Abstract
The baboon provides a natural non-human primate model for photosensitive, generalized epilepsy. This study describes an implantation procedure for the placement of subdural grid and strip electrodes for continuous video-EEG monitoring in the epileptic baboon to evaluate the generation and propagation of ictal and interictal epileptic discharges. Subdural grid, strip and depth electrodes were implanted in six baboons, targeting brain regions that were activated in functional neuroimaging studies during photoparoxysmal responses. The baboons were monitored with continuous video-EEG monitoring for 2–21 (mean 9) days. Although the animals were tethered, the EEG signal was transmitted wirelessly to optimize their mobility. Spontaneous seizures, interictal epileptic discharges (IEDs), and responses to intermittent light stimulation (ILS) were assessed. Due to cortical injuries related to the electrode implantation and their displacement, the procedure was modified. Habitual myoclonic and generalized tonic-clonic seizures were recorded in three baboons, all associated with a generalized ictal discharge, but were triggered multiregionally, in the frontal, parietal and occipital cortices. IEDs were similarly expressed multiregionally, and responsible for triggering most generalized spike-and-wave discharges. Generalized photoparoxysmal responses were activated only in one baboon, while driving responses recorded in all three photosensitive baboons were 2.5 times the stimulus rate. In contrast to previous intracranial investigations in this model, generalized ictal and interictal epileptic discharges were triggered by parietal and occipital, in addition to the frontocentral cortices. Furthermore, targeted visual areas responded differently to ILS in photosensitive than nonphotosensitive baboons, but further studies are required before mechanisms can be implicated for ILS-induced activation of the epileptic networks.
Keywords: Continuous Video-EEG, Intracranial Electrodes, Baboon, Photosensitivity, Idiopathic Generalized Epilepsy
Introduction
Epilepsy represents hyperexcitability of cortical-subcortical networks in the brain. The underlying mechanisms include inherited channelopathies, developmental or acquired brain injuries with altered connectivity or organization of the cortex resulting in decreased inhibition. Almost 40% of people with epilepsy have generalized epilepsy, based upon a simultaneous involvement of both hemispheres during an ictal or interictal epileptic discharge (www.epilepsyfoundation.org/about/statistics.cfm). Although generalized epilepsies are thought to be genetic in origin, and, in some cases, have been linked to inherited channelopathies, the mechanisms underlying generalized epilepsies are mostly unknown. Recently, blood oxygen level dependent (BOLD) functional MRI studies have delineated potential nodes of epileptic networks underlying generalized spike-and-wave discharges and absence seizures in humans (Aghakhani et al., 2004; Gotman et al., 2005; Bai et al., 2010), or in human photosensitivity (Moeller et al., 2009). One limitation of functional neuroimaging in humans with generalized epilepsies is the inability to record the electrophysiological activity using intracranial electrodes contributing regional BOLD changes. In generalized epilepsies, animal models are needed for the evaluation of electrophysiological activity using invasive electroencephalography (EEG).
The baboon offers a natural model of photosensitive, idiopathic generalized epilepsy (IGE), with myoclonic and generalized tonic clonic seizures that occur spontaneously or can be provoked by intermittent light stimulation (ILS; Killam, 1979). Photoparoxysmal and –convulsive responses could be reliably recorded and monitored with invasive, intracerebral depth electrodes (Fischer-Williams et al., 1968; Wada et al., 1972; Naquet et al., 1975). One of the shortcomings of these earlier studies was the inability to record continuously and, hence, record spontaneous seizures. These studies suggested that while ILS resulted in a driving response of the visual cortices, photoparoxysmal and –convulsive responses, as well as spontaneous interictal epileptic discharges (IEDs) appeared to be generated by the primary motor cortices, with secondary activation of the prefrontal cortices and thalamus (Fischer-Williams, 1968; Silva-Barrat et al., 1986). Although lesion studies demonstrated the occipital lobes were necessary to elicit photoparoxysmal or –convulsive responses, they were not implicated in the generation of the ictal or interictal epileptic discharges. This is in contrast to photosensitivity in humans, with photoparoxysmal responses being generated predominantly in the parietooccipital regions (Kasteleijn-Nolst Trenité, 1989; Takasaka et al., 1989). In later studies utilizing intracortical microelectrodes to record paroxysmal visual evoked potentials, researchers suggested a mitigating role of the parietal lobes in photosensitivity (Ménini et al., 1980). Similarly, preliminary H215O-PET studies evaluating the cerebral blood flow (CBF) response to ILS, demonstrated regional activations in the parietooccipital regions in photosensitive baboons, in addition to activations in motor and premotor cortices (Szabó et al., 2011), a pattern that reflects activations on BOLD-MRI studies in photosensitive humans (Moeller et al., 2009).
The goal of this study was to develop the methodology for implantation and maintenance of subdural grid and strip electrodes in epileptic baboons for long-term, continuous video-EEG monitoring. The ability to record continuously allows recording not only of photoparoxysmal or –convulsive responses, but also of spontaneous ictal and interictal epileptic discharges, resulting in a more accurate characterization of the underlying epilepsy. Subdural electrodes were implanted as they provide a better spatial resolution for the localization of potential cortical generators and cortico-cortical propagation of than depth electrodes. Using this approach, we aimed to reevaluate the baboon model of photosensitive epilepsy with respect to frontal predominance of provoked or spontaneous ictal and interictal epileptic discharges.
Methods
This study was approved by the Institutional Animal Care and Use Committees of the University of Texas Health Science Center at San Antonio (UTHSCSA) and the Texas Biomedical Research Institute (TBRI). We implanted six baboons, four females and two males, mean age 8 (+/−2) years old (Table 1). All of the baboons were of the Papio h. anubis or a hybrid subspecies. Five baboons had witnessed seizures, and subsequently underwent a scalp EEG in order to classify their epilepsy and photosensitivity. Three of the baboons were photosensitive, and epileptic (B2,B4,B6); the remaining two were epileptic, but not photosensitive (B3,B5). The control animal did not have a history of seizures, and her scalp EEG study was unremarkable (B1). The baboons were housed and treated in accordance with the “Guide for the Care and Use of Laboratory Animals” (Grossblatt, 1997).
Table 1.
