Chronic ultraviolet B (UVB) irradiation (290-320 nm) of the skin results in the degradation of extracellular matrix macromolecules, elastosis, formation of wrinkles [1] and enhanced risk for skin cancer [2] . We have previously found that chronic UVB irradiation of human and mouse skin induces pronounced angiogenesis of cutaneous blood vessels [3; 4]. Moreover, targeted overexpression of vascular endothelial growth factor (VEGF)-A enhanced the sensitivity to UVB-induced cutaneous photodamage [5], whereas transgenic overexpression of the angiogenesis inhibitor thrombospondin-1 in the epidermis completely prevented UVB-induced skin damage [3]. Together, these findings indicate that the cutaneous blood vasculature plays a critical role in the mediation of photodamage. In contrast, the role of cutaneous lymphatic vessels in the response to UVB irradiation has remained unknown.
Lymphatic vessels play a crucial role in the maintenance of tissue fluid homeostasis [6], and our recent results obtained in mouse models indicate that impairment of lymphatic vessel function is involved in the mediation of acute UVB-induced skin damage in mice because of increased leakiness of lymphatic vessels that was induced by enhanced levels of VEGF-A [7; 8]. To investigate the consequences of chronic UVB irradiation of human skin on cutaneous lymphatic vessels, we obtained skin samples from the face (sun-exposed skin; 2-mm punch biopsies from the crow's feet area not including wrinkles) and from the buttocks (non-sunexposed skin; 4-mm punch biopsies) of 17 healthy Korean volunteers. All procedures involving human subjects were approved by the Institutional Review Board of Seoul National University Hospital, and all subjects provided written informed consent. Facial skin samples were divided into 3 groups, according the the previously described scoring system for the severity of photodamage in Korean individuals [9]: Group 1: grades 1-3 (n=6); group 2: grades 4-5 (n=6); and group 3: grades 6-7 (n=5). Grade1 corresponds to absence of wrinkles whereas grade 7 corresponds to severely wrinkled skin [9].
We performed double immunofluorescence stains for the panvascular marker CD31 (BD Biosciences, Pharmingen, San Diego, CA) and for the lymphatic-specific hyaluronan receptor LYVE-1 (kindly provided by Dr. D. Jackson, Oxford, UK) as described [10]. By fluorescence microscopy, the density of LYVE-1 positive lymphatic vessels was apparently reduced in the dermis of samples obtained from photodamaged skin (Fig. 1 B,C) as compared to non-photodamaged skin (Fig. 1A). In contrast, no major difference in lymphatic vessel density was found by qualitative fluorescence microscopy analysis between all of the samples obtained from non-sunexposed buttock skin (Fig.1D-F). We next quantitatively measured the density of lymphatic vessels by calculating the average number of LYVE-1-positive lymphatic vessels per area unit (mm2) in the papillary and upper reticular dermis of the whole specimens by computer-assisted morphometric vessel analysis using the IP-Lab software (Scanalytics, Fairfax, VA) as described [10]. Although this method does not completely exclude the possibility that a tortuous vessel is sectioned - and thus counted - more than once, most vessels were clearly separated from each other. This analysis confirmed that the density of lymphatic vessels was significantly decreased in photodamaged facial skin (53% decrease for grades 4-5 and 47% decrease for grade 6-7) as compared to non-photodamaged facial skin (grades 1-3; Fig. 2). No significant differences of the lymphatic vessel density in non-sunexposed buttock skin were found between the three groups, and the vessel density in facial skin without photodamage was comparable to the lymphatic vessel density in the buttock skin (Fig. 2).
Figure 1.
Rarefication of lymphatic vessels in photodamaged facial skin. Double immunofluorescence stains for the panvascular marker CD31 (red) and the lymphatic vessel marker LYVE-1 (green) reveal a strong decrease of LYVE-1 positive lymphatic vessels in chronically photodamaged skin (grades 4-5, B; grades 6-7, C) as compared to skin with little or no photodamage (grades 1-3, A). In contrast, no major differences were found between samples obtained from non-sunexposed skin (buttock skin) of the same individuals (D-F). Scale bars = 100 μm.
Figure 2.
