Abstract
The blood and lymphatic vasculature play an important role in skin homeostasis. Angiogenesis and lymphangiogenesis – the growth of new vessels from existing ones - have received tremendous interest because of their role in promoting cancer spread. However, there is increasing evidence that both vessel types also play a major role in acute and chronic inflammatory disorders. Vessels change their phenotype in inflammation (vascular remodeling). In inflamed skin, vascular remodeling consists of a hyperpermeable, enlarged network of vessels with increased blood flow, and influx of inflammatory cells. During chronic inflammation, the activated endothelium expresses adhesion molecules, cytokines, and other molecules that lead to leukocyte rolling, attachment and migration into the skin. Recent studies reveal that inhibition of blood vessel activation exerts potent anti-inflammatory properties. Thus, anti-angiogenic drugs might be used to treat inflammatory conditions. In particular, topical application of anti-angiogenic drugs might be ideally suited to circumvent the adverse effects of systemic therapy with angiogenesis inhibitors. Our recent results indicate that stimulation of lymphatic vessel growth and function unexpectedly represents a novel approach for treating chronic inflammatory disorders.
INTRODUCTION
Inflammation is one of the body’s major defense mechanisms against pathological insults such as infection, physical or chemical injury. Acute inflammation is terminated by well understood mechanisms restoring homeostasis. In contrast, chronic inflammatory diseases are self-perpetuating conditions which often result in a generalized systemic inflammation affecting several different organs.
Blood and lymphatic vessels play pivotal roles under physiological conditions: the cardiovascular network is the first organ system to develop. Its major functions include the supply of oxygen and nutrients, and the disposal of metabolic waste products. In the adult, physiological angiogenesis is indispensable for the normal wound healing process, the menstrual and hair cycle, the response to ischemia and for endometrial growth (Carmeliet, 2003). The lymphatic vasculature is involved in intestinal fat absorption and immune surveillance, and it drains excess tissue fluid back to the blood circulation. The formation of new capillaries from preexisting vessels - angiogenesis and lymphangiogenesis - has received tremendous interest, mainly because of the presumed role in enhancing tumor progression and metastasis (Carmeliet, 2003; Hirakawa et al., 2005b; Karpanen and Alitalo, 2008; Mumprecht and Detmar, 2009). However, vascular remodeling is also a hallmark of many inflammatory diseases including chronic airway inflammation, rheumatoid arthritis, inflammatory bowel disease, atherosclerosis, and the chronic inflammatory skin disease psoriasis (Bainbridge et al., 2006; Baluk et al., 2005; Danese et al., 2006; Detmar et al., 1994). In these conditions, levels of the angiogenic growth factor vascular endothelial growth factor (VEGF)-A are elevated in the inflamed tissue (Detmar et al., 1994; Kanazawa et al., 2001; Koch et al., 1994). Interestingly, the main vascular changes during inflammation consist of vascular enlargement, whereas tumor growth is mainly associated with sprouting angiogenesis. However, vascular hyperpermeability and endothelial cell proliferation are common to both types of angiogenesis. The effect of blocking VEGF-A and angiogenesis is extensively investigated in human cancers but warrants further investigation in inflammatory processes.
BLOOD AND LYMPHATIC VESSELS UNDER PHYSIOLOGICAL CONDITIONS
The cutaneous blood vascular architecture consists of a lower and an upper horizontal plexus. The capillary loops extend from the latter (Braverman, 1989). The lymphatic vessels of the skin also form two plexuses in vicinity of the blood vascular plexuses. Branches from the superficial lymphatic vessel plexus protrude into the dermal papillae and drain into larger lymphatic vessels in the lower dermis and the superficial zone of the subcutaneous tissue. For more details regarding the cutaneous vessel anatomy please see (Skobe and Detmar, 2000). The structure of blood vascular endothelial cells varies with their anatomical location (Aird, 2007). The resting cutaneous blood vessels contain a continuous monolayer of endothelial cells with a continuous basement membrane (Figure 1). The blood vascular endothelial cells are covered with pericytes and form tight and adherens junctions. Under non-activated conditions, quiescent endothelial cells do not interact with leukocytes and inhibit coagulation, and there is no major extravasation of blood proteins into the surrounding tissue (Pober and Sessa, 2007).
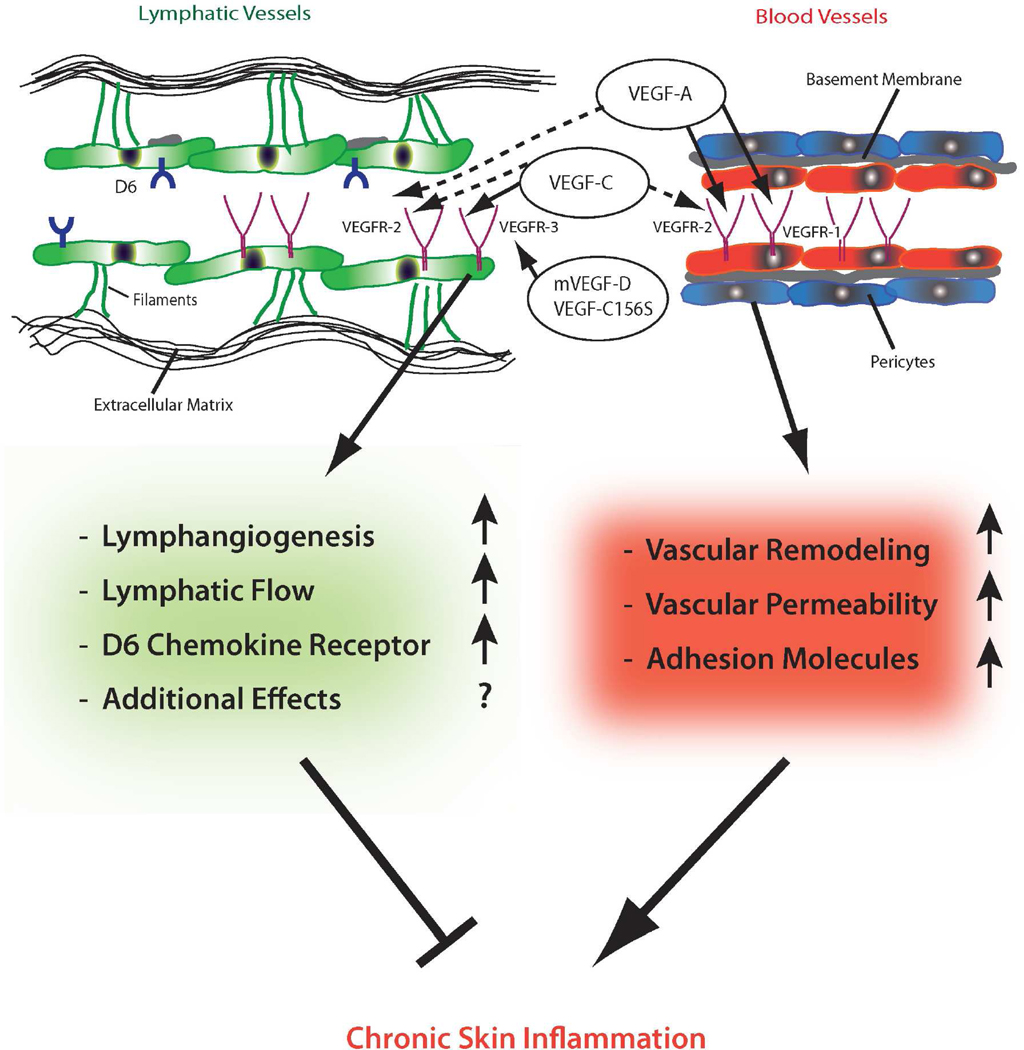
Figure 1. Schematic overview of the proposed role of blood and lymphatic vessels in chronic skin inflammation.
