Abstract
Glutamatergic pathways dominate information processing in the brain, but these are not homogeneous. They include two distinct types: Class 1, which carries the main information for processing, and Class 2, which serves a modulatory role. Identifying the Class 1 inputs in a circuit can lead to a better understanding of its function. Also, identifying Class 1 inputs to a thalamic nucleus tells us its main function (e.g., LGN is the relay of retinal Class 1 input), and such identification leads to a division of thalamic relays into first and higher order: the former receives Class 1 inputs from subcortical sources; the latter, from layer 5 of cortex, which it then relays to another cortical area. When a cortical area directly connects with another, it often has a parallel, transthalamic connection through these higher order relays. This leads to a novel appreciation of cortical functioning and raises many new questions.
Class 1 and Class 2 Glutamatergic Inputs
We have made the case in recent years that each glutamatergic pathway participating in thalamic and cortical circuits can be identified as a member of one of two classes, which we now refer to as Class 1 and Class 2 [1;2]. We originally suggested this classification for thalamic circuitry [3], where we then called them driver (Class 1) and modulator (Class 2) inputs, and we have argued that the Class 1 inputs represent the main information route, whereas the Class 2 inputs serve as modulators much like the conventional modulatory inputs using ACh, NA, 5-HT, etc., as neurotransmitters. An example of a Class 1 input carrying the main information to be relayed is the retinal input to the LGN, and of a Class 2 input is the corticogeniculate projection from layer 6 that serves to modulate retinogeniculate transmission.
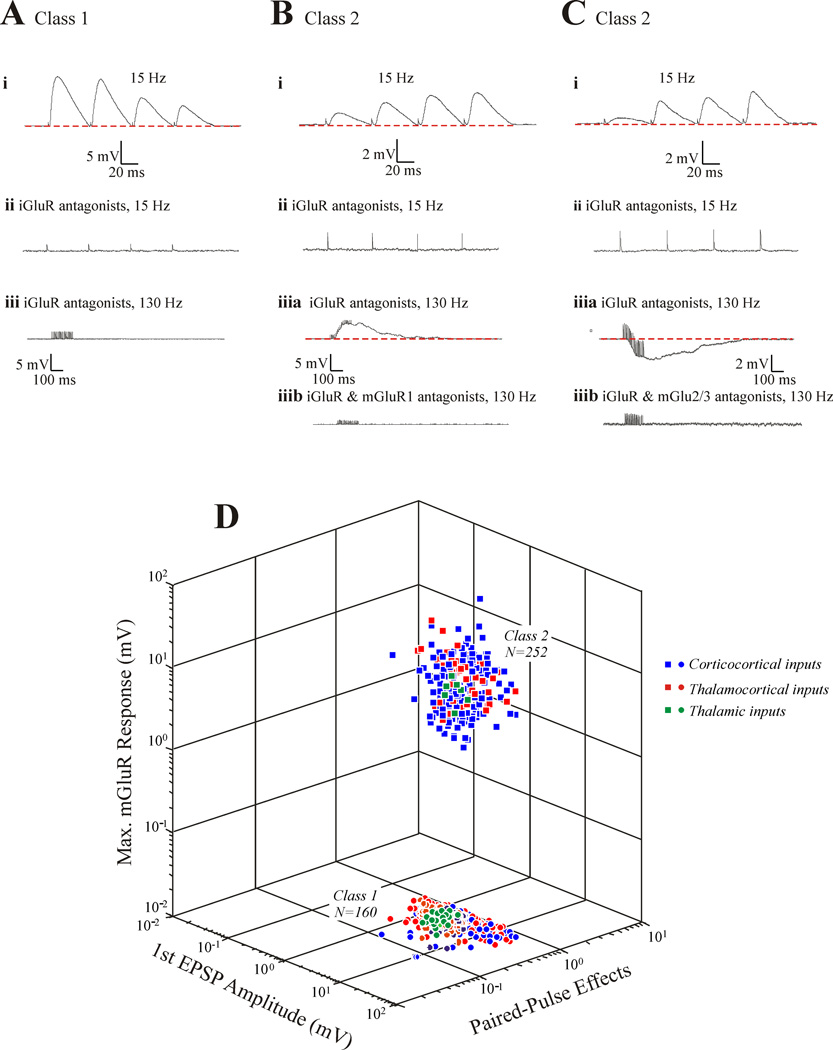
Table 1 and Figure 1A–C show some of the parameters that may be used to identify the two classes of glutamatergic input. Class 1 inputs: activate only iGluRs, whereas Class 2 inputs also activate metabotropic glutamate receptors; show a generally depressing paired-pulse pattern, indicating a higher probability of transmitter release, whereas Class 2 inputs show a facilitating pattern indicating a lower release probability; produce larger initial EPSPs; and are evoked in a less graded manner [4–13].* Anatomical differences also exist (see Table 1). An important point for Class 2 inputs is that they can activate Group I mGluRs, which depolarize and thus excite, or Group II mGluRs, which hyperpolarize and thus inhibit, or both (Figure 1B,C). Figure 1D shows the robustness of this classification scheme and also shows that the parameters graphed here for each of the Classes are the same for cortical and thalamic circuitry. These are also the only two classes of glutamatergic input so far seen.
