Abstract
Preclinical studies and clinical analyses have implicated the mammalian target of rapamycin (mTOR) pathway in the progression of prostate cancer, suggesting mTOR as a potential target for new therapies. mTOR, a serine/threonine kinase, belongs to two distinct signaling complexes: mTORC1 and mTORC2. We previously showed that the synthetic organoselenium compound, p-XSC, effectively inhibits viability and critical signaling molecules (e.g., androgen receptor, Akt) in androgen responsive (AR) and androgen independent (AI) human prostate cancer cells. Based on its inhibition of Akt, we hypothesized that p-XSC modulates mTORC2, an upstream regulator of the kinase. We further hypothesized that combining p-XSC with rapamycin, an mTORC1 inhibitor, would be an effective combinatory strategy for the inhibition of prostate cancer. The effects of p-XSC and rapamycin, alone or in combination, on viability and mTOR signaling were examined in AR LNCaP prostate cancer cells and AI C4-2 and DU145 cells. Phosphorylation of downstream targets of mTORC1 and mTORC2 was analyzed by immunoblotting. The interaction of mTORC1- and mTORC2-specific proteins with mTOR was probed through immunoprecipitation and immunoblotting. p-XSC inhibited phosphorylation of mTORC2 downstream targets, Akt and PCKα, and decreased the levels of rictor, an mTORC2-specific protein, co-immunoprecipitated with mTOR in C4-2 cells. The combination of p-XSC and rapamycin more effectively inhibited viability and mTOR signaling in C4-2, LNCaP, and DU145 cells than either agent individually.
Keywords: Prostate cancer, selenium, mTOR, combination therapy
INTRODUCTION
Despite advances in androgen deprivation therapy, prostate cancer recurrence and the development of androgen independence remain major challenges to prostate cancer survivorship. Hormone refractory prostate cancer is difficult to treat even though resistant cells express androgen receptors and rely on those receptors for growth and progression. Thus, an urgent need exists for the development of effective treatments against androgen independent (AI) prostate cancer by perhaps targeting androgen receptors or other cell proliferative targets. Specific mechanisms that account for the transition of androgen responsive (AR) tumors to AI tumors are not well understood but cell survival strategies that rely on signaling between androgen receptors and the PI3K/Akt/mTOR pathway may be critical for maintaining growth of prostate cancer cells. A study by Wu et al (1) suggest that repression of mTOR signaling results in a compensatory increase in androgen receptor function and thus promotes prostate cancer cell survival during deprivation therapy. Thus, agents that can modulate this signal pathway may be useful against AI prostate disease.
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that is an important transducer for cell growth and survival due to its involvement in protein synthesis, cell cycle progression, ribosome biogenesis and nutrient uptake (2). Additionally, mTOR signaling may be a key regulator of cancer cell growth as dysregulation of this pathway has been observed in a number of cancer types including prostate (3). Clinical analyses have shown increased expression and constitutive activity of components of the mTOR pathway in prostatic intraepithelial neoplasia (PIN) and prostate tumors compared with those of normal tissue (4-6). mTOR is a component of two compositionally and functionally distinct signaling complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 is rapamycin-sensitive and includes the mTOR catalytic subunit as well as proteins raptor and mLST-8/GβL. mTORC1 regulates cap-dependent translation initiation through phosphorylation of downstream targets 40S ribosomal protein S6 kinase (p70S6K) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) (3). mTORC2 is composed of mTOR, rictor, mSin1, mLST-8/GβL, and PRR5 proteins. This complex activates Akt by phosphorylating serine 473 (7) and also plays a role in cytoskeletal organization through phosphorylation of PKCα (8).
