previously implicated as an important target for gut inflammation [34], [35], [36]. Moreover, PXR haplotypes have been implicated in childhood IBD [34]. Certain drugs used in the clinic, such as rifaximin and prednisone, either activate or induce PXR expression and are associated with a beneficial outcome in intestinal immune homeostasis [37], [38]. Baicalein/baicalin has been previously shown to abrogate intestinal inflammation in a mouse model of colitis, although the mechanisms were not established [10], [11]. In this context, we have shown that baicalein activates human and likely, mouse PXR, albeit the latter has been inferred through in vivo studies on gut inflammation only. We confirm that Cdx2, a known intestinal cell differentiation factor [39], is induced by baicalein. Additionally, Cdx2 directly induces PXR promoter activity and mRNA expression in intestinal cells. Altogether, we provide compelling evidence that baicalein exerts its effects on inflammation through a Cdx2/PXR pathway.
We also showed that baicalin, a glucuronide-modified analogue of baicalein, is unable to induce Cdx2 and/or PXR and that it does not activate PXR in vitro or in vivo. The apparent anti-inflammatory effects of oral baicalin in mice are largely due to its conversion to the aglycone, baicalein, through the actions of microbial β-glucuronidases [40]. Indeed, even the effects of oral baicalin are modest compared with baicalein. Furthermore, oral baicalein at doses sufficient to suppress intestinal inflammation does not induce PXR target genes in the liver. Thus, we propose that oral baicalein could achieve concentrations in intestinal cells that could sufficiently abrogate inflammation locally. However, upon systemic absorption, baicalein is completely metabolized and excreted as its glucuronide or sulfate conjugate. We also demonstrate that baicalein is unable to induce PXR target genes in the liver, which is consistent with data showing that baicalin is unable to either induce or activate PXR. These data suggest that baicalein will not induce systemic drug interactions through PXR at the doses used to abrogate intestinal inflammation in mice. The clinical and translational relevance of our results are consistently supported by published data, demonstrating that baicalein is significantly metabolized in both humans and rodents. The glucuronide metabolites are major metabolites in both species [2], [41], [42].
We demonstrated that Cdx2 binds to a single element on the proximal promoter of PXR. Our data show that a careful analysis of specific sites may reveal important and functional gene regulatory elements. These data could be used to further determine the molecular mechanisms governing Cdx2-mediated PXR transduction. However, Cdx2 may function independently as a suppressor of inflammation, perhaps through non-transcriptional mechanisms [30], [43], and/or induce multiple morphogenetic changes that counter-balance the effects of mucosal inflammation [31]. These possibilities warrant further mechanistic studies. Finally, it is to be noted that PXR inhibits NF-κB activity [44]. Mutual repression between steroid and xenobiotic receptor and NF-κB signaling pathways links xenobiotic metabolism and inflammation, which could play an important independent role in abrogating intestinal inflammation and perhaps in conjunction with the newer observations implicating Cdx2. In this context, we have not assessed the impact of baicalein on activation of other nuclear receptors (e.g., liver X receptor or LXR, farnesoid X receptor or FXR), which independently affect intestinal inflammatory pathways. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease [45]. Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation [46].
On the basis of our findings, we propose that knowledge of baicalein’s in vivo clinical pharmacology could aid its use in patients with inflammatory bowel disease. However, the effects of baicalein on drug absorption as a result of nontargeted effects could still be operational [47]. Accordingly, it would be feasible and warrant the synthesis of potent PXR-activating baicalein analogs while still maintaining or even gaining metabolic lability (i.e., instability due to presence of increased sites for glucuronidation) [8]. Conversely, re-formulations of baicalein for colonic delivery and release may also be feasible [48].
Materials and Methods
The cell lines were procured and cultured according to the guidelines of American Type Culture Collection (ATCC, Rockville, MD, USA) or as per the information provided with the respective cell line. Cell lines, and Reagents, In Silico Binding Site Analysis, Molecular Docking, Gene Reporter Assay and PXR knock-down assay details are mentioned in File S1.