TABLE 1.
Class 1 and 2 Properties
| Class 1 (Driver) | Class 2 (Modulator) |
|---|---|
| Activates only ionotropic receptors | Activates metabotropic receptors |
| Synapses show paired-pulse depression (high p)† | Synapses show paired-pulse facilitation |
| Large EPSPs | Small EPSPs |
| Less convergence onto target | More convergence onto target |
| Thick axons | Thin axons |
| Large terminals on proximal dendrites | Small terminals on distal dendrites |
| Dense terminal arbors | Delicate terminal arbors |
See text for slight variation called “Class 1C”
Figure 1.
Some properties of Class 1 and 2 inputs in thalamus and cortex, showing synaptic responses evoked in postsynaptic cell in response to electrical activation of Class 1 or 2 afferents. The data are from slice preparations of mouse brain [4–13]. A: Some Class 1 properties. At lower frequency stimulation (15 Hz), the evoked EPSPs begin with one of large amplitude followed by a pattern of paired-pulse depression (i). These responses at this frequency are completely abolished with bath application of antagonists to iGluRs (ii). High frequency stimulation (10 shocks at 130 Hz) in the presents of ionotropic glutamate receptor antagonists evokes no response, suggesting that mGluRs are not activated by this input. B: Some Class 2 properties, conventions as in A. Here a train of stimuli at 15 Hz evokes a small EPSP showing paired-pulse facilitation (i); responses at 15 Hz are blocked by addition of ionotropic glutamate receptor antagonists (ii); and subsequent high frequency stimulation evokes a sustained EPSP (iiia) that is blocked by further addition of an mGluR1 antagonist (iiib). C: More Class 2 properties, which are the same as shown in B, except that the mGluRs activated are Group II and thus produce a sustained IPSP (iii). C: Three-dimensional scatterplot showing distribution of parameters for Class 1 and 2 inputs. The parameters shown are the ratio of the amplitude of the second EPSP evoked in a train to that of the first (Paired-Pulse effects), the amplitude of the first EPSP evoked in a train (1st EPSP Amplitude), and the maximum voltage change evoked in the 300 msec period after high frequency stimulation in the presence of ionotropic glutamate receptor antagonists [Maximum mGluR Response (mV)]. From [1].
First and Higher Order Thalamic Relays
Identifying the Class 1 input to a thalamic nucleus largely defines the function of that nucleus, and so we define the LGN as a relay of retinal input. This logic, in turn, led us to divide the thalamus into two major types: first order and higher order [2;14]. First order nuclei receive their Class 1 input from a subcortical source, and higher order, from layer 5 of cortex. Higher order thalamic nuclei are thus defined as relaying information between cortical areas.
