Abstract
The evolutionary importance of hybridization and introgression has long been debated1. We used genomic tools to investigate introgression in Heliconius, a rapidly radiating genus of neotropical butterflies widely used in studies of ecology, behaviour, mimicry and speciation2-5 . We sequenced the genome of Heliconius melpomene and compared it with other taxa to investigate chromosomal evolution in Lepidoptera and gene flow among multiple Heliconius species and races. Among 12,657 predicted genes for Heliconius, biologically important expansions of families of chemosensory and Hox genes are particularly noteworthy. Chromosomal organisation has remained broadly conserved since the Cretaceous, when butterflies split from the silkmoth lineage. Using genomic resequencing, we show hybrid exchange of genes between three co-mimics, H. melpomene, H. timareta, and H. elevatus, especially at two genomic regions that control mimicry pattern. Closely related Heliconius species clearly exchange protective colour pattern genes promiscuously, implying a major role for hybridization in adaptive radiation.
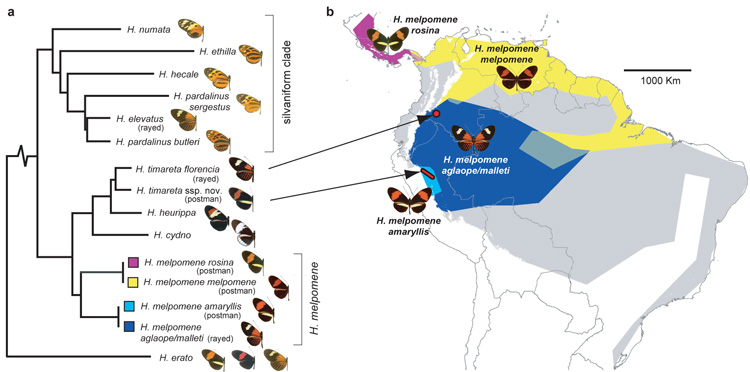
The butterfly genus Heliconius (Nymphalidae: Heliconiinae) is associated with a suite of derived life-history and ecological traits, including pollen-feeding, extended life-span, augmented ultraviolet colour vision, ‘trap-lining’ foraging behavior, gregarious roosting and complex mating behaviours, and provides outstanding opportunities for genomic studies of adaptive radiation and speciation4, 6. The genus is best known for the hundreds of different colour pattern races seen among its 43 species, with repeated examples of both convergent evolution among distantly related species and divergent evolution between closely related taxa3. Geographic mosaics of multiple colour pattern races, such as in Heliconius melpomene (Fig. 1), converge to similar mosaics in other species, and this led to the hypothesis of mimicry2. Heliconius are unpalatable and Müllerian mimicry of warning colour patterns enables species to share the cost of educating predators3. Divergence in wing pattern is also associated with speciation and adaptive radiation due to a dual role in mimicry and mate selection3, 5. A particularly recent radiation is the melpomene-silvaniform clade, where mimetic patterns often appear polyphyletic (Fig. 1a). Most species in this clade occasionally hybridise in the wild with other clade members7. Gene genealogies at a small number of loci indicate introgression between species8, and one non-mimetic species, H. heurippa, has a hybrid origin9. Adaptive introgression of mimicry loci is therefore a plausible explanation for parallel evolution of multiple mimetic patterns in the melpomene-silvaniform clade.
Figure 1. Distribution, mimicry and phylogenetic relationships of sequenced taxa.
a Phylogenetic relationship of sequenced species and subspecies in the ‘melpomene- silvaniform clade’ of Heliconius. H. elevatus falls in the ‘silvaniform’ clade, but its colour pattern mimics melpomene-timareta clade taxa. b Geographic distribution of ‘postman’ and ‘rayed’ H. melpomene races studied here (blue, yellow and purple), and the entire distribution of H. melpomene (grey). The H. timareta races investigated have limited distributions indicated by arrows (red) and mimic sympatric races of H. melpomene. H. elevatus and the other silvaniform species are distributed widely across the Amazon basin (Supplementary Information 22).
