Abstract
Klotho is highly expressed in the kidney and a soluble form of Klotho functions as an endocrine substance that exerts multiple actions including the modulation of renal solute transport and the protection of the kidney from a variety of insults in experimental models. At present, the Klotho database is still largely preclinical, but the anticipated forthcoming impact on clinical nephrology can be immense. This manuscript puts these potentials into perspective for the clinician. There is renal and systemic Klotho deficiency in both acute kidney injury (AKI) and chronic kidney disease (CKD). Klotho plummets very early and severely in AKI and represents a pathogenic factor that exacerbates acute kidney damage. In CKD, Klotho deficiency exerts a significant impact on progression of renal disease and extra renal complications. In AKI, soluble Klotho levels in plasma and/or urine may serve as an early biomarker for kidney parenchymal injury. Restoration by exogenous supplementation or stimulation of endogenous Klotho may prevent and/or ameliorate kidney injury and mitigate CKD development. In CKD, Klotho levels may be an indicator of early disease and predict the rate of progression, and presence and severity of soft tissue calcification. The correction of Klotho deficiency may delay progression and forestall development of extra renal complications in CKD. Rarely does one find a molecule with such broad potential applications in nephrology. Klotho can possibly emerge on the horizon as a candidate for an unprecedented sole biomarker and intervention. Nephrologists should monitor the progress of the preclinical studies and the imminently emerging human database.
Keywords: acute kidney injury, biomarker, cardiac hypertrophy, chronic kidney disease, FGF23, Klotho, prognosis, secondary hyperparathyroidism
Introduction
Klotho was originally identified as an anti-aging protein but was subsequently discovered to have a multitude of biologic actions [1–3]. Klotho is expressed in multiple tissues and organs, but by far, its highest expression is in the kidney [1]. Despite this exceedingly nephrocentric origin, it was not until the last several years that Klotho's role in kidney function and disease became fully apparent, a cognizance that jettisoned Klotho into the spotlight in nephrology research. Klotho has extreme pleiotropic actions, including cytoprotection, through its ability to confer anti-oxidation [4], anti-apoptosis [5] anti-senescence [5], protection of vasculature [6], promotion of angiogenesis and vascularization [7], inhibition of fibrogenesis [3, 8] and preservation of stem cells [2]. All of the above properties of Klotho can potentially mediate its renoprotective effects demonstrated in animal models. In addition, Klotho inhibits phosphate absorption and promotes phosphaturia, thus preventing phosphate overload, which may contribute to the prevention of vascular calcification, in addition to its renoprotective effects [9, 10].
Several recent reviews comprehensively addressed the physiology of Klotho in aging [11], renal calcium, phosphate and potassium transport [12], its pathophysiologic role in acute kidney injury (AKI) development and chronic kidney disease (CKD) progression and its complications [13]. This manuscript is devoted primarily to discussing the potential diagnostic, prognostic and therapeutic roles of Klotho in acute kidney disease and CKD. The human database has been slow to emerge due to reagent limitations but progress in securing definitive human data is certainly in the very near future. Recombinant Klotho administration is already used in animal models and maneuvers are available to enhance endogenous Klotho so therapeutic applications are quite tangible in addition to the diagnostic uses.
Brief account of Klotho biology
Klotho was identified in 1997 when its locus was unintentionally interrupted while generating a murine model of hypertension. The resultant phenotype resembled premature multiorgan degeneration characterized by shortened life span, hypogonadism, growth retardation, skin atrophy, vascular calcification, osteopenia, pulmonary emphysema, cognition impairment, hearing loss and hyperphosphatemia [1]. Conversely, transgenic Klotho-overexpressing mice (Tg-Kl) have about double the plasma Klotho levels and 20–30% longer average life span than wild-type (WT) mice [14]. These findings secured Klotho as an aging-suppressor gene and spawned two decades of aging research. Klotho is expressed in multiple tissues but is particularly high in the kidney, predominantly in renal tubular cells [1, 10]. This highly nephrocentric expression pattern was known from the beginning, but Klotho did not take center stage in renal research until recently.