Cutaneous blood vessels contain a monolayer of endothelial cells (red) with a continous basement membrane (gray). Pericytes (blue) cover the blood vascular endothelial cells (BEC). In contrast, lymphatic endothelial cells (LEC, green) lack mural cells and have only a rudimentary basement membrane. They are linked to the extracellular matrix via fibrillin-containing anchoring filaments (green). The lumen of lymphatic vessels is significantly wider and the wall is thinner than that of blood vessels. BEC express VEGFR-1 and VEGFR-2, whereas LEC express VEGFR-2 and VEGFR-3. VEGF-A – which binds both VEGFR-1 and VEGFR-2 - can directly induce blood and lymphatic vascular remodeling. Chronic stimulation of the blood vasculature by VEGF-A leads to vascular remodeling, increased vascular permeability, increased expression of adhesion molecules and chronic skin inflammation. VEGF-C binds to VEGFR-3 and – after proteolytic processing - might also bind to VEGFR-2 (dashed arrows). In contrast, mouse VEGF-D (mVEGF-D) and VEGF-C156S are specific ligands for VEGFR-3. Stimulation of VEGFR-3 leads to lymphangiogenesis and increases lymphatic flow. An expanded network of lymphatic vessels inhibits chronic skin inflammation. Additional effects of lymphatic vessels – such as binding of chemokines (e.g. to the D6 chemokine receptor) – might contribute to the reduction of chronic inflammation.
In contrast to blood vascular endothelial cells, the endothelial cells of lymphatic capillaries overlap, lack tight junctions and mural cells, have only a rudimentary or no basement membrane, and are linked to the extracellular matrix by fibrillin-containing anchoring filaments (Figure 1). Therefore, tissue fluid - containing cells and macromolecules - can directly enter the lymphatic capillaries. The lumen of lymphatic vessels is significantly wider and the wall is thinner than that of blood vessels. The fluid entering the initial lymphatic capillaries is drained to pre-collecting and collecting lymphatic vessels which contain a basement membrane, smooth muscle cells, and backflow-preventing valves (similar to veins). Finally, the fluid is returned to the blood circulation in the jugular region.
BLOOD AND LYMPHATIC VESSELS IN INFLAMMATION
Blood vessels and, to a lesser extent, lymphatic vessels contribute essentially to the cardinal signs of inflammation: dilated blood vessels with increased flow underlie the “rubor” and “calor”; the excess exsudate caused by hyperpermeable blood vessels exceeding the drainage capacity of fluid by lymphatic vessels results in “tumor”. Finally, “dolor” and “functio laesa” are subsequent processes following vascular activation and influx of leukocytes.
Activation of the endothelium by inflammatory mediators (such as VEGF-A, TNF-α, IL-6, IL-1β and others) leads to the up-regulation of adhesion molecules such as E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell-adhesion molecule-1 (VCAM-1), which enables the interaction with leukocytes (Jackson et al., 1997). In chronic inflammatory diseases, the vasculature remains activated, enlarged and hyperpermeable, and it sustains the accumulation of fluid (edema) and cells. Considerable amounts of plasma proteins extravasate from the blood into the tissue during inflammation (Feng et al., 1999). Increased interstitial fluid pressure in inflamed skin leads to the opening of the overlapping lymphatic endothelial cells and to the entry of cell- and macromolecule-rich fluid. The mechanisms controlling the widening of the lymphatic lumen are currently unknown, as is the function of dilated lymphatic vessels. Lymphatic vessels remained dilated in a mouse model of chronic airway inflammation even when inflammation was resolved (Baluk et al., 2005). Therefore, it remains unclear whether the increase in interstitial pressure is the sole driving force of lymphatic vessel dilation. Lymphatic vessels are also a direct source of cytokines and chemokines (Gunn et al., 1998).
INFLAMMATORY SKIN DIESEASES WITH VASCULAR INVOLVEMENT
A multitude of diseases are linked to an insufficient or overactive vasculature (Carmeliet, 2003). Among them are many inflammatory diseases (Table 1). The inflammatory skin diseases associated with prominent remodeling of the vasculature range from UV damage, bullous pemphigoid, contact dermatits to rosacea and psoriasis (Brown et al., 1995; Gomaa et al., 2007; Kunstfeld et al., 2004; Yano et al., 2002). Vascular remodeling is controlled by pro- and anti-angiogenic mediators. An imbalance leads to vessel growth or regression.
Table 1.
Inflammatory diseases with vascular involvement
| Disease state | References |
|---|---|
| Skin diseases | |
| Psoriasis | (Braverman, 1972; Kunstfeld et al., 2004) |
| Rosacea | (Gomaa et al., 2007) |
| Atopic dermatitis | (Agha-Majzoub et al., 2005; Chan, 2008; Zhang et al., 2006) |
| Alopecia areata | (Simonetti et al., 2004) |
| UV damage | (Yano et al., 2002) |
| Bullous pemphigoid | (Brown et al., 1995) |
| Dermatitis herpetiformis | (Brown et al., 1995) |
| Erythema multiforme | (Brown et al., 1995) |
| Systemic sclerosis | (Abraham et al., 2009; Distler et al., 2004) |
| Others | |
| Inflammatory bowel disease | (Chidlow et al., 2007) |
| Rheumatoid arthritis | (Paleolog, 2002) |
| Atherosclerosis | (Hansson, 2005) |
| Asthma | (Bailey et al., 2009; Feltis et al., 2006; Ribatti et al., 2009) |
Psoriasis
Psoriasis is probably the chronic inflammatory skin condition for which changes in the vasculature are best described. The finding that microvascular abnormalities are a characteristic feature, and happen at the onset of psoriasis has been recognized since more than 50 years (Braverman, 1972; Szodoray, 1955; Telner and Fekete, 1961). Already before the epidermal hyperplasia develops, the skin capillaries become tortuous and expanded. The redness of the skin lesions is caused by the close vicinity of the tortous vessels in regions of thinned epithelium. The lymphatic vasculature is also dilated in the superficial dermis, as recognized by electron microscopy, and recently by the detection of specific markers for lymphatic vessels (Braverman, 1972; Kunstfeld et al., 2004).