Rapamycin selectively inhibits mTORC1 kinase activity and thus may induce autophagy to enable cells to recycle nutrients by breaking down expendable substrates. Clinically, Rapamycin is commonly used to prevent kidney graft rejection and as a component of arterial stents (9). Based on the preclinical effectiveness of mTOR inhibitors against a variety of cancer types, rapamycin-based mTOR inhibitors are being tested alone or in combination in over 150 ongoing trials for the treatment of a broad spectrum of malignancies including AI prostate cancer (10). However, some clinical trials that have used rapamycin as a monotherapy, including a phase I/II trial for the treatment of AI prostate cancer have reported disappointing results (11). Preclinical studies show that rapamycin in combination with other chemotherapeutic agents or signal pathway inhibitors has enhanced efficacy against prostate cancer (12-15). These studies re-enforce the clinical finding that combination regimens show significant tumor-responses than single agent therapies.
Preclinical studies in several laboratories including our own provide evidence supporting the potential use of organoselenium for treatment of late stage prostate cancer (16). Various selenium-containing compounds have been shown to modulate key targets mechanistically linked to prostate cancer progression and the development of AI disease (16-19). We previously showed that the synthetic organoselenium compound, 1,4-phenylenebis(methylene)selenocyanate (p-XSC), can modulate the androgen receptor and Akt signaling cascades in human prostate cancer cells (20).
The current study expands our understanding of the molecular targets of p-XSC by demonstrating its direct regulatory effects on components of the mTOR signaling pathway. Our previous findings determined that p-XSC inhibits Akt expression which suggested that inhibition of prostate cancer by p-XSC may be mediated by the mTORC2 signaling pathway. We hypothesize that p-XSC in combination with rapamycin has a significant advantage over each agent alone and provides a complementary composite for the treatment of prostate cancer through inhibition of multiple critical cell signaling pathways. Coupled with rapamycin, various forms of organoselenium enhance or manifest a synergistic effect against prostate cancer when combined with other chemopreventive or chemotherapeutic agents (21-24). The current study demonstrates that p-XSC preferentially inhibits mTORC2 signaling in AI human prostate cancer cells. In addition, p-XSC in combination with rapamycin is superior to each agent alone in inhibiting prostate cancer cell growth. Further examination of the effects of combining p-XSC and rapamycin in prostate cancer cells shows that the combination inhibits downstream targets of both mTOR signaling complexes, likely contributing to their synergistic effects on cell viability primarily during androgen independent growth.
MATERIALS AND METHODS
Reagents and cell lines
Rapamycin was purchased from ALEXIS Biochemicals-Enzo Life Sciences (Plymouth Meeting, PA). p-XSC was synthesized as described previously (25). Androgen independent (AI) LNCaP C4-2 cells were obtained from Dr. Warren D.W. Heston, The Lerner Research Institute, The Cleveland Clinic Foundation, Ohio. Androgen responsive (AR) LNCaP cells and AI DU145 cells were obtained from the American Type Culture Collection (Manassas, VA).
Cell culture and treatments
C4-2 cells were maintained in RPMI-1640 medium supplemented with 10% Fetal Bovine Serum (FBS). LNCaP cells were maintained under the same conditions but with heat inactivated FBS. DU145 cells were cultured in Eagles minimum essential medium with Earle's balanced salt solution and 10% FBS. All cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and were routinely passaged when they were 70-80% confluent. Cells were plated in 10 cm dishes (1 million cells/plate) or 96-well plates (5,000 cells/well) depending on the assay, grown for 24 or 48 hr and then treated with p-XSC, rapamycin, or a combination of p-XSC and rapamycin at doses described below. The vehicle for both p-XSC and rapamycin was DMSO.
