Abstract
In the leech Helobdella, the ectoderm exhibits a high degree of morphological homonomy between body segments, but pattern elements in lateral ectoderm arise via distinct cell lineages in the segments of the rostral and midbody regions. In each of the four rostral segments, a complete set of ventrolateral (O fate) and dorsolateral (P fate) ectodermal pattern elements arises from a single founder cell, op. In the 28 midbody and caudal segments, however, there are two initially indeterminate o/p founder cells; the more dorsal of these is induced to adopt the P fate by BMP5-8 emanating from the dorsalmost ectoderm, while the more ventral cell assumes the O fate. Previous work has suggested that the dorsoventral patterning of O and P fates differs in the rostral region, but the role of BMP signaling in those segments has not been investigated. We show here that suppression of dorsal BMP5-8 signaling (which effects a P-to-O fate change in the midbody) has no effect on the patterning of O and P fates in the rostral region. Furthermore, ectopic expression of BMP5-8 in the ventral ectoderm (which induces an O-to-P fate change in the midbody) has no effect in the rostral region. Finally, expression of a dominant-negative BMP receptor (which induces a P-to-O fate change in the midbody) fails to affect O/P patterning in the rostral region. Thus, the rostral segments appear to use some mechanism other than BMP signaling to pattern O and P cell fates along the dorsoventral axis. From a mechanistic standpoint, the OP lineage of the rostral segments and the O-P equivalence group of the midbody and caudal segments constitute distinct developmental modules that rely to differing degrees on positional cues from surrounding ectoderm in order to specify homonomous cell fates.
Keywords: dorsoventral patterning, segmentation, BMP, leech, Helobdella
Introduction
It is generally understood that segmented body plans arise from the iterated deployment of a set of patterning pathways along the anteroposterior (AP) axis. But evidence from vertebrate and protostome systems indicates that segmental patterning processes can also exhibit regional differences along that axis. In Drosophila, for example, the deployment of segment polarity genes and dorsoventral (DV) patterning genes in the head segments differs from that in the more posterior thoracic and abdominal segments (Gallitano-Mendel and Finkelstein, 1997; Urbach and Technau, 2003).
To further explore regional differences in DV patterning mechanisms, we compared the role of BMP signaling in the DV patterning of ectoderm in rostral (head) and midbody (trunk) regions of the leech Helobdella, a clitellate annelid. The leech body plan consists of a non-segmental prostomium at its anterior end, followed by 32 segments, which arise from a posterior growth zone. The four rostral segments and the seven caudal segments form specialized front and rear suckers, respectively, and their ventral nerve cord ganglia do not separate during development. The fused ventral ganglia of the four rostral segments, together with a prostomium-derived dorsal ganglion, make up what is known as the “brain” in the leech (Muller et al., 1981). In contrast, the 21 midbody segments, though still showing segment-specific differences (for example in the occurrence of nephridia, reproductive organs and some segment-specific neuronal phenotypes) are similar in external morphology, and their individual ganglia become separated by connective nerves.
Segmentation of the leech is a cell lineage-dependent process (summarized in Fig. 1). Segmental ectoderm and mesoderm arise from five bilateral pairs of teloblasts, large lineage-restricted stem cells that constitute the posterior growth zone. Each teloblast undergoes repeated rounds of asymmetric cell division to generate a column of segmental founder cells (primary blast cells); each column of blast cells is also known as a bandlet. The five bandlets on each side merge in parallel to form a germinal band which ultimately gives rise to the ipsilateral segmented tissues. Within a bandlet, each primary blast cell follows a stereotyped and lineage-specific cell division pattern and eventually gives rise to a nearly identical set of differentiated descendants (Shankland, 1987b; Shankland, 1987c; Weisblat and Shankland, 1985; Zackson, 1984). Hence, segmentation of the leech mesoderm and ectoderm originates with the iterated production of blast cells by the teloblasts. In this process, the earliest-born blast cells contribute to the anteriormost segments whereas later-born blast cells contribute to progressively more posterior segments.
Fig. 1.
Development of the lateral segmental ectoderm in Helobdella. A. A summary of Helobdella early development (animal pole/prospective dorsal views except as noted). During cleavage, teloblasts (shading in stages 6b and 7) arise from macromere D′ of the 8-cell embryo (stage 4a). The end of teloblastogenesis is marked by the division of OP proteloblast into two equipotent O/P teloblasts. The right OP proteloblast in stage 6b and the resulting O/P teloblasts in stage 7 are outlined with red. The teloblasts undergo asymmetric cell divisions, adding cells posteriorly to the left and right germinal bands (gb), which coalesce along the AP axis to form the germinal plate (gp). B. Developmental origins of the OP lineage and the O-P equivalence group. The right OP proteloblast and its descendants during stages 6b and 7 are shown in these panels; anterior is to the top, and dorsal is to the left. Nuclei or mitotic chromosomes are marked with cyan. During stage 6b, the OP proteloblast undergoes four asymmetric cell divisions and gives rise to four op primary blast cells; the first-born op blast cell is designated as cell op1, the second as op2, and so on. The OP proteloblast then undergoes a symmetric cell division and gives rise to two equivalent O/P teloblasts; this marks the transition from stage 6b to stage 7. Each of the O/P teloblasts resumes asymmetric cell division to produce two parallel o/p bandlets in stage 7. C. In early stage-8 germinal band, the op blast cell clones (orange) occupy the anteriormost section of the lateral ectoderm, with the q bandlet lying dorsally and the n bandlet lying ventrally. Behind the posteriormost op clones are the two bandlets arising from the O/P teloblasts. Fates of the initially equipotent o/p blast cells (pink) are positionally specified. Blast cells in the more dorsal O/P-derived bandlet (p bandlet) adopt the P fate (green), while those in the more ventral one (o bandlet) adopt the O fate (red); these cells are then designated as o or p blast cells, respectively.
Among the five teloblasts on each side, the M teloblast gives rise to the mesoderm while the N teloblast, the two O/P teloblasts, and the Q teloblast give rise to ventral, ventrolateral, dorsolateral and dorsal sectors of the ectoderm, respectively. Embryological experiments suggest that the developmental fates of most teloblast lineages are specified in a cell-autonomous manner (Blair, 1982; Blair and Weisblat, 1982; Stuart et al., 1989; Torrence et al., 1989). However, the two ipsilateral O/P lineages form a developmental equivalence group, and become committed to distinct dorsolateral (P) and ventrolateral (O) fates by positional cues (Ho and Weisblat, 1987; Huang and Weisblat, 1996; Keleher and Stent, 1990; Kuo and Shankland, 2004b; Shankland and Weisblat, 1984; Weisblat and Blair, 1984).
A variety of cell-cell interactions have been implicated in the patterning of the O and P lineages (Ho and Weisblat, 1987; Shankland and Weisblat, 1984), but a key factor is the ability of the dorsally situated q bandlet to induce P fates in the adjacent (i.e. dorsolateral) o/p bandlet (Huang and Weisblat, 1996; Kuo and Shankland, 2004b). More recent studies have shown that the secreted protein BMP5-8 functions as this Q-derived P-inducing factor in the midbody segments (Kuo and Weisblat, 2011). In its dependency on BMP signaling, the patterning of the two O/P lineages in the leech embryo appears to be homologous to the DV patterning of neuroectoderm in other bilaterian taxa (Mizutani and Bier, 2008).