Angiogenesis in psoriasis
It is of interest that the main drivers of angiogenesis in psoriasis are derived from the epidermis (Malhotra et al., 1989). Macrophages and fibroblasts are additional sources of angiogenic factors, including VEGF-A. VEGF-A is probably the most important growth factor leading to blood and lymphatic vascular remodeling in psoriasis, and is currently the best described inducer of inflammation-driven vascular remodeling (Detmar et al., 1994; Ferrara et al., 2003). Additional angiogenic mediators, including hypoxia-inducible factor, TNF-α, IL-1, IL-6, IL-8, IL-17, IL-18, angiopoietins, and many others are involved (Bernardini et al., 2003; Heidenreich et al., 2009). Table 2 summarizes the differential pro- and anti-angiogenic effects of important cytokines and chemokines involved in the pathogenesis of psoriasis. VEGF-A binds to VEGFR-1 and VEGFR-2. VEGFR-1 is expressed on blood vessels, whereas VEGFR-2 is expressed on both blood and lymphatic vessels (Figure 1). VEGFR-1 can be expressed by monocytes / macrophages, whereas VEGFR-2 is expressed at least by a subset of T-cells (Edelbauer et al., 2010; Sawano et al., 2001). Hence, VEGF-A can directly lead to blood and lymphatic vessel activation, and directly affects the attraction of inflammatory cells. The receptor tyrosine kinase VEGFR-2 is thought to be the main mediator of VEGF-A-driven endothelial cell proliferation, differentiation, and sprouting (Adams and Alitalo, 2007). In contrast, the role of VEGFR-1 in the adult organism is less clear. In embryogenesis, VEGFR-1 - which has a higher affinity for VEGF-A than VEGFR-2 but lower kinase activity – likely sequesters VEGF-A to prevent excess signaling and increased angiogenesis through VEGFR-2 (Fong et al., 1995; Hiratsuka et al., 1998).
Table 2.
Cytokines and chemokines with potential pro- or anti-angiogenic activity in psoriasis
Thus, the remodeling of the vasculature in lesional psoriatic skin might depend on factors derived from the epidermis, whereas blood vascular remodeling is essential for nutrients supply of the overlying, hyperproliferative epidermis. Indeed, epidermal vegf-a−/− mice do not show epidermal hyperplasia after repeated tape stripping (Elias et al., 2008). Targeting both the epidermis and the dermal vasculature might therefore represent a valuable treatment strategy for psoriasis. VEGF-A serum levels correlate positively with disease severity in psoriasis patients, and negatively with the treatment success of standard therapies, implicating a role of VEGF-A in disease maintenance and progression (Bhushan et al., 1999; Mastroianni et al., 2005; Nielsen et al., 2002). Therefore, VEGF-A could serve as a biomarker for psoriasis activity.
Besides the morphological changes in the cutaneous vasculature, it has been increasingly recognized that these vessels are activated, and that they have an increased expression of adhesion molecules such as VCAM-1, ICAM-1 and E-selectin (Springer, 1994), sustaining the accumulation of infiltrating inflammatory cells.
Angiogenesis in mouse models of inflammation
Many insights into the proinflammatory role of VEGF-A stem from animal models: Homozygous keratin 14 (K14)/VEGF-A transgenic (Tg) mice – that overexpress mouse VEGF-A164 in the epidermis - spontaneously develop a chronic inflammatory skin disease with many features of human psoriasis at an age of approximately 6 months (Xia et al., 2003). Besides the vascular changes, the homozygous K14-VEGF-A Tg mice also show epidermal hyperplasia, altered keratinocyte differentiation, the typical infiltration of CD11b+ and CD4+ cells, the intraepidermal localization of CD8+ T cells, the presence of corneal microabscesses, and the typical Koebner phenomenon (Xia et al., 2003). Importantly, the K14-VEGF-A Tg mice are sensitive to standard anti-psoriatic therapies such as treatment with betamethasone and cyclosporine A, and they develop a Th17-like disease phenotype, similar to human psoriasis (Canavese et al., 2010; Hvid et al., 2008). Interestingly, many current treatment modalities for psoriasis have anti-angiogenic effects, such as targeted phototherapy with a laser, vitamin D3 analogues, TNF-α antagonists, methotrexate, cyclosporine A, and corticosteroids (Avramidis et al.; Canete et al., 2004; Cornell and Stoughton, 1985; Hernandez et al., 2001; Hirata et al., 1989; Oikawa et al., 1990), whereas their effect on the lymphatic vasculature remains elusive. Indeed, the vasoconstrictive potency of corticosteroids was even shown to correlate with clinical activity in psoriasis (Cornell and Stoughton, 1985).
In hemizygous K14-VEGF-A Tg mice, chronic inflammatory skin lesions can be induced by delayed-type hypersensitivity reactions (Kunstfeld et al., 2004), and we have previously used this model to discover that topical application of a small molecule inhibitor of VEGF receptor (VEGFR) kinases results in potent anti-inflammatory effects that were subsequently also found in other models of inflammation (Halin et al., 2008). Specific inhibition of VEGF-A also ameliorated psoriasis-like symptoms in a mouse model of psoriasis - where the epidermal specific deletion of c-Jun and JunB leads to the disease (Schonthaler et al., 2009). Besides VEGF-A, another member of the same family of growth factors, namely placental growth factor (PlGF), also plays a major role in cutaneous angiogenesis, inflammation, and edema formation (Oura et al., 2003). K14-PlGF Tg mice are characterized by an increased inflammatory response, with more pronounced vascular enlargement, edema, and inflammatory cell infiltration as compared with wild-type mice. In contrast, mice deficient in PlGF show less inflammation, diminished inflammatory angiogenesis, and edema (Oura et al., 2003). Last but not least, the importance of angiogenesis for inflammation is underscored by the finding that deficiency of the endogenous angiogenesis inhibitor thrombospondin-2 resulted in prolonged and enhanced cutaneous delayed-type hypersensitivity reactions (Lange-Asschenfeldt et al., 2002). Together, these results indicate an important role of angiogenesis and blood vascular activation in sustaining chronic inflammation. In contrast, the role of the lymphatic vasculature in chronic inflammation has remained unclear.
Lymphangiogenesis in psoriasis
Lymphatic vessels are the conduit for leukocytes from the site of inflammation to secondary lymphoid organs. The current literature suggests that chemokines expressed by lymphatic vessels (in particular CCL21) lead the way of leukocytes to lymphatic vessels, and that the migration in the interstitium depends on forward flow of polymerizing actin but is integrin independent (Alvarez et al., 2008; Lammermann et al., 2008; Ohl et al., 2004; Pflicke and Sixt, 2009).
It has been reported that the lymphatic vasculature plays an active role in corneal and kidney transplant rejection, in part by facilitating dendritic cell transport to draining lymph nodes (Cursiefen et al., 2004; Kerjaschki et al., 2004). On the other hand, specific blockade of VEGFR-3, a receptor for the lymphangiogenic growth factors VEGF-C and VEGF-D, which is mainly expressed on the lymphatic endothelium in the adult (Kaipainen et al., 1995), enhanced the mucosal edema in a mouse model of chronic airway inflammation (Baluk et al., 2005), increased the severity of inflammation in a mouse model of chronic inflammatory arthritis (Guo et al., 2009), and also prolonged the course of inflammatory ear swelling in a mouse model of chronic skin inflammation (Huggenberger et al., 2010). Additionally, the inhibition of VEGF-C/-D by sVEGFR-3 significantly decreased lymph flow in a model of bacterial skin inflammation (Kataru et al., 2009), whereas the genetic overexpression of soluble VEGFR-3 in the skin of mice resulted in a lymphedema-like phenotype (Makinen et al., 2001a).