Since the discovery of Klotho [1], another two related paralogs, β-Klotho [15], which is involved in bile acid and energy metabolism, and γ-Klotho [16], with a yet to be defined function, have been described leading to the revised naming of Klotho as α-Klotho. For this discussion, we will simply use the old nomenclature of Klotho. Full-length Klotho is a single-pass transmembrane protein that functions as a co-receptor for fibroblast growth factor-23 (FGF23) which promotes negative phosphate balance by inhibiting 1,25(OH)2 vitamin D3 synthesis and inducing phosphaturia (Figure 1). The strikingly similar phenotypes observed in Klotho- and FGF23-deficient mice indicate that Klotho and FGF23 share common downstream effects [17] and in vitro studies further prove that Klotho functions as an obligatory co-receptor for FGF23 to form a complex with FGFR's and FGF23 (Figure 1) [18]. The extracellular domain is cleaved by membrane-anchored protease ADAM10/17 (Figure 1) [19] and released into the extracellular space including the blood, urine and cerebrospinal fluid [9, 10, 20]. The soluble ectodomain of Klotho, usually called soluble Klotho, possesses glycosidase activity that trims sugar chains on ion channels and transporters on the cell surface, regulating their activity and/or cell surface retention time. It has been shown that this is one of the major mechanisms whereby soluble Klotho modulates calcium channels (TRPV5) [21], Na-phosphate co-transporters-2a (NaPi-2a) [10] and the potassium channel (ROMK) [22]. Another shorter length Klotho protein only encompassing the Kl1 domain in blood circulation is the secreted Klotho protein encoded by an alternative spliced transcript at Klotho gene exon 3 [23]. But its biological function is poorly known. Thus, the term ‘soluble Klotho’, the extracellular domain of membrane Klotho in plasma or urine, is exchangeably referred as plasma or urine Klotho in this manuscript [19].
Fig. 1.

Schema for Klotho gene, transcripts and proteins. Rodent and human Klotho spans 50 kb and consists of five exons. Two transcripts, secreted and membrane forms of Klotho, are generated through alternative RNA splicing. The internal splice donor site is in exon 3. The resultant alternatively spliced transcript after exon 3 (grey) with an in-frame translation stop codon is introduced. The short protein product, secreted Klotho, contains only Kl1 and is released from the cell. The longer Klotho encoded by the membrane form of the Klotho transcript is a single-pass transmembrane protein anchored in the cell surface. The extracellular domain of membrane Klotho containing Kl1 and Kl2 repeats is shed and cleaved by ADAM10/17, and released into bloodstream. Thus, the circulation harbors two forms of Klotho—one is the ectodomain derived from cleavage of the extracellular domain of membrane Klotho and another is the secreted protein derived from an alternatively spliced Klotho transcript. Transmembrane Klotho works with FGFRs as co-receptor for FGF23 signal transduction.
Clinical significance of Klotho in AKI
Klotho deficiency
Animal experiments clearly showed transient renal Klotho deficiency in AKI from a variety of causes including ischemia–reperfusion injury (IRI) [24, 25], ureteric obstruction [3], lipopolysaccharide (model of sepsis) [26], hypovolemia [26] and nephrotoxins including cisplatin [27, 28] and folic acid [28], indicating that renal Klotho downregulation in AKI is likely a general phenomenon after acute pre-, intrinsic- and post-renal insults. Longitudinal observation of the IRI-AKI model showed that both Klotho mRNA and protein started to fall on the first day and returned to near baseline around day 3 and 4, respectively [24]. While changes in kidney morphology and an increase in neutrophil gelatinase-associated lipocalin (NGAL) are detectable after 5 h, renal Klotho protein levels were drastically and sustainably decreased beginning at 3 h, suggesting that renal Klotho protein may be one of the earliest biomarkers for kidney injury, at least in a rodent IRI-AKI model [25]. In a post-renal model of AKI from unilateral ureteral obstruction (UUO), animals developed reduced renal Klotho expression accompanied by increased TGFβ1 and fibrogenic markers [8]. Although there is no human study to determine renal Klotho in AKI patients, the current animal studies showed that Klotho downregulation in AKI may be a common and general phenomenon of kidney damage, regardless of etiology (Figure 2).
Fig. 2.
Proposed model of beneficial roles of Klotho in AKI: after acute exposure to insults such as ischemia, nephrotoxin or obstruction, Klotho is reduced in the kidney, plasma and urine. Oxidative stress and cytokine production also directly suppress renal Klotho expression. The low Klotho level may be a pathological intermediate for exacerbation of kidney damage. If kidney damage is mild, the kidney undergoes normal tissue regeneration to restore normal kidney morphology. In more serious injury, reparative regeneration is replaced by fibrosis, which further worsens renal Klotho deficiency. Administrations of exogenous Klotho protein can effectively prevent and limit kidney injury, promote normal healing and prevent-fibrosis.
The measurement of plasma and urine Klotho is more meaningful than renal Klotho to monitor the Klotho status in humans. Unfortunately, to date, there are limited human data. In rodents, plasma soluble Klotho was dramatically decreased beginning at 3 h and started to recover at around 48 h reaching baseline levels by 7 days, which paralleled changes in renal Klotho [25]. Nephrotoxic AKI animals induced by both folic acid [28] and cisplatin [27, 28] have decreased plasma Klotho.
