Abstract
To identify a novel amplified cancer gene a systematic screen of 975 human cancer DNA samples, 750 cell lines and 225 primary tumors, using the Affymetrix 10K SNP microarray was undertaken. The screen identified 193 amplicons. A previously uncharacterized amplicon located on 6p21.2 whose 1 Mb minimal common amplified region contained eight genes (GLO1, DNAH8, GLP1R, C6orf64, KCNK5, KCNK17, KCNK16, and C6orf102) was further investigated to determine which gene(s) are the biological targets of this amplicon. Real time quantitative PCR (qPCR) analysis of all amplicon 6p21.2 genes in 618 human cancer cell lines identified GLO1, encoding glyoxalase 1, to be the most frequently amplified gene [twofold or greater amplification in 8.4% (49/536) of cancers]. Also the association between amplification and overexpression was greatest for GLO1. RNAi knockdown of GLO1 had the greatest and most consistent impact on cell accumulation and apoptosis. Cell lines with GLO1 amplification were more sensitive to inhibition of GLO1 by bromobenzylglutathione cyclopentyl diester (BBGC). Subsequent qPCR of 520 primary tumor samples identified twofold and greater amplification of GLO1 in 8/37 (22%) of breast, 12/71 (17%) of sarcomas, 6/53 (11.3%) of nonsmall cell lung, 2/23 (8.7%) of bladder, 6/93 (6.5%) of renal and 5/83 (6%) of gastric cancers. Amplification of GLO1 was rare in colon cancer (1/35) and glioma (1/94). Collectively the results indicate that GLO1 is at least one of the targets of gene amplification on 6p21.2 and may represent a useful target for therapy in cancers with GLO1 amplification.
INTRODUCTION
Genomic amplification (in the literature used interchangeably with gene amplification) may be defined as a somatically acquired increase in copy number of a restricted region of the genome. It is often found in cancer cells as a mechanism of increasing the amount of the transcript and therefore transcript levels of dominantly acting cancer genes (Schwab, 1998; Myllykangas and Knuutila, 2006; Bignell et al., 2007).
Amplifications can lead to neoplastic transformation and malignant progression by conferring clonal growth advantage to a cell. Such advantage can be mediated through protein coding genes (Schwab, 1999; Futreal et al., 2004; Albertson, 2006) and, less frequently, by nonprotein coding sequences (Ota et al., 2004). This advantage may relate to a cell’s core proliferative and antiapoptotic properties or a cell’s ability to adapt to its environment, such as the tissues of metastatic organs or exposure to cytotoxic agents.
It is usually the case that the amplified region in a particular cancer contains more than one gene. In comparison to cancer-causing rearrangements or point mutations which usually occur within the cancer gene, it has been difficult, in many cases, to unambiguously determine which gene is the “driver” and which are the “passenger” genes. This is important, because defining the driver directs strategies for drug discovery aimed at inhibiting the cancer gene.
Although driver genes for some amplified regions have been identified and have successfully been targeted in the laboratory or even the clinical setting, for many amplified regions the driver cancer gene is unknown. Here we have carried out a large scale systematic search for copy number changes in cancer cells and demonstrate that GLO1, encoding glyoxalase 1, is an amplified cancer gene in many types of human cancer.
MATERIALS AND METHODS
Samples
The details of each sample used are available at the Cancer Genome Project web site [http://www.sanger.ac.uk/cgi-bin/genetics/CGP/CGHviewer.cgi]. A summary of cancer types used in real time qPCR screen is in Table S1 (Supporting Information). All primary tumors were reviewed by a pathologist and assessed as being >80% tumor by microscopic inspection. Primary cancers by histological types are listed in Table S2 (Supporting Information). Cell lines were cultured using the suppliers’ recommended conditions, grown into confluence and the nucleic acids extracted as described below.
DNA Extraction
DNA was extracted from the cell lines using the Qiagen “blood and cell culture” DNA Maxi Kit and following manufacturer’s instructions (Qiagen GmbH, Hilden, Germany).
Genomic SNP-Based Microarrays
Copy number analysis was carried out using the Affymetrix 10K SNP and SNP6 array as previously described (Bignell et al., 2004; Greenman et al., 2010). The copy number was determined by first calculating the sum of the perfect match features from the array minus the sum of the mismatch features for each SNP (PM-MM). This process was carried out for all SNPs on the array, and the values were then “normalized” by calculating each individual SNP’s PM-MM value as a percentage of the total for all of the SNPs on the array. This accounts for differences between runs caused by variations in hybridization efficiency. The normalized value for each SNP from the array was then divided by the value for the same SNP from a “normal reference sample” to give a ratio. The “normal reference sample” was calculated from the average of the normalized values (above) for each SNP across a set of four control DNAs that were run at the same time as the test DNAs (usually running 24 samples per day: 4 reference and 20 test samples). Therefore, if a SNP is from a diploid region of the test sample, it will give a reading of one when divided by the normal reference sample, representing a DNA copy number of two. An amplified region was arbitrarily defined as a DNA region that contained three or more consecutive SNPs with their fluorescent ratio greater than three (level of amplification). If two amplified regions were separated by one or two SNPs the two regions were merged into one, assuming that the bridging SNPs reported falsely negative levels of amplification. Details of the study protocols and results are available at http://www.sanger.ac.uk/cgi-bin/genetics/CGP/cghviewer/CghHome.cgi
Real Time PCR
The gene dosage quantification was carried out using the comparative CT method as previously described (Pfaffl et al., 2002). The β-actin gene (ACTB) served as an endogenous control and a triplicate of the lymphoblastoid cell line NCI-BL2126 DNA was used as a calibrator for estimating the relative quantity of the DNA (RQ). Primers were designed using the Primer3 software (Rozen and Skaletsky, 2000). The experiments were performed on the ABI 7900 machine (Applied Biosystems, Foster City, CA) using the QuantiTect SYBR Green PCR kit (QIAGEN GmbH, Hilden, Germany). The reaction volume was 20 μL and contained 10 μL of QuantiTect SYBR Green PCR kit, 1.2 μL of forward and reverse primer mix (25 ng/μL), 8 μL of DNA (0.4 ng/μL) and 0.8 μL of double distilled water. An initial denaturation step of heating to 95 °C for 15 min was followed by 40 cycles of denaturation at 95 °C for 15 sec, annealing at 60 °C for 30 sec and extension at 72 °C for 30 sec. A dissociation stage with 95 °C, 60 °C, and 95 °C each for 15 sec followed to detect the formation of primer dimers. Quantification of the copy number of each gene in each sample was performed twice using two independent pairs of primers listed in Table S3 (Supporting Information). In addition, duplicates of each sample were used in all experiments with each given pair of primers. A gene was called amplified if the relative quantity of that gene was greater than two.
