Abstract
Background
The epidemic of obesity and diabetes is increasing within the USA and worldwide. We have previously shown that body mass index has increased significantly in autosomal dominant polycystic kidney disease (ADPKD) subjects seen at our center in more recent years. However, the impact of Type II diabetes in ADPKD patients has not been well studied.
Methods
This retrospective cohort study compared clinical characteristics in 44 pre-renal transplant patients with ADPKD and diabetes and 88 age- and sex-matched non-diabetic patients with ADPKD who were seen at the University of Colorado between 1977 and 2008. The primary outcomes in this study were renal volume determined by renal ultrasonography, renal function assessed by estimated glomerular filtration rate and time to onset of end-stage renal disease or death by Kaplan–Meier analyses.
Results
Diabetic patients had significantly larger kidney volumes than those with ADPKD alone [geometric mean (95% confidence interval (CI)]: 2456 (1510–3992) versus 1358 (1186–1556) cm3, P = 0.02. Among those whose age at hypertension diagnosis was known, the diabetic ADPKD patients had earlier median (95% CI) age at onset of hypertension compared to those with ADPKD alone: 32.5 (28–40) versus 38 (35–42) years, P = 0.04. Diabetic ADPKD patients tended to have an earlier median age of death than those with ADPKD alone.
Conclusions
Patients with ADPKD and type II diabetes have larger renal volumes, earlier age at diagnosis of hypertension and may die at a younger age compared to those patients with ADPKD alone. This study emphasizes the importance of diabetes risk management in ADPKD.
Keywords: ADPKD, chronic kidney disease, diabetes, hypertension, renal failure
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common potentially lethal genetic disorder affecting 1 in 400 live births [1]. Currently, ADPKD accounts for between 5 and 10% of individuals initiating renal replacement therapy, thus understanding the factors that impact renal disease progression in ADPKD and implementation of measures to preserve renal function and delay onset of end-stage renal in this population is essential.
As the epidemic of obesity and diabetes continues to increase within both the USA and worldwide, the number of patients afflicted with both ADPKD and diabetes is likely to increase. In this regard, information from our own ADPKD center indicated that body mass index increased significantly in female ADPKD patients in the time interval 1992–2001 compared to 1985–1992 [2]. In a more recent study of ADPKD patients with normal renal function, 14% of the patients met the National Heart, Lung and Blood Institiute's Adult Panel III criteria for the metabolic syndrome [3] while International Diabetes Federation criteria were fulfilled by 22% of patients [4], thus indicating that several features of the metabolic syndrome are present in some ADPKD patients who have normal renal function.
A previous small study of two kindreds with ADPKD demonstrated delayed onset of renal insufficiency among four family members with ADPKD and type II diabetes compared to eight non-diabetic individuals with ADPKD alone [5]. In this study, the authors suggested that treatment with the oral sulfonylurea, glibenclamide, might block fluid secretion into cysts by blocking ATP-dependent potassium channels and the cystic fibrosis transmembrane conductance regulator (CFTR). However, significant variability in age at onset of end-stage renal disease (ESRD) is common even among members of the same family who carry the same gene mutation [6]. A lower prevalence of diabetes has been described among Polish patients with ADPKD compared to non-ADPKD-affected siblings, although the type of diabetes was not specified in the siblings [7]. As expected, age was a significant risk factor for diabetes in both ADPKD-affected and non-affected individuals in this study.
To date, only one very small study has addressed the impact of type II diabetes in patients with ADPKD. Thus, the objective of the current study was to compare the clinical characteristics of ADPKD patients with diabetes to non-diabetic ADPKD individuals in our longitudinal ADPKD database.
Materials and methods
ADPKD study subjects
The ADPKD clinical database at the University of Colorado Denver was searched and 1340 subjects seen between 1977 and 2008 were identified with information regarding the presence or absence of type II diabetes mellitus. Among these subjects, 44 had type II diabetes that was diagnosed by their physician. Clinical data were available for 22 of the subjects with ADPKD and diabetes with more limited medical history data only available in the remaining 22 diabetic ADPKD subjects. Eighty-eight subjects with ADPKD alone who were matched by age and sex to the subjects with ADPKD and diabetes were selected as a control group. All subjects met Ravine's criteria for diagnosis of ADPKD [8]. All subjects gave informed consent and the study protocol was approved by the Colorado Multiple Institutional Review Board.
Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [9].