The measurement of urinary Klotho maybe a feasible surrogate [25]. Urinary Klotho in rodents was remarkably decreased on the first day post-IRI and began to rise by the second day to nearly normal levels by 7 days [25]. Furthermore, AKI patients had undetectable or notably lower urinary Klotho protein than normal healthy volunteers [25]. Larger cohorts with prospective design are required to define the time course, specificity and sensitivity of urinary and plasma Klotho.
Diagnostic role
Because of the relatively poor sensitivity, delayed response and lack of specificity of the traditional diagnostic markers of AKI [29, 30], several novel biomarkers have been developed to improve early diagnosis and predication of outcome of AKI. Among those novel biomarkers, urine or/and plasma interleukin (IL)-18, NGAL and kidney injury molecule-1 (KIM-1) have received the foremost attention. IL-18 and NGAL are very sensitive biomarkers for early detection of kidney damage [31], whereas KIM-1 is more specific, and less sensitive compared with NGAL [32]. Thus, the combination of those biomarkers could overcome some of their drawbacks and enhance their diagnostic value. While NGAL, KIM-1 and IL-18 are elevated, soluble Klotho in plasma is suppressed in AKI; therefore, combinational measurement of two divergent alterations, for instance NGAL/Klotho or KIM-1/Klotho ratio, would theoretically amplify the sensitivity for the early detection of AKI.
The prognostic value of plasma and urine-soluble Klotho to predict the outcome of AKI deserves to be explored. Ohyama et al. [26] showed that 10–15% volume phlebotomy, which presumably represents a pre-renal insult, does not decrease renal Klotho mRNA and protein. Unfortunately, this study did not examine urine and plasma Klotho and did not compare the different outcome of renal function and histology between the different degrees of blood loss. At present, one cannot determine whether there is a continuum of Klotho reduction or whether there is a distinct cut-off of Klotho levels that mark acute tubular necrosis. Undoubtedly, plasma Klotho measurement should be explored as a potential principal biomarker for distinguishing pre-renal from intrinsic AKI that can potentially serve as a renal equivalent of troponin for cardiac damage.
In a nephrotoxic AKI model induced by folic acid, low renal Klotho mRNA remained for 7 days even when serum creatinine had returned to near-baseline levels [28], suggesting that renal Klotho mRNA recovery may be delayed compared with renal function recovery, perhaps reflecting some persistent structural damage masked by functional compensation. Whether retarded recovery of renal Klotho expression is a predicator of progression to CKD from AKI is undefined [28]. In the UUO model, downregulation of Klotho expression as well as renal fibrosis was more dramatic in heterozygous Klotho-deficient mice (Kl+/−) than WT mice, suggesting that Klotho deficiency may be a marker and a pathogenic intermediate for renal fibrosis and deterioration of renal function (Figure 2) [8].
Therapeutic potential
A critical question is whether the transient endocrine and renal Klotho deficiency play an important role in kidney disease development and progression. It is conceivable that Klotho may be protecting the kidney against acute damage, promoting renal tissue regeneration and preventing or retarding progression to CKD by inhibiting renal fibrogenesis (Figure 2). The current animal database suggests that Klotho repletion may be a good strategy for treating AKI in two capacities. First is the amelioration of the acute damage. Tg-Kl mice that have higher renal and plasma-soluble Klotho levels [14] are more resistant to ischemic kidney injury than WT mice [25]. In the same ischemic model, recombinant Klotho, when given early in a timely fashion, can significantly reduce renal damage [25]. When normal rats were subjected to IRI, adenovirus-mediated Klotho gene delivery also resulted in significant improvement of serum creatinine, amelioration of renal histological changes and diminution of apoptotic cells in the kidneys [24]. Unfortunately, the efficacy of Klotho to alleviate acute damage diminishes dramatically when given after the acute insult, thereby placing serious limitation on its clinical utility for the treatment of AKI. However, it may still be a good agent for the prevention of acute injury in a high-risk population for developing AKI, such as a patient with atherosclerosis undergoing abdominal aortic aneurysm repair or coronary bypass, or in situations where the timing of the renal insult is predictable such as with nephrotoxins.
Nephrotoxicity is a serious side effect of cisplatin, a widely used anti-tumor agent, and unlike ischemia, the timing of the exposure of kidneys to nephrotoxins is known, thus allowing a practical window for Klotho administration. Cisplatin-induced AKI was exaggerated in Kl+/− mice and ameliorated in Tg-Kl mice. NGAL expression in the kidney was significantly higher in Kl+/− and lower in Tg-Kl compared with WT mice [27], indicating that Klotho may be a good prophylactic agent to prevent nephrotoxicity induced by cisplatin.