The above description holds for the midbody and caudal segments, but the homologous O and P cell fates in the four rostral segments arise through a distinct pattern of cell lineage (Shankland, 1987a). The OP proteloblast, which is the progenitor of the lateral ectoderm in all 32 body segments, undergoes four teloblast-like asymmetric divisions to produce ‘op’ blast cells before dividing equally into the sibling O/P teloblasts (Fig. 1B). The four op blast cells generate both dorsolateral and ventrolateral ectoderm for the four rostral segments. Morphologically, the set of pattern elements produced by a single op blast cell in the rostral region of the body plan is largely indistinguishable from the sum of an o blast cell clone and a p blast cell clone in the midbody (Kuo and Shankland, 2004a; Shankland, 1987a), and hence can be described as containing both O and P cell fates.
Several lines of evidence suggest that there are mechanistic differences in the patterning O and P cell fates between the rostral and midbody regions. In contrast to the midbody, the O and P sublineages of the rostral region are initially segregated along the AP axis rather than the DV axis (Shankland, 1987a). Moreover, cell ablation experiments indicate that the interaction between these sublineages differs from that in the midbody in several regards (Kuo and Shankland, 2004a). Finally, ablation of the q bandlet eliminates the expression of the gene Hau-Six1/2A in the p bandlet of the midbody, but has no obvious effect on the expression of this gene in the op blast cell clones of the rostral region (Quigley et al., 2010).
Here, we explicitly compared the role of BMP signaling in the O/P patterning of rostral and midbody regions. By knocking down the dorsally expressed Hau-BMP5-8, mis-expressing Hau-BMP5-8 in ventral territory, and expressing a dominant-negative BMP receptor, we demonstrated that BMP signaling is not required for the patterning of the OP lineage, although it plays a major role in the development of the O-P equivalence group.
Materials and Methods
Experimental manipulations of embryos
Experiments were performed on embryos obtained from breeding laboratory colonies of Helobdella sp. (Austin). Operated embryos were cultured at 23°C in a buffered saline medium supplemented with antibiotics.
Lineage tracer labeling was accomplished by pressure injecting the target cell with a 1:1 mixture of 100 mg/ml tetramethylrhodamine dextran, lysine fixable (RDA; Molecular Probes, Eugene, OR) or fluorescein dextran, lysine fixable (FDA; Molecular Probes, Eugene, OR) and 4% Fast Green (Sigma, St. Louis, MO) in 200 mM KCl. Morpholino oligomers (MOs) and synthetic mRNAs were prepared as previously described (Kuo and Weisblat, 2011), and were diluted from stock to the desired concentration with 10 mg/ml RDA or FDA in 0.5% phenol red solution (Sigma, St. Louis, MO). MOs were injected at 1 mM in the micropipette, and mRNA at 1 μM.
Operated embryos were raised separately in 24-well culture plates, and at stage 10 were fixed and counterstained with 2.5 μg/ml Hoechst 33258 at 4°C overnight. Fixed embryos were dissected, mounted on slides in buffered glycerol, and viewed by fluorescence microscopy to examine the set of differentiated pattern elements that had arisen from the labeled cell lineage.
Molecular markers and constructs
Whole-mount in situ hybridization (WMISH) was performed as described elsewhere (Weisblat and Kuo, 2009). Riboprobes for WMISH to Hau-Six1/2A were as described elsewhere (Quigley et al., 2010). A cDNA fragment encoding a partial coding region of Hau-tyrosine hydroxylase (Hau-TH) was PCR-amplified from first strand random primed stage 11 cDNA using the following primers: 5′-AAACAAGTTTGACCCGGAACT-3′ (forward) and 5′-TCAACTTAGACATAACTTGCG-3′ (reverse); these primers were designed based on sequence information retrieved from the H. robusta genome database (JGI, DOE). The amplicon was subcloned into pGEM-T Easy (Promega, Madison, WI), and the resultant plasmid used as a template to synthesize a riboprobe for Hau-th.
Molecular constructs for mRNA injections of Hau-BMP5-8, Hau-myostatin1, Hau-ALK3/6 and Hau-ALK3/6K253R were as described elsewhere (Kuo and Weisblat, 2011). Hau-ALK4/5/7ΔC, a kinase-deficient form of the non-BMP type I TGFβ receptor (Chang et al., 1997; Dyson and Gurdon, 1997; Mahony et al., 1998), was used as a negative control for the dominant negative BMP receptor construct, Hau-ALK3/6K253R. We have determined that Hau-alk4/5/7 and genes encoding its putative ligands are not expressed in Helobdella embryos at stage 8 (data not shown), and thus the expression Hau-ALK4/5/7ΔC should have no effect. The Hau-alk4/5/7 ΔC construct was obtained by PCR amplification of a cDNA fragment encoding the N′ region of Hau-ALK3/5/7 (including signal peptide, extracellular cysteine-rich and transmembrane domains), using the following primers: 5′-GGGATCCGAACCAAAATGTCTGAAATGTTTTTCAA-3′ (forward; underline marks an introduced BamHI site); 5′-GGGAATTCCTCGGTGTTAGTGGTACTGGCGACGGA-3′ (reverse; underline marks an introduced EcoRI site). The amplicon was then subcloned into the pCS107 backbone, and the resultant plasmid used as a template for mRNA synthesis.
GenBank accession numbers for cDNA clones of Hau-alk4/5/7 and Hau-tyrosine hydroxylase are JN565061 and JN565062, respectively.
Immunohistochemistry
To visualize FDA lineage tracer under brightfield microscopy following WMISH, embryos were incubated in 1:1000 dilution of alkaline phosphatase (AP)-conjugated monoclonal anti-fluorescein antibody (Roche) in blocking solution (1% BSA in phosphate-buffered saline with 0.1% Tween-20) overnight at 4°C. Prior to anti-fluorescein labeling, embryos were treated with 0.1 M glycine pH 2.2 for 5 minutes to inactivate the AP activity of conjugated anti-digoxigenin antibody used for WMISH detection. Coloration for anti-fluorescein staining was carried out using Fast Red (Sigma) as an alternative AP substrate. In room temperature, it took less than 5 minutes for the staining to reach the desired intensity. Following coloration, embryos were washed several times and then mounted in 80% glycerol in buffered saline for observation.
Results
Serial homology between the OP lineage and the O/P lineages
The O and P pattern elements of the midbody were first described in H. triserialis (Shankland and Weisblat, 1984), and the OP pattern elements of the rostral region were later characterized in the same species (Shankland, 1987a) as well as in Helobdella sp. (Austin) (Kuo and Shankland, 2004a). The combined O and P pattern elements of a midbody segment are nearly identical to the OP pattern elements of a rostral segment as described below.
The normal set of OP pattern elements includes four clusters of ganglionic neurons designated as the crescent (CR), posteroventral (PV), anterodorsal (AD) and wedge-shaped (WE) clusters, four discrete ganglionic neurons (pz1-4), and the packet glial cells. Peripheral pattern elements include the identified neurons oz1, oz2, pz5, pz6-10, LD1 and LD2, as well as the distalmost cell of the nephridial tubule (nt) in segments with nephridia or of a transient tubule (tt) seen in segments without nephridia. There are also readily detectable pattern elements in the epidermis, including three islands of rounded cells known as cell florets 1–3 (cf1-3).