Sequencing of GLO1
The sequencing was performed using the standard Cancer Genome Project sequencing pipeline [http://www.sanger.ac.uk/genetics/CGP/] (Davies et al., 2005b, 2006).
Quantification of Gene Expression Using Real Time PCR
RNA extraction and reverse transcription reaction
Total RNA was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA) and dissolved in DEPC-treated water (Ambion, Austin, TX). The RNA quality was assessed by agarose electrophoresis and stored in −80 °C.
The cDNA was obtained by two-step reverse transcription using SuperScript III reverse transcriptase kit (Invitrogen, Carlsbad, CA with Amplification Grade DNAse I (Sigma-Aldrich Corporation, St. Louis, MO) and random nonamer primers (Sigma-Aldrich Corporation, St. Louis, MO) as per manufacturer’s instructions.
Real time PCR
The CT method was used similarly to the genomic real time PCR described above. The ACTB gene served as an endogenous control and a mixture of normal cDNAs (brain, ovary, placenta, skeletal muscle, heart muscle, lung, testis, liver, spleen, small intestine, colon, pancreas, prostate, leukocyte, thymus, prostate and kidney obtained from AMS Biotechnology, Abingdon, Oxon, United Kingdom) was used as a calibrator for estimating the relative quantity (RQ) of the mRNA of interest. The primers used are listed in Table S4 (Supporting Information).
Affymetrix HT-HU133 Gene Expression Array
Gene expression was analyzed on HT-HU133 chips (Affymetrix, Santa Clara, CA), following standard protocols. Data quality test and normalization were performed using the affy (Gautier et al., 2004) and affyQCReport* packages implemented in R. The quality of each array was assessed by checking the mean and standard deviation of overall expression, ratios of detectable probes, expression information of control probes (housekeeping genes) and the correlation among samples from same tissue. Background correction of each array was using robust multiarray average (Bolstad et al., 2003; Irizarry et al., 2003a,b). The data were normalized by the quantile method and expression level of each gene was log transformed. Gene expression level higher than 0.8 folds of average tissue expression were labeled as over expressed.
Gene Knockdown Using siRNA
All experiments were performed in flat-bottomed transparent 96-well plates (Standard clear Corning and Costar 96 Well Plate, Corning Inc, Life Sciences, Acton, MA). On Day 1, cells were seeded at a density of 5,000 cells per well and grown in their normal media. On Day 2, the cells were transfected with siRNA (siGENOME SMART pool reagent; Dharmacon, Lafayette, Colorado) or siCONTROL (scrambled, non-targeting siRNA siCONTROL; Dharmacon, Lafayette, Colorado). The concentrations of siRNA and siCONTROL in the media were 80 nm for each cell line and gene. Dharmafect 1 lipid transfecting agent was used in all experiments (0.3 μL Dharmafect 1 per 100 μL of total transfection volume). The level of knockdown was determined at 48 hr after the transfection. It was calculated as the relative quantity (RQ) of mRNA of a gene in siRNA-treated cells, divided by the RQ of mRNA of the same gene in scrambled siRNA (siCONTROL)-treated cells and was expressed as percentage knockdown. A minimum knockdown of 60% was obtained for each gene and cell line. The apoptosis and the cell accumulation assays were performed on Day 4. Each experiment was performed in triplicate. The significance of the difference of the apoptotic indexes and the estimated cell numbers between the siRNA treated and control samples was measured using the one-tailed t test.
Cell Accumulation Assay
Cell accumulation was estimated using the CyQUANT Cell Proliferation Assay Kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Briefly, medium was removed from the cell culture plate wells using vacuum suction. Fluorescence was measured using the SpectraFluor PLUS plate reader (Tecan, Mannedorf, Switzerland) at 485 nm and emission detection at 530 nm. The average background fluorescence, measured in wells containing CyQUANT solution but no cells, was subtracted from each experimental measurement.
Apoptosis Assay
The apoptosis index was determined using the DNA fragmentation-based assay Cell Death Detection ELISAPLUS (Roche, Basel, Switzerland) according to the manufacturer’s instructions. The absorbance was measured using the Spectra-Fluor PLUS plate reader (Tecan, Mannedorf, Switzerland) at 405 nm. The average background absorbance (measured in wells containing all of the ELISA reagents but no cytoplasmic fraction) was subtracted from each experimental measurement. The apoptotic index was calculated by dividing the replicate average “apoptosis absorbance” by the replicate average “cell quantity fluorescence”. The “cell quantity absorbance” was determined using the CyQUANT kit (see the “Cell accumulation assay” section) in plates that were seeded and treated in exactly the same way as the plates for Cell Death Detection ELISAPLUS assay. Both CyQUANT and Death Detection ELISAPLUS assays were performed in parallel as a part of each functional experiment [RNAi knockdown experiments and bromobenzylglutathione cyclopentyl diester (BBGC) inhibition experiments].
Inhibition of GLO1 Using BBGC
On Day 1, the cells were seeded in concentration of 5,000 cells per well (Standard clear Corning and Costar 96-well plate, Corning Inc, Life Sciences, Acton, MA) using their normal media. On Day 2, the medium was removed by suction and was replaced with complete medium (with serum and antibiotic) containing BBGC (Thornalley et al., 1996). There were six replicates of each of the following concentrations of BBGC: 0, 1, 2, 2.5, 5, 10, 15, 20, and 25 μm. On Day 3, cell accumulation and apoptosis assays were performed as described above. Dose response curves were then constructed and IC50 determined. IC50 represents the concentration of a drug that is required for 50% inhibition of cell accumulation in vitro.
RESULTS
Identification of a Novel Amplicon on 6p21.2
To identify novel amplified cancer genes in human cancer genome-wide screening for copy number change in 975 human cancer DNA samples, including 750 cell lines and 225 primary tumors, using Affymetrix 10K SNP microarrays was undertaken. An amplified region was arbitrarily defined as a DNA region that contained three or more consecutive SNPs with the fluorescent ratio of a given sample and a normal reference greater than three (see also Materials and Methods). Where regions of amplification (in two or more different samples) overlapped or were immediately contiguous they were counted as a single amplicon.
The screen identified 334 amplified regions falling into 193 amplicons (See Supporting Information S5). The sizes of amplified regions ranged from 140 kb to 16 Mb with an average amplicon size of 2.5 Mb (although there is under-representation of small amplicons in this screen due to limited resolution of the array). The spread of the amplified regions around their minimum common amplified region (MCAR) varied widely. For example, the ratios of the mean size of amplified regions of individual samples and the size of the smallest common amplified region was smallest for the E2F3 and EGFR amplicons (2.6 and 2.7, respectively) and largest for the MYC amplicon (52.5).