Aside from the morbidity and mortality associated with AKI in the immediate period of injury, there is mounting evidence that there are long-term consequences after AKI [33]. Haploinsufficient Kl+/− mice have lower Klotho and more renal fibrosis than WT mice after UUO [8], suggesting that Klotho may potentially be a therapeutic agent to prevent renal fibrosis after AKI.
Clinical significance of Klotho in CKDs
Klotho deficiency
Renal Klotho transcript is markedly decreased in CKD patients with diverse etiologies such as obstructive nephropathy, rejected transplanted kidneys, diabetic nephropathy, minimal–change nephritic syndrome, IgA nephropathy and chronic glomerulonephritis [34, 35]. However, these studies did not examine renal or systemic Klotho protein levels. By using one recently developed ELISA kit, Yamazaki et al. examined plasma soluble Klotho in healthy subjects and found a correlation of the plasma Klotho level with serum creatinine, BUN and FGF23 [36], suggesting that plasma-soluble Klotho levels may be associated with renal function, although true or surrogate glomerular filtration rate (GFR) was not measured. Other publications examining plasma-soluble Klotho in a small CKD population using the same kit showed conflicting results: 3–4-fold higher [37] or no change [38] in early CKD or a mild-to-moderate decrease in end-stage renal disease (ESRD) patients [39]. Thus, larger cohorts are required, and most importantly, validation of this ELISA kit is critical to further investigate plasma Klotho levels in early CKD and ESRD patients.
Urinary Klotho levels of CKD patients were shown to significantly decrease at a very early stage and was sustainably reduced with the progression of CKD [9, 39]. Moreover, Klotho levels in the plasma, urine and kidney were decreased in parallel in the CKD rodent model [9]. A large body of animal data clearly showed endocrine and renal Klotho deficiency in a variety of CKD animal models. Animals with CKD induced by subtotal (5/6th) nephrectomy [40] or by unilateral nephrectomy plus contralateral ischemic reperfusion injury (Npx + IRI) [9] have low renal Klotho protein and mRNA. In addition, apolipoprotein E-deficient uremic mice induced by Npx and electrocautery also have low renal Klotho expression [41]. In addition to renal ablation models of CKD, other models including chronic glomerulonephritis [42], diabetes (streptozotocin-induced [43], leptin deficiency [44] and spontaneous non-insulin-dependent type II diabetes) and hypertension (spontaneous hypertension and volume-dependent hypertension) [40, 45] are dramatically reduced in renal Klotho mRNA or/and protein expression, suggesting that a decrease in renal Klotho is a universal phenotype in CKD.
Diagnostic potential
CKD can be occult and insidious at onset and thus presents a challenge to make an early diagnosis. Once detected, it is difficult to predict progression based on the clinical and laboratory methods currently available. Moreover, advances in the diagnosis and treatment of CKD have been thwarted by the lack of unique, sensitive, reliable, quantifiable and easily measured biomarkers that correlate with the presence, progression and complications of CKD. Currently, clinical markers such as serum creatinine and albuminuria lack sensitivity and specificity and are poor predictors of chronic progression [46]. GFR was, and still is, the gold standard for assessing kidney function, but GFR may be near normal in patients with severe injury and compensatory hyperfiltration. Cystatin C is an alternative but still suffers from the same limitations as plasma creatinine and estimated glomerular filtration rate (eGFR) [47].
Animal studies have shown the decline in Klotho levels in the plasma, urine and kidneys with a decrease in creatinine clearance in CKD [9]. In a human cross-sectional study, the renal Klotho mRNA level is significantly decreased in early diabetic nephropathy (eGFR ≥ 60 ml/min/1.73 m2) but not in IgA nephropathy, and minimal-change disease with a eGFR ≥ 60. In the latter two entities, Klotho mRNA expression was decreased when eGFR was ≤60 [43]. A correlation was noted between renal Klotho mRNA levels and severity of all three of these entities [43].
There is a graded decrease in the urine-soluble Klotho level in patients with a variety of advancing stages of CKD [9]. Urine-soluble Klotho levels sharply drop in the early stages of CKD and sustainably declined with a decrease in eGFR in CKD and ESRD patients [9]. Similarly, human urinary Klotho excretion, determined by Klotho ELISA kit [36], is significantly decreased and the amount of urinary Klotho is correlated with eGFR [39]. These observations suggest that urinary-soluble Klotho may be a good biomarker for CKD.