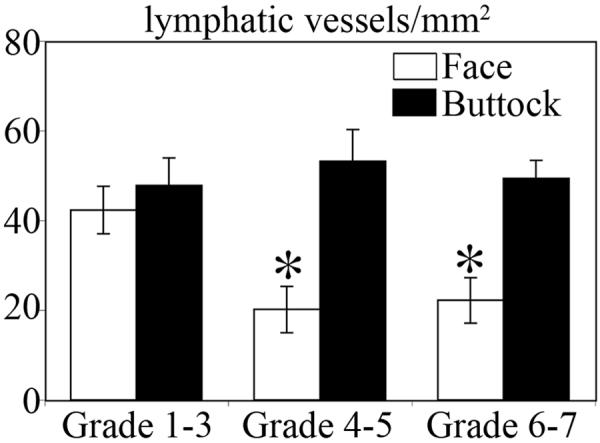
Decreased density of lymphatic vessels in photodamaged skin. Computer-assisted morphometric vessel analysis revealed a significant decrease of lymphatic vessel density in chronically photodamaged skin (grades 4 to 7) as compared to skin with little or no photodamage (grades 1-3). No significant differences in lymphatic vessel density were found in non-sunexposed skin (buttock skin) of the same individuals.
The pronounced decrease of lymphatic vessels observed in severely photodamaged facial skin suggests that impairment of lymphatic function might contribute to the mediation of cutaneous photodamage. In support of this concept, we recently found that blockade of the lymphatic-specific growth factor receptor VEGFR-3 resulted in the prolongation of acute UVB-induced edema formation in mice [7]. Thus, the decrease of lymphatic vessels in chronically photodamaged human skin might lead to impaired tissue drainage of fluids and proteins that have extravasated due to the UVB-induced leakage of blood vessels. The exact cellular and molecular mechanisms responsible for the decrease of lymphatic vessels in photoaged human skin remain to be elucidated. Because the endothelial cells of cutaneous lymphatic capillaries are attached to interstitial collagen and elastic fibers via fibrillin-containing anchoring filaments, degradation of elastic fibers in chronically UVB-damaged skin likely leads to reduced attachment of lymphatic endothelium to the extracellular matrix and thus might lead to rarefication of lymphatic vessels. It will be of great interest to investigate whether promotion of lymphatic growth and/or function by specific lymphangiogenesis factors such as VEGF-C or VEGF-D - that activate the lymphatic-specific receptor VEGFR-3 - or other recently identified growth factors [6], might attenuate UVB-induced cutaneous photodamage by increasing the number and/or the draining function of cutaneous lymphatic vessels.
Acknowledgements
This work was supported by National Institutes of Health grants CA69184 and CA92644, American Cancer Society Research Project Grant 99-23901, the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co. Ltd. Agreement, Swiss National Fund grant 3100A0-108207, Austrian Science Foundation grant S9408-B11, Cancer League Zurich, and Commission of the European Communities grant LSHC-CT-2005-518178 (all grants to M.D.) and Korea Science and Engineering Fund grant R11-2002-001-03001-0 through the Center for Aging and Apoptosis Research at Seoul National University (J.H.C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gilchrest BA. Skin aging and photoaging: an overview. J Am Acad Dermatol. 1989;21:610–3. doi: 10.1016/s0190-9622(89)70227-9. [DOI] [PubMed] [Google Scholar]
- 2.Kripke ML. Ultraviolet radiation and immunology: something new under the sun--presidential address. Cancer Res. 1994;54:6102–5. [PubMed] [Google Scholar]
- 3.Yano K, Oura H, Detmar M. Targeted overexpression of the angiogenesis inhibitor thrombospondin-1 in the epidermis of transgenic mice prevents ultraviolet-B-induced angiogenesis and cutaneous photo-damage. J Invest Dermatol. 2002;118:800–5. doi: 10.1046/j.1523-1747.2002.01752.x. [DOI] [PubMed] [Google Scholar]
- 4.Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol. 2005;152:115–121. doi: 10.1111/j.1365-2133.2005.06368.x. [DOI] [PubMed] [Google Scholar]
- 5.Hirakawa S, Fujii S, Kajiya K, Yano K, Detmar M. Vascular endothelial growth factor promotes sensitivity to ultraviolet B-induced cutaneous photodamage. Blood. 2005;105:2392–9. doi: 10.1182/blood-2004-06-2435. [DOI] [PubMed] [Google Scholar]
- 6.Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- 7.Kajiya K, Detmar M. An important role of lymphatic vessels in the control of UVB-induced edema formation and inflammation. J Invest Dermatol. 2006;126:919–21. doi: 10.1038/sj.jid.5700126. [DOI] [PubMed] [Google Scholar]
- 8.Kajiya K, Hirakawa S, Detmar M. Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function. Am J Pathol. 2006;169:1496–503. doi: 10.2353/ajpath.2006.060197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung JH, Lee SH, Youn CS, Park BJ, Kim KH, Park KC, Cho KH, Eun HC. Cutaneous photodamage in Koreans: influence of sex, sun exposure, smoking, and skin color. Arch Dermatol. 2001;137:1043–51. [PubMed] [Google Scholar]
- 10.Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, Lin C, Fiebiger E, Wei X, et al. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–57. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]