Most commonly, an amplicon was found in only one sample (107 amplicons). The amplicon defined by the highest number of samples (39) was the 8q24 amplicon with MYC as the only gene contained within its smallest common amplified region. Only three other amplicons were defined by ten or more samples: 2p24 (MYCN, 16 samples), 7p12 (EGFR, 10 samples), and 11q13 (CCND1, 18 samples). Some well-known amplicons were detected less frequently than expected. For example, the amplicon on 17q21 carrying ERBB2 was detected only in two samples. This reflected the relatively low sensitivity of the Affymetrix 10 k assay to detect small-sized amplicons and it was subsequently superseded by the Affymetrix SNP6 assay (http://www.sanger.ac.uk/cgi-bin/genetics/CGP/cghviewer/CghHome.cgi).
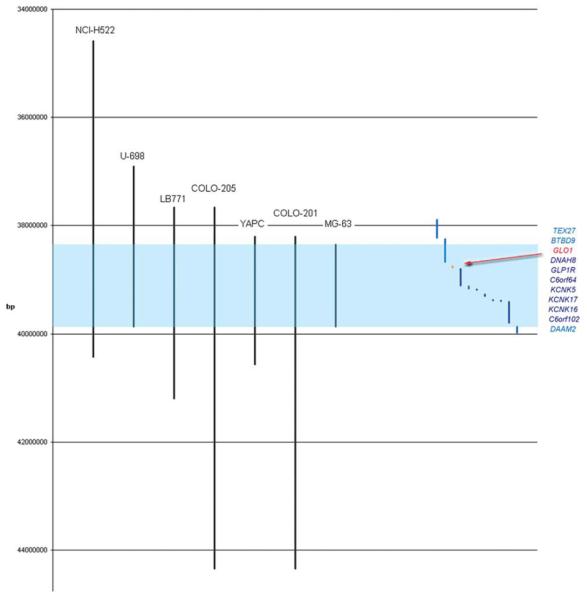
A set of criteria was used to select an amplicon most likely to yield a novel amplified cancer gene. This was weighted toward small MCAR’s containing few genes; occurrence in a large number of samples including primary tumors and cancer cell lines (amenable to RNAi manipulation); high homogeneity of cancer types; high-level amplification and absence of known cancer genes on the amplicon. An amplicon located on 6p21.2 was identified as best fulfilling these criteria. Its MCAR was ~1 Mb in size and harbored eight genes: GLO1, DNAH8, GLP1R, C6orf64, KCNK5, KCNK17, KCNK16, and C6orf102 (Fig. 1). The MCAR was defined by seven cancer cell lines (COLO-201 and COLO-205†, colorectal; NCI-H522, nonsmall cell carcinoma of lung; LB771, squamous cell carcinoma of the upper aerodigestive tract; YAPC, pancreatic cancer; U-698, acute lymphoblastic leukemia; and MG-63, osteosarcoma). In screening using the Affymetrix 10 K Array, more cell lines were found harboring the amplicon located on 6p21.2 than amplicons for many known cancer genes such as ERBB2 and MDM2. Four of the seven cell lines were known to exhibit adherent growth in vitro and were therefore expected to be amenable to functional interrogation using RNAi. The level of amplification across the amplified region in these seven cell lines ranged from two- to sevenfold.
Figure 1.
Schematic representation of the amplicon located on 6p21.2 as determined by the affymetrix 10K array screen of 975 human cancer samples. The vertical axis represents the genetic distance in base pairs (bp). The black vertical lines represent regions of amplification (three-fold or greater) of individual cancer samples. Light blue is shaded the minimum common amplified region (MCAR). On the right are plotted genes that map either completely of partially outside the MCAR (light blue) or into the MCAR (dark blue). GLO1 is marked in red. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Analysis of Frequency of Amplification of Genes Mapping to the 6p21.2 Amplicon Identifies GLO1 as the Most Likely Target of this Amplicon
To identify the most likely target gene of the amplicon on 6p21.2, real time quantitative genomic PCR (qPCR) was chosen as a sensitive and specific assay to determine the frequency of amplification of each gene mapping to this amplicon in a large number (N = 618) of human cancer cell lines. Each sample was assayed with at least two different set of primers for each gene. As a matched normal DNA was not available for the majority of samples, a screen of a panel of 90 human control DNAs extracted from EBV-immortalized lymphoblastoid human cell lines was also performed using the same primer pairs. In these control DNAs no copy number greater than two detected (data not shown). To exclude the possibility that gains were due to extended copy number increases, including gains of the whole chromosome or chromosome arm, a control gene, OPN5, located 10 Mb centromericly of the boundary of the 6p21.2 amplicon, was also assayed through the series of cancer cell lines and controls. This centromeric locus was found amplified more than twofold in only 0.3% of samples with no sample containing greater than threefold gain (data not shown). These control experiments demonstrated that the real time qPCR assays are specific assay for detection of the 6p21.2 amplicon.
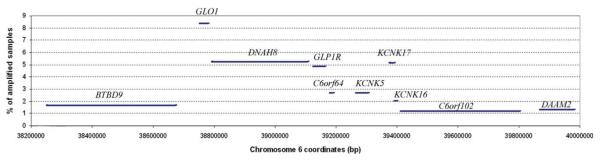
GLO1 was found to be most frequently amplified among the 6p21.2 amplicon genes. Amplification of more than twofold was found in 8.4% (49/536) and more than threefold in 2.4% (14/577) of cancers. Amplification of the remaining genes was found to be less frequent (Fig. 2). In Table 1 are listed P values of the comparison of amplification frequencies of each gene and the nonamplified 6p reference gene, OPN5, as determined by the Fisher and χ2 tests, for threefold and twofold amplification, respectively. When the amplification frequencies of individual genes were compared with the amplification of frequency of OPN5, the difference was most significant for GLO1, regardless of whether two- or threefold gains were considered (χ2 test; P = 3 × 10 −11 and P = 2 ×10 −4, respectively). When the amplification frequency of the second most commonly amplified gene, DNAH8, was compared with OPN5 the difference was also significant, but less than in the case of GLO1: χ2 test; P = 1 ×10 −6 and P = 3 ×10 −2 for two- and threefold amplification, respectively. Direct comparison of twofold and greater amplification frequencies of GLO1 and DNAH8 demonstrates that these also differ significantly (χ2 test; P = 0.021). Despite lower frequencies of amplification this difference is still significant when amplifications greater than threefold are considered (χ2 test; P = 0.034). Fourfold and greater amplifications were rare. GLO1 was amplified more than fourfold in five cell lines, KCNK17 in three, DNAH8, GLP1R, C6orf64, KCNK5, C6orf102 and DAAM2 in two, and KCNK16 in one cell line. In no cell line was there amplification of BTBD9 greater than fourfold.
Figure 2.