Similarly, reduced soluble Klotho in plasma is correlated with a decline in eGFR in both CKD patients [48] and ESRD patients on hemodialysis [49]. More importantly, and interestingly, plasma Klotho starts to decline in CKD Stage 2, much earlier than any other blood parameters, suggesting that plasma Klotho may be a novel biomarker for the early diagnosis of CKD [48]. Pavik et al. found a significant decrease in plasma-soluble Klotho levels in autosomal dominant polycystic kidney disease (ADPKD) at CKD stages 1 and 2, suggesting that plasma Klotho reduction occurs early in ADPKD [38]. Furthermore, plasma Klotho levels are inversely correlated with cyst volume and kidney size, but the casual relationship between the Klotho decline and increment of cyst volume remains to be illustrated [38].
Prognostic potential
One study found low renal Klotho protein and mRNA in early diabetic nephropathy (GFR ≥ 60) [43]. Interestingly, low renal Klotho is associated with a higher urine calcium-to-creatinine ratio, a finding compatible with the anti-calciuric effects of Klotho [12]. In ADPKD patients with early CKD, subjects with a low renal threshold of phosphate excretion (Tmp/GFR < 0.6 mmol/L) have lower plasma-soluble Klotho, suggesting that low plasma Klotho may contribute to defects in urinary phosphate excretion in early CKD [38] and impair the compensatory increase in phosphate excretion triggered by increased plasma FGF23. High Klotho expression in the kidney is also found to be associated with high fractional excretion of phosphate in urine and low plasma phosphate in CKD animals [9]. Klotho deficiency may initiate or aggravate dysregulated mineral metabolism (Figure 3B) which is involved in CKD progression and development of extra-renal complications [50]. Based on many animal studies and a few clinical epidemiological observations, Klotho deficiency is associated with complications such as secondary hyperparathyroidism, CKD mineral bone disease, cardiac hypertrophy and vascular calcification [9, 51, 52]. One small-scale study examined the effect of cinacalcet, an allosteric modulator of calcium-sensing receptor on plasma Klotho in hemodialyzed patients with secondary hyperparathyroidism [49]. During treatment, there were moderate improvements in mineral parameters including plasma Pi, PTH and FGF23, slightly decreased plasma Ca and a transient slight reduction in plasma-soluble Klotho [49]. There were no significant associations between the changes in plasma soluble Klotho and change in other mineral parameters [49]. Thus, the clinical value of Klotho determination in monitoring complications in CKD needs to be confirmed in a large-scale study.
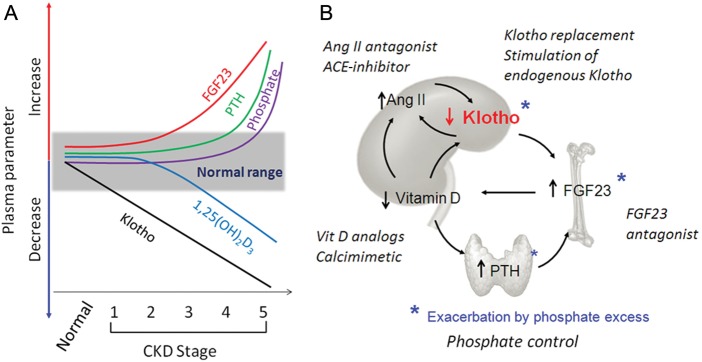
Fig. 3.
Proposed time profile of disturbances in mineral metabolism and phosphate-regulating hormones and therapeutic strategies in CKD. (A) Changes in parameters of mineral metabolism with CKD progression. Normal range is shown in the grey box. The x-axis represents a decline in renal function from normal to Stage 1–5 of CKD. Decreased plasma Klotho is proposed as an early event, followed by an increase in FGF23 and a decrease in vitamin D levels in that order. The elevation of plasma PTH occurs later with hyperphosphatemia appearing only in advanced CKD. (B) Potential therapeutic strategies targeting Klotho. Five principal mineral disturbances in CKD: Klotho deficiency, excess FGF23, secondary hyperparathyroidism, vitamin D deficiency and phosphate toxicity, and over-stimulation of Ang II are shown as an interacting and self-amplifying vicious downhill spiral. Hyperphosphatemia further reduces Klotho expression and increases plasma FGF23 and PTH. The final outcome is the severe downregulation of Klotho in the kidney and plasma. Shown in italics are potential means to interrupt these pathways at specific target points. Each maneuver has the potential of arresting the spiral with a direct or indirect impact on endogenous Klotho expression in the kidney. For a given CKD patient, a particular combination of prescriptions can be tailored to best suit that individual.