Renal volumes were measured by standard abdominal ultrasound. Renal volume was calculated as the volume of a modified ellipse for each kidney using the following formula: volume = π/6 × length × width × depth. Length, width and depth were measured in centimeters. Length and width were obtained from longitudinal images acquired in planes ranging from sagital to coronal, whereas depth was obtained from transverse images of the mid-kidney acquired in the plane perpendicular to the longitudinal plane. Total renal volume was measured by summing the volumes of both kidneys.
Statistical analysis
Due to the skewed nature of eGFR and total renal volumes, a natural log transformation was applied and the transformed data were used in analysis of variance with age and sex as covariates. Results are reported as geometric mean and 95% confidence interval (CI). Kaplan–Meier survival analysis was used to calculate survival time to diagnosis of hypertension, onset of ESRD and death. The survival analyses included all 44 of the patients with ADPKD and diabetes. Chi-square or Fisher's exact analysis was used to determine categorical variables. Mixed models were used to estimate the decline in eGFR and to estimate the increase in serum creatinine per year in the ADPKD with diabetes and ADPKD patient groups. In all analyses, P ≤ 0.05 was considered significant.
Results
The characteristics of the 22 study patients with clinical data are depicted in Table 1. The incidence of non-transplant-related diabetes among those with relevant information in the ADPKD database was 44/1340 (3.3%), although it should be noted that many subjects in the University of Colorado database are children or young adults. All but 1 of the 22 diabetic subjects with available clinical data and medical history managed their diabetes by oral anti-glycemic agents or by diet. The remaining subject required insulin for diabetes management. Exclusion of the patient on insulin therapy from data analysis did not affect the outcome so results are presented in Table 1 based on inclusion of all 22 subjects and of all 44 subjects in survival analyses.
Table 1.
Characteristics of 22 diabetic patients with ADPKD who had available clinical data and 88 age- and sex-matched patients with ADPKD alonea
| Parameter | ADPKD + diabetes (n = 22) | ADPKD (n = 88) | P-value |
|---|---|---|---|
| Age (years) | 47.8 ± 10.8 | 47.8 ± 10.6 | NS |
| Male/female (% male) | 8/14 (36.4) | 35/53 (39.8) | NS |
| Body mass indexb | 33.6 ± 7.9 | 26.2 ± 4.6 | 0.0008 |
| Diabetes duration (years) | 3.7 ± 4.4 | ||
| eGFRb (mL/min/1.73 m2) | 46.9 (37.1–59.1) | 39.8 (34.8–45.6) | NS |
| Total renal volumec (cm3) | 2456 (1510–3992) | 1358 (1186–1556) | 0.02 |
| Hypertension (%) | 21/22 (95.4) | 78/88 (88.6) | NS |
| Age at hypertension diagnosisd,e (years) | 32.5 (28–40) | 38.0 (35–42) | 0.04 |
| ESRD (%) | 4/22 (18.2) | 31/88 (35.2) | NS |
| Deceased (%) | 1/22 (4.5) | 13/88 (14.8) | NS |
NS, not significant.
Body mass index was available for 19 patients with ADPKD and diabetes.
Geometric mean (95% CI).
Age at diagnosis of hypertension was known in 24 patients with ADPKD and diabetes.
Median age at hypertension diagnosis (95% CI).
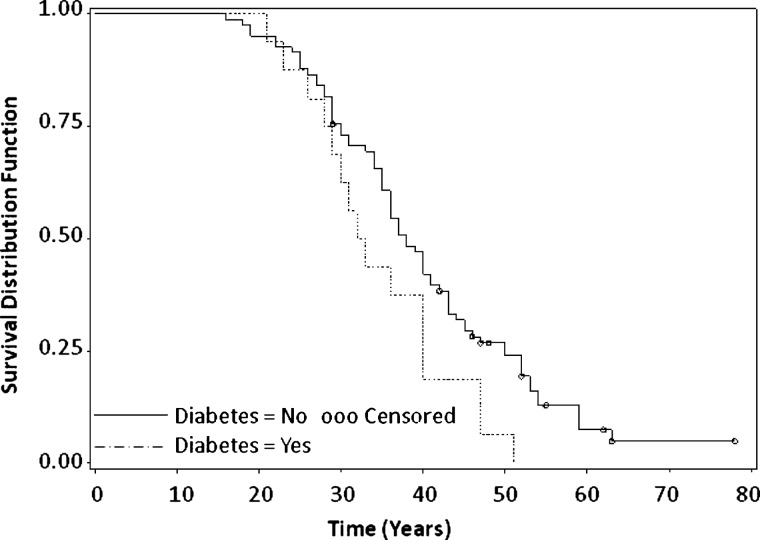
Patients with ADPKD and diabetes had significantly higher BMI (P = 0.0008) and larger renal volumes than those with ADPKD alone (P = 0.02). There were no statistical differences in the occurrence of hypertension, ESRD or death between patients with ADPKD and diabetes and those with ADPKD alone. However, among those patients with known age at diagnosis of hypertension, those with ADPKD and diabetes had earlier median age (CI) at diagnosis of hypertension, 32.5 years (28–40), compared to those with ADPKD alone, 38 years (35–42), P = 0.04 as depicted in Figure 1. Available data from 24 patients (19 patients with clinical data and 5 patients with limited medical history information) with ADPKD and diabetes was utilized to compute median age at hypertension diagnosis.