Demographics and Overview of Scalp/Invasive EEG Studies
| Demographics at Implantation | Results of Previous Scalp EEG | Results of Invasive Video-EEG | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baboon | Age | Weight (kg) | Sex | IEDs | Seizures | Driving | PS | Days | IEDs | Seizures | Driving | PS |
| B1 | 10 | 18.8 | F | N | N | Y | N | 7 | N | N | Y | N |
| B2 | 6.5 | 26.8 | M | Y | Y | Y | Y | 4 | Y | Y (atyp) | Y (x2.5) | N |
| B3 | 8 | 20 | F | Y | Y | Y | N | 3 | Y | Y | NA | NA |
| B4 | 10.5 | 23 | M | Y | Y | Y | Y | 2 | Y | N | Y (x2.5) | Y |
| B5 | 4.5 | 12 | F | Y | Y | Y | N | 14 | Y | Y | Y | N |
| B6 | 7 | 14.5 | F | Y | Y | Y | Y | 21 | Y | Y | Y (x2.5) | N |
IEDs interictal epileptic discharges, F(emale), M(ale), N(o), Y(es), atyp(ical), PS photosensitivity, x2.5 (2.5 times the stimulus rate)
Surgical implantation
The baboons were initially sedated with intramuscular ketamine (10 mg/kg) and intravenous diazepam (10 mg). Following intubation, isoflurane at 1.5–2% inspiratory MAC was administered (Datex-Ohmeda Aestiva 5, WI, USA). Because of the risk for perioperative increases in intracranial pressure noted during initial surgeries, the baboons were hyperventilated to a pCO2 below 30 mm Hg and pretreated with mannitol. The baboons were placed in a prone position with their necks extended. Two symmetric 4–5 cm diameter craniotomies were performed in all but one animal. Subdural grid and strip electrodes (PMT, MN, USA) were implanted bilaterally in all five epileptic baboons, and unilaterally in the control animal (Figure 1b). Two 1×4 contact depth electrodes were placed orbitofrontally four animals, particularly as strips were implicated in a frontal lobe hemorrhage in one baboon. One 4×6 contact grid was implanted in the control animal, ere implanted in one baboon, two 3×6 grids in two animals, and two 3×5 grids in three animals. The grids were placed over the frontoparietal convexity centered across the primary sensorimotor sulci. Occipitoparietal 1×4 strips were implanted bilaterally in all animals, covering the medial occipital cortical areas. Both the orbitofrontal depth electrodes and occipitoparietal strips targeted brain regions which were most consistently activated on conditional and correlational H215O-PET analyses of ILS responses in PS and control baboons (Szabó et al., 2007; Szabó et al., 2011). The electrode cables were tunneled under the scalp of the baboon and extended subcutaneously between the shoulder blades to the level of the thoracic spine, where they exited into a backpack. The electrode cables were connected to the tether through adaptors. The animals underwent postoperative skull X-rays, which in all, but two cases, demonstrated successful implantation, except for the displacement of one orbitofrontal depth electrode in each case. The baboons were extubated and monitored for one to five days in a veterinary intensive care setting. The signal was transmitted via 32–64 pins bundled in the tether through a modified connector to the amplifier (AirEEG, Nihon-Kohden, Japan), which was fixed atop a “Lazy Susan”, which rotated with baboons’ movements (Figure 1c). The signal was transmitted either through a cable or wireless transmission system.
Figure 1. Distribution of IEDs and Motor Responses in B6 Superimposed on Skull X-Rays.
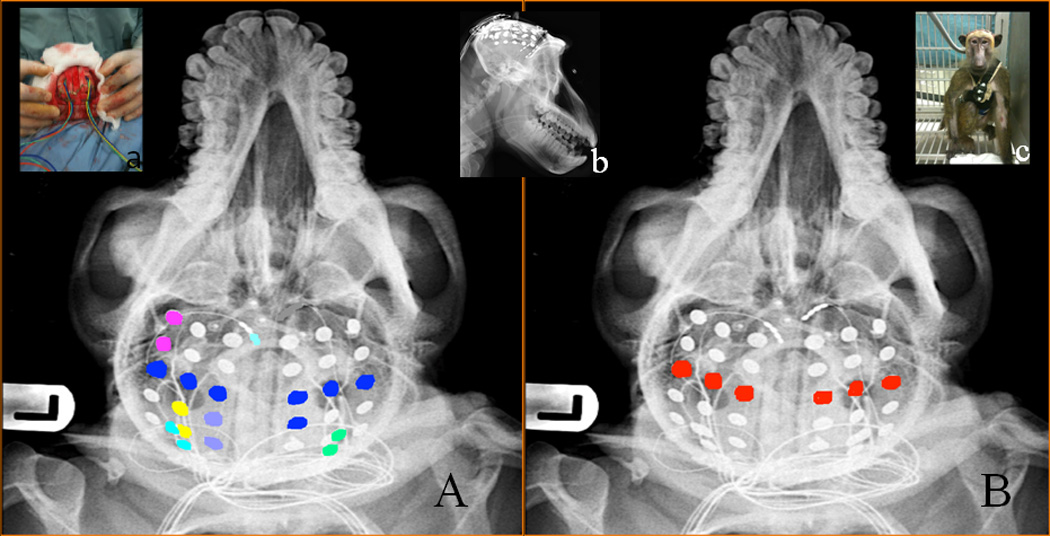
(A) Distribution of IEDS (each color represents regional IED population), (B) Motor responses (red); Inserts (a) implantation of grid electrodes, (b) lateral view of the head with electrodes, and (c) photograph of B6 a few hours after implantation.
Video-EEG Monitoring
Video-EEG recordings were performed using Neurofax 9200 (Nihon-Kohden, Japan) for 2–21 (mean 9) days. The recordings in three baboons were limited due to brain injury related to electrode placement and/or displacement of the subdural electrodes (B2,B3,B4). Two of these baboons had to be euthanized due to inability to thrive (B2,B4). One of the baboons (B2) was euthanized one week post-implantation, due to left-sided weakness and inability to thrive, while another baboon (B4) was euthanized 3 days after implantation, because of to progressive lethargy and inability to thrive. In both B3 and B4, the intracranial electrodes became displaced in to the subgaleal and subcutaneous area after dislodging the skull flap. In B3, there was no evidence of neurological injury and the skull flap returned to its original position. She was kept alive for a year after implantation and was utilized in other studies. B4 sustained cortical injury with subdural hemorrhages. In both of these cases, the grids became dislocated with abrupt head movements and seizure related falls. The other three baboons (B1,B5,B6) were monitored as scheduled, the control animal for 7 days, the second for 14 days and the third for 3 weeks. One of them (B6) had a transient left foot drop, which resolved within 48 hours. ILS was performed on a weekly basis. The ILS protocol has been described previously, during which the baboons are exposed to frequencies of 1–30 Hz (Szabó et al., 2004). The baboon was transferred to a primate chair requiring light sedation with intramuscular ketamine 5 mg/kg. Bipolar electrocortical stimulation was performed using an Ojemann Stimulator (50 Hz, 0.5 msec pulse duration, current output 0.5–3 mA) in two baboons (B5,B6) following ILS to identify primary motor cortex.