Transient Transfection Assay
293T cells were seeded at 70% confluence into 96-well plates. After an overnight incubation, transient transfection was performed using Lipofectamine 2000 reagent according to the manufacturer’s recommendation and as described in File S1. Details of cell viability assay, DPX2 based PXR transactivation and Cdx2 siRNA transfection assays are described in File S1.
Chromatin Immunoprecipitation (ChIP) Assay
We used a fast ChIP method as previously published [49]. For complete procedure, see File S1. Details of the PCR conditions and primer sequences are described in Table S1.
Electrophoretic Mobility Shift Assay (EMSA)
The gel shift assays were performed as previously described [26]. For complete procedure, see File S1. Probe sequences for EMSA are described in Table S2.
PXR Promoter Deletion Constructs
Three 6.04-kb deletion constructs (−3.81 kb, −2.77 kb and −0.72 kb) containing different lengths of the 5′-flanking sequences of the PXR gene were prepared for the luciferase transcription assay of the human PXR promoter and Site-Directed Mutagenesis were carried out as described in File S1.
PXR Mouse Models
Pxr-null (Pxr−/−) mice (8–10 weeks of age) and humanized (hPXR) PXR mice (mice that express the human PXR gene in a Pxr-null background) were generously provided by Dr. Jeff Staudinger (University of Kansas, Lawrence, KS) and Dr. Wen Xie (University of Pittsburgh, Pittsburgh, PA), respectively [50], [51]. Wild-type controls of the same age, gender and strain (C57BL/6) were purchased from Jackson Laboratory (Bar Harbor, Maine). In a subset of experiments, both Pxr-null and hPXR mice were backcrossed to wild-type controls, and after at least 5 generations, wild-type litters were obtained to serve as controls in our experiments. Animal experiments were carried out following Institutional Animal Care and Use guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of the Albert Einstein College of Medicine, New York.
DSS Mouse Model of Colitis
Mice were fed with 4% (wt/vol) DSS (MW 36–50 KDa, MP Biomedical LLC, Solon, OH) dissolved in sterile, distilled water (vehicle control) ad libitum for the duration of the experiment (days 0–9). Flavonoids were administered to the animals (n = 8–15/genotype/treatment group) by oral gavage (20 mg/kg/day in distilled water, respectively) for 3 days prior to the DSS treatment; the flavonoid administration continued until the end of the DSS treatment (days −3–9), as described previously [10]. For the β-glucuronidase inhibitor study, mice (n = 10/treatment group) were exposed to Inh1 (10 µg twice a day) on day −3 by oral gavage [19].
Clinical and Histological Scores of Colitis
Clinical signs of colitis, such as rectal bleeding, diarrhea, bloody stool and histological score were assessed and reported as a scored event as described previously [19].
RNA Isolation and Real-Time Quantitative (q) PCR
Total RNA was isolated using previously published methods [52], and RT-qPCR was carried out as described in File S1. Gene expression changes were calculated using the comparative Ct method with β-actin as the reference gene and vehicle treated cells (or indicated as control) as the calibrator.
Semi-quantitative, Immunoprecipitation, Western Blot Analysis, β-Glucuronidase Enzyme Assay and Immunohistochemistry
For complete procedure, see File S1.
Statistical Analysis
Statistical analyses were performed by unpaired Student’s t-test. For comparison of more than two groups of data, a 1-way ANOVA was performed. P values less than.05 were considered to be statistically significant (GraphPad Prism version 4).
Supporting Information
Baicalein, but not baicalin, activates and induces PXR in vitro . (A) The DPX2 assay (see Materials and Methods) was used to assess PXR transactivation and (B) cell viability. (C) Individual colon cancer cells lines with varying abundance of Cdx2 protein were used to determine the effect of flavonoids (baicalein, baicalin) on induction of PXR mRNA. Bottom panel shows expression of Cdx2 protein in these cell lines. (D) PXR transactivation assay in 293T cells co-expressing Cdx2 and −3.81 kb PXR reporter. Histogram and data points, mean ± SEM.