A Heliconius melpomene melpomene stock from Darién, Panama (Fig. 1) was inbred via five generations of sib mating. A single male was sequenced to 38x coverage (after quality filtering) using combined 454 and Illumina technologies (Supplementary Information 1-8). The complete draft genome assembly of 269 Mb consists of 3,807 scaffolds with an N50 of 277 kb and contains 12,657 predicted protein-coding genes. RAD linkage mapping was used to assign and order 83% of the sequenced genome onto the 21 chromosomes (Supplementary Information 4). These data permit a considerably improved genome-wide chromosomal synteny comparison with the silkmoth Bombyx mori10, 11. Using 6,010 orthologues identified between H. melpomene and B. mori we found that 11 of 21 H. melpomene linkage groups show homology to single B. mori chromosomes and ten linkage groups have major contributions from two B. mori chromosomes (Fig. 2a and Supplementary Information 8), revealing several previously unidentified chromosomal fusions. These fusions on the Heliconius lineage most likely occurred after divergence from the sister genus Eueides4, which has the lepidopteran modal karyotype of n=3112. Three chromosomal fusions are evident in Bombyx (Fig. 2a, B. mori chromosomes 11, 23 and 24), as required for evolution of the Bombyx n=28 karyotype from the ancestral n=31 karyotype. Heliconius and Bombyx lineages diverged in the Cretaceous >100 MYA11, so the chromosomal structures of Lepidoptera genomes have remained highly conserved compared to those of flies or vertebrates13, 14. In contrast, small-scale rearrangements were frequent. In the comparison with Bombyx, we estimate 0.05-0.13 breaks/Mb/MY, and with the Monarch butterfly, Danaus plexippus, 0.04-0.29 breaks/Mb/MY. Although lower than previously suggested for Lepidoptera15, these rates are comparable to Drosophila (Supplementary Information 8).
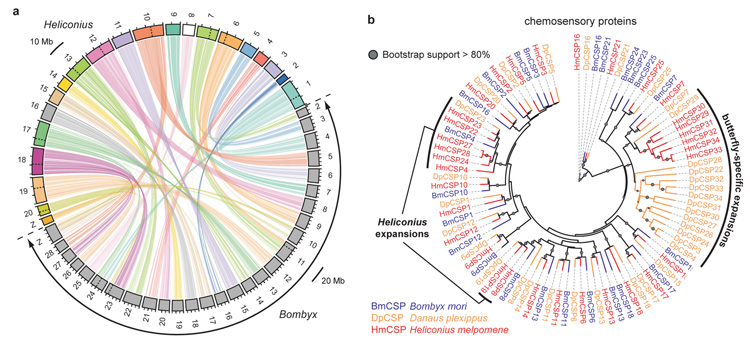
Figure 2. Comparative analysis of synteny and expansion of the chemosensory genes.
a Maps of the 21 Heliconius chromosomes (above) and of the 28 Bombyx chromosomes (below in grey) based on positions of 6010 orthologue pairs demonstrate highly conserved synteny and a shared n=31 ancestor (Supplementary Information 8). Dotted lines within chromosomes indicate major chromosomal fusions. b Maximum likelihood tree showing expansion of the chemosensory protein genes (CSP) in two butterfly genomes.
The origin of butterflies was associated with a switch from nocturnal to diurnal behaviour, and a corresponding increase in visual communication16. Heliconius have increased visual complexity through expression of a duplicate UV opsin6, in addition to the long wavelength, blue, and UV-sensitive opsins in Bombyx. We might therefore predict reduced complexity of olfactory genes, but in fact Heliconius and Danaus17 genomes have more chemosensory proteins (CSPs) than any other insect genome: 33 and 34 CSPs respectively (Supplementary Information 9), versus 24 in Bombyx and 3- 4 in Drosophila18. Lineage-specific CSP expansions were evident in both Danaus and Heliconius (Fig. 2b). In contrast, all three lepidopteran genomes possess similar numbers of odorant binding proteins and olfactory receptors (Supplementary Information 9). Hox genes are involved in body plan development and show strong conservation across animals. We identified four additional Hox genes located between the canonical Hox genes pb and zen, orthologous to shx genes in B. mori (Supplementary Information 10)19. These Hox gene duplications in the butterflies and Bombyx share a common origin, and are independent of the two tandem duplications known in dipterans (zen2, bcd). Immunity-related gene families are similar across all three lepidopterans (Supplementary Information 11), contrasting with extensive duplications and losses within dipterans20.