All four surviving baboons (B1,B3,B5,B6) were monitored after electrode removal for 10 days to 18 months. All of the animals recovered well after the surgery, except one baboon that harbored a chronic infection of a surgical scar.
Video-EEG recordings were reviewed by the principal investigator (CAS). The control baboon’s study was reviewed to characterize awake and sleep states and normal ILS responses. The video-EEG studies were reviewed for the remaining baboons, initially with the goal of classifying and enumerating seizures, then to characterize IEDs both in wakefulness and sleep, and finally, to evaluate the response to ILS in each baboon.
Pathological Evaluation
Pathological evaluation was performed in five baboons (B1,B2,B4,B5,B6) in order to identify cortical injury or chronic inflammatory response. The examination included gross pathology as well as histopathology using hematoxylineosin stains. In two cases (B2,B4), the examination was performed acutely in the setting of a necropsy. In the remaining 3 cases (B1,B5,B6), the examination was performed 10 days to 4 months after the electrodes were removed.
Results
Normal Awake and Sleep
The awake posterior background of the control baboon (B1, Figure 2) was 6–8 Hz, consisting of sharply contoured activity (250–400 microvolts in amplitude) maximal on the most caudal three contacts of the occipital strip. The posterior background waxed and waned in drowsiness, with intermixed lower amplitude fast frequency activities and episodic suppression (<50 microvolts). In restful wakefulness or drowsiness, the sensorimotor cortices demonstrated a 50–100 microvolt 20–25 Hz activity. In sleep, 100–200 microvolt spindle activity of approximately 12–15 Hz was visible in the frontocentral regions, with the occipital regions demonstrating 0.5–3 second paroxysms of 25–30 Hz intermixed with 5–6 Hz activity, separated by periods of suppression. Frontally, discrete paroxysms of 75–200 microvolt activity of 30–50 Hz frequency, lasting 3–10 seconds, emerged with a waxing and waning in amplitude and frequency. These were often associated with high amplitude bursts (500–600 microvolts) of 10 Hz discharges lasting about 0.5 seconds. Other activities associated with sleep included 4–6 Hz frontally predominant saw-tooth waves, but these could not be clinically correlated with rapid eye movements. There was no difference in the awake or asleep background activity between the control animal and B3-B6.
Figure 2. Awake Background in the Asymptomatic, Control Animal.
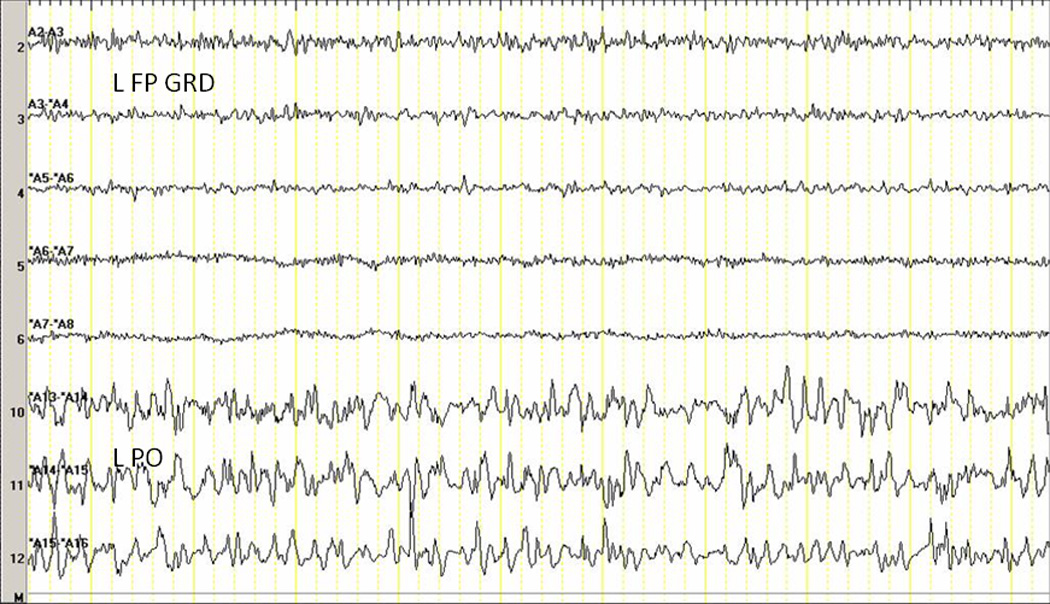
L FP GRD left frontoparietal grid, L PO left parieto-occipital strip
Spontaneous Seizures
Spontaneous seizures were recorded in three (B3,B5,B6) of five epileptic baboons (Table 1). Generalized tonic-clonic seizures were recorded in two female baboons (B5,B6; Table 2). In B5, two generalized tonic-clonic seizures were recorded, one with onset in the medial parietal (58 seconds duration) and the other in the left orbitofrontal region (70 seconds duration). In the B6, 26 generalized tonic-clonic were seizures recorded, 20 to 32 seconds duration (Figure 3). All of the GTCS were associated with a generalized suppression followed by the evolution of a paroxysmal fast activity, which gradually increased in amplitude and slowed, converting to generalized spike-and-wave discharges. The onset of the seizures was characterized by a more localized transient, which appeared to originate in the frontal, central or occipital regions.
Table 2.