(PDF)
Cdx2 knockdown in LS174T colon cancer cells. (A) LS174T cells co-transfected with GFP expressing plasmid and scrambled siRNA or si-Cdx2 with images captured under appropriate filters (Phase contrast and GFP). The middle panel shows fold Cdx2 mRNA expression by RT-qPCR. β-actin was used as internal control. Gene expression changes were calculated using the comparative Ct method with β-actin as the reference gene and scrambled siRNA transfected cells as the calibrator. The bottom panel shows a western blot of Cdx2 from nuclear extract from the same cells. Histogram, mean ± SEM. Scale bar, 100 µm; ** P<0.02
(PDF)
Cdx2 directly targets and induces the PXR promoter. (A) Schematic diagram illustrating possible mechanisms by which Cdx2 induces PXR mRNA, through its (1) direct action or (2, 3) indirect action(s) on the PXR promoter. BS: Cdx2 binding site on the PXR promoter. (B) Western blot of cell lysates from two clones (c1, c2) of HT-29/Cdx2-ER cells (obtained after sequential passage) with (+) or without (-) exposure to 4-OHT (hydroxytamoxifen) for Cdx2 and β-actin. The right panel shows time-dependent induction of PXR mRNA as assessed by RT-qPCR. (C) Immunoprecipitation blot of PXR from HT-29/Cdx2-ER cells exposed to cycloheximide and/or 4-OHT. Right panel shows fold expression of PXR mRNA by RT-qPCR. β-actin was used as internal or loading control for western blots. Gene expression changes were calculated using the comparative Ct method with β-actin as the reference gene and vehicle treated cells (or indicated as 0) as the calibrator. Histogram, mean ± SD.
(PDF)
Cdx2 directly targets and induces the PXR promoter. (A) Left panel shows western blot of Cdx2 in cell lysates prepared from DLD-1 pGIPZ(c) (control cells) and shCdx2 infected cells. Right panel shows fold mRNA expression of Cdx2 and PXR by RT-qPCR from the same experiment. (B) Immunoprecipitation blot of PXR from control DLD-1 pGIPZ(c) and shCdx2 infected cells exposed to cycloheximide and/or 4-OHT. Right panel shows fold expression of PXR mRNA by RT-qPCR. β-actin was used as internal or loading control for western blots. Gene expression changes were calculated using the comparative Ct method with β-actin as the reference gene and vehicle treated cells (or indicated as 0) as the calibrator. Histogram, mean ± SD.
(PDF)
PXR knockdown in LS174T colon cancer cells. (A) LS174T cells co-transduced with GFP expressing plasmid and scrambled shRNA or shPXR with images captured under appropriate filters (Phase contrast and GFP). The middle panel shows fold PXR mRNA expression by RT-qPCR. β-actin was used as internal control. Gene expression changes were calculated using the comparative Ct method with β-actin as the reference gene and scrambled shRNA transfected cells as the calibrator. The bottom panel shows a western blot for PXR from nuclear extract from the same cells. Histogram, mean ± SEM. Scale bar, 100 µm; ** P<0.02
(PDF)
Baicalin does not decrease SN-38 glucuronide (SN-38G) emission spectra upon incubation with mouse feces. (A) SN-38G emission spectra in HEPES buffer. (B) Co-incubation of mouse feces with vehicle (HEPES buffer), Inh1 (10 µM), or Baicalin (10 µM).
(PDF)
Cdx2 binds to binding Site1 (BS1) oligonucleotide probe by electrophoretic mobility shift assay (EMSA). (A) Black arrows demonstrate bands upon incubation of respective probes with and without LS174T colon cancer cell line nuclear extract. The lower arrow indicates band common to the positive control and PXR probe lane. (B) Black arrow (bold) demonstrates loss of band upon addition of 50-fold excess of cold PXR probe. (C) Black arrow (bold) demonstrates supershift band, identical to that observed in positive control lane, with the addition of Cdx2 antibody. (D) Black arrow (bold) demonstrates loss of band upon incubation of LS174T nuclear extracts with BS1 mutant oligo. -, negative control oligo; +, positive control oligo; wt, wild-type or PXR probe sequence from PXR promoter containing BS1; mt, PXR probe sequence mutant of BS1.