Identifying Class 1 and 2 Inputs in Thalamic and Cortical Circuits
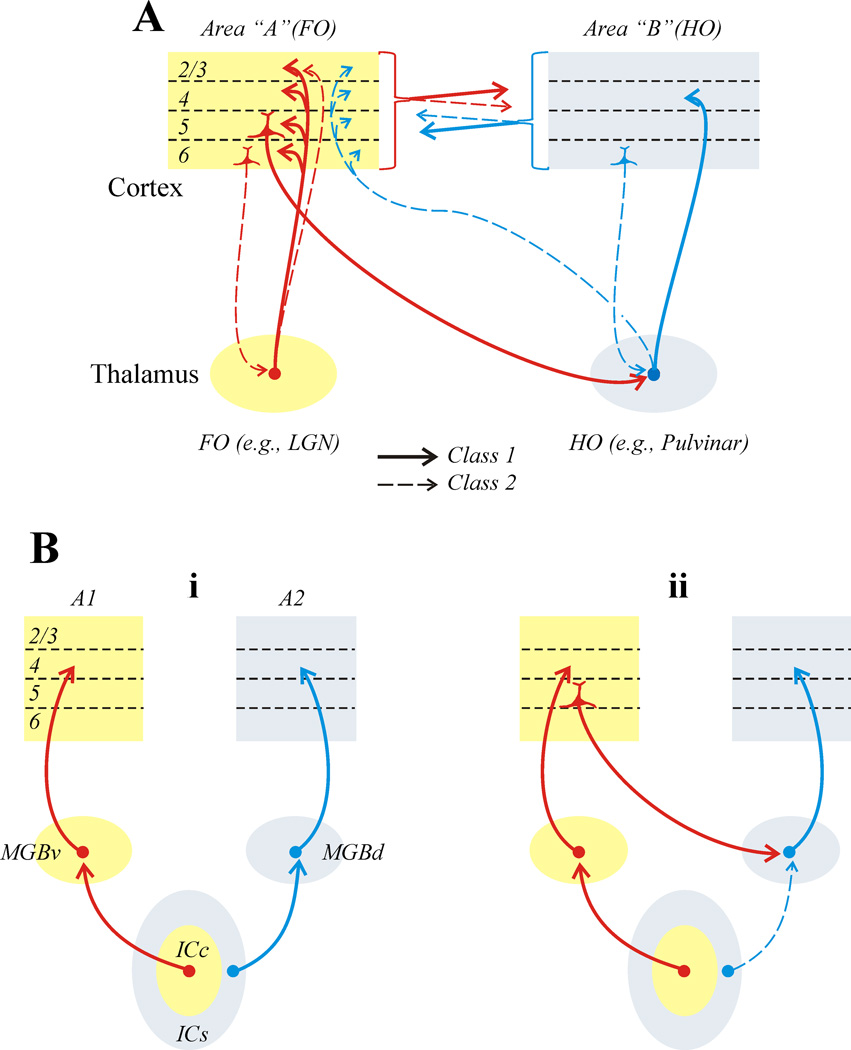
Figure 2A shows the various thalamic and cortical circuits that have been identified as Class 1 or 2. Several points are worth emphasizing:
A clear Class 1 (or information bearing) pathway can be followed from layer 5 of one cortical area through a higher order thalamic relay to another cortical area [10;12;13].
Cortical areas are also reciprocally connected directly, and these connections involve both Class 1 and 2 inputs in a complex laminar pattern (for details, see [4;5]).
The transthalamic path appears to be organized as a feedforward information route, because the higher order thalamic relay provides Class 1 input to the higher order cortical area but only Class 2 input to the first order cortical area [7]. In contrast, the direct connections seem quite similar in both directions, providing no clear hierarchical order [4;5]. The transthalamic pathway does provide a hierarchical order.
The main thalamic inputs, at least from first order thalamic nuclei to their first order cortical areas, show a curious pattern of Class 1 inputs exclusively to layers 4–6, but layers 2/3 receive predominantly (~80%) Class 2 inputs [6;8]. In the geniculostriate projection of monkeys and cats, one cell type (W for cats and K for monkeys) innervates layers 2/3, whereas the others (X and Y for cats and M and P for monkeys) mainly innervate layer 4 [15–18]. This suggests the possibility that W or K inputs may be modulatory, putting a different spin on ideas of parallel processing in vision.
Figure 2.
Identification of various pathways as Class 1 or 2. A: Pathways in mouse thalamic and cortical circuits [4–13]. B: Example from auditory thalamocortical relationships of impact of classification on understanding of circuits [9]. The dominant view is that the MGBv and MGBd represent two parallel streams of information relayed to cortex from the ICc and ICs, respectively (i). Identification of inputs shows that the input from ICs to MGBd is Class 2, and thus modulates input to that nucleus from layer 5 of cortex (ii).