Certain pattern elements are not reliably identifiable when the entire OP lineage is labeled with fluorescent dextran due to the density of labeled cells [although these pattern elements are readily visible when labeling is limited to sublineages of the op blast cell clone (Kuo and Shankland, 2004a)]. Among the cells that can be consistently identified when the entire OP lineage is labeled, the CR, PV and AD neuron clusters in the ventral nerve cord are O fate pattern elements, i.e. they derive exclusively from the O lineage in the midbody during normal development. In contrast, the WE neuron cluster in the ventral nerve cord and cell floret 3 are P fate pattern elements (Fig. 2). Thus, in the work presented here, these five pattern elements were scored to determine the status of O and P fate specification in op blast cell clones.
Fig. 2.
The OP lineage-derived pattern elements in the rostral region contain both O-type and P-type pattern elements. A. A single op primary blast cell, injected with a fluorescent dextran tracer, gave rise to both O-type (CR, PV, and AD; red) and P-type (cf3 and WE; green) pattern elements in the stage 10 embryo. B. Schematics of the generic O and P pattern elements for midbody segments. In the OP lineage, the entire segmental complement of pattern elements arises from a single op primary blast cell. In the separate O and P lineages of the midbody and caudal regions, the O and P pattern elements comprise the descendants of separate o and p blast cells. Red represents the O pattern elements and green represents the P pattern elements. Prominent and consistently identifiable pattern elements are indicated in bold. CR: crescent neuron cluster; PV: posterior ventral neuron cluster; AD: anterior dorsal neuron cluster; WE: wedge-shape neuron cluster; cfl-3: cell florets 1–3; mpg: medial packet glia; LD1-2: lateral dopaminergic neurons 1–2; tt/nt: transient tubule/nephridial tubule. Anterior is to the top; dorsal is to the left. Segmental ganglia of the ventral nerve cord are outlined with dotted lines, and the ventral midline is marked with dashed line. Scale bar: 30 microns.
Region-specific difference in the requirement for BMP signaling
Previous work has shown that Hau-BMP5-8 expressed by the q bandlet is an important positional cue for P fate specification in the adjacent o/p bandlet in the midbody region (Kuo and Weisblat, 2011). Hau-bmp5-8 is also expressed by the q bandlet in the future rostral region of the germinal band, and this expression is readily apparent at the time of op blast cell production (Fig. 3). Thus, Q-derived Hau-BMP5-8 could pattern O and/or P cell fates within the op blast cell clones as well. To test this hypothesis, we sought to manipulate the DV polarity of the BMP signal in the rostral region by either knocking down or mis-expressing Hau-bmp5-8.
Fig. 3.
Hau-bmp5-8 is expressed in the Q lineage blast cells in both the rostral and midbody regions of the germinal band. A. Expression of Hau-bmp5-8 in a stage 6b embryo is revealed by WMISH. During this stage, each of the left and right OP proteloblasts (OPL and OPR) produces four primary op blast cells. Animal-pole view is shown; also visible are the left and right N teloblasts (NL and NR). B. Higher-power view of the boxed area in panel A. Hau-bmp5-8 transcript was detected in the very first q blast cell of the left and right germinal bands (arrowheads), as well as in a pair of cell clusters in the non-segmental region (dashed circles). C. Hau-bmp5-8 is expressed in the q bandlet throughout the entire length of the germinal band in a late stage 7 embryo. FDA lineage tracer was injected into the right OP proteloblast immediately before it divided to form the O/P teloblasts and later visualized immunohistochemically (red; see Materials and Methods). The anterior boundary of tracer labeling thus marks the anteriormost o and p blast cell clones, and the op blast cell clones are located in the unlabeled region anterior to the boundary (double-headed arrow). Scale bar: 150 μm (A,C);40μm (B).
Hau-BMP5-8 knockdown
To determine whether dorsally secreted Hau-BMP5-8 is required to pattern O and P fates in the rostral region, we examined the pattern elements produced by the OP lineage following introduction of a translation-blocking antisense morpholino oligomer (ASMO) complementing the translation initiation site of Hau-bmp5-8 (Hau-bmp5-8 ASMO). Rescuing experiments described elsewhere have validated the specificity of this Hau-bmp5-8 ASMO (Kuo and Weisblat, 2011).
To ensure that the entire q bandlet, including its firstborn anteriormost blast cell, received Hau-bmp5-8 ASMO, we injected the Q teloblast within one hour after its birth, i.e. before it underwent its first blast cell-producing division. The ipsilateral OP proteloblast was then injected with the fluorescent lineage tracer RDA In a second set of experiments, proteloblast OPQ″, the immediate progenitor of the Q teloblast and the OP proteloblast, was injected with Hau-bmp5-8 ASMO, and then its daughter OP injected with RDA. The results from these two protocols were indistinguishable.
In these experiments, the midbody O/P lineages gave rise exclusively to O pattern elements, such as CR, AD, and PV neuron clusters (Fig. 4B, F). P pattern elements, such as the wedge-shaped neuron cluster, and cell floret 3, were missing altogether from the midbody. In contrast, the rostral op blast cell clones gave rise to largely normal sets of O- and P-type pattern elements, suggesting that dorsal expression of Hau-BMP5-8 is not necessary for the specification of P cell fates in the rostral region (Fig. 4B, F). A striking feature of these embryos was the sharp boundary between the rostral and midbody segments in terms of their lateral ectoderm pattern elements. When a control MO was injected, no such a boundary was observed, and the labeled lateral ectoderm in all segments exhibited complete sets of O and P pattern elements (Fig. 4B, F). Thus, there is a clear regional difference in the developmental requirement for BMP5-8, and this difference correlates precisely with the transition from unpaired op blast cell clones to paired o/p blast cell clones.
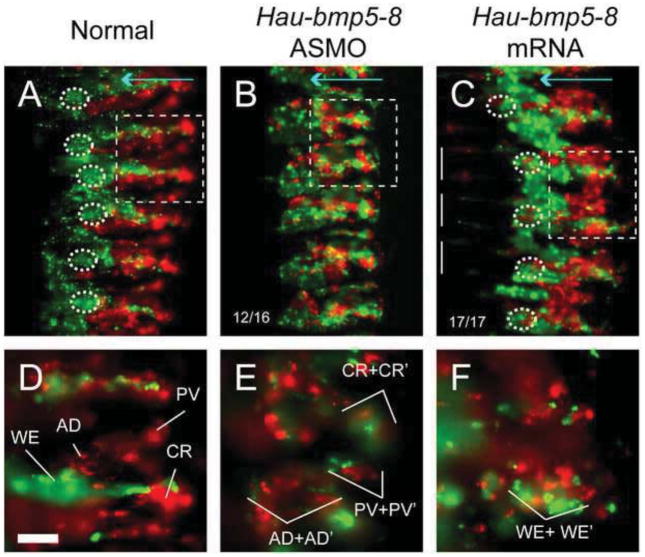
Fig. 4.