(PDF)
Cdx2 binds to binding Site2 (BS2) oligonucleotide probe by electrophoretic mobility shift assay (EMSA). (A) Black arrows demonstrate bands upon incubation of respective probes with and without LS174T colon cancer cell line nuclear extract. The lower arrow indicates band common to the positive control and PXR probe lane. (B) Black arrow (bold) demonstrates loss of band upon addition of 50-fold excess of cold PXR probe. (C) Black arrow (bold) demonstrates supershift band, identical to that observed in positive control lane, with the addition of anti-Cdx2 antibody. (D) Black arrow (bold) demonstrates loss of band upon incubation of LS174T nuclear extracts with BS2 mutant oligo. -, negative control oligo; +, positive control oligo; wt, wild-type or PXR probe sequence from PXR promoter containing BS2; mt, PXR probe sequence mutant of BS2.
(PDF)
Primer sequences for ChIP assay.
(DOC)
Probe Sequences for EMSA.
(DOC)
File contains the following: Supporting Information Methods including primer sequences and shRNA sequences, Supporting Information Results and Supporting Information References.
(DOC)
Acknowledgments
The authors thank Dr. Jeff Staudinger (University of Kansas, Lawrence, KS) and Dr. Wen Xie (University of Pittsburgh, PA) for providing the Pxr−/− and hPXR mice, respectively and Professor Matthew Redinbo (University of North Carolina at Chapel Hill, Chapel Hill, NC) for providing critical comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Supported in part by The Damon Runyon Clinical Investigator Award CI15–02, PSC Partners Seeking a Cure Award and National Institutes of Health Grant R01CA161879 to SM and a Program for Changjiang Scholars and Innovative Research Team in University, Ministry of Education of China (IRT1071). Postdoctoral Fellowship to WD from the Institute of Chinese Materia Medica, Shanghai University of TCM, Shanghai, China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Shimizu I. Sho-saiko-to: Japanese herbal medicine for protection against hepatic fibrosis and carcinoma. Journal of gastroenterology and hepatology. 2000;15 doi: 10.1046/j.1440-1746.2000.02138.x. [DOI] [PubMed] [Google Scholar]
- 2.Srinivas NR. Baicalin, an emerging multi-therapeutic agent: pharmacodynamics, pharmacokinetics, and considerations from drug development perspectives. Xenobiotica. 2010;40:357–367. doi: 10.3109/00498251003663724. [DOI] [PubMed] [Google Scholar]
- 3.Zhang HY, Chen LL, Li XJ, Zhang J. Evolutionary inspirations for drug discovery. Trends Pharmacol Sci. 2010;31:443–448. doi: 10.1016/j.tips.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Ahn HC, Lee SY, Kim JW, Son WS, Shin CG, et al. Binding aspects of baicalein to HIV-1 integrase. Mol Cells. 2001;12:127–130. [PubMed] [Google Scholar]
- 5.Chang WS, Lee YJ, Lu FJ, Chiang HC. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993;13:2165–2170. [PubMed] [Google Scholar]
- 6.Kim DH, Jang IS, Lee SW. Bacteroides J-37, a human intestinal bacterium, produces alpha-glucuronidase. Biol Pharm Bull. 1997;20:834–837. doi: 10.1248/bpb.20.834. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki K, Taura F, Shoyama Y, Morimoto S. Molecular characterization of a novel beta-glucuronidase from Scutellaria baicalensis georgi. J Biol Chem. 2000;275:27466–27472. doi: 10.1074/jbc.M004674200. [DOI] [PubMed] [Google Scholar]
- 8.Neves MP, Cidade H, Pinto M, Silva AM, Gales L, et al. Prenylated derivatives of baicalein and 3,7-dihydroxyflavone: synthesis and study of their effects on tumor cell lines growth, cell cycle and apoptosis. Eur J Med Chem. 2011;46:2562–2574. doi: 10.1016/j.ejmech.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 9.Tian H, Zhang X, Wu C, Chen L, Ying R, et al. Effects of Baicalin and Octreotide on the serum TNF-alpha level and apoptosis in multiple organs of rats with severe acute pancreatitis. Inflammation. 2009;32:191–201. doi: 10.1007/s10753-009-9120-8. [DOI] [PubMed] [Google Scholar]
- 10.Hong T, Jin GB, Cho S, Cyong JC. Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med. 2002;68:268–271. doi: 10.1055/s-2002-23143. [DOI] [PubMed] [Google Scholar]