Therapeutic potential
There are many common features between the phenotype of the Klotho-deficient mice and CKD such as cardiovascular calcification, hypertension, cardiac hypertrophy, hyperphosphatemia and high blood FGF23 [13, 52] which raises the possibility that Klotho may be a potential therapeutic agent for complications in CKD. Animal data clearly indicate that Klotho deficiency is not a mere biomarker for CKD but is actually pathogenic for progression [9]. When the Klotho-deficient state in rodent CKD is rescued by genetic overexpression of Klotho, the renal function and morphologic lesion were much improved [9], indicating that the repletion of Klotho is beneficial in CKD.
Although the causes of CKD are multifactorial, renal fibrosis appears to be a monotonous final common pathway. Interestingly, Klotho supplementation significantly attenuates renal fibrosis after UUO induction and suppresses the expression of fibrosis markers and TGF-β1 target genes such as Snail and Twist [3], suggesting that Klotho is a potential therapeutic agent to suppress renal fibrosis and to retard chronic progression from acute kidney damage to CKD [8]. In addition to suppression of renal fibrosis, acceleration of kidney regeneration by preservation of stem cells [2], improvement of endothelial function and angiogenesis [7] and restoration of renal microcirculation [7] are all potential effects of Klotho on the kidney. In animal models and cultured cells, Klotho directly protects the vasculature from high-phosphate-induced calcification above and beyond its effect on phosphate balance [9].
Thus far, there are no published data to directly document the therapeutic effect of Klotho in humans. Establishing whether Klotho protein has undeniably important potential for the treatment of CKD complications is one of many predictably logical, but comprehensive next steps in the development of the Klotho plan for clinical practice.
Conclusion
Multiple efforts have been devoted to identify biomarkers with high sensitivity and specificity, and indeed, significant progress has been made. Biomarkers should have utility in the early diagnostic as well as prognostic value. Compared with NGAL, KIM and IL-18, all of which are entering the clinical realm, Klotho measurement in AKI and CKD patients is still in its infancy. Hopefully, Klotho measurement may provide the following clinical applications in AKI and CKD: (i) early detection of the presence of AKI, (ii) identification of risk of progression post-AKI to CKD, (iii) early detection of the presence of CKD and (iv) prediction of progression, extra-renal complications and mortality in CKD (Table 1). These potential applications are all based on preclinical data at the moment. Prior to proceeding to securing the clinical database, there are many critical questions to answer. What is the normal range of plasma and urinary-soluble Klotho levels in humans? Are there age, gender, ethnic and dietary dependence and adjustments? What kind of assay for plasma and urine-soluble Klotho is ideal and how should this be validated?
Table 1.
Potential applications of Klotho in clinical nephrology
| AKI | CKD | |
|---|---|---|
| As a biomarker | ||
| Diagnostic | • Detect intrinsic renal damage | • Detect early CKD |
| Prognostic | • Predict recovery • Progression to CKD | • Predict progression to ESRD, complication or death |
| As a therapeutic agent | ||
| Prevention | • Prevent acute damage • Prevent progression to CKD | • Prevent progression and complications |
| Treatment | • Acute renoprotection or renal regeneration | • Anti-phosphotoxicity |
| • Delayed allograft dysfunction | • Anti-proteinuria | |
| • Nephrotoxic injury | • Retard progression | |
| • Other high-risk scenarios | ||
Emerging preclinical data suggest that AKI is a transient and CKD is in a sustained state of renal and systemic Klotho deficiency, and Klotho deficiency plays a pathogenic role in the kidney dysfunction, recovery and progression to CKD (Figure 2). Renal damage including renal fibrosis can trigger a spiraling vortex of Klotho deficiency that further worsens CKD progression and complications (Figure 3B). These studies strongly pose the potential utility of endogenous Klotho restoration or exogenous Klotho replacement as therapeutic options in both AKI and CKD. Recombinant Klotho administration is efficacious in animal studies, so the means of production and safety is not likely to be a large obstacle. But prior to launching clinical trials, it is important to generate appropriate indications for Klotho treatment based on animal experiments. One needs to define specific etiologies, stages of kidney diseases and level of endogenous Klotho to identify the likely responders. One also needs to confirm whether Klotho could prevent or attenuate extra-renal complications in CKD. In addition to the direct administration of Klotho protein, stimulation or un-suppression of Klotho production might be an alternative way to increase endogenous Klotho, especially when residual kidney function is somewhat preserved. In the CKD setting, control of plasma Pi by restricting Pi intake, supplementation of vitamin D [53] and inhibition of angiotensin II (Ang II) [54] directly and indirectly elevate Klotho expression in in vitro or/and in vivo animal studies (Figure 3B). In the AKI setting, peroxisome proliferator-activated receptor-γ agonist [55] and anti-oxidants [4] also increase Klotho expression (Figure 2). Determination of whether those traditional maneuvers modulate Klotho levels in kidney disease is priority.