Manipulation of BMP signaling activity affected specification of O and P fates in the midbody but not the the rostral regions. A. O and P pattern elements in the germinal plate of a normal stage 10 embryo revealed by injection the OP proteloblast with fluorescent lineage tracer. Cell clones op1 and op2 are not labeled in this specimen, due to the timing of the injection. In panels A–D, dashed circles indicate cell floret 3, a P pattern element; the horizontal cyan arrows mark the extent of the right hemiganglia in ventral nerve cord, pointing from ventral midline to the lateral edge of the ganglia. In all panels, the orange arrowheads and green arrowheads mark the R4-M1 and M1–M2 boundaries respectively (the posteriormost op clone, the op4 clone, straddles R4 and Ml segments, and the first o and p clones straddle M1 and M2 segments). B. In an embryo injected with Hau-bmp5-8 ASMO in the QR teloblast, the P pattern element cell floret 3 is missing from the O/P-derived segments, but is present in OP-derived segments (dashed circles). C. In an embryo injected with Hau-bmp5-8 mRNA in the NR teloblast, the P pattern elements are present in all segments. The CNS O pattern elements are missing in the O/P-derived segments; see panel G for details. D. Injection of Hau-alk3/6K253R mRNA into proteloblast OPR produced a similar effect to that of Hau-bmp5-8 ASMO injection. E–H. High-power views of the regions boxed with red in panels A–D. Pattern elements arising from the op4 cell are marked with white; pattern elements arising from cells o1 and p1 are marked with green. In Hau-bmp5-8 morphants (F) and dominant-negative BMP receptor expren ssing embryos (H), the CNS P pattern elements, WE neuron clusters are present in the op4-derived segment but are missing in the segments derived from the o1 and p1 clones; in contrast, the O pattern elements are present in all segments. The focal planes of panels F and H are chosen to highlight the more ventrally situated P pattern element WE cluster, so the dorsally situated AD neuron clusters are out of focus in these panels. In embryos mis-expressing Hau-BMP5-8 ventrally (G), the O pattern elements (CR, PV and AD clusters) are present in the op4-derived segment but are missing from the segment derived from o1 and p1. The normal positions of missing pattern elements are indicated with hollow type. Scale bar: 90 μm (A–D); 30 μm (E–H).
Because the precursor of the O/P teloblast pair, the OP proteloblast, was injected with RDA in these experiments, the two O/P lineages were labeled with the same tracer. As a result, we were not able to discern the lineal origins of labeled O pattern elements in the midbody region. It was previously shown that Hau-BMP5-8 knockdown led to a P-to-O fate change in both blast cell division pattern and gene expression patterns (Kuo and Weisblat, 2011), but a corresponding change in definitive pattern elements has not been reported. Thus, it remained possible that the ectopic O-fated cells arising from the nominal p bandlet die later in development and do not contribute to the differentiated pattern elements.
To determine whether both ipsilateral O/P lineages contributed O pattern elements in the experiments described above, we next injected one O/P teloblast with RDA and the other with FDA after the ipsilateral Q teloblast had been injected with Hau-bmp5-8 ASMO. In contrast to control embryos, in which differentially labeled O/P teloblasts gave rise to distinct sets of O and P pattern elements (Fig. 5A), both O/P teloblasts gave rise exclusively to O pattern elements in embryos co-injected with Hau-bmp5-8 ASMO, and the O pattern elements in these segments were a mosaic of FDA- and RDA-labeled cells (Fig. 5B). These data indicated that Hau-BMP5-8 knockdown caused a P-to-O fate change in the dorsal o/p bandlet, consistent with the conclusions of our previous study in which O and P cell fates were assigned on the basis of blast cell division pattern and gene expression (Kuo and Weisblat, 2011).
Fig. 5.
Hau-BMP5-8 knockdown and over-expression produced transfating in the O-P equivalence group of the midbody region. O and P lineages were differentially labeled by injecting the two ipsilateral O/P teloblasts with distinct tracers (RDA and FDA). The images shown here are of germinal plates dissected from stage 9 embryos. A. In normal embryos, the O (red) and P (green) lineages each give rise to a distinct set of pattern elements. Dashed circles mark the P-derived pattern element cell floret 3. B. In embryos in which the Q lineage was treated with Hau-bmp5-8 ASMO, the two ipsilateral O/P teloblasts gave rise to similar sets of intermingled pattern elements characteristic of normal O lineage and cell floret 3 was not observed C. In embryos misexpressing Hau-bmp5-8 in the N lineage, the two ipsilateral O/P teloblasts gave rise to similar sets of intermingled P pattern elements. Certain peripheral neural pattern elements arose in single copies per segment from either one or the other of the two O/P teloblast lineages, rather than as duplicated pattern elements. White bars mark individual segments. Note that the two prominent nerve outgrowth in each of the three labeled segments had distinct lineage origins. The horizontal cyan arrows in A–C mark the extent of the right hemiganglia in ventral nerve cord, pointing from ventral midline to the lateral edge of the ganglia. D–F. High-power views of the regions boxed with red dashed line in panels A–C, showing CNS pattern elements with the ventral ganglia. For simplicity, only a few selected prominent pattern elements are indicated here. In a normal embryo (D), crescent cell cluster (CR) anterior-dorsal cell cluster (AD) and posterior-ventral cell cluster (PV) are O-derived pattern elements; wedge-shape cell cluster (WE) is a P-derived pattern element. In a Hau-bmp5-8 morphant (E), both RDA- and FDA-labeled lineages contribute to O pattern elements (CR, AD, and PV); P pattern elements are missing. In an embryo mis-expressing Hau-BMP5-8 (F), both lineages contribute to the P pattern element WE, resulting in expanded cell clusters. For all panels, anterior is to the top and ventral midline is to the right. Scale bar: 30 μm (A–C); 10 μm (D–F).
As a further assay for the role of Hau-BMP5-8 in patterning the O and P pattern elements, we examined the segmental distribution of individually identified dopaminergic neurons following knockdown of Hau-BMP5-8. In Helobdella and other glossiphoniid leeches, each segment contains three bilateral pairs of dopaminergic peripheral neurons: MD (a Q pattern element), LD1 (a P-type pattern element), and LD2 (an O-type pattern element) (Stuart et al., 1987). We were able to specifically label these neurons in juveniles by WMISH for Hau-tyrosine hydroxylase (Hau-th), which encodes an enzyme involved in dopamine biogenesis. Immediately after the birth of the Q teloblasts, Hau-bmp5-8 ASMO was injected into the right Q teloblast and a control MO into the left. In the rostral region, the distribution patterns of Hau-th+ neurons on both sides were identical and appeared normal (Fig. S1). In the midbody, LD1 neurons were present on the left side but absent on the right; MD and LD2 neurons, on the other hand, were present on both sides (Fig. S1A). Since Hau-BMP5-8 knockdown appears to produce a P-to-O fate change in the midbody region (see above), we had expected to observe duplicated Hau-th+ LD2 neurons in those segments. However, no such duplications were observed, suggesting that local competition between duplicated neurons may allow only one of the pair to survive and/or express this neurotransmitter phenotype. Such competition has been reported previously for the left and right homologs of certain Helobdella peptidergic neurons (Blair et al., 1990; Martindale and Shankland, 1990; Shankland and Martindale, 1989). In any case, the distinct effect of Hau-BMP5-8 knockdown on the formation of the LD1 neuron in the rostral and midbody regions further supported the differing role of Hau-BMP5-8 in these two body regions.