Results of Long-term Video-EEG Monitoring in B5 and B6
| Baboon | Interictal Epileptic Discharges | Seizures | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Location | Seizure Type |
Stimulus | # | EEG Onset | Duration | Day | ||||
| Day 1 | Day 7 | Day 14 | Day 21 | |||||||
| B5 | LOF 1/2 | LOF NA | LOF 1/2 | GTCS | Spontaneous | 2 | LG 15–16, 21/22; LOF 1/2 |
58, 70 | 4, 13 | |
| LOF 1/2 LG 7/8 |
LOF NA LG 7/8 |
LOF 1/2 LG 7/8 |
Tonic | Ketamine- induced |
16 | LF 1/2; LG 8/14 |
13–27 | Every sedation |
||
| LO 3/4 | LO 2, 3/4 | LO 2, 3/4 | R Focal motor→ GTCS |
ECS-induced | 1 | LG 7/8 and LG 13/14 |
50 | ECS | ||
| LG 8/14 LG 14/20 |
LG 8/14 LG 14/20 |
LG 8/14 LG 14/20 |
R Focal motor→ GTCS |
ECS-induced | 1 | LG 14/15 and LG 20/21 |
74 | ECS | ||
| LG 15/16 LG 21/22 |
LG 15/16 LG 21/22 |
LG 15/16 LG 21/22 |
WCS | Spontaneous | 49 | RO, LO | 44–205 | 1–3, 8– 11 |
||
| RO 3 | RO 2, 3 | RO NA | WCS | Spontaneous | 49 | LG 9 | 50–171 | 3–4 | ||
| B6 | GEN LG 4,10/11, 15/16 RG 3,9,15 |
GEN LG 4,10,16 RG 3,9,15 |
GEN LG 4,10,16 RG 3,9,15 |
GEN LG 10/16 LG 10/11, 15/16→GEN RG 2/3, 9/10 →GEN |
GTCS | Spontaneous | 26 | LG 2/3 →GEN LO 3 →GEN RO 2 →GEN LG 15/16 or RG 2/3 →GEN |
20–32 | 1–6,13, 16,20 |
| LG 2/3, 11/12 or 17/18 |
LG 2/3, 11/12 or 17/18 |
LG 2/3, 11/12 or 17/18 |
LG 2/3, 11/12 or 17/18 |
MS | Spontaneous Arousal |
144 | LG 12/13, RG 3 | <0.5 | 13–21 | |
| LO 3/4 or RO 2, 3/4 |
LO 3/4 or RO 2, 3/4 |
LO 3/4 or RO 2, 3/4 |
LO NA RO 2 |
GTCS | ECS | 1 | LG 10/11 and LG 16, RG 3 |
15 | 7 | |
| LOF 1/2, RO 3 LO 3/4, ROF NA |
LOF 1/2 LG 10/15 ROF NA |
LOF NA ROF NA |
LOF NA, ROF NA |
WCS | Spontaneous | 2 | LOF 1/2 LG8/9,14/15RG 3/4 |
15–17 | 1 | |
LOF left orbitofrontal, LG left grid, RG right grid, LO left occipital, RO right occipital, GEN(eralized), GTCS generalized tonic-clonic seizure, MS myoclonic seizure, ECS electrocortical stimulation, WCS ictal EEG pattern without clinical signs, NA not available, # (of seizures).
Figure 3. EEG Onset of a GTCS in B6.
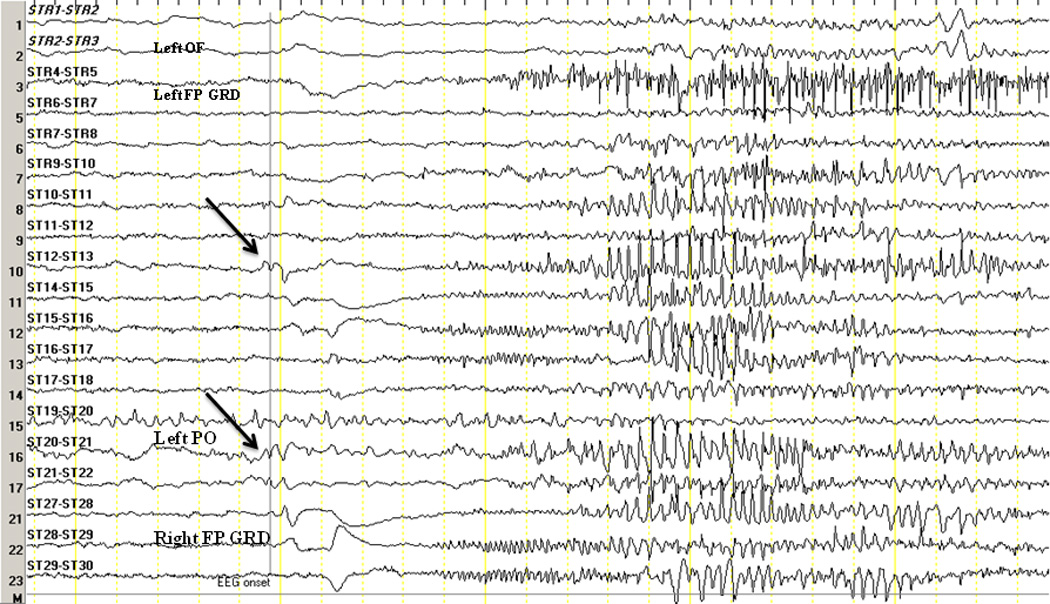
The arrows indicate EEG onset characterized by an initial transient in the right parietal region (ST13/21). OF orbitofrontal depth electrode, FP GRD frontoparietal grid, PO parieto-occipital strip. The left-sided grid was not connected at the time.
Myoclonic seizures were noted in two baboons (B3,B6). Due to their brevity, myoclonic seizures were difficult to identify on video recordings unless they were repetitive. In B3, we relied on muscle artifact, in B6 on cable artifacts. The discharge underlying myoclonic seizures consisted of polyspikes with frequency of 15–20 Hz, lasting no longer than 300 msec, localized to the motor cortices. In B3, the myoclonic seizures were, in part, triggered by a focal right parietal ictal, otherwise subclinical, discharge. In B6, the myoclonic seizures occurred about one minute after each arousal, and were associated with a polyphasic discharge in the motor cortices. Myoclonic jerks affecting the trunk were also recorded in B5, but these were associated with regional parieto-occipital ictal discharges without involvement of the sensorimotor cortices, and hence may have been subcortical.
The most frequent seizures were EEG events consisting of ictal epileptic discharges which evolved temporally, spatially and in frequency, without any observable clinical symptoms (B2,B3,B5,B6). Common to all of these ictal events was a focal or regional onset. The most common origin of the ictal epileptic discharges included the parietal lobes (B2,B3,B5,B6), occipital lobes (B5) and orbitofrontal cortices (B6). The discharges were usually relatively brief (i.e. shorter than 30 seconds), but occipital lobe seizures could last up to 205 seconds without clinical symptoms.