Graph of amplification frequencies as determined by real time qPCR in 618 human cancer samples for genes located on amplicon 6p21.2. Amplifications of twofold or greater are considered. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
TABLE 1.
Results of the Comparison of Amplification Between OPN5 and 6p21.2 Genes
| >2 |
>3 |
|||
|---|---|---|---|---|
| OPN5 | OPN5 | |||
| 2/573 (0.3%) | p, Chi-sq | 0 | p, Fisher | |
| BTBD9 | 9/530 (1.7%) | 2.43E-02 | 2/530 (0.4%) | 1.42E-01 |
| GLO1 | 49/536 (8.4%) | 2.59E-11 | 14/577 (2.4%) | 2.10E-04 |
| DNAH8 | 30/568 (5.0%) | 9.70E-07 | 5/595 (0.8%) | 2.85E-02 |
| GLP1R | 26/532 (4.7) | 3.41E-06 | 7/551 (1.3%) | 7.16E-03 |
| C6orf64 | 16/589 (2.6%) | 1.34E-03 | 3/602 (0.5%) | 9.15E-02 |
| KCNK5 | 15/557 (2.6%) | 1.45E-03 | 2/570 (0.3%) | 1.58E-01 |
| KCNK17 | 26/520 (4.8%) | 1.32E-06 | 5/541 (0.9%) | 2.19E-02 |
| KCNK16 | 11/546 (2.0%) | 1.02E-02 | 3/554 (0.5%) | 1.33E-02 |
| C6orf102 | 7/576 (1.2%) | 9.90E-02 | 2/586 (0.3%) | 1.63E-01 |
| DAAM2 | 8/598 (1.3%) | 6.83E-02 | 3/604 (0.5%) | 5.18E-02 |
In this table the frequencies of amplification (for threshold of >2-fold and >3-fold) of genes mapping to the amplicon on 6p21.2 are listed together with the statistical significance of the difference between amplification frequencies of OPN5 and respective genes. P-values significant after Benjamini-Hochberg correction with a FDR of 10% are indicated in bold. The amplification frequencies were calculated as ratios of the number of samples with amplification confirmed in two independent experiments and the total number of informative samples.
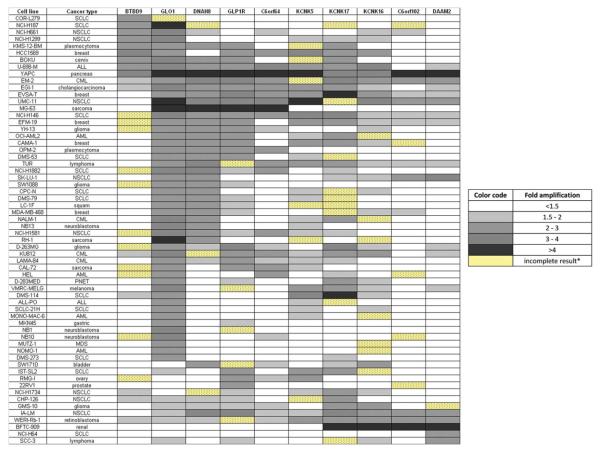
The pattern of amplification across the 6p21.2 amplicon is illustrated in Figure 3. In total, there were 63 samples with twofold and greater amplification of at least one gene, of which 49 (78%) had amplified GLO1. Eighty-six percent of cases with amplification of GLO1 also carried amplification of one or more additional 6p21.2 genes. In one case (YAPC), the amplification extended across the whole 6p21.2 amplicon. In eight cases (16% of cases with GLO1 amplification) the amplicon extended toward the p-telomere and in 31 cases (63% of cases with GLO1 amplification) the amplicon extended toward the centromere. In nine samples amplification of only GLO1 and no other gene from 6p21.2 smallest common amplified region was detected. Affymetrix SNP6 array results for these samples were reviewed and in five cases the finding could be confirmed. In one case the results were of insufficient quality to be interpreted and in the remaining three cases the Affymetrix SNP6 array failed to detect the increased copy number. These findings suggest that GLO1 is the main 6p21.2 amplification target and that in some samples the amplification can be very focal, targeting only a single gene.
Figure 3.
Cell Lines with amplification of one or more amplicon 6p21.2 genes as determined by real time qPCR. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
GLO1 amplification varied between cell lines derived from cancers of different histological types. Among cancer types with more than 20 analyzed samples, a frequency of amplification greater than 5% was seen in breast (19.2%), small cell lung cancer (15.5%), acute myeloid leukemia (15%), sarcoma (10.7%), nonsmall cell lung cancer (9.5%), neuroblastoma (7.5%), acute lymphoblastic leukemia (7.1%) and glioma (6.7%). No amplification was seen, among others, in colon (N = 30), ovary (N = 17), bladder (N = 14), esophagus (N = 13), Ewing (N = 12), and thyroid (N = 11) cancer cell lines. The complete list of cancers and their GLO1 amplification frequencies, including cancers with n < 20, is given in (Supporting Information Table S1).
GLO1 Is Amplified in Primary Human Cancers
To determine whether GLO1 amplification also occurs in primary cancers a screen of DNA extracted from primary cancer samples was undertaken. As for cell lines, relative quantities of the GLO1 gene were determined in two independent experiments and amplification was called if the relative quantity (RQ) in both experiments was greater than two. In total 520 primary tumors were investigated yielding 492 informative results (i.e., samples for which RQ from both experiments were available).
Similar to the results of the cancer cell line screen, the amplification of GLO1 in primary cancers was generally of low level. Only eight samples (1.6%) had GLO1 amplification greater than threefold with half of these occurring in sarcomas in which threefold or greater amplification was present in 5.6% of samples. Twofold or greater amplification occurred in 22% (8/37) of breast, 17% of sarcomas (12/71), 11.3% of non-small cell lung cancers (6/53), 8.7% of bladder (2/23), 6.5% (6/93) of renal, and 6% of gastric (5/83) cancers (Supporting Information Table S2). Amplification of GLO1 was rare in colon cancer (1/35, 2.9%) and glioma (1/94, 1%).
Expression Analysis of Human Cancer Cell Lines Supports GLO1 as the Most Likely Target of the Amplification on 6p21.2
Expression analysis of GLO1 and other genes mapped to the smallest common amplified region of amplicon 6p21.2 was undertaken to determine whether the expression pattern of GLO1 is consistent with GLO1 being an amplification target and whether other genes may also exhibit similar pattern. The target gene(s) would be expected to have generally increased level of expression and the level of expression to correlate with the level, or at least with the presence of amplification. In addition to genes mapping to the MCAR of 6p21.2 (GLO1, DNAH8, GLP1R, C6orf64, KCNK5, KCNK17, KCNK16, and C6orf102) the flanking genes (DAAM2 and BTBD9) were also included in the expression studies.