Mis-expression of Hau-BMP5-8 in the ventral territory
In the midbody, mis-expression of Hau-bmp5-8 in the n bandlet (the ventralmost column of blast cells in the germinal band) causes an O-to-P fate change in the nominal o bandlet (Kuo and Weisblat, 2011). To test whether ectopic expression of Hau-bmp5-8 can induce similar O-to-P fate changes within the rostral OP lineage, the N teloblast was first injected with synthetic Hau-bmp5-8 mRNA, and then the OP proteloblast with RDA. In all operated embryos, both O and P pattern elements were observed in the rostral, op-derived clones, but only the P pattern elements were found in the midbody (Fig. 4C, G). In contrast, when N teloblasts were injected with Hau-myostatin1 mRNA (encoding a non-BMP TGFβ super-family ligand, which signals through a different set of receptors and SMADs), normal O and P pattern elements were observed in all segments (data not shown). By differentially labeling the two ipsilateral O/P teloblasts, we found that both ipsilateral O/P teloblasts gave rise to the P pattern elements in embryos mis-expressing Hau-BMP5-8 in the N lineage (Fig. 5C). Thus, these results indicated that ectopic, ventral expression of Hau-bmp5-8 caused an O-to-P fate change in the midbody but failed to disrupt O/P patterning in the rostral region.
Patterning of the OP lineage is independent of responsiveness to BMP signaling
The results presented above indicated that the patterning of O and P cell fates within the OP lineage does not require the polarized BMP5-8 signal provided by the Q lineage. Next we considered the possibility that some or all of these cell fates are specified by a different BMP signal, e.g. the broadly expressed BMP2/4 homologs (Kuo and Weisblat, 2011). To test this hypothesis, we sought to inhibit BMP signaling indiscriminately by expressing a dominant-negative BMP receptor in the OP lineage.
Among the BMP receptors present in Helobdella genome, Hau-alk3/6 is the only type I receptor expressed in the segmental ectoderm of the germinal bands and germinal plate, whereas both type II receptors (which partner with type I receptors to form active receptor complexes) are broadly expressed (Kuo and Weisblat, 2011). Hence, over-expressing dominant negative Hau-ALK3/6 alone should inhibit all BMP signaling in the segmental ectoderm, independent of the particular ligand or its source. For this purpose, we injected the OP proteloblast with a mixture of RDA and Hau-alk3/6K253R mRNA, which encodes a kinase-deficient version of the Hau-ALK3/6 receptor. In these embryos only O pattern elements were found in the midbody o- and p-derived clones, while complete sets of O and P pattern elements were observed in the op-derived clones (Fig. 4D, H). Similar to the Hau-BMP5-8 knockdown experiments, the transition between the op blast cell clones and their midbody counterparts was sharp, and the boundary was always in segment M1, which contains descendants of the posteriormost op blast cell and the anteriormost o and p blast cells. These results were independent of the amount of mRNA injected (which ranged from 0.1–1 μM in the pipette). In control experiments, OP proteloblasts were injected with Hau-alk4/5/7ΔC mRNA, encoding a kinase-deficient receptor for activin/nodal/myostatin-type TGFβ ligands. Neither Hau-ALK4/5/7 nor its putative ligand is normally expressed in the segmental tissue. As expected, ectopically expressing this irrelevant receptor did not affect the distribution of O and P pattern elements in either the rostral or midbody region (data not shown).
Thus, a general reduction in BMP signaling had a profound effect on the specification of P cell fates in the midbody, but little or no effect on the specification of either P or O fates in the rostral region. We cannot rule out the possibility that a low level of BMP signaling persisted following expression of the dominant-negative receptor, but our data suggest BMP signaling activity itself is dispensable for the normal patterning of op blast cell clones.
Molecular differentiation of OP sublineages
Although the progeny of op blast cells assume their normal fates in the absence of BMP signaling, we did observe a change in the pattern of gene expression when BMP signaling was reduced. Hau-Six1/2A (a SIX-class homeodomain transcription factor) is expressed in the p bandlet of midbody segments, and in the rostral region is expressed in the op.p sublineage, i.e., that arising from the posterior daughter of the op blast cell, which gives rise to a combination of O-type and P-type progeny (Quigley et al., 2010). Thus, expression of Hau-Six1/2A specifically marks a subset of op progeny, but in contrast to the midbody, Hau-Six1/2A expression is not a general marker of P fates in the rostral region.
We previously showed that Hau-Six1/2A is positively regulated by BMP5-8 in the p bandlet of the midbody region (Kuo and Weisblat, 2011). To see if BMP signaling is also required for expression of Hau-Six1/2A within the op clones, we examined the expression of this gene after co-injecting Hau-alk3/6K253R mRNA and RDA into the right OP proteloblast soon after its birth. Hau-alk4/5/7ΔC mRNA and FDA were co-injected into the left OP proteloblast as a control. In contrast to the p bandlet, where expression of the dominant-negative receptor led to a loss of detectable expression, Hau-Six1/2A RNA was detected in op clones expressing the kinase-deficient BMP-specific receptor, and showed its normal cellular distribution. The level of Hau-Six1/2A expression in these clones was significantly reduced relative to the contralateral control, however (Fig. 6C). The lineal origins of Hau-Six1/2A expressing cells were ascertained with tracer labeling (data not shown).
Fig. 6.
The effect of inhibiting BMP signaling on Hau-Six1/2A expression differs between the op blast cell clones and the o and p bandlets. A. Normal expression of Hau-Six1/2A in a late stage 7 embryo. Arrows indicate the Hau-Six1/2A expressing cells in the four op clones; arrowheads indicate the p bandlet expressing Hau-Six1/2A. B. Enlarged view of the boxed area in A, showing Hau-Six1/2A expression in op.pa and op.pp cells of the four-cell op clones. C. In all 28 embryos injected with Hau-alk3/6K253R mRNA in the right OP proteloblast and a control mRNA in the left OP, expression of Hau-Six1/2A was undetectable in the p bandlet of right side (red open arrowhead), but remained detectable, at a reduced level, in the op.pa and op.pp cells of right germinal band (red open arrows). On the left side, expression of Hau-Six1/2A in the p bandlet (black arrowhead) and in op.pa and op.pp cells (black arrows) was normal. D. In all 30 embryos injected with Hau-bmp5-8 ASMO in the right NOPQ proteloblast and an irrelevant control MO in the left NOPQ proteloblast, Hau-Six1/2A transcript was not detected in the p bandlet of the right germinal band (red open arrowhead) but was normal in the p bandlet of left side (black arrowhead). Expression level of Hau-Six1/2A in cells op.pa and op.pp on the right side (red open arrows) were somewhat lower than that on the left side (black arrows). E. In 10 of 12 embryos injected with Hau-bmp5-8 ASMO in the right Q teloblast and a control MO in the left Q teloblast, Hau-Six1/2A was expressed in the p bandlet of left side (black arrowhead), but not in the right germinal band (red open arrowhead). In contrast, the expression levels of Hau-Six1/2A in cells op.pa and op.pp on the right side (red arrows) were similar to those on the left side (black arrows). Scale bar = 150 μm (A, C, D, E); 45 μm (B).