Symptomatic seizures
One of the male baboons (B2) with a history of generalized myoclonic and tonic-clonic seizures and generalized interictal epileptic discharges (IEDs) on scalp EEG, developed a partial status epilepticus with onset in the right parietal region shortly after implantation. Prior to the onset of the seizures, he already demonstrated a left hemiparesis, mainly affecting his arm. The partial seizures were associated with decreased responsiveness and head turning to the left accompanied by left face and arm clonic activity. Over a 48-hour period, over 200 seizures were recorded, occurring every 5 to 6 minutes and were 24 to 305 (mean 65) seconds in duration. Only seizures lasting longer than 45 seconds were associated with motor symptoms. The seizures became better controlled on phenytoin and midazolam, occurring once every 10–20 minutes with 12 to 96 (mean 41) second duration before remitting. The seizures originated from the right parietal lobe, which was traumatized by the implanted electrodes as demonstrated on necropsy. As the focal seizures remitted, the expected generalized spike-and-wave discharges emerged, though none of the generalized motor seizures were recorded.
Provoked seizures
Eighteen ketamine-induced tonic seizures were recorded in B5, lasting 13 to 17 seconds in duration and occurred 5–15 minutes after intramuscular ketamine administration. The ictal discharge was characterized by bihemispheric rhythmical discharges of 10–12 Hz frequency, originating occipitally, frontally or from the primary sensorimotor cortices. One of them was a typical right occipital discharge lasting 306 seconds, without any overt clinical correlate. Electrocortical stimulation was performed in two animals (B5,B6) in order to localize the primary motor cortices. Partial motor seizures, some of which generalized secondarily, were triggered in the motor regions of each baboon at or above 1.0 mA. The motor thresholds in both cases were 0.7 mA for face and hand regions (Figure 1B).
Interictal Epileptic Discharges
IEDs were generalized and multiregional in all of the epileptic baboons (Table 2, Figure 1A). Most of the IEDs were expressed regionally, or in homologous regions of both hemispheres with lateralization. They tended to be maximal in the sensorimotor, orbitofrontal, frontal, parietal and occipital cortices. The discharges were usually isolated and rarely repetitive (usually only in sleep or due to ketamine). Generalized IEDs were maximally expressed in the sensorimotor cortices, but were frequently triggered by more focal discharges in the same regions, frontally, parietally or occipitally. However, IEDs that were visible on scalp electrodes were generalized in distribution.
Responses to ILS
ILS was successfully performed in five baboons (B1,B2,B4,B5,B6), repeated 2 to 4 times in three of them (B1,B5,B6). Generalized photoparoxysmal responses were noted only in B4, consisting of individual, generalized IEDs activated within 2–3 seconds after stimulus onset. These discharges were expressed simultaneously in parieto-occipital and frontocentral regions, with secondary propagation to the orbitofrontal areas. While focal frontocentral and parito-occipital discharges were also noted, these appeared randomly and were not increased during ILS. In B6, ILS-induced unilateral occipital spikes occasionally propagated to the ipsilateral sensorimotor cortices in a time-locked fashion (Figure 4). On the other hand, photic driving responses were recorded in all five animals exposed to ILS, including the three photosensitive baboons (B2,B4,B6), one nonphotosensitive, epileptic baboon (B5), and the control baboon (B1). The photic driving response in the two nonphotosensitive animals corresponded to the delivered ILS frequency, whereas the driving rate in photosensitive baboons was about 2.5 times the ILS frequency. In other words, a 3 Hz stimulus produced a 7.5 Hz driving response, 6 Hz produced a 15 Hz driving response, while a 30 Hz stimulus produced a 75 Hz driving response. The driving response was restricted to the occipital regions at frequencies at or below 6 Hz in two baboons, but spreading to the frontoparietal cortices in all baboons at 9–30 Hz.
Figure 4. Driving Response in a Photosensitive Baboon.
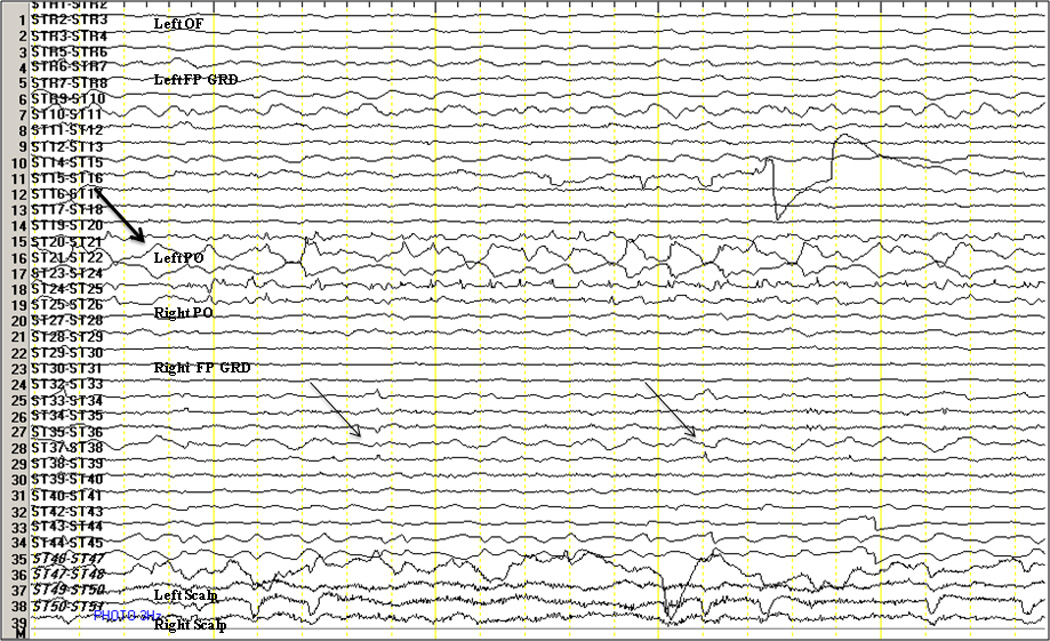
The top arrow indicates an 8 Hz occipital driving response to 3 Hz ILS lateralized to the right occipital region in B6. The two arrows below indicate propagation of the right occipital discharges to the ipsilateral motor and premotor areas. L(eft), R(ight), OF orbitofrontal depth electrode, FP GRD frontoparietal grid, PO parieto-occipital strip.
Cortical Histopathology
In the two animals (B2,B4), that had to be euthanized post-impantation, one animal demonstrated intraparenchymal hemorrhages induced by the right frontal and parietal lobes related to penetrating injuries with subdural strips and venous compression, respectively, while the other developed bilateral subdural hematomas related to the displacement of the subdural grids (B4). The control animal’s pathological examination revealed meningeal inflammation, perivascular cuffing and venous distention of peripheral vessels, with evidence of focal parenchymal damage near to the posterior edge of the grid, near to the cable departure from the skull. Focal parenchymal damage was also evident in the same region in B5 after being monitored for 2 weeks, only in this case there was associated perivascular edema, satellitosis and gitter cells suggesting inflammation. Nonetheless, in the last implanted baboon (B6) with three weeks of recording, only meningeal inflammation was noted with minimal evidence of subpial neuronal necrosis, without parenchymal injury.