In the first set of experiments, gene expression was determined using real time qRT-PCR in a panel of 23 human cancer cell lines. The results are summarized in Table S3. The expression of most genes (DNAH8, GLP1R, KCNK17, KCNK16, C6orf102, and DAAM2) was generally lower than in reference sample (RNA from mixed normal tissues), suggesting that they are unlikely targets of this amplicon. In contrast, BTBD9, GLO1, C6orf64, and KCNK5 were frequently overexpressed (RQ >1.5-fold the mixed normal human tissues control) in this panel of cell lines. For BTBD9, GLO1, and C6orf64 the correlation of expression and amplification was high (correlation coefficient 0.88, 0.65, and 0.82, respectively), and the expression was higher in cell lines with amplification than in cell lines without amplification (t test: P < 0.017, P < 0.014, and P < 0.014, respectively) (Table 2). In addition, over half (57%) of the cell lines overexpressed KCNK5 and most (57%) samples with 6p21.2 amplification also overexpressed this gene. However, the correlation between amplification and overexpression of KCNK5 was poor (correlation coefficient 0.42), and there was no statistically significant difference in expression between samples with and without amplification (P < 0.13; one tailed t test). These results are consistent with the indication from the physical mapping data that GLO1 is a target of the amplicon on 6p21.2.
TABLE 2.
Summary of Results of the Expression Studies of Genes Mapping to Amplicon 6p21.2
| Gene | % OE | %AOE | CC of A&E | t-test EA vs EnA |
|---|---|---|---|---|
| BTBD9* | 14/23 (61%) | 6/6 (100%) | 0.880 | 0.017 |
| GLO1 | 21/23 (91%) | 7/7 (100%) | 0.646 | 0.014 |
| DNAH8 | 0 | 0 | −0.022 | 0.126 |
| GLP1R | 1/22 (5%) | 0 | −0.154 | 0.159 |
| C6orf64 | 13/23 (57%) | 7/7 (100%) | 0.823 | 0.014 |
| KCNK5 | 13/23 (57%) | 4/7 (57%) | 0.426 | 0.133 |
| KCNK17 | 1/23 (4%) | 0 | 0.089 | 0.300 |
| KCNK16 | 2/23 (9%) | 0 | −0.219 | 0.095 |
| C6orf102-KIF6 | 0 | 0 | −0.135 | 0.106 |
| DAAM2 a | 1/23 (4%) | 0 | −0.241 | 0.167 |
% OE, percentage of samples with expression greater than 1.5-fold the reference (mixed normal tissues cDNA); %AOE, percentage of samples with amplification that also overexpressed the respective gene; CC of A&E, correlation coefficient between expression and amplification (cDNA and DNA RQs). T-test EA vs. EnA, P-value of the t-test testing the null hypothesis: “there is no difference between the level of expression in samples with (EA, expression in nonamplified) and without amplification (EnA, expression in nonamplified).
BTBD9 and DAAM2 lie outside the smallest common amplified region of amplicon 6p21.2
In the second set of experiments we analyzed gene expression in 501 human cancer cell lines using Affymetrix HT-HU133 expression arrays. The results showed that GLO1 was overexpressed in 50 (10.0%) samples, while KCNK5 and C6orf64 were found overexpressed in 43 (8.6%) and 28 (5.6%) samples, respectively. No overexpression was identified for the remaining genes from the 6p21.2 amplicon. Gene overexpression and amplification occurred concurrently in 12, 4, and 3 samples for GLO1, C6orf64, and KCNK5, respectively. Concurrence of amplification and overexpression is significantly different between GLO1 and C6orf64 (Fisher’s exact test, P = 0.046), as well as between GLO1 and KCNK5 (Fisher’s exact test; P = 0.034). Taken together, the results suggest that, among the amplicon 6p21.2 genes, the association between amplification and overexpression is greatest for GLO1. The data are therefore consistent with the physical mapping results in indicating that GLO1 is the most likely target of the amplification.
RNAi Knockdown of GLO1 Has the Greatest and Most Consistent Effect on Apoptosis and Cell Accumulation among the Genes Mapping to the Amplicon on 6p21.2
Knockout of a target gene that is overexpressed is expected to show the greatest reduction of proliferation and increase in apoptosis among the genes residing on the investigated amplicon. Of the seven cell lines carrying the 6p21.2 amplicon, identified by Affymetrix 10K SNP array, four grew as adherent cell cultures (LB771, squamous cell carcinoma of the upper aerodigestive tract; MG63, osteosarcoma; NCI-H522, nonsmall cell lung cancer and YAPC, esophageal carcinoma), and were therefore amenable to lipid transfection with siRNA (Ovcharenko et al., 2005). Based on the results of the physical mapping experiments and expression studies, four genes were selected for knockdown: BTBD9, C6orf64, GLO1 and KCNK5. The remaining genes were considered unlikely targets of the 6p21.2 amplicon.
The results of the knockdown studies are summarized in Figure 4. Knockdown of GLO1 led to the greatest and most consistent increase of the apoptotic index. Knockdown of C6orf64 led to some increase of the apoptotic index in LB771 and NCI-H522, but these failed to reach statistical significance. The effect of KCNK5 knockdown on apoptotic index was inconsistent. For example, in the case of NCI-H522 the apoptotic index was significantly greater and in the case of YAPC significantly lower than the control. Knockdown of BTBD9 did not cause a significant decrease in apoptotic index in any cell line.
Figure 4.
The influence of amplicon 6p21.2 gene knockdown on cell accumulation and apoptosis. Genes BTBD9, GLO1, C6orf64 and KCNK5 were knocked down using siRNA (80 nm) [BLUE bars] and the resulting cell accumulation and apoptotic indexes were measured 72 hr after treatment. Each experiment was performed in triplicate. Scrambled (nontargeting) siRNA was used as a control [RED bars]. Each cell line used in these experiments carried an amplicon that included GLO1. Amplification of GLO1 as, determined by real time qPCR, was: LB771 2fold, MG63 6.4-fold, NCI-H522 6.1-fold, YAPC 5.1-fold. The measurements of cell growth and apoptotic indexes of the siRNA-treated samples were expressed relative to their scrambled controls. The controls were given value of “1”. The significance of difference for each gene knockdown was assessed using t test (two-tailed, unequal variance assumed). The P values of the t tests are listed under the respective experiments (listed above names of the knocked-down genes). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Similarly for the cell accumulation assay, knockdown of BTBD9 had little impact on the cell accumulation, although an increase in LB771 was seen. A reduction in cell accumulation was observed following KCNK5 knockdown in NCI-H522 and YAPC. Similarly, knockdown of C6orf64 led to a reduction of growth of YAPC. Knockdown of GLO1 had the greatest and most consistent impact on cell accumulation: the reduction of cell accumulation following GLO1 knockdown occurred in all cell lines except LB771 where it failed to reach statistical significance.