Other examples of how this classification of glutamatergic inputs can change our views of the functioning of circuits exist. One involves processing of auditory information through thalamus. Figure 2Bi shows the prevailing view, involving two parallel routes of information flow: a lemniscal system involving a path from the ICc through MGNv to A1, and a paralamniscal system from the ICs through MGNd to A2. But do these really represent two distinct information paths? A classification of these glutamatergic inputs provides a very different picture (Figure 2Bii). Whereas the lemniscal input to MGNv is indeed Class 1, the paralemniscal input to MGNd is Class 2 [9]. This suggests that the ICs input to MGNd, rather than providing the main information to be relayed, instead modulates the circuit from layer 5 of A1 through MGNd. Conventional wisdom also calls for parallel thalamocortical processing of visual and somatosensory information analogous to that shown in Figure 2Bi. For the visual system, the proposed parallel routes are retina to LGN and superior colliculus to pulvinar; for somatosensation, they are the principal nucleus of the Vth nerve to VPN and the spinal nuclei of the Vth nerve to POm. At issue is whether the above inputs to the higher order relays (i.e., pulvinar and POm) are Class 1 or Class 2. That is, do these inputs carry main information to be relayed to cortex or do they modulate the relay of layer 5 inputs?
Implications for Corticocortical processing
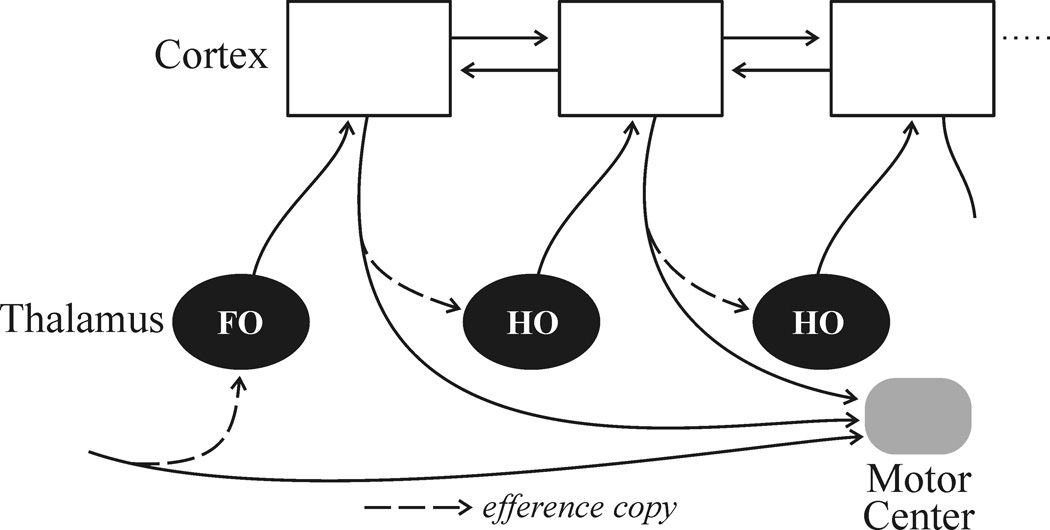
If we follow the logic that information is processed mainly by projections of Class 1 glutamatergic inputs, a novel picture emerges for the role of thalamus in corticocortical communications [1;2;14]. Figure 3 illustrates some of the main points.
A clear information route between cortical areas exists as a transthalamic pathway involving higher order thalamic relays. It appears that often, and perhaps always, when cortical areas have a direct connection, they also have a parallel one through thalamus.
Many and perhaps all of the Class 1 axons that innervate thalamus branch, and the extrathalamic branch innervates brainstem and sometimes spinal regions that appear to have a motor function (reviewed in [14]). Thus, for example, retinogeniculate axons branch to also innervate midbrain structures such as the superior colliculus and pretectum, which are involved in eye movements, pupillary control, etc. The axons from layer 5 of cortex that innervate higher order thalamic relays also branch to innervate targets associated with motor control [19–21]. The significance of this anatomical fact is far from clear, but as we have argued in detail elsewhere, it appears that the message sent to thalamus for relay to cortex is a copy of a motor message, and in this sense, the branched Class 1 inputs to thalamus may be regarded as efference copies [14]. This could serve to inform cortical areas higher in the hierarchy about motor commands issued from lower centers, which could provide information needed to disambiguate sensory stimuli that are self-induced (e.g., by eye movements) from those that are initiated in the environment.