Regional differences at the molecular level were also observed following knockdown of BMP5-8 by ASMO injection, and the results varied depending on which cell was injected. In one set of experiments, Hau-bmp5-8 ASMO was injected into the NOPQ proteloblast, which generates all four ectodermal teloblasts and also several cells that contribute non-segmental progeny at the anterior end of the germinal band (Bissen and Weisblat, 1989; Smith and Weisblat, 1994); this led to a partial reduction of Hau-Six1/2A levels in the op clones (Fig. 6D). But in other experiments, when Hau-bmp5-8 ASMO was injected into the Q teloblast immediately after its birth, Hau-Six1/2A expression in the op clones appeared normal (Fig. 6E). In all experiments, BMP5-8 knockdown caused a complete loss of Hau-Six1/2A expression in the p bandlet, but Hau-Six1/2A expression in the op clone was never completely eliminated. Note that we failed to detect Hau-Six1/2a expression in op clones following the injection of Hau-bmp5-8 ASMO into proteloblast NOPQ in a previous study (Kuo and Weisblat, 2011). This was due to a shorter coloration reaction time for Hau-Six1/2A in situ hybridization in the previous study; extended color reaction times resulted in a more sensitive detection of Hau-Six1/2A expression in the present study.
Our data suggested that Hau-Six1/2A expression is differentially dependent on BMP signaling in the op clones (rostral region) as compared to the p bandlet (midbody region). In the midbody, there seemed to be an absolute requirement of the Q-derived BMP5-8 for expression of Hau-Six1/2A in the p bandlet. By contrast, in the op clones of the rostral region, Hau-Six1/2A expression is only partially dependent on BMP signaling, so that a low level of expression persisted when BMP signaling was inhibited. In any case, the Hau-Six1/2A expression in op clones appeared to be of no consequence for the specification of O or P cell fates, as shown in the previous section.
In sum, these results further support our general finding that the development of the OP lineage in the rostral region and the O/P lineages in the midbody region differ in terms of the mechanism and consequences of BMP signaling.
Discussion
In the work described here, we have compared the patterning mechanisms by which different regions of the leech embryo generate homonomous sets of cells known collectively as O (ventrolateral) and P (dorsolateral) pattern elements. The OP proteloblast gives rise to the lateral ectoderm of all 32 body segments. But in each rostral segment the O and P pattern elements all arise from a single op blast cell (Kuo and Shankland, 2004a; Shankland, 1987a), whereas in midbody and caudal segments the O and P pattern elements arise separately, from a pair of initially equipotent o/p blast cells, which become specified to respective O and P fates by hierarchical cell interactions (Kuo and Weisblat, 2011; Shankland, 1984).
We showed here that these two regions of the leech body also have fundamentally different requirements for patterning signals from surrounding tissues. In the midbody, the indeterminacy of the o/p blast cells is broken when the P fate is induced in response to BMP5-8 originating from the Q (dorsal) lineage (Huang and Weisblat, 1996; Kuo and Shankland, 2004b; Kuo and Weisblat, 2011). In contrast, our present findings show that neither the difference in BMP signaling between dorsal and ventral territories nor the BMP signaling activity itself is required to specify O and P fates in the rostral region. The op blast cells of the rostral region produced their normal O and P pattern elements following three distinct experimental manipulations that alter BMP signaling, all of which have a dramatic effect on O/P patterning in the midbody. Furthermore, expression of Hau-Six1/2A is a molecular marker of certain specific OP sublineages (Quigley et al., 2010), and we showed that perturbations of BMP signaling do not alter the cellular pattern of Hau-Six1/2A expression within the op clone, though they may effect a reduction in expression. Thus, BMP signaling has no essential role in patterning op clones.
Dorsoventral patterning mechanism of the rostral region
During normal development of the OP lineage, the first two rounds of division in the primary op blast cell clone produce two granddaughter cells that have P-type fates (op.ap and op.pp) interspersed with two granddaughter cells that have predominantly O-type fates (op.aa and op.pa) (Kuo and Shankland, 2004a; Shankland, 1987a). These two classes of granddaughter occupy equivalent DV positions within the germinal band. Thus, in contrast to the positioning of O and P precursors as DV neighbors in the midbody, cells with O and P fates alternate as AP neighbors in the four most rostral segments. The O- and P-type sublineages of the rostral region eventually segregate along the DV axis when they generate their respectively ventrolateral and dorsolateral definitive progeny, but that process has not yet been described.
This difference in the spatial arrangement of O and P precursors between the rostral and midbody regions has led previously to the suggestion that the specification of ventrolateral (O) and dorsolateral (P) fates within the op clone may not depend on positional cues deployed along the DV axis (Kuo and Shankland, 2004a; Shankland, 1987a). This hypothesis is suppported by our present finding that polarized BMP5-8 signals emanating from the dorsal ectodermal (Q) lineage are dispensable for specifying O and P fates within op clones.
Prior work has shown that the granddaughters of the op blast cell have already undergone some degree of fate specification when they are still aligned along the AP axis. If a single O-type granddaughter (op.pa) is ‘isolated’ by ablation of the three other cells in its clone, it still generates its normal subset of O-type descendants (Kuo and Shankland, 2004a). In contrast, when one or both O-type granddaughters (op.aa and/or op.pa) are ablated, the P-type sublineages (op.ap and op.pp) regulate and produce some O-type descendants in addition to their normal fates. Those findings suggest that the O-type granddaughters of op blast cells are committed to the O pathway - at least in an operational sense - at a time in development when O vs. P specification is still malleable in the prospective P-type cells. A stepwise commitment of O vs. P fates also occurs in the midbody, but there it is the o blast cells clones that exhibit plasticity of cell fates in response to ablation of cells in the prospective P lineage (Shankland and Weisblat, 1984).
The differential expression of Hau-Six1/2A (in the op.p clone, but not the op.a clone) reveals molecular differences between these op sublineages, and further highlights the differences in patterning processes between the midbody (where Hau-Six1/2A expression is both a marker and a determinant of P fates (Quigley et al., 2010) and the rostral region (where op.a and op.p each contribute a mix of O and P pattern elements). In addition, the spatiotemporal pattern of Hau-Six1/2A expression within the op blast cell clones is largely independent of BMP signaling, in contrast to the midbody, where Hau-Six1/2A expression requires BMP signaling. Following the expression of a dominant negative BMP receptor, Hau-Six1/2A was still present in the op.p clone, although at a lower level. This reduction suggests that BMP signaling affects, but is not required for Hau-Six1/2A expression within the rostral region.
We observed no reduction in Hau-Six1/2A expression by the op.p clone following BMP5-8 knockdown in the q bandlet, nor did Quigley et al. when the q bandlet was ablated entirely (Quigley et al., 2010). But Hau-Six1/2A expression in op.p clones was reduced when BMP5-8 was knocked down in the entire NOPQ lineage. Since Hau-bmp5-8 is normally expressed in non-segmental cells (e.g. Fig. 3B), we speculate that BMP5-8 produced by one or more of the NOPQ-derived non-segmental cells may up-regulate expression of Hau-Six1/2A in op.p clones. Alternatively, BMP ligands other than BMP5-8 (e.g. Hau-BMP2/4a, Hau-BMP2/4b) could up-regulate Hau-Six1/2A in op.p clones.