Interestingly, the deficiency of the chemokine receptor D6 in mice – that is expressed on lymphatic vessels, and likely degrades pro-inflammatory chemokines – leads to a chronic inflammatory skin disease resembling human psoriasis after treatment with phorbol esters (Jamieson et al., 2005; Nibbs et al., 2001).
Lymphatic vessels also have an increased density in arthritic joints of mice and men, and are further increased after standard infliximab therapy (Polzer et al., 2008; Zhang et al., 2007). In inflamed tissues, the lymphangiogenic growth factors VEGF-C and VEGF-A are secreted by immune cells such as macrophages, and by resident tissue cells such as keratinocytes and fibroblasts. After proteolytic processing of the propeptides, the mature VEGF-C also binds and activates VEGFR-2 which, besides its expression on the blood vascular endothelium, is also expressed on lymphatic vessels (Joukov et al., 1997; Kriehuber et al., 2001; Makinen et al., 2001b; Wirzenius et al., 2007). Inflammation-induced lymphangiogenesis can be directly regulated by VEGF-A/VEGFR-2 and VEGF-C/VEGF-D/VEGFR-3 signaling, and might be modulated by the attraction of inflammatory cells secreting lymphangiogenic factors (Baluk et al., 2005; Kataru et al., 2009; Wuest and Carr, 2010). However, VEGF-A-induced lymphatic vessels might be less functional than those induced by VEGF-C or VEGF-D/VEGFR-3 signaling (Kajiya et al., 2006; Nagy et al., 2002). Recently, it was reported that the inflamed lymphatic endothelium expresses ICAM-1, and that it might directly interact with CD11b expressing dendritic cells, resulting in a reduced capacity of dendritic cells to stimulate T-cell proliferation (Podgrabinska et al., 2009). These results highlight the active participation of lymphatic endothelial cells in regulating inflammatory processes.
We have recently found that the establishment of chronic inflammatory skin lesions is associated with impaired lymphatic function and concomitantly decreased lymph flow using an in vivo near-infrared imaging approach in mice (Huggenberger et al., 2010). More importantly, we found, for the first time, that specific activation of lymphatic vessels by Tg overexpression of VEGF-C or of the VEGFR-3-specific ligand mVEGF-D, as well as the intradermal injection of the VEGFR-3-specific mutant VEGF-C156S protein, inhibited chronic skin inflammation in the K14-VEGF-A Tg mouse model (Huggenberger et al., 2010). The reduction in skin inflammation was accompanied by a decreased inflammatory cell infiltrate and normalized epidermal differentiation. It will be of great interest to see whether the application of VEGF-C and activation of lymphatic vessels also exert anti-inflammatory effects in other chronic inflammatory diseases, such as arthritis and inflammatory bowel disease.
Inflammation-induced lymphangiogenesis might therefore represent an endogenous counter-regulatory mechanism aimed at limiting edema formation and inflammation.
Rosacea
Besides psoriasis, rosacea is also characterized by pronounced vascular alterations. The potential mechanisms contributing to the pathogenesis of rosacea include innate immunity, reactive oxygen species, UV radiation, microbes, and vascular alterations (Yamasaki and Gallo, 2009). Blood flow is increased and dermal dilation of blood vessels is visible in lesional rosacea skin (Marks and Harcourt-Webster, 1969; Sibenge and Gawkrodger, 1992). VEGF-A levels, angiogenesis, and lymphangiogenesis have been reported to be increased in lesional skin of rosacea patients (Gomaa et al., 2007). This is in line with the clinical flushing episodes and the erythema observed in patients. Interestingly, UV irradiation exacerbates rosacea, likely by stimulating keratinocytes to produce VEGF-A (Brauchle et al., 1996). In contrast, the role of lymphatic vessels in rosacea is currently unknown. A number of patients show skin edema reminiscent of lymphedema, and at the phymous stage, there is a pronounced lymphedema of the skin. Together, these findings implicate an important role of impaired lymphatic function in rosacea pathogenesis.
Cutaneous UVB damage
A single dose of ultraviolet B (UVB; 290–320 nm) irradiation induces epidermal thickening, dilation, and hyperpermeability of blood vessels, edema, and erythema (Berton et al., 1997; Pearse et al., 1987). UVB irradiation up-regulates several pro-angiogenic molecules, such as basic fibroblast growth factor, interleukin-8, and VEGF-A, whereas the anti-angiogenic proteins such as thrombospondin-1 are down-regulated (Bielenberg et al., 1998; Kramer et al., 1993; Strickland et al., 1997; Yano et al., 2004). The repeated exposure of human skin to UVB radiation results in the degradation of extracellular matrix, increased elastosis, a reduction of dermal blood and lymphatic capillaries, wrinkle formation, and ultimately in an increased risk for epithelial skin cancers (Chung et al., 2002; Kajiya et al., 2007; Kligman, 1979, 1989; Kripke, 1994). The reduction of blood and lymphatic vessels most likely is the consequence of extracellular matrix degradation that no longer supports the vessel maintenance (Chung and Eun, 2007; Kajiya et al., 2007).
Mice that overexpress VEGF-A are more sensitive to UVB irradiation than wild-type mice (Hirakawa et al., 2005a). Conversely, we previously found that systemic blockade of VEGF-A reduces UVB-induced inflammation and vascular enlargement without inhibiting tissue repair (Hirakawa et al., 2005a). In line with these findings, overexpression of the angiogenesis inhibitor thrombospondin-1 in epidermal keratinocytes of Tg mice potently prevented UVB-induced photodamage (Yano et al., 2002). These data underscore a damage-mediating role of angiogenesis and blood vascular hyperpermeability in UVB-induced skin damage.
Importantly, we recently found that chronic UVB exposure of mouse skin results in dilated lymphatic vessels that are leaky (Kajiya et al., 2006). Furthermore, inhibition of the lymphatic endothelium-specific VEGFR-3 by a monoclonal antibody significantly prolonged UVB-induced inflammatory edema formation and cell infiltration (Kajiya and Detmar, 2006), whereas activation of VEGFR-3 by the specific activator VEGF-C156S or mouse VEGF-D reduced edema and inflammation (Huggenberger et al., 2011; Kajiya et al., 2009). While VEGF-A is up-regulated after UVB irradiation, VEGF-C is down-regulated (Kajiya et al., 2009). This finding might explain the increased permeability of blood vessels, and the reduced lymphatic drainage function after UVB exposure. Together, these data indicate that inhibition of blood vessel activation / angiogenesis or stimulation of lymphatic function might represent novel approaches to prevent cutaneous photodamage.