Fig. 1.
Time to diagnosis of hypertension. From left of Figure, dashed line represents ADPKD with diabetes and solid line represents ADPKD no diabetes. Open circles represent censored values in Kaplan–Meier analysis. Patients with ADPKD and diabetes had a significantly earlier age at hypertension diagnosis [32.5 (28–40) years] compared to patients with ADPKD alone [38 (35–42) years], P = 0.04.
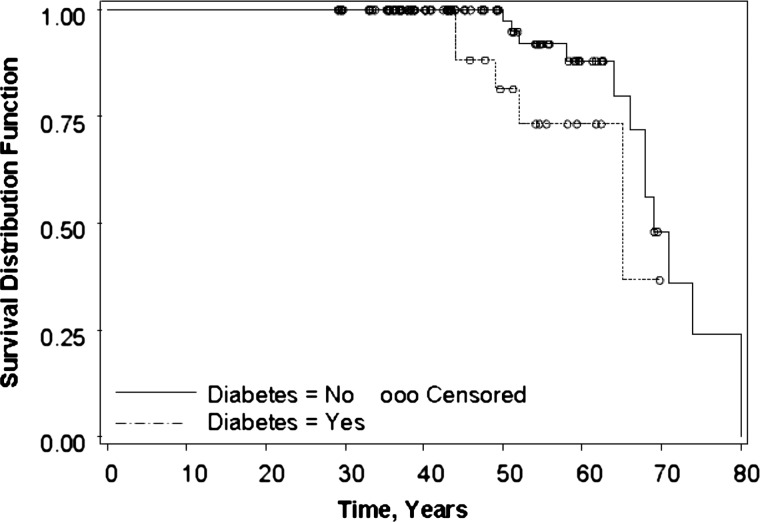
Based on inclusion of all 44 patients with ADPKD and diabetes (14 male patients and 30 female patients), median age at onset of ESRD did not differ between patients with ADPKD and diabetes and those with ADPKD alone as shown in Figure 2. There was a trend toward earlier age at death in ADPKD patients with diabetes [65 years (52 to no upper limit)] compared to patients with ADPKD alone [69 years (66–80)] as depicted in Figure 3, although this did not reach significance, P = 0.09.
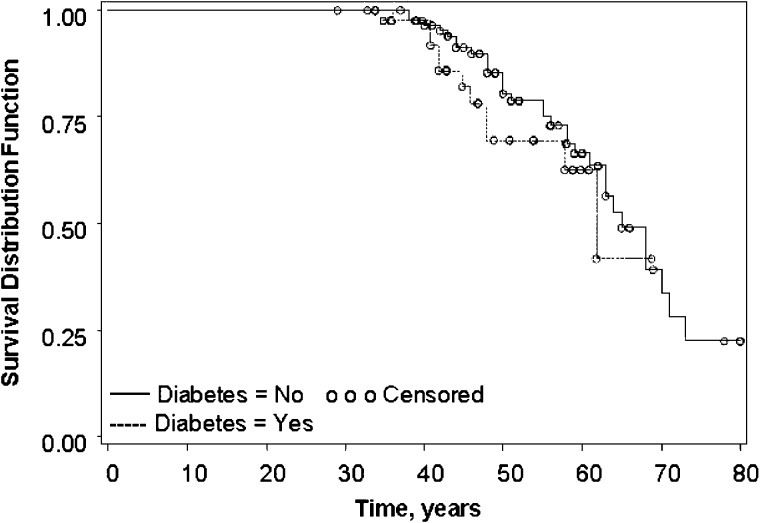
Fig. 2.
Time to onset of ESRD. From left of figure, dashed line represents ADPKD with diabetes and solid line represents ADPKD no diabetes. Open circles represent censored values in Kaplan–Meier analysis. There was no significant difference in time to onset of ESRD between patients with ADPKD and diabetes [63 (49 to no upper limit) and those with ADPKD alone [65 (61–71) years], P = 0.92.
Fig. 3.