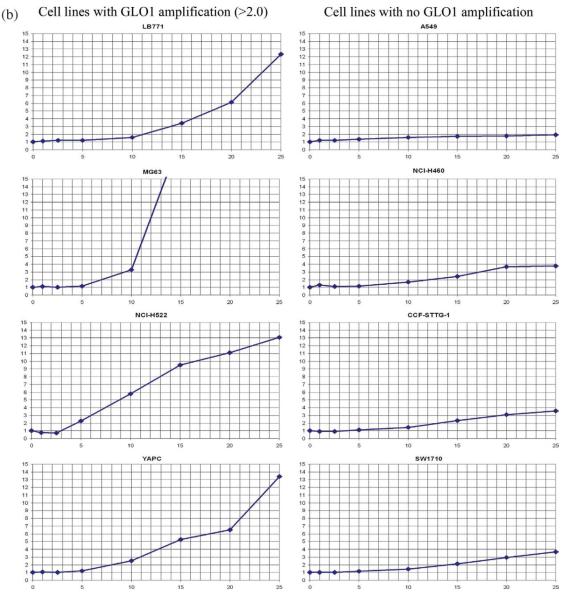
Inhibition of GLO1 With BBGC Has a Greater Effect on Apoptosis and Cell Accumulation in Cell Lines With Than Without GLO1 Amplification
A potent GLO1 competitive inhibitor, BBGC, was developed by Lo and Thornalley (1993). Sakamoto et al. (2001) reported BBGC induced apoptosis specifically occurring in cells with high activity of GLO1. We selected four cell lines with amplification and overexpression of GLO1, and four cell lines without to determine their sensitivity to GLO1 inhibition with BBGC. Apoptotic index and cell accumulation were measured after 24-hr incubation in media containing BBGC in concentrations ranging from 0 to 25 μm. The results are summarized in Table 3 and Figure 5 (Supporting Information). All cell lines showed a reduction of cell accumulation following treatment with BBGC in concentrations as low as 5 μm. However, there was substantial variation in sensitivity to this drug. Cell lines with a GLO1 gene dosage of twice the normal or more were more sensitive. The average BBGC IC50 for these cell lines was 7.6 μM (standard error 1.4 μm) (Table 3 and Fig. 5a). The average BBGC IC50 of cell lines with normal GLO1 gene dosage was 19 μM (standard error 1.8 μm) (Table 3 and Fig. 5a). This was statistically significant (P = 0.0026; t test, equal variance assumed).
TABLE 3.
Summary of GLO1 Inhibition With BBGC in Cell Lines With and Without GLO1 Amplification
| Cell line | IC50 μM |
mRNA RQ |
DNA RQ |
|---|---|---|---|
| MG53 | 3.8 | 9.5 | 6.4 |
| NCI-H522 | 7 | 30 | 6.1 |
| YAPC | 10 | 11.8 | 5.1 |
| DMS114 | x | 29 | 2.4 |
| LB771 | 9.5 | 4.4 | 2.1 |
| DMS273 | x | X | 2.5 |
| A549 | 23.5 | 5.9 | 1.4 |
| NCI-H460 | 19.8 | 0.9 | 0.8 |
| CCF-STTG-1 | 17.5 | 3 | 1 |
| SW1710 | 15 | 7.5 | 1.7 |
| NCI-H226 | x | 1.6 | 0.6 |
| NCI-H23 | x | 11.6 | 0.9 |
IC50, concentration of a drug (μM) that is required for 50% inhibition of cell accumulation in vitro; RQ - relative quantity as determined using real time qPCR; x, not available.
Figure 5.
(a) Cell growth after 24 hr following GLO1 inhibition with BBGC. The horizontal axis represents concentration of BBGC in the culture medium in μm. The vertical axis represents cell accumulation expressed as percentage of the control. The control contained no BBGC in the medium. (b) Apoptosis at 24 hr following GLO1 inhibition with BBGC. The horizontal axis represents concentration of BBGC in the culture medium in μm. The vertical axis represents fold change in the apoptotic index relative to the control. The control contained no BBGC in the medium. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The higher sensitivity to BBGC of cell lines with GLO1 amplification was also reflected in the apoptotic index which rose more steeply with rising concentration of BBGC in cell lines with GLO1 amplification (Fig. 5b). The apoptotic index doubled with an average concentration of 7.8 μM (standard error 1.3 μm) in cell lines with and 14.8 μM (standard error 1.9 μm) in cell lines without GLO1 amplification. This difference was statistically significant (P = 0.026; t test, equal variance assumed).
DISCUSSION
In this article the results of a systematic search for the target of a novel genomic amplification in human cancer are presented. The search was designed based on the premise that during the evolution of cancer, amplicons are selected for based on the biological advantage that the gene(s) residing on the amplicons confer on cells (Schwab, 1999; Albertson, 2006). Amplicons are often extended genomic regions that carry several genes, even although only one may be under selection and confer clonal growth advantage on the cancer cell. However, in this case the gene which is conferring selective advantage is likely to be most frequently amplified. Because of the large number of cancer samples required to detect a difference in the amplification frequencies of individual genes on an amplicon real time qPCR was used as a highly sensitive and specific technique amenable to high throughput screening.
The screening of a set of 618 human cancer cell lines using real time qPCR identified GLO1 as the most frequently amplified gene with 8.4% of the informative samples having twofold or greater amplification. When compared with the control gene, OPN5, located ~10 Mb in the centromeric direction from the 6p21.2 amplicon, the frequency of amplification of most amplicon 6p21.2 genes was significantly higher (Table 1). However the P value of the difference was four orders of magnitude greater for GLO1 than any other amplicon 6p21.2 gene (Table 1). These differences of amplification frequencies were maintained if threefold instead of twofold amplification was considered, although due to the lower frequency of amplifications the P values were all several magnitudes lower. Moreover, when amplification frequencies of GLO1 and the other amplicon 6p21.2 genes were directly compared, the amplification frequency of GLO1 was significantly greater than that of the other genes both for two- and threefold level of amplification. When a panel of 90 lymphoblastoid cell line controls was screened for GLO1 copy numbers, none had a relative quantity of GLO1 equal to or greater than two. Taken together, the data demonstrate significantly greater amplification frequency of GLO1 as compared with other genes on amplicon 6p21.2. The results therefore indicate that GLO1 is a target of the 6p21.2 amplification.