Technical Modifications
Based upon complications and pathological findings our procedure was incrementally modified until the successful implantation of B6. Initially, pediatric grids and strips were used, but due to the relatively tighter subdural space, and subsequent pressure of the electrodes on the cortical veins and parenchyma (B1), these electrodes (the contacts and plastic sheaths) were decreased to half of their original thickness (PMT, MN, USA). Subdural electrodes were replaced by depth electrodes orbitofrontally, due to penetrating parenchymal trauma resulting in a localized hemorrhagic injury to one frontal lobe (B2). The mobility of the baboons and their seizure-related falls resulted in displacement of the electrodes and secondary damage (B3,B4) led to fixation of the bone flaps with titanium clamps. Finally, tension of grid cables exiting the skull, that lifted the posterior edges of the grids and damaged the cables, resolved by using grids with centrally exiting cables, which were subsequently channeled through a craniotomy in the center of the skull flap (B6, Figure 1a).
Discussion
This is the first intracranial evaluation of the photosensitive epilepsy of baboons in several decades. Modifications of previous protocols (Fischer-Williams et al., 1968; Wada et al., 1972; Naquet et al., 1975) included the adaptation of continuous video-EEG monitoring to record spontaneous generalized myoclonic and tonic-clonic seizures, and the implementation of a combination of subdural grid, strip and depth electrodes instead of merely depth electrodes to improve spatial resolution of ictal or interictal epileptic discharges on the cortical surface. Furthermore, cerebral blood flow (CBF) studies were utilized to generate targets for electrode placement (Szabó et al., 2007; Szabó et al., 2011). Finally, improvement of temporal sampling rates also offers the opportunity to evaluate the propagation of ictal and interictal epileptic discharges, and potentially record high frequency oscillations, which may not only underlie CBF increases, but also play a role in the abnormal synchronization of functionally connected cortices (Parra et al., 2003). By adapting these technological innovations, our goal was to better characterize both ictal and interictal EEG in the epileptic baboon. While this data is preliminary, serving mainly as proof of concept, it offers new directions for future development of an EEG platform to study this important natural, nonhuman primate model of epilepsy.
The greatest challenge was the adaptation of intracranial electrodes to fit the baboon’s subdural space, without applying increased pressure on the underlying cortex or comprimising venous circulation, yet providing resilience to withstand seizure-related falls from the top of the cage and other abrupt movements. Variable degrees of cortical injury related to the electrodes were noted on histopathology in all but the last baboon. The injuries ranged from intraparenchymal or subdural hemorrhages related to electrode displacement, to subtle histological changes associated with meningeal inflammation related to long-term instrumentation. Unfortunately, regardless of the electrode type, long-term monitoring with intracranial electrodes can cause inflammation and subsequent neuronal injury, which may be associated with alterations of cortical excitability (Hirschler et al., 2010), either by reducing the cortical EEG signal or increasing cortical irritability. Hence, caution must be exercised in the interpretation of the electroclinical findings. Recording from brain regions not implicated in the expression of generalized ictal and interictal epileptic discharges can be used to control for electrode-related increases in hyoprexcitability. The recording of high frequency oscillations may also help to differentiate interictal epileptic discharges from other less specific epileptiform activity (Cendes and Engel, 2011). Nonetheless, it is essential to interpret the video-EEG data in context of available CBF data and electrophysiological findings in asymptomatic control animals.
Despite the relatively uniform ictal and interictal scalp EEG findings in these baboons, it is clear that invasive EEG may unravel a large number of subphenotypes, based upon clinical and subclinical seizure characterization and the distribution of IEDs. But despite the variability of the clinical and EEG findings, it is evident that this epilepsy model is generalized as it is multiregional, with generalized ictal and interictal epileptic discharges frequently following lateralized or focal discharges in a time-locked manner. In contrast to Naquet’s hypothesis suggesting that the epilepsy was primarily frontocentral, we showed that ictal and interictal epileptic discharges can arise in the parietal and occipital regions in addition to the frontocentral cortices (Naquet et al., 1975; Ménini and Silva-Barrat, 1998). There may be several reasons why our data diverge from EEG findings acquired four decades ago (Fischer-Williams et al., 1968; Wada et al., 1972; Corcoran et al., 1972). One reason may be the differences between epileptic phenotypes among baboon subspecies. The data collected in earlier studies pertained to Papio h. papio collected from Senegal. The animals in this study belonged to Papio h. anubis and its hybrids collected in Kenya and Tanzania, and crossbred in primate colonies in the United States (Balzamo et al., 1975). While phenotypic differences, such as differences in degrees of photosensitivity, could provide a possible explanation, scalp EEG studies demonstrated a surprising stability of the electroclinical phenotypes among the subspecies (Killam et al, 1967; Szabó et al., 2005). Another potential difference, as mentioned above, may be related to cortical irritation caused by subdural electrodes themselves. Supporting this possibility is the fact that the electrodes caused cortical injury with functional deficits in one baboon, subsequently giving rise to a partial status epilepticus. Once the focal status resolved, his habitual generalized interictal epileptic discharges emerged. Arguing against the interference by ictal or interictal activity related to cortical injury, was the stability of the location, frequency and amplitude of multiregional ictal and interictal discharges in the two animals with the most prolonged recordings, and the lack of parenchymal histopathology in the last implanted baboon. Finally, the most important difference of our approach to earlier studies was the use of mainly subdural electrode arrays, which have better spatial resolution, particularly for evaluating cortico-cortical propagation, than depth electrodes.
One shortcoming of our study was our inability to activate seizures with ILS. Three of the epileptic baboons were diagnosed as photosensitive by their scalp EEG recordings. All of the PS baboons included in this study had myoclonic seizures or generalized IEDs time-locked to stimulus onset on prior scalp EEG studies. Regardless, generalized photoparoxysmal responses were recorded in only one animal before his electrodes were displaced. But even the generalized IEDs induced by ILS were expressed simultaneously in the parieto-occipital and frontocentral regions, a distribution which was not described earlier depth electrode studies in baboons (Fischer-Williams et al., 1968), yet resembling scalp EEG findings in humans (Kasteleijn-Nolst Trenité, 1989; Takasaka et al., 1989). More regional or focal photoparoxysmal responses were not identified, but in one animal there was evidence of parieto-occipital responses preceding focal frontocentral spikes, also suggesting a posterior to anterior propagation pattern. As the number of trials and spatial sampling were limited, further studies need to be performed. The absence of photoparoxysmal responses in the remaining photosensitive baboons may have been related to their treatment with antiepileptic medications prior to implantation, which can suppress photosensitivity for weeks after they are discontinued (Harding et al., 1978).