Detection of protein expression and phosphorylation by immunoblotting
Immunoblotting was performed as previously described to determine changes in downstream targets of mTOR (26). Briefly, cells were grown in 10 cm dishes for 48 hr and treated with p-XSC (5 and 10 μM), rapamycin (10 nM), or a combination of p-XSC and rapamycin (2.5 or 5 μM p-XSC with 1 or 10 nM rapamycin) for 90 min, harvested by scraping and washed with PBS containing phosphatase inhibitors (New England Biolabs, Ipswich, MA). The doses of p-XSC chosen were previously reported by us to inhibit Akt phosphorylation at 90 minute post incubation (20). The doses of rapamycin used in the combination studies (1 and 10 nM) were considerably lower than the doses of p-XSC as it is a potent and specific inhibitor of mTORC1 signaling and therefore individually inhibits mTORC1 target phosphorylation at such concentrations without notable effects on mTORC2 targets. Protein extraction was carried out using cell lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin) with freshly added 1 mM phenylmethylsulfonyl fluoride (PMSF). Equal amounts of protein (50 μg) were separated by electrophoresis on either 10 or 12% SDS-polyacrylamide gels and transferred to PVDF membranes. The following primary antibodies were used at dilutions ranging from 1:500 to 1:1000 for immunoblotting: Akt, phospho-Akt (Ser473), p70S6K, phospho-p70S6K (Thr 389), RPS6, phospho-RPS6 (Ser 235/236), rictor, raptor, and mTOR from Cell Signaling Technology (Beverly, MA), and PKCα and phospho-PKCα (Ser 657) from Millipore (Billerica, MA). HRP-conjugated anti-mouse and anti-rabbit secondary antibodies (Cell Signaling, Beverly, MA) were used at a final dilution of 1:3000. Antigen-antibody reactions were developed using ECL reagents from Amersham (Piscataway, NJ) and density was analyzed using VisionWorks™ software (UVP, Inc. Upland, CA). All immunoblotting experiments were repeated three times. The results are presented as representative blots from single experiments.
Analysis of protein complex components by immunoprecipitation
In order to investigate the interactions of mTOR with its mTORC1 and mTORC2 binding partners, raptor and rictor, respectively, we immunoprecipitated mTOR from C4-2 cell lysates. C4-2 cells were grown in 10 cm for 48 hr and then treated with p-XSC (5 and 10 μM), rapamycin (10 nM), or a combination of p-XSC and rapamycin (5 μM p-XSC with 10 nM rapamycin) for 15, 30, 60, 90 min and 6 hr. After treatment, cells were washed with cold PBS and 500 μl of cell lysis buffer was added to each plate. The cells were incubated on ice for 5 min, harvested by scraping, transferred to microfuge tubes, and sonicated on ice by three 10-sec bursts using a Sonic Dismembrator Model 100 (Fisher Scientific, Pittsburgh, PA). Samples were then centrifuged (12,000 × g) at 4°C for 10 min and the supernatant fractions were transferred to fresh tubes. Equal amounts of protein (200 μg) from each sample were incubated with an anti-mTOR primary antibody (Cell Signaling, Beverly, MA) at a final dilution of 1:100 and rocked overnight at 4°C. Next, 20 μl of a Protein A agarose bead slurry (Invitrogen, Carlsbad, CA) was added and samples were rocked at 4°C for additional 3 hr. The protein-antibody-bead complexes were then collected by centrifugation (5,000 × g for 5 minutes) and washed with cell lysis buffer. Samples were resuspended in 25 μl of 3X SDS-PAGE sample buffer, separated by electrophoresis on 7.5% SDS-polyacrylamide gels, and transferred to PVDF membranes for immunoblotting analysis.
MTT assay for cell viability
C4-2, LNCaP, and DU145 cells were plated (5000 cells/well) in triplicate in 96 well format. After 24 hr, cells were treated with a range of doses of p-XSC (0.625, 1.25, 2.5, 5, 10, and 20 μM), rapamycin (0.1, 1, 10, 100, and 1000 nM), or a combination of the two agents including each dose of p-XSC with each dose of rapamycin. After 48 hr, MTT assays were performed as previously described to determine cell viability (27). Analyses were repeated three times and data averaged. Results are expressed as percent viability compared to the vehicle-only control.
Median effect analysis for combined effects
Synergistic effects of p-XSC and rapamycin were determined using a constant molar ratio of p-XSC to rapamycin (5000:1). Maintaining this ratio, we assessed the viability of C4-2 using a dose range of each agent as follows: 2.5, 5, 10, 20, and 40 μM p-XSC with 0.5, 1, 2, 4, and 8 nM rapamycin, respectively. The molar ratio of 5000:1 was chosen initially because a strong enhancement of inhibition was seen when C4-2 cells were treated for 48 hr with a combination of 5 μM p-XSC and 1 nM rapamycin. Also, the dose of p-XSC is within a physiological attainable concentration that is a relatively nontoxic level for this organoselenium compound and for rapamycin, one that is clinically achievable (11, 28). C4-2 cells were grown in triplicate in 96 well plates (5000 cells/well) for 24 hr, treated with the aforementioned combinations of p-XSC and rapamycin for 48 hr, and evaluated by MTT assay for cell viability.