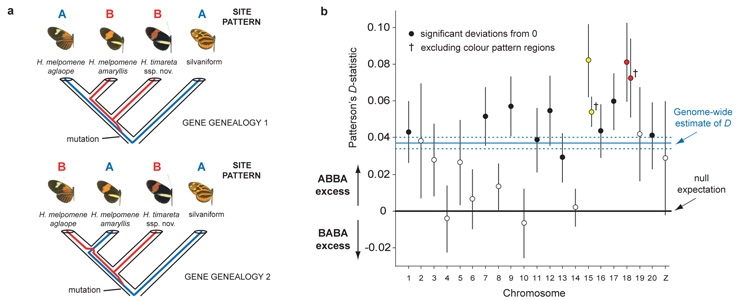
The Heliconius reference genome enabled rigorous tests for introgression among melpomene-silvaniform clade species. We used RAD resequencing to reconstruct a robust phylogenetic tree based on 84 individuals of H. melpomene and its relatives,sampling on average 12 Mb, or 4% of the genome (Fig 1a, Supplementary Information 12, 13, 18). We then tested for introgression between the sympatric co-mimetic postman races of H. melpomene aglaope and H. timareta ssp. nov. (Fig. 1) in Peru, employing ‘ABBA-BABA’ single nucleotide sites and Patterson’s D-statistics (Fig. 3a), originally developed to test for admixture between Neanderthals and modern humans21, 22 (Supplementary Information 12). Genome-wide we found an excess of ABBA sites, giving a significantly positive Patterson’s D = 0.037 ± 0.003 (two tailed Z-test for D = 0, P = 1 × 10−40), indicating greater genome-wide introgression between the sympatric mimetic taxa H. m. amaryllis and H. timareta ssp. nov., than between H. m. aglaope and H. timareta ssp. nov., which do not overlap spatially (Fig. 1b). These D-statistics yield an estimate of 2-5% of the genome exchanged21 between the two taxa (Supplementary Information 12). Eleven of the 21 chromosomes have significantly positive D-statistics (Fig. 3b,); interestingly, the strongest signals of introgressions were found on two chromosomes containing the known mimicry loci B/D and N/Yb (Fig. 3b, Supplementary Information 15).
Figure 3. Four-taxon ABBA-BABA test of introgression.
a ABBA and BABA nucleotide sites employed in the test are derived (– – B –) in H. timareta, compared with the silvaniform outgroup (– – – A), but differ among H. melpomene amaryllis and H. melpomene aglaope (either ABBA or BABA). As this almost exclusively restricts attention to sites polymorphic in the ancestor of H. timareta and H. melpomene, equal numbers of ABBA and BABA sites22 are expected under a null hypothesis of no introgression, as depicted in the two gene genealogies. b Distribution among chromosomes of Patterson’s D-statistic ± s.e., which measures excess of ABBA vs. BABA sites22, here for the comparison H. m. aglaope; H. m. amaryllis; H. timareta ssp. nov.; silvaniform. Chromosomes containing the two colour pattern regions (B/D red; N/Yb yellow) have the two highest D-statistics; the combinatorial probability of this occurring by chance is 0.005. The excess of ABBA sites (0 < D < 1) indicates introgression between sympatric H. timareta and H. melpomene amaryllis.
Perhaps the best known case of Müllerian mimicry is the geographic mosaic of ~30 bold postman and rayed colour pattern races of H. melpomene (Fig. 1b, Supplementary Information 22), which mimic a near-identical colour pattern mosaic in H. erato (Fig. 1a), among other Heliconius. Mimicry variation is generally controlled by a few loci with major effects. Mimetic pattern differences between the postman H. melpomene amaryllis and rayed H. melpomene aglaope races studied here (Fig 1a) are controlled by the B/D (red pattern) and N/Yb (yellow pattern) loci23, 24. These loci are located on the same two chromosomes showing the strongest D- statistics in our RAD analysis (Fig. 3b). To test whether mimicry loci might be introgressed between co-mimetic H. timareta and H. melpomene (Fig. 1a)7, we resequenced the colour pattern regions B/D (0.7 Mb) and N/Yb (1.2 Mb), and 1.8 Mb of unlinked regions across the genome, from both postman and ray-patterned H. melpomene and H. timareta from Peru and Colombia, and six silvaniform outgroup taxa (Fig. 1a, Supplementary Information 12). To test for introgression at the B/D mimicry locus we compared rayed H. m. aglaope and postman H. m. amaryllis as the ingroup with postman H. timareta ssp. nov. (as in Fig. 3a) and found large, significant peaks of shared fixed ABBA nucleotide sites combined with an almost complete lack of BABA sites (Fig. 4b). This provides evidence that blocks of shared sequence variation in the B/D region were exchanged between postman H. timareta and postman H. melpomene, in the genomic region known to determine red mimicry patterns between races of H. melpomene23, 24 (Fig. 4a).