CONCLUSIONS AND OUTLOOK
There are numerous drugs for the treatment of inflammatory disorders but none of these drugs was intentionally developed to directly modulate the vascular endothelium, although many clinically used therapeutics also target the vasculature. There is now extensive evidence that targeting the activated, remodeled blood vessels might represent a novel and promising therapeutic approach for treating chronic inflammatory diseases – not only of the skin. The status of vascular activation might also be used as a biomarker for the intensity and activity of inflammatory diseases. Importantly, our recent findings indicate that activation of lymphatic vessels might serve as a novel strategy for treating chronic inflammatory disorders such as psoriasis, rosacea, chronic airway inflammation, rheumatoid arthritis, inflammatory bowel disease, atherosclerosis, and others.
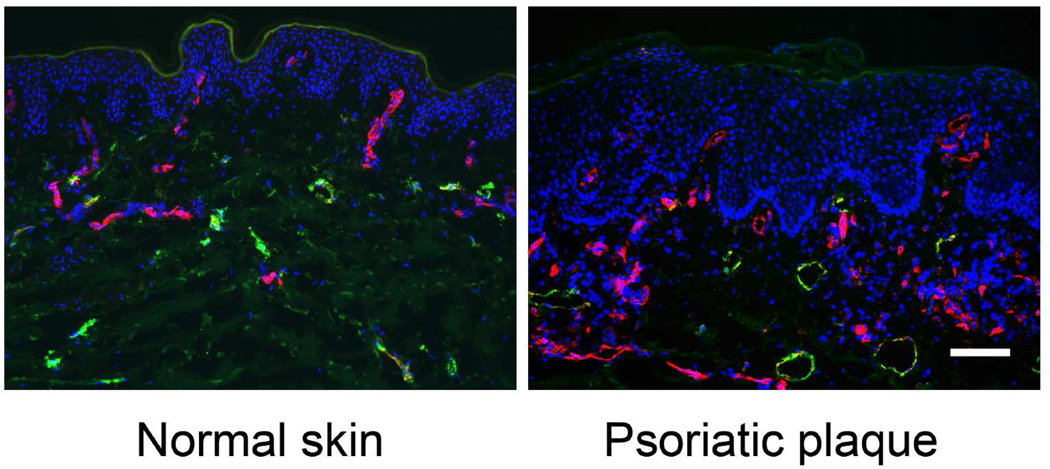
Figure 2. Blood and lymphatic vessel expansion in psoriatic skin lesion.
The number and size of CD31+/LYVE-1− blood vessels (red) is increased in lesional psoriatic skin vs. normal skin of healthy donors. CD31+/LYVE-1+ lymphatic vessel size (green) is also increased in lesional skin of psoriasis patients. Nuclear staining is shown in blue (Hoechst); bar, 100 µm.
Acknowledgements
Work in the authors’ lab has been supported by National Institutes of Health grant CA69184, Swiss National Science Foundation grants 3100A0-108207 and 31003A_130627, Commission of the European Communities grant LSHC-CT-2005-518178, Oncosuisse and Krebsliga Zurich (to M.D.).
Abbreviations
- K14
keratin 14
- Tg
transgenic
- VEGF(R)
vascular endothelial growth factor (receptor)
Footnotes
Conflict of Interest:
The authors declare no conflict of interest.
REFERENCES
- Abraham DJ, Krieg T, Distler J, Distler O. Overview of pathogenesis of systemic sclerosis. Rheumatology (Oxford) 2009;48(Suppl 3):iii3–iii7. doi: 10.1093/rheumatology/ken481. [DOI] [PubMed] [Google Scholar]
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Agha-Majzoub R, Becker RP, Schraufnagel DE, Chan LS. Angiogenesis: the major abnormality of the keratin-14 IL-4 transgenic mouse model of atopic dermatitis. Microcirculation. 2005;12:455–476. doi: 10.1080/10739680591003297. [DOI] [PubMed] [Google Scholar]
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin MA, Rabquer BJ, Mansfield PJ, Ruth JH, Marotte H, Haas CS, et al. Interleukin 18 induces angiogenesis in vitro and in vivo via Src and Jnk kinases. Ann Rheum Dis. 2010;69:2204–2212. doi: 10.1136/ard.2009.127241. [DOI] [PubMed] [Google Scholar]
- Angiolillo AL, Kanegane H, Sgadari C, Reaman GH, Tosato G. Interleukin-15 promotes angiogenesis in vivo. Biochem Biophys Res Commun. 1997;233:231–237. doi: 10.1006/bbrc.1997.6435. [DOI] [PubMed] [Google Scholar]
- Avramidis G, Kruger-Krasagakis S, Krasagakis K, Fragiadaki GI, Kokolakis G, Tosca A. The Role of Endothelial Cell Apoptosis in the Effect of Etanercept in Psoriasis. Br J Dermatol. 2010 doi: 10.1111/j.1365-2133.2010.09935.x. [DOI] [PubMed] [Google Scholar]
- Bae J, Park D, Lee YS, Jeoung D. Interleukin-2 promotes angiogenesis by activation of Akt and increase of ROS. J Microbiol Biotechnol. 2008;18:377–382. [PubMed] [Google Scholar]
- Bailey SR, Boustany S, Burgess JK, Hirst SJ, Sharma HS, Simcock DE, et al. Airway vascular reactivity and vascularisation in human chronic airway disease. Pulm Pharmacol Ther. 2009;22:417–425. doi: 10.1016/j.pupt.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Bainbridge J, Sivakumar B, Paleolog E. Angiogenesis as a therapeutic target in arthritis: lessons from oncology. Curr Pharm Des. 2006;12:2631–2644. doi: 10.2174/138161206777698747. [DOI] [PubMed] [Google Scholar]
- Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BenEzra D, Hemo I, Maftzir G. In vivo angiogenic activity of interleukins. Arch Ophthalmol. 1990;108:573–576. doi: 10.1001/archopht.1990.01070060121061. [DOI] [PubMed] [Google Scholar]
- Bernardini G, Ribatti D, Spinetti G, Morbidelli L, Ziche M, Santoni A, et al. Analysis of the role of chemokines in angiogenesis. J Immunol Methods. 2003;273:83–101. doi: 10.1016/s0022-1759(02)00420-9. [DOI] [PubMed] [Google Scholar]
- Berton TR, Mitchell DL, Fischer SM, Locniskar MF. Epidermal proliferation but not quantity of DNA photodamage is correlated with UV-induced mouse skin carcinogenesis. J Invest Dermatol. 1997;109:340–347. doi: 10.1111/1523-1747.ep12335984. [DOI] [PubMed] [Google Scholar]
- Bhushan M, McLaughlin B, Weiss JB, Griffiths CE. Levels of endothelial cell stimulating angiogenesis factor and vascular endothelial growth factor are elevated in psoriasis. Br J Dermatol. 1999;141:1054–1060. doi: 10.1046/j.1365-2133.1999.03205.x. [DOI] [PubMed] [Google Scholar]
- Bielenberg DR, Bucana CD, Sanchez R, Donawho CK, Kripke ML, Fidler IJ. Molecular regulation of UVB-induced cutaneous angiogenesis. J Invest Dermatol. 1998;111:864–872. doi: 10.1046/j.1523-1747.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- Brauchle M, Funk JO, Kind P, Werner S. Ultraviolet B and H2O2 are potent inducers of vascular endothelial growth factor expression in cultured keratinocytes. J Biol Chem. 1996;271:21793–21797. doi: 10.1074/jbc.271.36.21793. [DOI] [PubMed] [Google Scholar]
- Braverman IM. Electron microscopic studies of the microcirculation in psoriasis. J Invest Dermatol. 1972;59:91–98. doi: 10.1111/1523-1747.ep12625852. [DOI] [PubMed] [Google Scholar]
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93:2S–9S. doi: 10.1111/1523-1747.ep12580893. [DOI] [PubMed] [Google Scholar]
- Brown LF, Harrist TJ, Yeo KT, Stahle-Backdahl M, Jackman RW, Berse B, et al. Increased expression of vascular permeability factor (vascular endothelial growth factor) in bullous pemphigoid, dermatitis herpetiformis, and erythema multiforme. J Invest Dermatol. 1995;104:744–749. doi: 10.1111/1523-1747.ep12606974. [DOI] [PubMed] [Google Scholar]
- Canavese M, Altruda F, Ruzicka T, Schauber J. Vascular endothelial growth factor (VEGF) in the pathogenesis of psoriasis--a possible target for novel therapies? J Dermatol Sci. 2010;58:171–176. doi: 10.1016/j.jdermsci.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Canete JD, Pablos JL, Sanmarti R, Mallofre C, Marsal S, Maymo J, et al. Antiangiogenic effects of anti-tumor necrosis factor alpha therapy with infliximab in psoriatic arthritis. Arthritis Rheum. 2004;50:1636–1641. doi: 10.1002/art.20181. [DOI] [PubMed] [Google Scholar]