Nonetheless, all three PS baboons did demonstrate altered ILS responses compared to the nonphotosensitive animals. The driving response in the PS baboons was 2.5 times the ILS rate, regardless of ILS frequency, and despite demonstration of responses conforming ILS frequencies prior scalp EEG studies. It is likely that this shift in driving frequency is, restricted to particular visual pathways, and hence, not detected by scalp electrodes. Nonetheless, as the superior medial occipital convexity, where the response was recorded, projects to parietal and frontal association areas (Yeterian and Pandya, 2010), this area may represent a node in the visual networks driving extrastriatal networks. The driving response appeared to synchronize these association cortices at frequencies of 15 Hz and higher, even in the control baboon. The observed frequency shift of the ILS response in the PS baboons and the synchronization of frontal and parietal cortices may be two factors contributing to the generation of the photoparoxysmal response in the baboon. A recent magnetoencephalography study in photosensitive humans demonstrated that ILS-induced phase clustering, or synchronization, of gamma frequency activity in the occipital and frontoparietal cortices was associated with the activation of photoparoxysmal responses (Parra et al., 2003). Unfortunately, in humans there are no intracranial EEG recordings of photoparoxysmal responses in association with idiopathic generalized epilepsy. Two cases of mesial temporal lobe seizures triggered by ILS were reported, but there was no regional variability described in the occipital driving responses (Benbadis et al., 1996; Isnard et al., 1998). In baboons, photoparoxysmal and –convulsive responses are maximally expressed at ILS frequencies of 20–25 Hz, compared to 12–18 Hz in humans (Naquet et al., 1975). At these ILS frequencies, the medial occipital lobe and functionally connected extrastriatal cortices were driven at 50–75 Hz. It is possible that the driving frequency recorded by the subdural electrodes also reflected averaged responses, and that even higher frequency discharges generated by the occipital lobe are responsible for synchronization and triggering of the epileptic network. Future invasive EEG studies need to rigorously evaluate hyperexcitability of occipital lobe circuits with a combination of subdural and microelectrodes, utilizing higher sampling rate to detect a wider spectrum of high frequency activity, including ripple and fast ripples, which may trigger the epileptic networks.
Conclusions
Continuous electrophysiological monitoring using subdural electrodes in the epileptic baboon is a potentially safe and effective procedure for characterizing electroclinical phenotypes. In contrast to earlier hypotheses suggesting that the photosensitive, generalized epilepsy of the baboon invoked primarily motor and premotor cortices (Naquet et al., 1975; Ménini and Silva-Barrat, 1998), this study demonstrated that multiple regions, including the occipital and parietal cortices are potentially epileptogenic and can provoke epileptic seizures. Furthermore, photosensitivity may be associated with an altered physiological response of the occipital lobe networks, instead of only altered seizure thresholds of the motor cortex as previously proposed (Naquet et al., 1975). Nonetheless, there is a need to optimize electrode coverage to target the complete cortical-subcortical network as demonstrated by functional neuroimaging studies (Szabó et al., 2007), using a combination of subdural grid, strip and depth electrodes. Prolonged video-EEG recording with electrodes for monitoring eye movements, truncal or appendicular muscle activity and cardiac functions, would be useful to evaluate the effect of sleep stages, diurnal and catamenial variability on ictal or interictal epileptic discharges, and conversely, the effect of seizures and IEDs on sleep and cardiac rhythms. Furthermore, EEG coherence analyses of high-frequency cortical rhythms could help model cortical connectivity and spatiotemporal propagation of spontaneous and provoked IEDs. Finally, the baboon model can be adapted for testing efficacy and electrophysiological mechanisms of action of future antiepileptic medications or other nonmedical therapies for idiopathic generalized epilepsy in humans (Killam et al., 1973; Meldrum et al., 1975).
Acknowledgements
This study was supported by National Institutes of Health (R21 NS065431 to CÁS, Ruth L. Kirshstein National Research Service Award (T32) NS066694 to FSS, and NS047755-01 to JTW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, Gotman J. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127:1127–1144. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, Desalvo M, Novotny EJ, Constable RT, Blumenfeld H. Dynamic time course of typical childhood absence seizures: EEG behavior, and functional magnetic resonance imaging. J Neurosci. 2010;30:5884–5893. doi: 10.1523/JNEUROSCI.5101-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzamo E, Bert J, Menini C, Naquet R. Excessive light sensitivity in Papio papio: Its variation with age sex, and geographic origin. Epilepsia. 1975;16:269–276. doi: 10.1111/j.1528-1157.1975.tb06057.x. [DOI] [PubMed] [Google Scholar]
- Benbadis SR, Gerson WA, Harvey JH, Lüders HO. Photosensitive temporal lobe epilepsy. Neurology. 1996;46:1540–1542. doi: 10.1212/wnl.46.6.1540. [DOI] [PubMed] [Google Scholar]
- Cendes F, Engel J., Jr Extending applications for high-frequency oscillations. Neurology. 2011;77:518–519. doi: 10.1212/WNL.0b013e318228c16f. [DOI] [PubMed] [Google Scholar]
- Corcoran ME, Cain DP, Wada JA. Photically induced seizures in the yellow baboon, papio cynocephalus. J Can Sciences Neurologiques. 1979;6:129–131. doi: 10.1017/s0317167100119511. [DOI] [PubMed] [Google Scholar]