Every cortical area for which relevant information is available possesses a layer 5 projection to motor centers (Figure 3). This applies even to primary sensory areas [19–21]. Thus the distinction between so-called “sensory” and “motor” areas of cortex is of limited use.
As Figure 3 indicates, it appears that often and perhaps always when cortical areas have a direct connection, they also have a transthalamic one arranged in parallel. This raises two main questions: What is different about the two pathways in terms of information content? Why is one of these paths relayed through thalamus, offering the possibility that this pathway may be modulated or gated in ways unavailable to the direct connection? It is beyond the scope of this account to suggest answers, which are no more than speculation at this point (but see [1]).
Figure 3.
Class 1 pathways involved in thalamocortical processing in the mouse [4–13]. Details in text.
Conclusions
The idea that glutamatergic pathways are heterogeneous, and that one of the two main classes of such input, Class 1, is the main carrier of information, leads to a novel way of analyzing brain circuits. Other classes may emerge as the classification continues. One of the consequences of this view is that Class 1 inputs define the basic role of a thalamic nucleus, and as such, we can thereby classify these nuclei as first order, if they receive their Class 1 inputs from a subcortical source, and higher order, if from layer 5 of cortex. The higher order relays appear to be an integral ink in a transthalamic corticocortical circuit that parallels direct corticocortical connections between areas. This sets up a novel theoretical framework for assessing the role of thalamus in cortical function.
Highlights.
Glutamatergic inputs in thalamic and cortical circuitry are either Class 1 or Class 2
We suggest that Class 1 is information bearing and Class 2 is modulatory
Class 1 inputs represent what a thalamic nucleus relays to cortex
A transthalamic information route exists between cortical areas
Many or all cortical areas directly connected have a parallel transthalamic route
Acknowledgments
The author’s laboratory has been supported by grants from the National Institutes of Health.
Abbreviations
- A1 & A2
first and second auditory cortical areas
- ACh
acetylcholine
- AD
anterodorsal nucleus
- GABA
γ-aminobutyric acid
- ICc & ICs
core & shell regions of the inferior colliculus
- iGluR
ionotropic glutamate receptor
- LGN
lateral geniculate nucleus
- MGBd & MGBv
dorsal and ventral divisions of the medial geniculate body
- mGluR
metabotropic glutamate receptor
- POm
posterior medial nucleus
- S1 & S2
first and second somatosensory cortical areas
- V1 & V2
first and second visual cortical areas
- VA
ventral anterior nucleus
- VL
ventral lateral nucleus
- VPL & VPM
lateral and medial ventral posterior nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Although not relevant to this more general overview, we have shown in certain pathways that subtypes exist among Class 1 afferents [4–8;11–13]. Class 1A inputs have a strictly all-or-none activation pattern, whereas Class 1B inputs have a pattern intermediate between all-or-none and the more graded pattern seen in Class 2 inputs, and Class 1C have a curious paired-pulse pattern whereby the first two EPSPs show facilitation and the remainder in a train show depression. We do not distinguish among these Class 1 inputs elsewhere in this account.
Reference List
- 1.Sherman SM, Guillery RW. Distinct functions for direct and transthalamic corticocortical connections. J. Neurophysiol. 2011;106:1068–1077. doi: 10.1152/jn.00429.2011. [DOI] [PubMed] [Google Scholar]
- 2.Sherman SM, Guillery RW. Exploring the Thalamus and its Role in Cortical Function. edn second. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- 3.Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: Distinguishing "drivers" from "modulators". Proc. Natl. Acad. Sci. USA. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Covic EN, Sherman SM. Synaptic properties of connections between the primary and secondary auditory cortices in mice. Cereb Cortex. 2011;21:2425–2441. doi: 10.1093/cercor/bhr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DePasquale R, Sherman SM. Synaptic properties of corticocortical connections between the primary and secondary visual cortical areas in the mouse. J. Neurosci. 2011;31:16494–16506. doi: 10.1523/JNEUROSCI.3664-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viaene AN, Petrof I, Sherman SM. Synaptic properties of thalamic input to the subgranular layers of primary somatosensory and auditory cortices in the mouse. J. Neurosci. 2011;31:12738–12747. doi: 10.1523/JNEUROSCI.1565-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viaene AN, Petrof I, Sherman SM. Properties of the thalamic projection from the posterior medial nucleus to primary and secondary somatosensory cortices in the mouse. Proc Nat Acad Sci Usa. 2011;108:18156–18161. doi: 10.1073/pnas.1114828108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Viaene AN, Petrof I, Sherman SM. Synaptic properties of thalamic input to layers 2/3 in primary somatosensory and auditory cortices. J. Neurophysiol. 2011;105:279–292. doi: 10.1152/jn.00747.2010. It had been implicitly assumed that thalamocortical inputs represent fundamental information streams and thus should all have Class 1 properties. This study, using an vitro slice preparation from the mouse, showed in the primary somatosensory and auditory pathways that most of the thalamic input to layers 2/3 is Class 2 and thus likely modulatory in nature.