Given that every cell in the 2-cell and 4-cell op-derived clone contacts the BMP5-8-expressing q bandlet, and that BMP2/4a and BMP2/4b are broadly expressed in the germinal bands (Kuo and Weisblat, 2011), it seems that the daughters and/or granddaughters of cell op must differ autonomously in their response to BMP signals. Thus, op.a does not express Hau-Six1/2A even when exposed to BMP ligands, but op.p does. However, the functional significance of BMP signaling and Hau-Six1/2A expression in the OP lineage is unclear, because the mechanism by which the O-and P-type granddaughters of the op cells become specified to their respective fates remains unknown. One possibility is that early blast cell divisions segregate cell-autonomous determinants which are then reinforced by the cell interactions described above. There is a precedent for such asymmetric blast cell divisions in the midbody region, where the positionally defined o and p blast cells appear to segregate anterior and posterior cell fates to their daughters in a cell-autonomous fashion (Seaver and Shankland, 2001) while their dorsoventral (i.e. P vs.O) fates are being determined by positional cues (Shankland and Weisblat, 1984; Weisblat and Blair, 1984). The fact that the initial O vs. P decisions in the op blast cell clone are oriented along the axis of blast cell division raises the possibility that those decisions could be specified by intrinsic factors to some significant degree. If this is the case, it suggests that the O and P sublineages of the rostral region never constitute an equivalence group, unlike their midbody homologs.
The OP lineage and O/P lineages are separate developmental modules
Teloblastic segmentation is an ancestral feature of clitellate annelids, and the stereotyped distributions of neural and epidermal cells referred to in leech as the O (ventrolateral) and P (dorsolateral) ectoderm can be readily identified in other clitellate taxa (Goto et al., 1999; Storey, 1989). However, despite this conservation of their morphological end product, the developmental mechanisms that pattern the O and P fates differ between species (Arai et al., 2001; Huang and Weisblat, 1996; Kuo and Shankland, 2004b) and, as shown here, between different body regions of an individual embryo.
This evolutionary and developmental plasticity indicates a dissociation between the patterning process and its morphological end products. One factor that can facilitate this dissociation is a modular organization of development. In a modular system, individual modules develop independently of one another and can therefore evolve independently as well (Kirschner and Gerhart, 1998; Wagner and Altenberg, 1996). There are clear differences in the sequence of cellular events that give rise to the serially homologous O and P pattern elements of the rostral and midbody regions (Kuo and Shankland, 2004a), and here we show that there is also a difference between these two regions in the role of BMP signaling in specifying those cell fates. Thus, the OP lineage of the rostral region and the O-P equivalence group of the midbody region appear to behave in both development and evolution as independent modular units.
Supplementary Material
Hau-BMP5-8 knockdown disrupted normal distribution patterns of lateral dopaminergic (LD) peripheral neurons (as revealed by WMISH for Hau-tyrosine hydroxylase; Hau-th) in the midbody but not in the rostral region. Black arrows indicate the LD1 neuron, a P pattern element; red arrows indicate the LD2 neuron, an O pattern element; open arrows indicate the expected position of missing Hau-th-expressing cells. Note that the bilateral pair of LD1 neurons was normally missing in the first rostral segment (Stuart et al., 1987). Dashed circles indicate the Q lineage-derived MD neurons. Dorsal view of late stage 11 embryo is shown. Following injecting Hau-bmp5-8 ASMO into the right NOPQ proteloblast and a control MO into the left NOPQ, both the left and right halves contained a normal set of LD neurons in the rostral region. In the midbody, the left side contained both types of LD neurons, but the right side lacks LD1 neuron. Scale bar: 150 μm.
Highlights.
Segmented leech body plan comprises rostral, midbody and caudal regions.
BMP signaling is required for DV patterning in midbody lateral ectoderm.
BMP signaling is dispensable for patterning rostral lateral ectoderm.
This regional difference reflects modular nature of segmental units in leech.
Acknowledgments
This work was supported by a NASA research grant NAG2-1349 to MS and an NIH R01 grant GM 074619 to DAW. In the beginning phase of this investigation, DHK was also supported in part by a Hartman Fellowship from the U.T. Austin Graduate Program in Zoology/EEB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arai A, Nakamoto A, Shimizu T. Specification of ectodermal teloblast lineages in embryos of the oligochaete annelid Tubifex: involvement of novel cell-cell interactions. Development. 2001;128:1211–1219. doi: 10.1242/dev.128.7.1211. [DOI] [PubMed] [Google Scholar]
- Bissen ST, Weisblat DA. The durations and compositions of cell cycles in embryos of the leech, Helobdella triserialis. Development. 1989;106:105–118. doi: 10.1242/dev.106.1.105. [DOI] [PubMed] [Google Scholar]
- Blair SS. Interactions between mesoderm and ectoderm in segment formation in the embryo of a glossiphoniid leech. Dev Biol. 1982;89:389–396. doi: 10.1016/0012-1606(82)90327-x. [DOI] [PubMed] [Google Scholar]
- Blair SS, Martindale MQ, Shankland M. Interactions between adjacent ganglia bring about the bilaterally alternating differentiation of RAS and CAS neurons in the leech nerve cord. J Neurosci. 1990;10:3183–3193. doi: 10.1523/JNEUROSCI.10-10-03183.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SS, Weisblat DA. Ectodermal interactions during neurogenesis in the glossiphoniid leech Helobdella triserialis. Dev Biol. 1982;91:64–72. doi: 10.1016/0012-1606(82)90008-2. [DOI] [PubMed] [Google Scholar]
- Chang C, Wilson PA, Mathews LS, Hemmati-Brivanlou A. A Xenopus type I activin receptor mediates mesodermal but not neural specification during embryogenesis. Development. 1997;124:827–837. doi: 10.1242/dev.124.4.827. [DOI] [PubMed] [Google Scholar]
- Dyson S, Gurdon JB. Activin signalling has a necessary function in Xenopus early development. Curr Biol. 1997;7:81–84. doi: 10.1016/s0960-9822(06)00030-3. [DOI] [PubMed] [Google Scholar]
- Gallitano-Mendel A, Finkelstein R. Novel segment polarity gene interactions during embryonic head development in Drosophila. Dev Biol. 1997;192:599–613. doi: 10.1006/dbio.1997.8753. [DOI] [PubMed] [Google Scholar]
- Goto A, Kitamura K, Arai A, Shimizu T. Cell fate analysis of teloblasts in the Tubifex embryo by intracellular injection of HRP. Dev Growth Differ. 1999;41:703–713. doi: 10.1046/j.1440-169x.1999.00469.x. [DOI] [PubMed] [Google Scholar]
- Ho RK, Weisblat DA. A provisional epithelium in leech embryo: cellular origins and influence on a developmental equivalence group. Dev Biol. 1987;120:520–534. doi: 10.1016/0012-1606(87)90255-7. [DOI] [PubMed] [Google Scholar]