- Fischer-Williams M, Poncet M, Riche D, Naquet R. Light-induced epilepsy in the baboon, Papio papio: cortical and depth recordings. Electroencephalography Clin Neurophysiol. 1968;25:557–569. doi: 10.1016/0013-4694(68)90235-6. [DOI] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Nat Acad Sci USA. 2005;102:15236–15240. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossblatt N. Guide for the Care and Use of Laboratory Animals. Washington D.C.: National Academy Press; 1997. [Google Scholar]
- Harding GFA, Herrick CE, Jeavons PM. A controlled study of the effect of sodium valproate on photosensitive epilepsy and its prognosis. Epilepsia. 1978;19:555–556. doi: 10.1111/j.1528-1157.1978.tb05036.x. [DOI] [PubMed] [Google Scholar]
- Hirschner Y, Polat U, Biegon A. Intracranial electrode implantation produces regional inflammation and memory deficits in rats. Exp Neurol. 2010;222:42–50. doi: 10.1016/j.expneurol.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnard J, Guénot M, Fischer C, Mertens P, Sindou M, Maugière F. A stereoelectroencephalographic (SEEG) study of light-induced mesiotemporal epileptic seizures. Epilepsia. 1998;39:1098–1103. doi: 10.1111/j.1528-1157.1998.tb01296.x. [DOI] [PubMed] [Google Scholar]
- Kasteleijn-Nolst Trenité DGA. Photosensitivity in epilepsy: Electrophysiological and clinical correlates. Acta Neurol Scand Suppl. 1989;125:3–149. [PubMed] [Google Scholar]
- Killam EK, Starck LG, Killam KF. Photic stimulation in three species of baboons. Life Science. 1967;6:1569–1574. doi: 10.1016/0024-3205(67)90165-8. [DOI] [PubMed] [Google Scholar]
- Killam EK, Matsuzaki M, Killam KF. Studies of anticonvulsant compounds in the Papio papio model of epilepsy. In: Sabelli H, editor. Chemical Modulation of Brain Function. New York: Raven Press; 1973. pp. 161–171. [Google Scholar]
- Killam EK. Photomyoclonic seizures in the baboon, Papio papio. Federation Proc. 1979;38:2429–2433. [PubMed] [Google Scholar]
- Meeren H, van Luijtelaar G, da Silva FL, Coenen A. Evolving concepts on the pathophysiology of absence seizures: The cortical focus theory. Arch Neurol. 2002;62:371–376. doi: 10.1001/archneur.62.3.371. [DOI] [PubMed] [Google Scholar]
- Meldrum BS, Anlezark G, Balzamo E, Horton RW, Trimble M. Photically induced epilepsy in Papio papio as a model for drug studies. In: Meldrum BS, Marsden CD, editors. Advances in Neurology. Vol 10. New York: Raven Press; 1975. pp. 119–128. [PubMed] [Google Scholar]
- Ménini C, Silva-Barrat C. The photosensitive epilepsy of the baboon: A model of generalized reflex epilepsy. In: Zifkin BJ, Andermann F, Beaumanoir A, Rowan AJ, editors. Reflex Epilepsies and Reflex Seizures: Advances in Neurology. Vol 75. Philadelphia: Lippincott-Raven Publishers; 1998. pp. 29–47. [PubMed] [Google Scholar]
- Ménini C, Stutzmann JM, Laurent H, Naquet R. Paroxysmal visual evoked potentials (PVEPs) in Papio papio. I. Morphological and topographical characteristics. Comparison with paroxysmal discharges. Electroencephalogr Clin Neurophysiol. 1980;50:356–364. doi: 10.1016/0013-4694(80)90003-6. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Ahlgrimm N, Wolff S, Muhle H, Granert O, Boor R, Jansen O, Gotman J, Stephani U, Siniatchkin M. fMRI activation during spike and wave discharges evoked by photic stimulation. NeuroImage. 2009;48:682–695. doi: 10.1016/j.neuroimage.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Naquet R, Catier J, Menini C. Neurophysiology of photically induced epilepsy in Papio papio. In: Meldrum BS, Marsden CD, editors. Advances in Neurology. Vol 10. New York: Raven Press; 1975. pp. 107–118. [PubMed] [Google Scholar]
- Nersesyan H, Herman P, Erdogan E, Hyder F, Blumenfeld H. Relative changes in cerebral blood flow and neuronal activity in local microdomains during generalized seizures. J Cereb Blood Flow & Metab. 2004;24:1057–1068. doi: 10.1097/01.WCB.0000131669.02027.3E. [DOI] [PubMed] [Google Scholar]
- Parra J, Kalitzin SN, Iriarte J, Blanes W, Velis DN, Lopes da Silva FH. Gamma-band phase clustering and photosensitivity: Is there an underlying mechanism common to photosensitive epilepsy and visual perception? Brain. 2003;126:1164–1172. doi: 10.1093/brain/awg109. [DOI] [PubMed] [Google Scholar]
- Silva-Barrat C, Ménini C, Bryére P, Naquet R. Multiunitary activity analysis of cortical and subcortical structures in paroxysmal discharges and grand mal seizures in photosensitive baboons. Electroencephalogr Clin Neurophysiol. 1986;64:455–468. doi: 10.1016/0013-4694(86)90079-9. [DOI] [PubMed] [Google Scholar]
- Szabó CÁ, Leland MM, Knape KD, Elliott JJ, Haines VL, Williams JT. Clinical and EEG phenotypes of epilepsy in the baboon (Papio hamadryas spp) Epilepsy Res. 2005;65:71–80. doi: 10.1016/j.eplepsyres.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Szabó CÁ, Narayana S, Kochunov PV, Franklin C, Knape KD, Davis MD, Fox PT, Leland MM, Williams JT. PET Imaging in the photosensitive baboon: A case-controlled study. Epilepsia. 2007;48:245–253. doi: 10.1111/j.1528-1167.2006.00949.x. [DOI] [PubMed] [Google Scholar]
- Szabó CÁ, Salinas FS, Narayana S. Duong TQ. Neuroimaging of Non-Human Primates TONIJ. Suppl 2-M9. Vol. 5. 2011. Functional PET Evaluation of the Photosensitive Baboon; pp. 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaka Y, Takamatsu K, Nakagawara M. Anterior-posterior relationships of EEG in photosensitive subjects: Coherence and cross-phase-spectral analysis. Japanese Journal of Psychiatry & Neurology. 1989;43:651–663. doi: 10.1111/j.1440-1819.1989.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Van Luijtelaar G, Sitnikova E. Global and focal aspects of absence epilepsy: The contribution of genetic models. Neuroscience and Behavioral Rev. 2006;30:983–1003. doi: 10.1016/j.neubiorev.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Wada JA, Terao A, Booker HE. Longitudinal correlative analysis of the epileptic baboon, Papio papio. Neurology. 1972;22:1272–1285. doi: 10.1212/wnl.22.12.1272. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Fiber pathways and cortical connections of preoccipital areas in rhesus monkeys. J Comp Neurol. 2010;518:3725–3751. doi: 10.1002/cne.22420. [DOI] [PubMed] [Google Scholar]