- 11.Kawashima K, Nomura A, Makino T, Saito K, Kano Y. Pharmacological properties of traditional medicine (XXIX): effect of Hange-shashin-to and the combinations of its herbal constituents on rat experimental colitis. Biol Pharm Bull. 2004;27:1599–1603. doi: 10.1248/bpb.27.1599. [DOI] [PubMed] [Google Scholar]
- 12.Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes & development. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 14.Kliewer SA. The nuclear pregnane X receptor regulates xenobiotic detoxification. J Nutr. 2003;133:2444S–2447S. doi: 10.1093/jn/133.7.2444S. [DOI] [PubMed] [Google Scholar]
- 15.Biswas A, Mani S, Redinbo MR, Krasowski MD, Li H, et al. Elucidating the 'Jekyll and Hyde' nature of PXR: the case for discovering antagonists or allosteric antagonists. Pharm Res. 2009;26:1807–1815. doi: 10.1007/s11095-009-9901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekins S, Chang C, Mani S, Krasowski MD, Reschly EJ, et al. Human pregnane X receptor antagonists and agonists define molecular requirements for different binding sites. Mol Pharmacol. 2007;72:592–603. doi: 10.1124/mol.107.038398. [DOI] [PubMed] [Google Scholar]
- 17.Mu Y, Zhang J, Zhang S, Zhou HH, Toma D, et al. Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increase warfarin clearance in rats. J Pharmacol Exp Ther. 2006;316:1369–1377. doi: 10.1124/jpet.105.094342. [DOI] [PubMed] [Google Scholar]
- 18.Wipf P, Gong H, Janjic JM, Li S, Day BW, et al. New opportunities for pregnane X receptor (PXR) targeting in drug development. lessons from Enantio- and species-specific PXR ligands identified from a discovery library of amino acid analogues. Mini Rev Med Chem. 2007;7:617–625. doi: 10.2174/138955707780859404. [DOI] [PubMed] [Google Scholar]
- 19.Wallace BD, Wang H, Lane KT, Scott JE, Orans J, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330:831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HS, Kim JY, Park MS, Zheng H, Ji GE. Cloning and expression of beta-glucuronidase from Lactobacillus brevis in E. coli and application in the bioconversion of baicalin and wogonoside. J Microbiol Biotechnol. 2009;19:1650–1655. doi: 10.4014/jmb.0904.04053. [DOI] [PubMed] [Google Scholar]
- 21.Li Y WQ, Yao X, Li Y. Induction of CYP3A4 and MDR1 gene expression by baicalin, baicalein, chlorogenic acid, and ginsenoside Rf through constitutive androstane receptor- and pregnane X receptor-mediated pathways. Eur J Pharmacol. 2010;640:46–54. doi: 10.1016/j.ejphar.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Liu YH, Mo SL, Bi HC, Hu BF, Li CG, et al. Regulation of human pregnane X receptor and its target gene cytochrome P450 3A4 by Chinese herbal compounds and a molecular docking study. Xenobiotica. 2011;41:259–280. doi: 10.3109/00498254.2010.537395. [DOI] [PubMed] [Google Scholar]
- 23.Park S, Choi J. Inhibition of beta-catenin/Tcf signaling by flavonoids. J Cell Biochem. 2010;110:1376–1385. doi: 10.1002/jcb.22654. [DOI] [PubMed] [Google Scholar]
- 24.Wu YL, Lian LH, Wan Y, Nan JX. Baicalein inhibits nuclear factor-kappaB and apoptosis via c-FLIP and MAPK in D-GalN/LPS induced acute liver failure in murine models. Chem Biol Interact. 2010;188:526–534. doi: 10.1016/j.cbi.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Aouabdi S, Gibson G, Plant N. Transcriptional regulation of the PXR gene: identification and characterization of a functional peroxisome proliferator-activated receptor alpha binding site within the proximal promoter of PXR. Drug Metab Dispos. 2006;34:138–144. doi: 10.1124/dmd.105.006064. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Venkatesh M, Li H, Goetz R, Mukherjee S, et al. Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. The Journal of clinical investigation. 2011;121:3220–3232. doi: 10.1172/JCI41514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qualtrough D, Hinoi T, Fearon E, Paraskeva C. Expression of CDX2 in normal and neoplastic human colon tissue and during differentiation of an in vitro model system. Gut. 2002;51:184–190. doi: 10.1136/gut.51.