In summary, we are sitting at a juncture where a wealth of preclinical data are awaiting clinical testing. It is quite conceivable that Klotho may be a unique protein which does not only serve as a potentially useful biomarker for kidney disease, but also functions as a renoprotective protein to alleviate kidney injury, promote kidney regeneration, to arrest or slow down progression and to ameliorate complications such as improvement of mineral metabolism, suppression of secondary hyperparathyroidism and attenuation of vascular calcification and cardiac remodeling in CKD (Table 1). Only well-collected and interpreted data will decide whether Klotho deserves to be the new ‘superstar’ in nephrology.
Funding
The authors were in part supported by the National Institutes of Health (R01-DK091392, R01-DK092461, R01-AG019712), the George M. O'Brien Kidney Research Center/UT Southwestern Medical Center (P30-DK-07938), American Heart Association (0865235F), and the Charles and Jane Pak Foundation, the Simmons Family Foundation, Norman Hackerman Advanced Research Program (010019-0043-2009).
Conflict of interest statement. None declared.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 3.Doi S, Zou Y, Togao O, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitobe M, Yoshida T, Sugiura H, et al. Oxidative stress decreases Klotho expression in a mouse kidney cell line. Nephron Exp Nephrol. 2005;101:e67–74. doi: 10.1159/000086500. [DOI] [PubMed] [Google Scholar]
- 5.Ikushima M, Rakugi H, Ishikawa K, et al. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827–832. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Kuro OM, Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell. 2012 doi: 10.1111/j.1474-9726.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukino K, Suzuki T, Saito Y, et al. Regulation of angiogenesis by the aging suppressor gene Klotho. Biochem Biophys Res Commun. 2002;293:332–337. doi: 10.1016/S0006-291X(02)00216-4. [DOI] [PubMed] [Google Scholar]
- 8.Sugiura H, Yoshida T, Shiohira S, et al. Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. Am J Physiol Renal Physiol. 2012 doi: 10.1152/ajprenal.00294.2011. [DOI] [PubMed] [Google Scholar]
- 9.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu MC, Shi M, Zhang J, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. Faseb J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuro-o M. A potential link between phosphate and aging—lessons from Klotho-deficient mice. Mech Ageing Dev. 2010;131:270–275. doi: 10.1016/j.mad.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang CL, Moe OW. Klotho: a novel regulator of calcium and phosphorus homeostasis. Pflugers Arch. 2011;462:185–193. doi: 10.1007/s00424-011-0950-5. [DOI] [PubMed] [Google Scholar]
- 13.Hu MC, Kuro OM, Moe OW. Secreted Klotho and chronic kidney disease. Adv Exp Med Biol. 2012;728:126–157. doi: 10.1007/978-1-4614-0887-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuro OM. Klotho and betaKlotho. Adv Exp Med Biol. 2012;728:25–40. doi: 10.1007/978-1-4614-0887-1_2. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Fujimori T, Hayashizaki Y, et al. Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta. 2002;1576:341–345. doi: 10.1016/s0167-4781(02)00281-6. [DOI] [PubMed] [Google Scholar]
- 17.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by Klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CD, Podvin S, Gillespie E, et al. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imura A, Iwano A, Tohyama O, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 21.Cha SK, Ortega B, Kurosu H, et al. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cha SK, Hu MC, Kurosu H, et al. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol. 2009;76:38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumura Y, Aizawa H, Shiraki-Iida T, et al. Identification of the human Klotho gene and its two transcripts encoding membrane and secreted Klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 24.Sugiura H, Yoshida T, Tsuchiya K, et al. Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant. 2005;20:2636–2645. doi: 10.1093/ndt/gfi165. [DOI] [PubMed] [Google Scholar]
- 25.Hu MC, Shi M, Zhang J, et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78:1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohyama Y, Kurabayashi M, Masuda H, et al. Molecular cloning of rat Klotho cDNA: markedly decreased expression of Klotho by acute inflammatory stress. Biochem Biophys Res Commun. 1998;251:920–925. doi: 10.1006/bbrc.1998.9576. [DOI] [PubMed] [Google Scholar]
- 27.Panesso MC, Shi M, Cho HJ, et al. Klotho ameliorates cisplatin nephrotoxicity. J Am Soc Nephrol. 2011;22:116A. [Google Scholar]