Survival time until death. From left of Figure, dashed line represents ADPKD with diabetes and solid line ADPKD represents no diabetes. Open circles represent censored values in Kaplan–Meier analysis. Patients with ADPKD and diabetes had a trend toward earlier median age at death [65 (52 to no upper limit) years] compared to patients with ADPKD alone [69 (66–80) years], but this difference did not reach significance, P = 0.09.
In order to compare the rate of decline in renal function in patients with ADPKD and diabetes to those with diabetes alone, all patients with at least two measurements of eGFR and serum creatinine were included in the analysis. Thirteen patients with ADPKD and diabetes were included and 71 with ADPKD alone. The mean observation period for patients with diabetes and ADPKD was 9.5 ± 6.9 years and for those with diabetes alone 10.9 ± 6.9 years. There were no significant differences in the slopes for eGFR decline between both groups (P = 0.9). In patients with ADPKD and diabetes, eGFR declined at a rate of −2.89 ± 1.24 mL/min/1.73 m2/year observed compared to −3.10 ± 0.49 mL/min/1.73 m2/year observed in patients with ADPKD. Age at first eGFR measurement was included in the model used for the analysis. In a similar manner, the rate of increase in serum creatinine was compared in both the diabetic and non-diabetic patient groups. Serum creatinine increased at a rate of 0.10 mg/dL/year observed in the ADPKD patients with diabetes compared to 0.20 mg/dL/year observed in the ADPKD patients without diabetes; the slopes were not statistically different, P = 0.36.
Discussion
The epidemic of obesity and metabolic syndrome fostered by modern dietary excess and lack of physical activity are increasing worldwide and as a consequence likewise the incidence of type II diabetes. Data from our own research center indicate a higher body mass index in women with ADPKD in more recent times [2], increasing the risk for type II diabetes in the ADPKD population. Similarly, a recent study in patients with ADPKD reported a 14% incidence of the components of metabolic syndrome in ADPKD comparable to that found in a healthy control population [4], underlining the fact that patients with ADPKD are at risk for type II diabetes. Based on a survey of our ADPKD database, we identified 44/1340 subjects with type II diabetes. This incidence of 3.3% is somewhat lower than the estimated overall US incidence of diabetes, 7.8% [10]. However, as the prevalence of diabetes increases with age, it should be noted that a significant number of subjects in our ADPKD database are children and young adults. An incidence of transplant-unrelated diabetes of ∼2% was previously reported based on a survey of 459 subjects from a Polish ADPKD registry [7].
In the current study, subjects with ADPKD and diabetes had significantly larger kidneys than age- and sex-matched subjects with ADPKD alone [total renal volume (CI): 2456 (1510–3992) versus 1358 (1186–1556) mL; P = 0.02]. Early in disease, diabetic patients are known to have increased kidney size secondary to mesangial expansion [11–13]. However, age at onset of ESRD did not differ significantly between the ADPKD diabetic and non-diabetic subjects. This contrasts with the outcome of an earlier study that suggested delayed onset of ESRD in patients with ADPKD and diabetes compared to subjects with ADPKD alone [5].
In the current study, the oral anti-glycemic agents utilized by the diabetic subjects were either sulfonylurea agents or metformin. McCarty et al. recently proposed that activation of AMP-activated kinase (AMPK) by agents such as metformin may be an effective strategy for management of ADPKD [14] by inhibition of mammalian target for rapamycin and cystic fibrosis transmembrane conductance regulator. Likewise, Beckenroth and Popovtzer [5] suggested that sulfonylurea therapy may decrease fluid secretion into cysts through inhibition of ATP-sensitive potassium channels and CFTR. Thus, oral anti-glycemic therapy may ameliorate renal disease progression to a certain extent in diabetic patients with ADPKD. This may partially compensate for the additional renal injury associated with diabetes and account for the similarity in age at ESRD between diabetic and non-diabetic patients with ADPKD. However, a longitudinal study would be necessary to test this hypothesis.
One significant finding in the current study was the earlier age at hypertension diagnosis in subjects with ADPKD and diabetes compared to those with ADPKD alone; 32.5 (28–40) versus 38 (35–42) years, P = 0.04 as shown in Figure 1. In addition, the trend toward earlier age at death in subjects with ADPKD and diabetes coupled with no difference in age at onset of ESRD or rate of renal function decline would suggest that death results from causes other than renal failure. In a previous study, cardiovascular disease was shown to dominate the clinical picture in ADPKD patients with diabetes [5]. Indeed, since introduction of renal replacement therapy, cardiovascular complications are the leading causes of death in patients with ADPKD [15, 16]. Similarly, among subjects with diabetes, the incidence of heart disease and stroke is approximately two to four times higher than in the general population [10]. Earlier onset of hypertension in patients with ADPKD and diabetes may exacerbate the risk for cardiovascular complications. Thus, subjects with both ADPKD and diabetes may be at significantly greater risk for cardiovascular complications and associated heightened risk for earlier death. In fact, a large recent study of ADPKD patients in the United Kingdom demonstrated that mortality was significantly higher in ADPKD patients with diabetes [17].