- Cao R, Farnebo J, Kurimoto M, Cao Y. Interleukin-18 acts as an angiogenesis and tumor suppressor. FASEB J. 1999;13:2195–2202. doi: 10.1096/fasebj.13.15.2195. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Chan LS. Atopic dermatitis in 2008. Curr Dir Autoimmun. 2008;10:76–118. doi: 10.1159/000131450. [DOI] [PubMed] [Google Scholar]
- Chidlow JH, Jr, Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol. 2007;293:G5–G18. doi: 10.1152/ajpgi.00107.2007. [DOI] [PubMed] [Google Scholar]
- Cho ML, Jung YO, Moon YM, Min SY, Yoon CH, Lee SH, et al. Interleukin-18 induces the production of vascular endothelial growth factor (VEGF) in rheumatoid arthritis synovial fibroblasts via AP-1-dependent pathways. Immunol Lett. 2006;103:159–166. doi: 10.1016/j.imlet.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Chung JH, Eun HC. Angiogenesis in skin aging and photoaging. J Dermatol. 2007;34:593–600. doi: 10.1111/j.1346-8138.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- Chung JH, Yano K, Lee MK, Youn CS, Seo JY, Kim KH, et al. Differential effects of photoaging vs intrinsic aging on the vascularization of human skin. Arch Dermatol. 2002;138:1437–1442. doi: 10.1001/archderm.138.11.1437. [DOI] [PubMed] [Google Scholar]
- Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63–67. [PubMed] [Google Scholar]
- Coughlin CM, Salhany KE, Wysocka M, Aruga E, Kurzawa H, Chang AE, et al. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest. 1998;101:1441–1452. doi: 10.1172/JCI1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino F, Torcia M, Aldinucci D, Ziche M, Almerigogna F, Bani D, et al. Interleukin 1 is an autocrine regulator of human endothelial cell growth. Proc Natl Acad Sci U S A. 1990;87:6487–6491. doi: 10.1073/pnas.87.17.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Cao J, Chen L, Liu Y, Maruyama K, Jackson D, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, et al. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180:1141–1146. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler O, Distler JH, Scheid A, Acker T, Hirth A, Rethage J, et al. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ Res. 2004;95:109–116. doi: 10.1161/01.RES.0000134644.89917.96. [DOI] [PubMed] [Google Scholar]
- Edelbauer M, Datta D, Vos IH, Basu A, Stack MP, Reinders ME, et al. Effect of vascular endothelial growth factor and its receptor KDR on the transendothelial migration and local trafficking of human T cells in vitro and in vivo. Blood. 2010;116:1980–1989. doi: 10.1182/blood-2009-11-252460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Arbiser J, Brown BE, Rossiter H, Man MQ, Cerimele F, et al. Epidermal vascular endothelial growth factor production is required for permeability barrier homeostasis, dermal angiogenesis, and the development of epidermal hyperplasia: implications for the pathogenesis of psoriasis. Am J Pathol. 2008;173:689–699. doi: 10.2353/ajpath.2008.080088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol. 1992;140:539–544. [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Ye J, Shen F, Zhu Y, Yeghiazarians Y, Zhu W, et al. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab. 2008;28:90–98. doi: 10.1038/sj.jcbfm.9600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathallah-Shaykh HM, Zhao LJ, Kafrouni AI, Smith GM, Forman J. Gene transfer of IFN-gamma into established brain tumors represses growth by antiangiogenesis. J Immunol. 2000;164:217–222. doi: 10.4049/jimmunol.164.1.217. [DOI] [PubMed] [Google Scholar]
- Feltis BN, Wignarajah D, Zheng L, Ward C, Reid D, Harding R, et al. Increased vascular endothelial growth factor and receptors: relationship to angiogenesis in asthma. Am J Respir Crit Care Med. 2006;173:1201–1207. doi: 10.1164/rccm.200507-1105OC. [DOI] [PubMed] [Google Scholar]
- Feng D, Nagy JA, Pyne K, Hammel I, Dvorak HF, Dvorak AM. Pathways of macromolecular extravasation across microvascular endothelium in response to VPF/VEGF and other vasoactive mediators. Microcirculation. 1999;6:23–44. [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Frater-Schroder M, Risau W, Hallmann R, Gautschi P, Bohlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987;84:5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately MK, Warrier RR, Honasoge S, Carvajal DM, Faherty DA, Connaughton SE, et al. Administration of recombinant IL-12 to normal mice enhances cytolytic lymphocyte activity and induces production of IFN-gamma in vivo. Int Immunol. 1994;6:157–167. doi: 10.1093/intimm/6.1.157. [DOI] [PubMed] [Google Scholar]
- Gomaa AH, Yaar M, Eyada MM, Bhawan J. Lymphangiogenesis and angiogenesis in non-phymatous rosacea. J Cutan Pathol. 2007;34:748–753. doi: 10.1111/j.1600-0560.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Zhou Q, Proulx ST, Wood R, Ji RC, Ritchlin CT, et al. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum. 2009;60:2666–2676. doi: 10.1002/art.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halin C, Fahrngruber H, Meingassner JG, Bold G, Littlewood-Evans A, Stuetz A, et al. Inhibition of chronic and acute skin inflammation by treatment with a vascular endothelial growth factor receptor tyrosine kinase inhibitor. Am J Pathol. 2008;173:265–277. doi: 10.2353/ajpath.2008.071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Heidenreich R, Rocken M, Ghoreschi K. Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol. 2009;90:232–248. doi: 10.1111/j.1365-2613.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez GL, Volpert OV, Iniguez MA, Lorenzo E, Martinez-Martinez S, Grau R, et al. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med. 2001;193:607–620. doi: 10.1084/jem.193.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuze-Vourc'h N, Liu M, Dalwadi H, Baratelli FE, Zhu L, Goodglick L, et al. IL-20, an anti-angiogenic cytokine that inhibits COX-2 expression. Biochem Biophys Res Commun. 2005;333:470–475. doi: 10.1016/j.bbrc.2005.05.122. [DOI] [PubMed] [Google Scholar]
- Hirakawa S, Fujii S, Kajiya K, Yano K, Detmar M. Vascular endothelial growth factor promotes sensitivity to ultraviolet B-induced cutaneous photodamage. Blood. 2005a;105:2392–2399. doi: 10.1182/blood-2004-06-2435. [DOI] [PubMed] [Google Scholar]
- Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005b;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata S, Matsubara T, Saura R, Tateishi H, Hirohata K. Inhibition of in vitro vascular endothelial cell proliferation and in vivo neovascularization by low-dose methotrexate. Arthritis Rheum. 1989;32:1065–1073. doi: 10.1002/anr.1780320903. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh MY, Chen WY, Jiang MJ, Cheng BC, Huang TY, Chang MS. Interleukin-20 promotes angiogenesis in a direct and indirect manner. Genes Immun. 2006;7:234–242. doi: 10.1038/sj.gene.6364291. [DOI] [PubMed] [Google Scholar]
- Huggenberger R, Siddiqui SS, Brander D, Ullmann S, Zimmermann K, Antsiferova M, et al. An important role of lymphatic vessel activation in limiting acute inflammation. Blood. 2011;117:4667–4678. doi: 10.1182/blood-2010-10-316356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggenberger R, Ullmann S, Proulx ST, Pytowski B, Alitalo K, Detmar M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J Exp Med. 2010;207:2255–2269. doi: 10.1084/jem.20100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvid H, Teige I, Kvist PH, Svensson L, Kemp K. TPA induction leads to a Th17-like response in transgenic K14/VEGF mice: a novel in vivo screening model of psoriasis. Int Immunol. 2008;20:1097–1106. doi: 10.1093/intimm/dxn068. [DOI] [PubMed] [Google Scholar]
- Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–465. [PubMed] [Google Scholar]
- Jain S, Gabunia K, Kelemen SE, Panetti TS, Autieri MV. The anti-inflammatory cytokine interleukin 19 is expressed by and angiogenic for human endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:167–175. doi: 10.1161/ATVBAHA.110.214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, et al. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol. 2005;6:403–411. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya K, Detmar M. An important role of lymphatic vessels in the control of UVB-induced edema formation and inflammation. J Invest Dermatol. 2006;126:919–921. doi: 10.1038/sj.jid.5700126. [DOI] [PubMed] [Google Scholar]
- Kajiya K, Hirakawa S, Detmar M. Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function. Am J Pathol. 2006;169:1496–1503. doi: 10.2353/ajpath.2006.060197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya K, Kunstfeld R, Detmar M, Chung JH. Reduction of lymphatic vessels in photodamaged human skin. J Dermatol Sci. 2007;47:241–243. doi: 10.1016/j.jdermsci.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya K, Sawane M, Huggenberger R, Detmar M. Activation of the VEGFR-3 pathway by VEGF-C attenuates UVB-induced edema formation and skin inflammation by promoting lymphangiogenesis. J Invest Dermatol. 2009;129:1292–1298. doi: 10.1038/jid.2008.351. [DOI] [PubMed] [Google Scholar]
- Kanazawa S, Tsunoda T, Onuma E, Majima T, Kagiyama M, Kikuchi K. VEGF, basic-FGF, and TGF-beta in Crohn's disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. The American journal of gastroenterology. 2001;96:822–828. doi: 10.1111/j.1572-0241.2001.03527.x. [DOI] [PubMed] [Google Scholar]
- Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol. 2008;28:1928–1936. doi: 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- Kligman AM. Perspectives and problems in cutaneous gerontology. J Invest Dermatol. 1979;73:39–46. doi: 10.1111/1523-1747.ep12532758. [DOI] [PubMed] [Google Scholar]
- Kligman AM. The treatment of photoaged human skin by topical tretinoin. Drugs. 1989;38:1–8. doi: 10.2165/00003495-198938010-00001. [DOI] [PubMed] [Google Scholar]
- Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong WL, et al. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol. 1994;152:4149–4156. [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Kramer M, Sachsenmaier C, Herrlich P, Rahmsdorf HJ. UV irradiation-induced interleukin-1 and basic fibroblast growth factor synthesis and release mediate part of the UV response. J Biol Chem. 1993;268:6734–6741. [PubMed] [Google Scholar]
- Kriehuber E, Breiteneder-Geleff S, Groeger M, Soleiman A, Schoppmann SF, Stingl G, et al. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke ML. Ultraviolet radiation and immunology: something new under the sun-- presidential address. Cancer Res. 1994;54:6102–6105. [PubMed] [Google Scholar]
- Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, et al. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- Lange-Asschenfeldt B, Weninger W, Velasco P, Kyriakides TR, von Andrian UH, Bornstein P, et al. Increased and prolonged inflammation and angiogenesis in delayed-type hypersensitivity reactions elicited in the skin of thrombospondin-2--deficient mice. Blood. 2002;99:538–545. doi: 10.1182/blood.v99.2.538. [DOI] [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001a;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001b;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R, Stenn KS, Fernandez LA, Braverman IM. Angiogenic properties of normal and psoriatic skin associate with epidermis, not dermis. Lab Invest. 1989;61:162–165. [PubMed] [Google Scholar]
- Marks R, Harcourt-Webster JN. Histopathology of rosacea. Arch Dermatol. 1969;100:683–691. [PubMed] [Google Scholar]
- Mastroianni A, Minutilli E, Mussi A, Bordignon V, Trento E, D'Agosto G, et al. Cytokine profiles during infliximab monotherapy in psoriatic arthritis. Br J Dermatol. 2005;153:531–536. doi: 10.1111/j.1365-2133.2005.06648.x. [DOI] [PubMed] [Google Scholar]
- Montrucchio G, Lupia E, Battaglia E, Passerini G, Bussolino F, Emanuelli G, et al. Tumor necrosis factor alpha-induced angiogenesis depends on in situ platelet-activating factor biosynthesis. J Exp Med. 1994;180:377–382. doi: 10.1084/jem.180.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumprecht V, Detmar M. Lymphangiogenesis and Cancer Metastasis. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–1506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JD, et al. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001;158:867–877. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen HJ, Christensen IJ, Svendsen MN, Hansen U, Werther K, Brunner N, et al. Elevated plasma levels of vascular endothelial growth factor and plasminogen activator inhibitor-1 decrease during improvement of psoriasis. Inflamm Res. 2002;51:563–567. doi: 10.1007/pl00012428. [DOI] [PubMed] [Google Scholar]