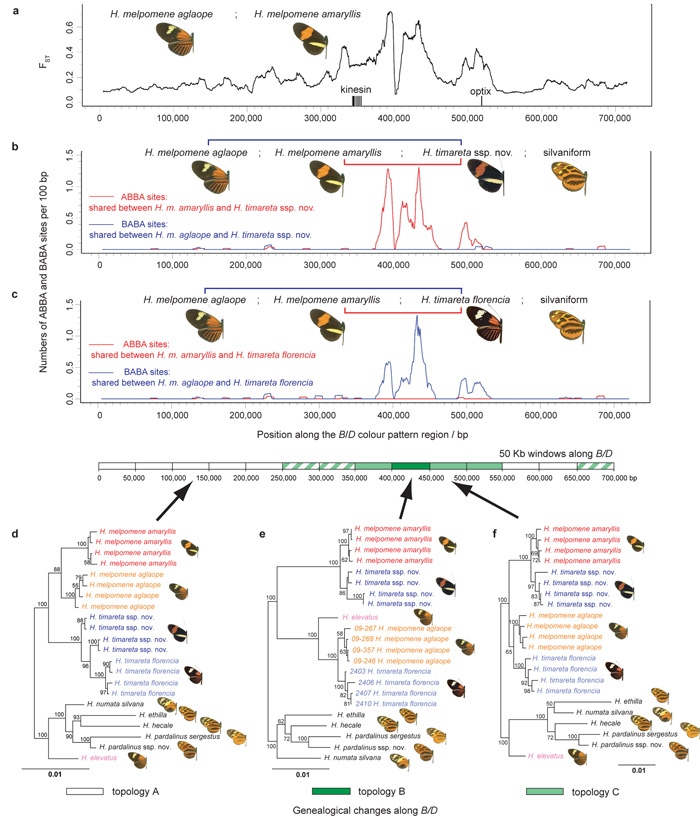
Figure 4. Evidence for adaptive introgression at the B/D mimicry locus.
a Genetic divergence between H. melpomene races aglaope (rayed) and amaryllis (postman) across a hybrid zone in N.E. Peru. Divergence, FST, is measured along the B/D region (Supplementary Information 14). FST peaks in the region known to control red wing pattern elements between the genes kinesin and optix23. b, c Distribution of fixed ABBA and BABA sites (see Fig. 4a) along B/D for two comparisons. Excesses of ABBA in b and BABA in c are highly significant (two-tailed Z-tests for D = 0; D = 0.90 ± 0.13, P = 5 × 10−14 and D = –0.91 ± 0.10, P = 9 × 10−24 respectively), indicating introgression. d, e, f, Genealogical change along B/D investigated with maximum likelihood based on 50 kb windows. Three representative tree topologies are shown. Topology A, the species tree, is found within the white windows. In topologies B (dark green window) and C (light green windows) taxa group by colour pattern rather than species. Within striped windows H. melpomene and/or H. timareta are paraphyletic but the taxa do not group by colour pattern. Support is shown for nodes with > 50% bootstrap support (Supplementary Information 19).