In conclusion, patients with ADPKD and diabetes have larger kidneys, earlier age at hypertension diagnosis and trend toward earlier age at death compared to patients with ADPKD alone. Exercise and healthy weight management should therefore be emphasized in patients with ADPKD.
Acknowledgements
This research was supported by grant numbers M01RR00051 and M01RR00069 from the General Research Centers Program, National Center for Research Resources (NCRR)/NIH; by NIH/NCRR Colorado Clinical and Translational Sciences Institute (CTSI) grant number ULI RR025780, by grant DK34039 from NIH (National Institute of Diabetes and Digestive and Kidney Diseases) and by the Zell Family Foundation.
Conflict of interest statement. None declared.
References
- 1.Ecder T, Fick-Brosnahan G, Schrier RW. Polycystic kidney disease. In: Schrier RW, editor. 2007. pp. 502–539. Diseases of the Kidney and Urinary Tract Vol. 1 Philadelphia, PA Lipincott Williams and Wilkins. [Google Scholar]
- 2.Schrier RW, McFann K, Johnson AM. Epidemiological study of kidney survival in autosomal dominant polycystic kidney disease. Kidney Int. 2003;63:678–685. doi: 10.1046/j.1523-1755.2003.00776.x. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 4.Pietzak-Nowacka M, Safranow K, Byra E, et al. Metabolic syndrome components in patients with autosomal dominant polycystic kidney disease. Kidney Blood Press Res. 2009;32:405–410. doi: 10.1159/000260042. [DOI] [PubMed] [Google Scholar]
- 5.Backenroth R, Popovtzer MM. Does type 2 diabetes mellitus delay renal failure in autosomal dominant polycystic kidney disease? Kidney Int. 2002;24:803–813. doi: 10.1081/jdi-120015682. [DOI] [PubMed] [Google Scholar]
- 6.Reed BY, McFann K, Bekheirnia MR, et al. Variation in age at ESRD in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2008;51:173–183. doi: 10.1053/j.ajkd.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietrzak-Nowacka M, Rozanski J, Safranow K, et al. Autosomal dominant polycystic kidney disease reduces the risk of diabetes mellitus. Arch Med Res. 2006;37:360–364. doi: 10.1016/j.arcmed.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Ravine D, Gibson RN, Walker RG, et al. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney 1. Lancet. 1994;343:824–827. doi: 10.1016/s0140-6736(94)92026-5. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 11.Wirta O, Pasternak A, Laippala P, et al. Glomerular filtration rate and kidney size after 6 years duration in non-insulin dependent diabetic subjects. Clin Nephrol. 1996;45:810–817. [PubMed] [Google Scholar]
- 12.Giordano M, Ciarambino T, Gesue L, et al. The ratio between kidney volume and function increases with the progression of nephropathy in type 2 diabetes. Clin Nephrol. 2009;72:247–251. doi: 10.5414/cnp72247. [DOI] [PubMed] [Google Scholar]
- 13.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarty MF, Barrosa-Aranda J, Contreras F. Activation of AMP-activated kinase as a strategy for managing autosomal dominant polycystic kidney disease. Med Hypotheses. 2009;73:1008–1010. doi: 10.1016/j.mehy.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 15.Fick GM, Johnson AM, Hammond WS, et al. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;5:2048–2056. doi: 10.1681/ASN.V5122048. [DOI] [PubMed] [Google Scholar]
- 16.Perrone RD, Ruthazer R, Terrin NC. Survival after end-stage renal disease in autosomal dominat polycystic kidney disease: Contribution of extrarenal complications to mortality. Am J Kidney Dis. 2001;38:777–784. doi: 10.1053/ajkd.2001.27720. [DOI] [PubMed] [Google Scholar]
- 17.Patch C, Charlton J, Roderick PJ, et al. Use of antihypertensive medications and mortality of patients with autosomal dominant polycystic kidney disease: a population-based study. Am J Kidney Dis. 2011;57:856–862. doi: 10.1053/j.ajkd.2011.01.023. [DOI] [PubMed] [Google Scholar]