- 9. Lee CC, Sherman SM. Topography and physiology of ascending streams in the auditory tectothalamic pathway. Proc. Nat Acad. Sci. Usa. 2010;107:372–377. doi: 10.1073/pnas.0907873107. Using an in vitro slice preparation from the mouse, this study refuted the notion that there are parallel auditory information streams from the inferior colliculus through the ventral and dorsal divisions of the medial geniculate body to first and secondary auditory cortical areas; instead, the input from the inferior colliculus to the dorsal division of the medial geniculate body has Class 2 properties and thus appears to modulate the information stream from layer 5 of the primary auditory cortical area through this higher order thalamic relay to the secondary cortical area.
- 10. Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13:84–88. doi: 10.1038/nn.2449. This in vitro slice study in the mouse provided clear evidence for a robust transthalamic pathway from primary somatosensory cortex through the posterior medial nucleus to the secondary somatosesnory cortex.
- 11.Petrof I, Sherman SM. Synaptic properties of the mammillary and cortical afferents to the anterodorsal thalamic nucleus in the mouse. J. Neurosci. 2009;29:7815–7819. doi: 10.1523/JNEUROSCI.1564-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CC, Sherman SM. Synaptic properties of thalamic and intracortical inputs to layer 4 of the first- and higher-order cortical areas in the auditory and somatosensory systems. J. Neurophysiol. 2008;100:317–326. doi: 10.1152/jn.90391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reichova I, Sherman SM. Somatosensory corticothalamic projections: Distinguishing drivers from modulators. J. Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- 14.Guillery RW, Sherman SM. Branched thalamic afferents: what are the messages that they relay to cortex? Brain Res Brain Res Rev. 2011;66:205–219. doi: 10.1016/j.brainresrev.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson JC, da Costa NM, Martin KA. The W cell pathway to cat primary visual cortex. J Comp Neurol. 2009;516:20–35. doi: 10.1002/cne.22085. [DOI] [PubMed] [Google Scholar]
- 16.Shostak Y, Ding Y, Casagrande VA. Neurochemical comparison of synaptic arrangements of parvocellular, magnocellular, and koniocellular geniculate pathways in owl monkey (Aotus trivirgatus) visual cortex. J. Comp. Neurol. 2003;456:12–28. doi: 10.1002/cne.10436. [DOI] [PubMed] [Google Scholar]
- 17.Ding Y, Casagrande VA. The distribution and morphology of LGN K pathway axons within the layers and CO blobs of owl monkey V1. Visual Neurosci. 1997;14:691–704. doi: 10.1017/s0952523800012657. [DOI] [PubMed] [Google Scholar]
- 18.Ferster D, LeVay S. The axonal arborizations of lateral geniculate neurons in the striate cortex of the cat. J. Comp. Neurol. 1978;182:923–944. doi: 10.1002/cne.901820510. [DOI] [PubMed] [Google Scholar]
- 19.Bourassa J, Deschênes M. Corticothalamic projections from the primary visual cortex in rats: A single fiber study using biocytin as an anterograde tracer. Neurosci. 1995;66:253–263. doi: 10.1016/0306-4522(95)00009-8. [DOI] [PubMed] [Google Scholar]
- 20.Bourassa J, Pinault D, Deschênes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: A single-fibre study using biocytin as an anterograde tracer. Eur. J. Neurosci. 1995;7:19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 21.Deschênes M, Bourassa J, Pinault D. Corticothalamic projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain Res. 1994;664:215–219. doi: 10.1016/0006-8993(94)91974-7. [DOI] [PubMed] [Google Scholar]