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregory PA, Lewinsky RH, Gardner-Stephen DA, Mackenzie PI. Regulation of UDP glucuronosyltransferases in the gastrointestinal tract. Toxicol Appl Pharmacol. 2004;199:354–363. doi: 10.1016/j.taap.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi T, Gu X, Golden HM, Suh E, Rhoads DB, et al. Cloning of the human claudin-2 5'-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1alpha. J Biol Chem. 2002;277:21361–21370. doi: 10.1074/jbc.M110261200. [DOI] [PubMed] [Google Scholar]
- 30.Coskun M, Troelsen JT, Nielsen OH. The role of CDX2 in intestinal homeostasis and inflammation. Biochim Biophys Acta. 2011;1812:283–289. doi: 10.1016/j.bbadis.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Gao N, Kaestner KH. Cdx2 regulates endo-lysosomal function and epithelial cell polarity. Genes & development. 2010;24:1295–1305. doi: 10.1101/gad.1921510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takakura Y, Hinoi T, Oue N, Sasada T, Kawaguchi Y, et al. CDX2 regulates multidrug resistance 1 gene expression in malignant intestinal epithelium. Cancer Res. 2010;70:6767–6778. doi: 10.1158/0008-5472.CAN-09-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takasuna K, Kasai Y, Kitano Y, Mori K, Kobayashi R, et al. Protective effects of kampo medicines and baicalin against intestinal toxicity of a new anticancer camptothecin derivative, irinotecan hydrochloride (CPT-11), in rats. Jpn J Cancer Res. 1995;86:978–984. doi: 10.1111/j.1349-7006.1995.tb03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glas J, Seiderer J, Fischer D, Tengler B, Pfennig S, et al. Pregnane X receptor (PXR/NR1I2) gene haplotypes modulate susceptibility to inflammatory bowel disease. Inflamm Bowel Dis. 2010. [DOI] [PubMed]
- 35.Sepe V, Ummarino R, D'Auria MV, Mencarelli A, D'Amore C, et al. Total synthesis and pharmacological characterization of solomonsterol A, a potent marine pregnane-X-receptor agonist endowed with anti-inflammatory activity. J Med Chem. 2011;54:4590–4599. doi: 10.1021/jm200241s. [DOI] [PubMed] [Google Scholar]
- 36.Wallace K, Cowie DE, Konstantinou DK, Hill SJ, Tjelle TE, et al. The PXR is a drug target for chronic inflammatory liver disease. J Steroid Biochem Mol Biol. 2010;120:137–148. doi: 10.1016/j.jsbmb.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng J, Shah YM, Ma X, Pang X, Tanaka T, et al. Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation. J Pharmacol Exp Ther. 2010;335:32–41. doi: 10.1124/jpet.110.170225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mencarelli A, Migliorati M, Barbanti M, Cipriani S, Palladino G, et al. Pregnane-X-receptor mediates the anti-inflammatory activities of rifaximin on detoxification pathways in intestinal epithelial cells. Biochem Pharmacol. 2010;80:1700–1707. doi: 10.1016/j.bcp.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Beck F, Stringer EJ. The role of Cdx genes in the gut and in axial development. Biochem Soc Trans. 2010;38:353–357. doi: 10.1042/BST0380353. [DOI] [PubMed] [Google Scholar]
- 40.Liu L, Deng YX, Liang Y, Pang XY, Liu XD, et al. Increased oral AUC of baicalin in streptozotocin-induced diabetic rats due to the increased activity of intestinal beta-glucuronidase. Planta Med. 2010;76:70–75. doi: 10.1055/s-0029-1185946. [DOI] [PubMed] [Google Scholar]
- 41.Guo XY, Yang L, Chen Y, Wang QF, Sun QS, et al. Identification of the metabolites of baicalein in human plasma. J Asian Nat Prod Res. 2011;13:861–868. doi: 10.1080/10286020.2011.599321. [DOI] [PubMed] [Google Scholar]
- 42.Hou YC, Lin SP, Tsai SY, Ko MH, Chang YC, et al. Flavonoid pharmacokinetics and tissue distribution after repeated dosing of the roots of Scutellaria baicalensis in rats. Planta Med. 2011;77:455–460. doi: 10.1055/s-0030-1250433. [DOI] [PubMed] [Google Scholar]