The interaction between the combined agents was evaluated for synergism using the median effect and combination index (CI) equations described by Chou and Talalay (29-31). The efficacies of a given agent on cell viability are described by the median effect equation:
where fa and fu represent the affected and unaffected fractions respectively, by a given dose (D). Dm is the dose that elicits the median effect and m is the coefficient of the sigmoidocity of the dose effect curve. We generated dose-effect curves and median effect plots [log(D) vs. log(fa/fu)] for p-XSC, rapamycin, and the combination of the two at the constant molar ratio of 5000:1. The parameters Dm and m were determined from the median effect plots. We assessed the fractional effects associated with each agent individually and in combination over a range of concentrations.
The nature of the interactive effects of p-XSC and rapamycin were evaluated by calculating the CI defined as:
where α = 0 and α = 1 for p-XSC and rapamycin indicating whether the compounds are mutually exclusive and non-exclusive, respectively. (Dx)1 and (Dx)2 are the doses of p-XSC and rapamycin alone required to achieve a given effect level (fa). (D)1 and (D)2 are the doses of p-XSC and rapamycin in combination that achieve the same fa. The value of CI reflects synergism when the calculated value is < 1, antagonism when it is > 1 and additivity when it is = 1. CI values were calculated for the combination of p-XSC and rapamycin over a range of effect levels (0.2 to 0.9) indicating strong to moderate synergy between the test compounds.
Statistical analysis
Histogram results are expressed as mean ± standard error. Statistical significance was evaluated using either one or two-factor analysis of variance (ANOVA). Differences were considered significant at p < 0.05.
RESULTS
Time-course effects of p-XSC on mTOR complex proteins
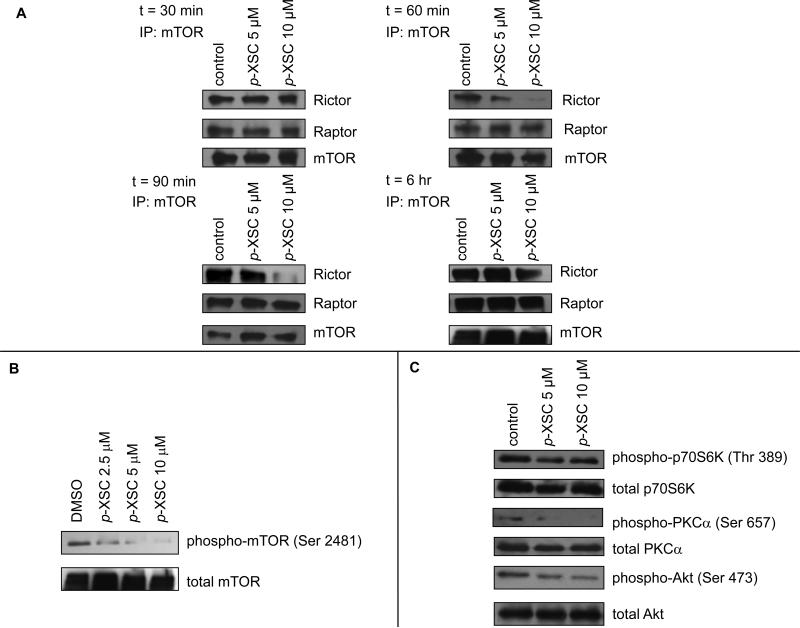
To determine whether p-XSC interferes with mTOR complex formation, we immunoprecipitated mTOR from C4-2 cells treated with 5 and 10 μM p-XSC and probed to identify whether raptor and rictor co-immunoprecipitated. Immunoprecipitation was conducted after 15, 30, 60, and 90 min of treatment as well as after 6 hr of treatment to evaluate how early p-XSC mediated changes occur. No change in rictor or raptor binding to mTOR was observed at 15 (data not shown) or 30 min (Figure 1A) post-treatment with p-XSC. p-XSC decreased the amount of mTORC2 binding protein, rictor, that coimmunoprecipitated with mTOR as early as 60 min after treatment (Figure 1A). This inhibition lasted for at least 90 min and began to return to control levels at 6 hr; rictor levels in cells after 6 hr of treatment with 10 μM p-XSC were still less than those in the control samples (Figure 1A). At 6 hr of treatment, p-XSC caused a dose-dependent decrease in the amount of immunoprecipitated Ser 2481 phospho-mTOR, an autophosphorylated form of mTOR suggested to be a biomarker for intact mTORC2 and an indicator of mTOR catalytic activity (Figure 1B) (32,33).