For a reciprocal test, we used the same H. melpomene races as the ingroup to compare with rayed H. timareta florencia at the B/D region. In this case, correspondingly large and significant peaks of BABA nucleotide sites are accompanied by virtual absence of ABBA sites (Fig. 4c) indicating that variation at the same mimicry locus was also shared between rayed H. timareta and rayed H. melpomene. Equivalent results in the N/Yb colour pattern region, controlling yellow colour pattern differences, are in the expected directions for introgression and highly significant for the test using postman H. timareta ssp. nov. (P = 6 × 10−34), although not significant with rayed H. timareta florencia (P = 0.13, Supplementary Information 17). In contrast hardly any ABBA or BABA sites are present in either comparison across 1.8 Mb in 55 genomic scaffolds unlinked to the colour pattern regions (Supplementary Information 21). These concordant, but reciprocal patterns, where fixed ABBA and BABA substitutions occur almost exclusively within large genomic blocks at two different colour pattern loci (449 and 99 sites for B/D and N/Yb respectively, Figs. 4b,c and Supplementary Information 17) would be very hard to explain via convergent functional site evolution or under coalescent fluctuations. Instead, our results imply that derived colour pattern elements have introgressed recently between both rayed and postman forms of H. timareta and H. melpomene.
To test whether colour pattern loci might be shared more broadly across the clade, we used sliding-window phylogenetic analyses along the colour pattern regions. For regions flanking and unlinked to colour pattern loci, tree topologies are similar to the overriding signal recovered from the genome as a whole (Supplementary Information 18). Races of H. melpomene and H. timareta each form separate monophyletic sister groups and both are separated from the more distantly related silvaniform species (Fig. 4d). By contrast, within the region of peak ABBA/BABA differences, the topologies switch dramatically. Races of H. melpomene and H. timareta group according to wing pattern, while the species themselves become polyphyletic (Figs. 4e,f, Supplementary Information 19, 20). Remarkably, the rayed H. elevatus, a member of the silvaniform clade according to genome average relationships (Fig. 1a, Supplementary Information 18), groups with rayed races of unrelated H. melpomene and H. timareta in small sections within both B/D and N/Yb colour pattern loci (Fig. 4e, Supplementary Information 19, 20). These results are again most readily explained by introgression and fixation of mimicry genes.
We have developed a de novo reference genome sequence that will facilitate evolutionary and ecological studies in this key group of butterflies. We have demonstrated repeated exchange of small (~100 kb) adaptive genome regions among multiple species in an adaptive radiation. Our genome-scale analysis provides considerably greater power than previous tests of introgression 8, 25, 26. As with H. heurippa9, our evidence suggests that H. elevatus was formed during a hybrid speciation event. The main genomic signal from this rayed species places it closest to H. pardalinus butleri (Fig. 1a), but colour pattern genomic regions resemble those of rayed races of H. melpomene (Fig. 4e and Supplementary Information 18-20). Colour pattern is important in mating behaviour in Heliconius5, and the transfer of mimetic pattern may have enabled the divergent sibling species H. elevatus to coexist with H. pardalinus across the Amazon. Although it was long suspected that introgression might be important in adaptive radiation1, our results from the most diverse terrestrial biome on the planet suggest that adaptive introgression is more pervasive than previously realized.
Methods summary
A full description of methods can be found in the Supplementary Information.
Supplementary Material
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Acknowledgements
We thank the governments of Colombia, Peru and Panama for permission to collect and export butterflies. Sequencing was funded by contributions from consortium members. We thank Moisés Abanto for assistance in raising the inbred line. Individual laboratories were funded by the Leverhulme Trust (CDJ), John Fell Fund and Christ Church College, Oxford (LCF), The Royal Society (MJ, CDJ), NSF (WOM, MK, RR, SM, ADB), NIH (MK, SLS, JY), CNRS (MJ), ERC (MJ, PWHH), Banco de la República and COLCIENCAS (ML), and the BBSRC (JM, CDJ, MB, R ff-C).