- 43.Aoki K, Kakizaki F, Sakashita H, Manabe T, Aoki M, et al. Suppression of colonic polyposis by homeoprotein CDX2 through its nontranscriptional function that stabilizes p27Kip1. Cancer Res. 2011;71:593–602. doi: 10.1158/0008-5472.CAN-10-2842. [DOI] [PubMed] [Google Scholar]
- 44.Zhou C, Tabb MM, Nelson EL, Grun F, Verma S, et al. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. The Journal of clinical investigation. 2006;116:2280–2289. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–472. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 46.Mencarelli A, Distrutti E, Renga B, D'Amore C, Cipriani S, et al. Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation. PLoS One. 2011;6:e22978. doi: 10.1371/journal.pone.0022978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin SC, Li C, Choi JS. Effects of baicalein, an antioxidant, on the bioavailability of doxorubicin in rats: possible role of P-glycoprotein inhibition by baicalein. Pharmazie. 2009;64:579–583. [PubMed] [Google Scholar]
- 48.De Amit K, Datta S, Mukherjee A. Design and in vitro evaluation of a bipolymeric delivery device for the amelioration of colonic diseases using a popular glucocorticoid as a model drug. Acta Pol Pharm. 2011;68:735–744. [PubMed] [Google Scholar]
- 49.Nelson JD, Denisenko O, Bomsztyk K. Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat Protocols. 2006;1:179. doi: 10.1038/nprot.2006.27. [DOI] [PubMed] [Google Scholar]
- 50.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong H, Singh SV, Singh SP, Mu Y, Lee JH, et al. Orphan nuclear receptor pregnane X receptor sensitizes oxidative stress responses in transgenic mice and cancerous cells. Molecular endocrinology. 2006;20:279–290. doi: 10.1210/me.2005-0205. [DOI] [PubMed] [Google Scholar]
- 52.Huang H, Wang H, Sinz M, Zoeckler M, Staudinger J, et al. Inhibition of drug metabolism by blocking the activation of nuclear receptors by ketoconazole. Oncogene. 2007;26:258–268. doi: 10.1038/sj.onc.1209788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baicalein, but not baicalin, activates and induces PXR in vitro . (A) The DPX2 assay (see Materials and Methods) was used to assess PXR transactivation and (B) cell viability. (C) Individual colon cancer cells lines with varying abundance of Cdx2 protein were used to determine the effect of flavonoids (baicalein, baicalin) on induction of PXR mRNA. Bottom panel shows expression of Cdx2 protein in these cell lines. (D) PXR transactivation assay in 293T cells co-expressing Cdx2 and −3.81 kb PXR reporter. Histogram and data points, mean ± SEM.
(PDF)
Cdx2 knockdown in LS174T colon cancer cells. (A) LS174T cells co-transfected with GFP expressing plasmid and scrambled siRNA or si-Cdx2 with images captured under appropriate filters (Phase contrast and GFP). The middle panel shows fold Cdx2 mRNA expression by RT-qPCR. β-actin was used as internal control. Gene expression changes were calculated using the comparative Ct method with β-actin as the reference gene and scrambled siRNA transfected cells as the calibrator. The bottom panel shows a western blot of Cdx2 from nuclear extract from the same cells. Histogram, mean ± SEM. Scale bar, 100 µm; ** P<0.02
(PDF)
Cdx2 directly targets and induces the PXR promoter. (A) Schematic diagram illustrating possible mechanisms by which Cdx2 induces PXR mRNA, through its (1) direct action or (2, 3) indirect action(s) on the PXR promoter. BS: Cdx2 binding site on the PXR promoter. (B) Western blot of cell lysates from two clones (c1, c2) of HT-29/Cdx2-ER cells (obtained after sequential passage) with (+) or without (-) exposure to 4-OHT (hydroxytamoxifen) for Cdx2 and β-actin. The right panel shows time-dependent induction of PXR mRNA as assessed by RT-qPCR. (C) Immunoprecipitation blot of PXR from HT-29/Cdx2-ER cells exposed to cycloheximide and/or 4-OHT. Right panel shows fold expression of PXR mRNA by RT-qPCR. β-actin was used as internal or loading control for western blots. Gene expression changes were calculated using the comparative Ct method with β-actin as the reference gene and vehicle treated cells (or indicated as 0) as the calibrator. Histogram, mean ± SD.