In 13 (21%) samples with 6p21.2 amplification, the amplification did not reach the twofold threshold at the GLO1 locus, suggesting that GLO1 may not be the sole target of amplification involving 6p21.2 region. One cell line (COR-L279) had amplification of BTBD9 and 12 cell lines had amplification of DNA segments of various sizes that were located in the centromeric direction relative to GLO1. It is possible therefore that these amplicons target genes other than GLO1, including possibly genes located outside the smallest common amplified region of 6p21.2, such as CCND3 and VEGF, both previously implicated in cancer development (Kerbel, 2008; Wlodarska et al., 2008). There is also the possibility that, despite not being at the epicenter of the amplification in a particular cell line, GLO1 is nevertheless the biological target of the amplicon.
Subsequent screening of 520 primary human cancer specimens using real time qPCR identified GLO1 amplification in all seven cancer types with frequencies ranging from 1% in gliomas to 22% in breast cancers. These data confirmed that amplification of GLO1 is not only found in cancer cell lines, but also occurs in primary cancers. Moreover, the frequencies of amplification of primary cancers and equivalent cell lines were comparable (Supporting Information Tables S1 and S2). For example, amplification of GLO1 was frequent in breast cancers, sarcomas and nonsmall cell lung cancers in both cell lines and primary cancers (19%, 11%, and 10%, and 22%, 17%, and 14% for the respective cell lines and primary cancers), and appears to be rare in colon cancer and glioma, both in cell lines and primary cancers. Overall the frequency of amplification greater than 3fold was higher in cell lines than in primary cancers (2.4% and 1.6%, respectively). This is likely a reflection of a greater cellular heterogeneity, both of tumor and normal cells, present in primary cancer specimens. The frequency of threefold or greater amplification was higher both in sarcoma cell lines and primary sarcomas (7.1% and 5.6%, respectively) than in other cancers.
RNAi silencing of GLO1 led most consistently to an increase in apoptosis and a reduction in cell accumulation. These data therefore suggest that GLO1 is the most indispensable of the four studied genes in the four cell lines with 6p21.2 amplification and together with the expression data complement the conclusion of the study of amplification frequencies of amplicon 6p21.2 genes.
Importantly, the effects of GLO1 knockdown using siRNA were consistent with those caused by inhibition with BBGC. For each cell line the cell accumulation curve (Fig. 5a) was mirrored by the apoptotic index curve (Fig. 5b), suggesting that the reduced cell accumulation is caused by the increased rate of apoptosis. Although all cell lines were sensitive to treatment with BBGC, significantly lower concentrations of BBGC were needed to induce apoptosis and consequently reduce cell accumulation in lines with GLO1 amplification, as compared with cell lines with normal GLO1 copy numbers. All cell lines with GLO1 amplification had high to moderate overexpression of GLO1 and three cell lines with no amplification had mild to moderate overexpression of GLO1 (A549, CCF-STTG-1 and SW1710). Interestingly, NCI-H23 was previously found by Sakamoto et al. (2001) to have high activity of GLO1, but had low sensitivity to GLO1 inhibition. We found that although this cell line had relatively high GLO1 expression, its GLO1 amplification was 0.9-fold. Therefore, it is possible that the level of dependence on increased levels of GLO1 is more closely related to GLO1 amplification than the relative level of expression or GLO1 activity.
Glyoxalase 1 is an enzyme present in cytosol. It catalyzes the conversion of reactive α-oxoaldehydes to corresponding α-hydroxyacids (Thornalley, 2003). Accumulation of the toxic substrates of GLO1 causes modification of DNA, RNA and proteins which may result in cell death (Thornalley, 1996). Indeed, apoptosis as the main biological consequence of GLO1 inhibition using BBGC has been demonstrated before (Thornalley et al., 1996; Sakamoto et al., 2000, 2001; Davies et al., 2005a). GLO1 was found to be overexpressed in several cancers by a number of groups (Davidson et al., 1999; Rulli et al., 2000, 2001). Sakamoto et al. (2000) found that GLO1 was significantly overexpressed in chemoresistant derivative leukemia cell lines as compared to their parental cell lines. The cells overexpressing GLO1 also had higher GLO1 enzyme activity (Sakamoto et al., 2000). Reduction of expression of GLO1 using troglitazone led to sensitization to doxycyclin of GLO1 overexpressing and a doxycyclin-resistant leukemia cell line K562 (Davies et al., 2005a). Ectopic overexpression of GLO1 in the NIH3T3 cell line leads to increased resistance to mitomycin and doxorubicin (Ranganathan et al., 1995). The mechanism of overexpression in cancer generally has not, however, been investigated and only one group has determined the copy number of GLO1 in a single colon cancer cell line (HT29) using Southern analysis and found no amplification (Ranganathan et al., 1993). Data from our experiments demonstrate, for the first time, a direct link between GLO1 gene amplification, overexpression and increased sensitivity to GLO1 inhibition. As a whole, cell lines with GLO1 amplification were significantly more sensitive to GLO1 inhibition than those without.
Collectively the physical mapping, expression, RNAi knockdown and BBGC inhibition results indicate that GLO1 is at least one of the targets of gene amplification on 6p21.2. Moreover, GLO1 may represent a useful target for therapy in cancers with GLO1 amplification.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Todd Golub and colleagues at the Broad Institute of Broad Institute of Massachusetts Institute of Technology and Harvard University, Cambridge, Massachusetts for the expression data.
Supported by: Wellcome Trust, Grant number: 077012/Z/05/Z; The Michael and Betty Kadoorie Cancer Genetics Research Programme.