- 28.Moreno JA, Izquierdo MC, Sanchez-Nino MD, et al. The inflammatory cytokines TWEAK and TNFalpha reduce renal Klotho expression through NFkappaB. J Am Soc Nephrol. 2011;22:1315–1325. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waikar SS, Betensky RA, Emerson SC, et al. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol. 2012;23:13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrier RW. Diagnostic value of urinary sodium, chloride, urea, and flow. J Am Soc Nephrol. 2011;22:1610–1613. doi: 10.1681/ASN.2010121289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative Biomarkers Predict Acute Kidney Injury and Poor Outcomes after Adult Cardiac Surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaidya VS, Ford GM, Waikar SS, et al. A rapid urine test for early detection of kidney injury. Kidney Int. 2009;76:108–114. doi: 10.1038/ki.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Berendoncks AM, Elseviers MM, Lins RL. Outcome of acute kidney injury with different treatment options: long-term follow-up. Clin J Am Soc Nephrol. 2010;5:1755–1762. doi: 10.2215/CJN.00770110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asai O, Nakatani K, Tanaka T, et al. Decreased renal alpha-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012;81:539–547. doi: 10.1038/ki.2011.423. [DOI] [PubMed] [Google Scholar]
- 35.Koh N, Fujimori T, Nishiguchi S, et al. Severely reduced production of Klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki Y, Imura A, Urakawa I, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398:513–518. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugiura H, Tsuchiya K, Nitta K. Circulating levels of soluble alpha-Klotho in patients with chronic kidney disease. Clin Exp Nephrol. 2011 doi: 10.1007/s10157-011-0511-4. [DOI] [PubMed] [Google Scholar]
- 38.Pavik I, Jaeger P, Ebner L, et al. Soluble Klotho and autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:248–257. doi: 10.2215/CJN.09020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akimoto T, Shiizaki K, Sugase T, et al. The relationship between the soluble Klotho protein and the residual renal function among peritoneal dialysis patients. Clin Exp Nephrol. 2012 doi: 10.1007/s10157-011-0582-2. [DOI] [PubMed] [Google Scholar]
- 40.Aizawa H, Saito Y, Nakamura T, et al. Downregulation of the Klotho gene in the kidney under sustained circulatory stress in rats. Biochem Biophys Res Commun. 1998;249:865–871. doi: 10.1006/bbrc.1998.9246. [DOI] [PubMed] [Google Scholar]
- 41.Yu J, Deng M, Zhao J, Huang L. Decreased expression of Klotho gene in uremic atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 2010;391:261–266. doi: 10.1016/j.bbrc.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 42.Haruna Y, Kashihara N, Satoh M, et al. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci USA. 2007;104:2331–2336. doi: 10.1073/pnas.0611079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asai O, Nakatani K, Tanaka T, et al. Decreased renal alpha-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012 doi: 10.1038/ki.2011.423. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y, Banerjee S, Dey N, et al. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (Serine)536 phosphorylation. Diabetes. 2011;60:1907–1916. doi: 10.2337/db10-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. J Am Med Assoc. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimamura Y, Hamada K, Inoue K, et al. Serum levels of soluble secreted alpha-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol. 2012 doi: 10.1007/s10157-012-0621-7. [DOI] [PubMed] [Google Scholar]
- 49.Komaba H, Koizumi M, Tanaka H, et al. Effects of cinacalcet treatment on serum soluble Klotho levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr645. [DOI] [PubMed] [Google Scholar]
- 50.Goldsmith DJ, Cunningham J. Mineral metabolism and vitamin D in chronic kidney disease–more questions than answers. Nat Rev Nephrol. 2011;7:341–346. doi: 10.1038/nrneph.2011.53. [DOI] [PubMed] [Google Scholar]
- 51.Krajisnik T, Olauson H, Mirza MA, et al. Parathyroid Klotho and FGF-receptor 1 expression decline with renal function in hyperparathyroid patients with chronic kidney disease and kidney transplant recipients. Kidney Int. 2010;78:1024–1032. doi: 10.1038/ki.2010.260. [DOI] [PubMed] [Google Scholar]
- 52.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011 doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsujikawa H, Kurotaki Y, Fujimori T, et al. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 54.de Borst MH, Vervloet MG, ter Wee PM, et al. Cross talk between the renin–angiotensin–aldosterone system and vitamin D-FGF-23-Klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22:1603–1609. doi: 10.1681/ASN.2010121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang R, Zheng F. PPAR-gamma and aging: one link through Klotho? Kidney Int. 2008;74:702–704. doi: 10.1038/ki.2008.382. [DOI] [PubMed] [Google Scholar]