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, et al. Interleukin- 17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Oikawa T, Hirotani K, Ogasawara H, Katayama T, Nakamura O, Iwaguchi T, et al. Inhibition of angiogenesis by vitamin D3 analogues. Eur J Pharmacol. 1990;178:247–250. doi: 10.1016/0014-2999(90)90483-m. [DOI] [PubMed] [Google Scholar]
- Oura H, Bertoncini J, Velasco P, Brown LF, Carmeliet P, Detmar M. A critical role of placental growth factor in the induction of inflammation and edema formation. Blood. 2003;101:560–567. doi: 10.1182/blood-2002-05-1516. [DOI] [PubMed] [Google Scholar]
- Paleolog EM. Angiogenesis in rheumatoid arthritis. Arthritis Res. 2002;4(Suppl 3):S81–S90. doi: 10.1186/ar575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001;167:1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- Patterson C, Perrella MA, Endege WO, Yoshizumi M, Lee ME, Haber E. Downregulation of vascular endothelial growth factor receptors by tumor necrosis factor-alpha in cultured human vascular endothelial cells. J Clin Invest. 1996;98:490–496. doi: 10.1172/JCI118816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse AD, Gaskell SA, Marks R. Epidermal changes in human skin following irradiation with either UVB or UVA. J Invest Dermatol. 1987;88:83–87. doi: 10.1111/1523-1747.ep12465094. [DOI] [PubMed] [Google Scholar]
- Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med. 2009;206:2925–2935. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol. 2009;183:1767–1779. doi: 10.4049/jimmunol.0802167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzer K, Baeten D, Soleiman A, Distler J, Gerlag DM, Tak PP, et al. Tumour necrosis factor blockade increases lymphangiogenesis in murine and human arthritic joints. Ann Rheum Dis. 2008;67:1610–1616. doi: 10.1136/ard.2007.083394. [DOI] [PubMed] [Google Scholar]
- Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K, et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63:5105–5113. [PubMed] [Google Scholar]
- Ribatti D, Puxeddu I, Crivellato E, Nico B, Vacca A, Levi-Schaffer F. Angiogenesis in asthma. Clin Exp Allergy. 2009;39:1815–1821. doi: 10.1111/j.1365-2222.2009.03385.x. [DOI] [PubMed] [Google Scholar]
- Ruegg C, Yilmaz A, Bieler G, Bamat J, Chaubert P, Lejeune FJ. Evidence for the involvement of endothelial cell integrin alphaVbeta3 in the disruption of the tumor vasculature induced by TNF and IFN-gamma. Nat Med. 1998;4:408–414. doi: 10.1038/nm0498-408. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- Sawano A, Iwai S, Sakurai Y, Ito M, Shitara K, Nakahata T, et al. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood. 2001;97:785–791. doi: 10.1182/blood.v97.3.785. [DOI] [PubMed] [Google Scholar]
- Schonthaler HB, Huggenberger R, Wculek SK, Detmar M, Wagner EF. Systemic anti-VEGF treatment strongly reduces skin inflammation in a mouse model of psoriasis. Proc Natl Acad Sci U S A. 2009;106:21264–21269. doi: 10.1073/pnas.0907550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibenge S, Gawkrodger DJ. Rosacea: a study of clinical patterns, blood flow, and the role of Demodex folliculorum. J Am Acad Dermatol. 1992;26:590–593. doi: 10.1016/0190-9622(92)70086-u. [DOI] [PubMed] [Google Scholar]
- Simonetti O, Lucarini G, Bernardini ML, Simoncini C, Biagini G, Offidani A. Expression of vascular endothelial growth factor, apoptosis inhibitors (survivin and p16) and CCL27 in alopecia areata before and after diphencyprone treatment: an immunohistochemical study. Br J Dermatol. 2004;150:940–948. doi: 10.1111/j.1365-2133.2004.05881.x. [DOI] [PubMed] [Google Scholar]
- Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. J Investig Dermatol Symp Proc. 2000;5:14–19. doi: 10.1046/j.1087-0024.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Strickland I, Rhodes LE, Flanagan BF, Friedmann PS. TNF-alpha and IL-8 are upregulated in the epidermis of normal human skin after UVB exposure: correlation with neutrophil accumulation and E-selectin expression. J Invest Dermatol. 1997;108:763–768. doi: 10.1111/1523-1747.ep12292156. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Kunkel SL, Elner VM, Martonyi CL, Koch AE, Polverini PJ, et al. Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol. 1992;141:1279–1284. [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- Szodoray L. [Nerval factors in the pathomechanism of psoriasis.] Arch Klin Exp Dermatol. 1955;201:581–586. [PubMed] [Google Scholar]
- Telner P, Fekete Z. The capillary responses in psoriatic skin. J Invest Dermatol. 1961;36:225–230. doi: 10.1038/jid.1961.36. [DOI] [PubMed] [Google Scholar]
- Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- Westerweel PE, Rabelink TJ, Rookmaaker MB, Grone HJ, Verhaar MC. RANTES is required for ischaemia-induced angiogenesis, which may hamper RANTES-targeted anti-atherosclerotic therapy. Thromb Haemost. 2008;99:794–795. doi: 10.1160/TH07-10-0628. [DOI] [PubMed] [Google Scholar]
- Wirzenius M, Tammela T, Uutela M, He Y, Odorisio T, Zambruno G, et al. Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting. J Exp Med. 2007;204:1431–1440. doi: 10.1084/jem.20062642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2010;207:101–115. S101–S102. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77–81. doi: 10.1016/j.jdermsci.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Kajiya K, Ishiwata M, Hong YK, Miyakawa T, Detmar M. Ultraviolet B-induced skin angiogenesis is associated with a switch in the balance of vascular endothelial growth factor and thrombospondin-1 expression. J Invest Dermatol. 2004;122:201–208. doi: 10.1046/j.0022-202X.2003.22101.x. [DOI] [PubMed] [Google Scholar]
- Yano K, Oura H, Detmar M. Targeted overexpression of the angiogenesis inhibitor thrombospondin-1 in the epidermis of transgenic mice prevents ultraviolet-B-induced angiogenesis and cutaneous photo-damage. J Invest Dermatol. 2002;118:800–805. doi: 10.1046/j.1523-1747.2002.01752.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lu Y, Proulx ST, Guo R, Yao Z, Schwarz EM, et al. Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthritis Res Ther. 2007;9:R118. doi: 10.1186/ar2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Matsuo H, Morita E. Increased production of vascular endothelial growth factor in the lesions of atopic dermatitis. Arch Dermatol Res. 2006;297:425–429. doi: 10.1007/s00403-006-0641-9. [DOI] [PubMed] [Google Scholar]