(PDF)
Cdx2 directly targets and induces the PXR promoter. (A) Left panel shows western blot of Cdx2 in cell lysates prepared from DLD-1 pGIPZ(c) (control cells) and shCdx2 infected cells. Right panel shows fold mRNA expression of Cdx2 and PXR by RT-qPCR from the same experiment. (B) Immunoprecipitation blot of PXR from control DLD-1 pGIPZ(c) and shCdx2 infected cells exposed to cycloheximide and/or 4-OHT. Right panel shows fold expression of PXR mRNA by RT-qPCR. β-actin was used as internal or loading control for western blots. Gene expression changes were calculated using the comparative Ct method with β-actin as the reference gene and vehicle treated cells (or indicated as 0) as the calibrator. Histogram, mean ± SD.
(PDF)
PXR knockdown in LS174T colon cancer cells. (A) LS174T cells co-transduced with GFP expressing plasmid and scrambled shRNA or shPXR with images captured under appropriate filters (Phase contrast and GFP). The middle panel shows fold PXR mRNA expression by RT-qPCR. β-actin was used as internal control. Gene expression changes were calculated using the comparative Ct method with β-actin as the reference gene and scrambled shRNA transfected cells as the calibrator. The bottom panel shows a western blot for PXR from nuclear extract from the same cells. Histogram, mean ± SEM. Scale bar, 100 µm; ** P<0.02
(PDF)
Baicalin does not decrease SN-38 glucuronide (SN-38G) emission spectra upon incubation with mouse feces. (A) SN-38G emission spectra in HEPES buffer. (B) Co-incubation of mouse feces with vehicle (HEPES buffer), Inh1 (10 µM), or Baicalin (10 µM).
(PDF)
Cdx2 binds to binding Site1 (BS1) oligonucleotide probe by electrophoretic mobility shift assay (EMSA). (A) Black arrows demonstrate bands upon incubation of respective probes with and without LS174T colon cancer cell line nuclear extract. The lower arrow indicates band common to the positive control and PXR probe lane. (B) Black arrow (bold) demonstrates loss of band upon addition of 50-fold excess of cold PXR probe. (C) Black arrow (bold) demonstrates supershift band, identical to that observed in positive control lane, with the addition of Cdx2 antibody. (D) Black arrow (bold) demonstrates loss of band upon incubation of LS174T nuclear extracts with BS1 mutant oligo. -, negative control oligo; +, positive control oligo; wt, wild-type or PXR probe sequence from PXR promoter containing BS1; mt, PXR probe sequence mutant of BS1.
(PDF)
Cdx2 binds to binding Site2 (BS2) oligonucleotide probe by electrophoretic mobility shift assay (EMSA). (A) Black arrows demonstrate bands upon incubation of respective probes with and without LS174T colon cancer cell line nuclear extract. The lower arrow indicates band common to the positive control and PXR probe lane. (B) Black arrow (bold) demonstrates loss of band upon addition of 50-fold excess of cold PXR probe. (C) Black arrow (bold) demonstrates supershift band, identical to that observed in positive control lane, with the addition of anti-Cdx2 antibody. (D) Black arrow (bold) demonstrates loss of band upon incubation of LS174T nuclear extracts with BS2 mutant oligo. -, negative control oligo; +, positive control oligo; wt, wild-type or PXR probe sequence from PXR promoter containing BS2; mt, PXR probe sequence mutant of BS2.
(PDF)
Primer sequences for ChIP assay.
(DOC)
Probe Sequences for EMSA.
(DOC)
File contains the following: Supporting Information Methods including primer sequences and shRNA sequences, Supporting Information Results and Supporting Information References.
(DOC)