Author contributions
Consortium leaders:
Chris D. Jiggins, W. Owen McMillan
Heliconius Genome Consortium PIs:
Richard ffrench-Constant, Marcus Kronforst, Mathieu Joron, James Mallet, Sean Mullen, Robert Reed, Mark Blaxter, Larry Gilbert, Mauricio Linares, Gerardo Lamas
Introgression study leader & corresponding author:
James Mallet
Lead investigators:
Kanchon Dasmahapatra, James Walters, Nicola Nadeau, Annabel Whibley, John Davey, Adriana Briscoe, Laura Ferguson, Daniel Hughes, Simon Martin, Camilo Salazar, James Lewis
Sequencing:
Stephen Richards, Steve Scherer, Alexi Balmuth, Marian Thomson, Karim Gharbi, Cathlene Eland, Mark Blaxter, Richard Gibbs, Yi Han, Joy Christina Jayaseelan, Christie Kovar, Tittu Mathew, Donna Marie Muzny, Fiona Ongeri, Ling-Ling Pu, Jiaxin Qu, Rebecca Lynn Thornton, Kim C. Worley, Yuan-Qing Wu
Assembly:
Aleksey Zimin, James Yorke, Steven Salzberg, Alexie Papanicolaou, Karl Gordon
RAD map and assembly verification:
John Davey, Simon Baxter, Mark Blaxter, Luana Maroja, Durrell D. Kapan, James Walters, Paul Wilkinson
Geographic distribution map:
Neil Rosser
Annotation:
James Walters, Daniel Hughes, Derek Wilson, Daniel Lawson, Katharina Hoff, Sebastian Adler, Paul Wilkinson
Genome browser and databases:
Daniel Hughes, James Lewis
Manual annotation and evolutionary analyses:
Olfactory proteins: Adriana Briscoe, Emmanuelle Jacquin-Joly, Furong Yuan
Hox genes: Laura Ferguson, Peter W. H. Holland, James Walters
Micro-RNAs: Alison Surridge, Tamas Dalmay, Daniel Mapleson, Simon Moxon
Immune genes: William Palmer, Francis Jiggins
P450 genes: Robert Jones and Ritika Chauhan
UGT genes: Heiko Vogel, Seung-Joon Ahn, David Heckel
Ribosomal Proteins: Yannick Pauchet
Manual annotation group: Simon Baxter, Mark Blaxter, Adriana Briscoe, Nicola Chamberlain, Brian Counterman, Laura Ferguson, Heather Hines, Chris Jiggins, Frank Jiggins, Mathieu Joron, Durrell Kapan, Marcus Kronforst, Jim Mallet, Arnaud Martin, Sean Mullen, Nicola Nadeau, William Palmer, Riccardo Papa, Megan Supple, Ayse Trolander, Annabel Whibley, Furong Yuan
Transposable elements: Brian Counterman, David Ray
Orthologue predictions: Dean Baker
Synteny: Annabel Whibley, John Davey, David Heckel, Karl Gordon
Introgression analysis: Kanchon Dasmahapatra, Nicola Nadeau, John Davey, Simon Martin, Camilo Salazar, Chris Jiggins, Mathieu Joron, James Mallet
Author Information The genome sequence has been submitted to European Nucleotide Archive with accession numbers HE667773-HE672081. The annotated genome is available on our genome browser at http://butterflygenome.org/ and will also be made available in the next release of ENSEMBL Genomes. Additional short read sequences have been submitted to the European Nucleotide Archive with accession numbers ERP000993 and ERP000991.
Reprints and permissions information is available at www.nature.com/ reprints.
The authors declare no competing financial interests. Readers are welcome to comment on the online version of this article at www.nature.com/nature.