Footnotes
COLO-201 and COLO-205 are synonymous cell lines and are likely to be derived from the same ancestoral cancer cell line (http://www.sanger.ac.uk/genetics/CGP/Genotyping/synlinestable.shtml)
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Albertson DG. Gene amplification in cancer. Trends Genet. 2006;22:447–455. doi: 10.1016/j.tig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Bignell GR, Huang J, Greshock J, Watt S, Butler A, West S, Grigorova M, Jones KW, Wei W, Stratton MR, Futreal PA, Weber B, Shapero MH, Wooster R. High-resolution analysis of DNA copy number using oligonucleotide microarrays. Genome Res. 2004;14:287–295. doi: 10.1101/gr.2012304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignell GR, Santarius T, Pole JC, Butler AP, Perry J, Pleasance E, Greenman C, Menzies A, Taylor S, Edkins S, Campbell P, Quail M, Plumb B, Matthews L, McLay K, Edwards PA, Rogers J, Wooster R, Futreal PA, Stratton MR. Architectures of somatic genomic rearrangement in human cancer amplicons at sequence-level resolution. Genome Res. 2007;17:1296–1303. doi: 10.1101/gr.6522707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Davidson SD, Cherry JP, Choudhury MS, Tazaki H, Mallouh C, Konno S. Glyoxalase I activity in human prostate cancer: a potential marker and importance in chemotherapy. J Urol. 1999;161:690–691. [PubMed] [Google Scholar]
- Davies GF, Roesler WJ, Juurlink BH, Harkness TA. Troglitazone overcomes doxorubicin-resistance in resistant K562 leukemia cells. Leuk Lymphoma. 2005a;46:1199–1206. doi: 10.1080/10428190500102555. [DOI] [PubMed] [Google Scholar]
- Davies H, Hunter C, Smith R, Stephens P, Greenman C, Bignell G, Teague J, Butler A, Edkins S, Stevens C, Parker A, O’Meara S, Avis T, Barthorpe S, Brackenbury L, Buck G, Clements J, Cole J, Dicks E, Edwards K, Forbes S, Gorton M, Gray K, Halliday K, Harrison R, Hills K, Hinton J, Jones D, Kosmidou V, Laman R, Lugg R, Menzies A, Perry J, Petty R, Raine K, Shepherd R, Small A, Solomon H, Stephens Y, Tofts C, Varian J, Webb A, West S, Widaa S, Yates A, Brasseur F, Cooper CS, Flanagan AM, Green A, Knowles M, Leung SY, Looijenga LH, Malkowicz B, Pierotti MA, Teh BT, Yuen ST, Lakhani SR, Easton DF, Weber BL, Goldstraw P, Nicholson AG, Wooster R, Stratton MR, Futreal PA. Somatic mutations of the protein kinase gene family in human lung cancer. Cancer Res. 2005b;65:7591–7595. doi: 10.1158/0008-5472.CAN-05-1855. [DOI] [PubMed] [Google Scholar]
- Davies H, Dicks E, Stephens P, Cox C, Teague J, Greenman C, Bignell G, O’Meara S, Edkins S, Parker A, Stevens C, Menzies A, Blow M, Bottomley B, Dronsfield M, Futreal PA, Stratton MR, Wooster R. High throughput DNA sequence variant detection by conformation sensitive capillary electrophoresis and automated peak comparison. Genomics. 2006;87:427–432. doi: 10.1016/j.ygeno.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Futreal PA, Coin L, Marshall M, Down T, Hubbard T, Wooster R, Rahman N, Stratton MR. A census of human cancer genes. Nat Rev Cancer. 2004;4:177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier L, Cope L, Bolstad BM, Irizarry RA. affy—Analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- Greenman C, Bignell GR, Butler A, Edkins S, Hinton J, Beare DM, Swamy S, Santarius T, Chen L, Widaa S, Futreal PA, Stratton MR. PICNIC: An algorithm to predict absolute allelic copy number variation with microarray cancer data. Biostatistics. 2010;11:164–175. doi: 10.1093/biostatistics/kxp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of affymetrix GeneChip probe level data. Nucleic Acids Res. 2003a;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003b;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo TW, Thornalley PJ. Inhibition of human leukaemia 60 cell growth by diethyl esterification of the glyoxalase II inhibitor S-p-nitrobenzoxycarbonylglutathione in vitro. Biochem Soc Trans. 1993;21:159S. doi: 10.1042/bst021159s. [DOI] [PubMed] [Google Scholar]
- Myllykangas S, Knuutila S. Manifestation, mechanisms and mysteries of gene amplifications. Cancer Lett. 2006;232:79–89. doi: 10.1016/j.canlet.2005.07.045. [DOI] [PubMed] [Google Scholar]
- Ota A, Tagawa H, Karnan S, Tsuzuki S, Karpas A, Kira S, Yoshida Y, Seto M. Identification and characterization of a novel gene, C13orf25, as a target for 13q31-q32 amplification in malignant lymphoma. Cancer Res. 2004;64:3087–3095. doi: 10.1158/0008-5472.can-03-3773. [DOI] [PubMed] [Google Scholar]
- Ovcharenko D, Jarvis R, Hunicke-Smith S, Kelnar K, Brown D. High-throughput RNAi screening in vitro: From cell lines to primary cells. RNA. 2005;11:985–993. doi: 10.1261/rna.7288405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan S, Walsh ES, Godwin AK, Tew KD. Cloning and characterization of human colon glyoxalase-I. J Biol Chem. 1993;268:5661–5667. [PubMed] [Google Scholar]
- Ranganathan S, Walsh ES, Tew KD. Glyoxalase I in detoxification: Studies using a glyoxalase I transfectant cell line. Biochem J. 1995;309(Part 1):127–131. doi: 10.1042/bj3090127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa: 2000. [DOI] [PubMed] [Google Scholar]
- Rulli A, Carli L, Romani R, Baroni T, Giovannini E, Rosi G, Talesa V. Expression of glyoxalase I and II in normal and breast cancer tissues. Breast Cancer Res Treat. 2001;66:67–72. doi: 10.1023/a:1010632919129. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Mashima T, Kizaki A, Dan S, Hashimoto Y, Naito M, Tsuruo T. Glyoxalase I is involved in resistance of human leukemia cells to antitumor agent-induced apoptosis. Blood. 2000;95:3214–3218. [PubMed] [Google Scholar]
- Sakamoto H, Mashima T, Sato S, Hashimoto Y, Yamori T, Tsuruo T. Selective activation of apoptosis program by S-p-bromobenzylglutathione cyclopentyl diester in glyoxalase I-overexpressing human lung cancer cells. Clin Cancer Res. 2001;7:2513–2518. [PubMed] [Google Scholar]
- Schwab M. Amplification of oncogenes in human cancer cells. Bioessays. 1998;20:473–479. doi: 10.1002/(SICI)1521-1878(199806)20:6<473::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Schwab M. Oncogene amplification in solid tumors. Semin Cancer Biol. 1999;9:319–325. doi: 10.1006/scbi.1999.0126. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Pharmacology of methylglyoxal: Formation, modification of proteins and nucleic acids, and enzymatic detoxification—A role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27:565–573. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Protecting the genome: Defence against nucleotide glycation and emerging role of glyoxalase I overexpression in multidrug resistance in cancer chemotherapy. Biochem Soc Trans. 2003;31:1372–1377. doi: 10.1042/bst0311372. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ, Edwards LG, Kang Y, Wyatt C, Davies N, Ladan MJ, Double J. Antitumour activity of S-p-bromobenzylglutathione cyclopentyl diester in vitro and in vivo. Inhibition of glyoxalase I and induction of apoptosis. Biochem Pharmacol. 1996;51:1365–1372. doi: 10.1016/0006-2952(96)00059-7. [DOI] [PubMed] [Google Scholar]
- Wlodarska I, Dierickx D, Vanhentenrijk V, Van RK, Pospisilova H, Minnei F, Verhoef G, Thomas J, Vandenberghe P, De Wolf-Peeters C. Translocations targeting CCND2, CCND3, and MYCN do occur in t(11;14)-negative mantle cell lymphomas. Blood. 2008;111:5683–5690. doi: 10.1182/blood-2007-10-118794. [DOI] [PubMed] [Google Scholar]