Figure 1.
The effects of p-XSC on mTOR pathway molecules. A. Levels of mTOR binding proteins raptor and rictor co-immunoprecipitated with mTOR from C4-2 cells treated with p-XSC for 30, 60, and 90 min and 6 hr. B. Immunoblot analysis of mTOR phosphorylation in C4-2 cells treated with p-XSC for 6 hr. C. Immunoblot analysis of phosphorylated downstream targets of mTOR in C4-2 cells treated with p-XSC for 90 min.
The effects of p-XSC on mTOR target proteins
We assessed p-XSC modulation of mTOR by immunoblotting the levels of phospho-p70S6K (Ser 235/236), phospho-Akt (Ser 473), and phospho-PKCα (Ser 657) in C4-2 cell lysates that had been treated with p-XSC for 90 min. p70S6K is a downstream target of mTORC1, whereas Akt and PKCα are downstream targets of mTORC2. p-XSC, at 5 and 10 μM decreased phospho-Akt (Ser 473) (as we had also seen previously, 20) and phospho (Ser 657)- PKCα (Figure 1C). p-XSC had little to no effect on the phosphorylation of mTORC1 target p70S6K (Figure 1C). These results indicate that p-XSC affects the ratio of phosphorylated to non-phosphorylated proteins as no change in total p70S6K, Akt, or PKCα was observed with treatment.
The effects of p-XSC and rapamycin on prostate cancer cell viability
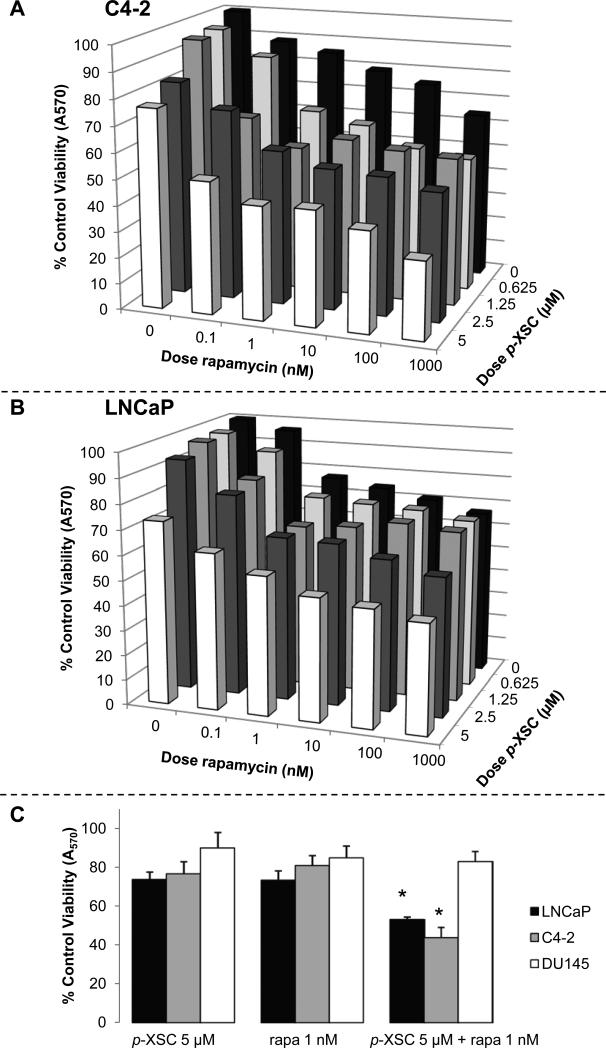
We initially examined the effects of mixtures of p-XSC and rapamycin on viability in the AI cell line, C4-2, and its AR parental cell line, LNCaP, by combining four doses of p-XSC (0.625, 1.25, 2.5, and 5 μM) with six doses of rapamycin (0.1, 1, 10, 100, 1000, and 10,000 nM). Data acquired at 48 hr of treatment showed that the combinations of p-XSC and rapamycin in the C4-2 cells, especially those with low-dose rapamycin (0.1, 1, and 10 nM), had significantly (p < 0.05) superior efficacy to inhibit growth compared with each agent individually (Figure 2A). For example, the combination of 0.1 nM rapamycin with 1.25, 2.5, and 5 μM p-XSC resulted in an 18.3, 12.3, and 26.8 percent increased inhibition, respectively. The addition of 1 nM rapamycin to 0.625, 1.25, 2.5, and 5 μM p-XSC resulted in 23, 32.2, 27.4, and 37.9 percent increased inhibition of C4-2 cell viability, respectively. The 10 nM dose of rapamycin enhanced inhibition mediated by 0.625, 1.25, 2.5, and 5 μM p-XSC by 19.7, 22.4, 30.1, and 