- Huang FZ, Weisblat DA. Cell fate determination in an annelid equivalence group. Development. 1996;122:1839–1847. doi: 10.1242/dev.122.6.1839. [DOI] [PubMed] [Google Scholar]
- Keleher GP, Stent GS. Cell position and developmental fate in leech embryogenesis. Proc Natl Acad Sci USA. 1990;87:8457–8461. doi: 10.1073/pnas.87.21.8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M, Gerhart J. Evolvability. Proc Natl Acad Sci USA. 1998;95:8420–8427. doi: 10.1073/pnas.95.15.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo DH, Shankland M. A distinct patterning mechanism of the O and P cell fates in the development of the rostral segments of the leech Helobdella robusta: implication for the evolutionary dissociation of developmental pathway and morphological outcome. Development. 2004a;131:105–115. doi: 10.1242/dev.00919. [DOI] [PubMed] [Google Scholar]
- Kuo DH, Shankland M. Evolutionary diversification of specification mechanisms within the O/P equivalence group of the leech genus Helobdella. Development. 2004b;131:5859–5869. doi: 10.1242/dev.01452. [DOI] [PubMed] [Google Scholar]
- Kuo DH, Weisblat DA. A new molecular logic for BMP-mediated dorsoventral patterning in the leech Helobdella. Curr Biol. 2011;21:1282–1288. doi: 10.1016/j.cub.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony D, Weis FM, Massagué J, Gurdon JB. XTrR-I is a TGFβ receptor and overexpression of truncated form of the receptor inhibits axis formation and dorsalising activity. Mech Dev. 1998;75:95–105. doi: 10.1016/s0925-4773(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Martindale MQ, Shankland M. Neuronal competition determines the spatial pattern of neuropeptide expression by identified neurons of the leech. Dev Biol. 1990;139:210–226. doi: 10.1016/0012-1606(90)90289-u. [DOI] [PubMed] [Google Scholar]
- Mizutani CM, Bier E. EvoD/Vo: the origins of BMP signalling in the neuroectoderm. Nat Rev Genet. 2008;9:663–677. doi: 10.1038/nrg2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller KJ, Nicholls JG, Stent GS, editors. Neurobiology of the Leech. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1981. [Google Scholar]
- Quigley IK, Schmerer MW, Shankland M. A member of the Six gene family promotes the specification of P cell fates in the O/P equivalence group of the leech Helobdella. Dev Biol. 2010;344:319–330. doi: 10.1016/j.ydbio.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Seaver EC, Shankland M. Establishment of segment polarity in the ectoderm of the leech Helobdella. Development. 2001;128:1629–1641. doi: 10.1242/dev.128.9.1629. [DOI] [PubMed] [Google Scholar]
- Shankland M. Positional determination of supernumerary blast cell death in the leech embryo. Nature. 1984;307:541–543. doi: 10.1038/307541a0. [DOI] [PubMed] [Google Scholar]
- Shankland M. Cell lineage in leech embryogenesis. Trends Genet. 1987a;3:314–319. [Google Scholar]
- Shankland M. Differentiation of the O and P cell lines in the embryo of the leech. I Sequential commitment of blast cell sublineages. Dev Biol. 1987b;123:85–96. doi: 10.1016/0012-1606(87)90430-1. [DOI] [PubMed] [Google Scholar]
- Shankland M. Differentiation of the O and P cell lines in the embryo of the leech. II Genealogical relationship of descendant pattern elements in alternative developmental pathways. Dev Biol. 1987c;123:97–107. doi: 10.1016/0012-1606(87)90431-3. [DOI] [PubMed] [Google Scholar]
- Shankland M, Martindale MQ. Segmental specificity and lateral asymmetry in the differentiation of developmentally homologous neurons during leech embryogenesis. Dev Biol. 1989;135:431–448. doi: 10.1016/0012-1606(89)90192-9. [DOI] [PubMed] [Google Scholar]
- Shankland M, Weisblat DA. Stepwise commitment of blast cell fates during the positional specification of the O and P cell lines in the leech embryo. Dev Biol. 1984;106:326–342. doi: 10.1016/0012-1606(84)90231-8. [DOI] [PubMed] [Google Scholar]
- Smith CM, Weisblat DA. Micromere fate maps in leech embryos: lineage-specific differences in rates of cell proliferation. Development. 1994;120:3427–3438. doi: 10.1242/dev.120.12.3427. [DOI] [PubMed] [Google Scholar]
- Storey KG. Cell lineage and pattern formation in the earthworm embryo. Development. 1989;107:519–531. [Google Scholar]
- Stuart DK, Blair SS, Weisblat DA. Cell lineage, cell death, and the developmental origin of identified serotonin- and dopamine-containing neurons in the leech. J Neurosci. 1987;7:1107–1122. doi: 10.1523/JNEUROSCI.07-04-01107.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart DK, Torrence SA, Law MI. Leech neurogenesis. I Positional commitment of neural precusor cells. Dev Biol. 1989;136:17–39. doi: 10.1016/0012-1606(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Torrence SA, Law MI, Stuart DK. Leech neurogenesis. II Mesodermal control of neuronal patterns. Dev Biol. 1989;136:40–60. doi: 10.1016/0012-1606(89)90129-2. [DOI] [PubMed] [Google Scholar]
- Urbach R, Technau GM. Segment polarity and DV patterning gene expression reveals segmental organization of the Drosophila brain. Development. 2003;130:3607–3620. doi: 10.1242/dev.00532. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Altenberg L. Complex adaptations and the evolution of evolvability. Evolution. 1996;50:967–976. doi: 10.1111/j.1558-5646.1996.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Weisblat DA, Blair SS. Developmental interderterminacy in embryos of the leech Helobdella triserialis. Dev Biol. 1984;101:326–335. doi: 10.1016/0012-1606(84)90146-5. [DOI] [PubMed] [Google Scholar]
- Weisblat DA, Kuo D-H. Helobdella (Leech): a model for developmental studies, Emerging Model Organisms: a Laboratory Manual. CSHL Press; Cold Spring Harbor, NY: 2009. pp. 245–267. [DOI] [PubMed] [Google Scholar]
- Weisblat DA, Shankland M. Cell lineage and segmentation in the leech. Philos Trans R Soc Lond B. 1985;312:39–56. doi: 10.1098/rstb.1985.0176. [DOI] [PubMed] [Google Scholar]
- Zackson SL. Cell lineage, cell-cell interaction, and segment formation in the ectoderm of a glossiphoniid leech embryo. Dev Biol. 1984;104:143–160. doi: 10.1016/0012-1606(84)90044-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hau-BMP5-8 knockdown disrupted normal distribution patterns of lateral dopaminergic (LD) peripheral neurons (as revealed by WMISH for Hau-tyrosine hydroxylase; Hau-th) in the midbody but not in the rostral region. Black arrows indicate the LD1 neuron, a P pattern element; red arrows indicate the LD2 neuron, an O pattern element; open arrows indicate the expected position of missing Hau-th-expressing cells. Note that the bilateral pair of LD1 neurons was normally missing in the first rostral segment (Stuart et al., 1987). Dashed circles indicate the Q lineage-derived MD neurons. Dorsal view of late stage 11 embryo is shown. Following injecting Hau-bmp5-8 ASMO into the right NOPQ proteloblast and a control MO into the left NOPQ, both the left and right halves contained a normal set of LD neurons in the rostral region. In the midbody, the left side contained both types of LD neurons, but the right side lacks LD1 neuron. Scale bar: 150 μm.