References
- 1.Seehausen O. Hybridization and adaptive radiation. Trends Ecol. Evol. 2004;19:198–207. doi: 10.1016/j.tree.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Bates HW. Contributions to an insect fauna of the Amazon valley. Lepidoptera: Heliconidae. Trans. Linn. Soc. Lond. 1862;23:495–566. [Google Scholar]
- 3.Turner JRG. Adaptation and evolution in Heliconius: a defense of neo-Darwinism. Ann. Rev. Ecol. Syst. 1981;12:99–121. [Google Scholar]
- 4.Brown KS. The biology of Heliconius and related genera. Ann. Rev. Entomol. 1981;26:427–456. [Google Scholar]
- 5.Jiggins CD, Naisbit RE, Coe RL, Mallet J. Reproductive isolation caused by colour pattern mimicry. Nature. 2001;411:302–305. doi: 10.1038/35077075. [DOI] [PubMed] [Google Scholar]
- 6.Briscoe AD, et al. Positive selection of a duplicated UV-sensitive visual pigment coincides with wing pigment evolution in Heliconius butterflies. Proc. Natl. Acad. Sci. USA. 2010;107:3628–3633. doi: 10.1073/pnas.0910085107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallet J. In: Speciation and Patterns of Diversity. Butlin RK, Schluter D, Bridle JR, editors. Cambridge University Press; Cambridge: 2009. pp. 177–194. [Google Scholar]
- 8.Kronforst MR. Gene flow persists millions of years after speciation in Heliconius butterflies. BMC Evol. Biol. 2008;8:98. doi: 10.1186/1471-2148-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salazar C, et al. Genetic evidence for hybrid trait speciation in Heliconius butterflies. PLoS Genet. 2010;6:e1000930. doi: 10.1371/journal.pgen.1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Silkworm Genome Consortium The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem. Molec. Biol. 2008;38:1036–1045. doi: 10.1016/j.ibmb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Pringle EG, et al. Synteny and chromosome evolution in the Lepidoptera: evidence from mapping in Heliconius melpomene. Genetics. 2007;177:417–426. doi: 10.1534/genetics.107.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson R. Lepidoptera Genetics. Pergamon Press; Oxford: 1971. [Google Scholar]
- 13.Deng Q, Zeng Q, Qian Y, Li C, Yang Y. Research on the karyotype and evolution of the Drosophila melanogaster species group. J. Genet. Genomics. 2007;34:196–213. doi: 10.1016/S1673-8527(07)60021-6. [DOI] [PubMed] [Google Scholar]
- 14.Kemkemer C, et al. Gene synteny comparisons between different vertebrates provide new insights into breakage and fusion events during mammalian karyotype evolution. BMC Evol. Biol. 2009;9:84. doi: 10.1186/1471-2148-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.d’Alençon E, et al. Extensive synteny conservation of holocentric chromosomes in Lepidoptera despite high rates of local genome rearrangements. Proc. Natl. Acad. Sci. USA. 2010;107:7680–7685. doi: 10.1073/pnas.0910413107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vane-Wright RI, Boppré M. Visual and chemical signalling in butterflies: functional and phylogenetic perspectives. Phil. Trans. Roy. Soc. Lond. B. 1993;340:197–205. [Google Scholar]
- 17.Zhan S, Merlin C, Boore JL, Reppert SM. The monarch butterfly genome yields insights into long-distance migration. Cell. 2011;147:1171–1185. doi: 10.1016/j.cell.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieira FG, Rozas J. Comparative genomics of the odorant-binding and chemosensory protein gene families across the Arthropoda: origin and evolutionary history of the chemosensory system. Genome Biol. Evol. 2011;3:476–490. doi: 10.1093/gbe/evr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chai CL, et al. A genomewide survey of homeobox genes and identification of novel structure of the Hox cluster in the silkworm, Bombyx mori. Insect Biochem. Molec. Biol. 2008;38:1111–1120. doi: 10.1016/j.ibmb.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Sackton TB, et al. Dynamic evolution of the innate immune system in Drosophila. Nat. Genet. 2007;39:1461–1468. doi: 10.1038/ng.2007.60. [DOI] [PubMed] [Google Scholar]
- 21.Green RE, et al. A draft sequence of the Neandertal genome. Science. 2010;328:710–722. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durand EY, Patterson N, Reich D, Slatkin M. Testing for ancient admixture between closely related populations. Molec. Biol. Evol. 2011;28:2239–2252. doi: 10.1093/molbev/msr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed RD, et al. optix drives the repeated convergent evolution of butterfly wing pattern mimicry. Science. 2011;333:1137–1141. doi: 10.1126/science.1208227. [DOI] [PubMed] [Google Scholar]
- 24.Nadeau NJ, et al. Evidence for genomic islands of divergence among hybridizing species and subspecies of Heliconius butterflies obtained by large-scale targeted sequencing. Phil. Trans. Roy. Soc. B. 2012;367:343–353. doi: 10.1098/rstb.2011.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim M, et al. Regulatory genes control a key morphological and ecological trait transferred between species. Science. 2008;322:1116–1119. doi: 10.1126/science.1164371. [DOI] [PubMed] [Google Scholar]
- 26.Song Y, et al. Adaptive introgression of anticoagulant rodent poison resistance by hybridization between Old World mice. Curr. Biol. 2011;21:1296–1301. doi: 10.1016/j.cub.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.