37.6 percent, respectively. In LNCaP cells, treatment with the same mixtures of p-XSC and rapamycin showed similar trends (Figure 2B). However, the combination was less effective than that observed in C4-2 cells; significant (p < 0.05) reductions in viability were only achieved for the combination of 2.5 μM p-XSC with 1 μM rapamycin or when 5 μM p-XSC was combined with 1 nM or higher dose of rapamycin. Figure 2C shows that while the mixture of 5 μM p-XSC and 1 nM rapamycin was significantly more effective at inhibiting both C4-2 and LNCaP cell viability than either agent individually, DU145 cells did not appear to be sensitive to the same combination.
Figure 2.
The effects of selected combinations of p-XSC and rapamycin on cell viability and IC50 values (t = 48 hr). A. C4-2 and B. LNCaP cells were treated for 48 hr with a range of doses of rapamycin (x-axis; 0.1 - 1000 nM) and p-XSC (z-axis; 0.625 – 5 μM) alone and in combination and assayed for viability using the MTT method. C. Comparison of the effects of 5 μM p-XSC and 1 nM rapamycin (rapa), alone and in combination, on LNCaP, C4-2, and DU145 cell viability. Cells were treated for 48 hr with 5 μM p-XSC and 1 nM rapa, alone and in combination, and assayed for viability using the MTT method (p < 0.05).
The C4-2 cells also achieved the most dramatic decreases in IC50 when p-XSC was combined with rapamycin. Table 1 compiles the calculated IC50 values that occurred when each agent at a range of doses was combined with a single dose of the other agent (represented graphically by each row and column in Figure 2A). For example, when 1 nM rapamycin was added to p-XSC, the IC50 decreased more than 2-fold. The addition of 5 μM p-XSC lowered the IC50 of rapamycin from the micromolar range to sub-nanomolar range.
Table 1.
IC50 values for p-XSC in combination with varying doses of rapamycin (0.1 - 1000 nM) and IC50 values for rapamycin in combination with single doses of p-XSC (0.625 - 5 μM) in C4-2 cells treated for 48 hr. MTT assays were used to generate the dose curves from which the IC50 values were calculated.
| [Rapa] (nM) | IC50 p-XSC (μM) | [p-XSC] (μM) | IC50 Rapa (nM) |
|---|---|---|---|
| 0 | 7.2 | 0 | 49000 |
| 0.1 | 5.9 | 0.625 | 1400 |
| 1 | 3.5 | 1.25 | 570 |
| 10 | 3.3 | 2.5 | 560 |
| 100 | 2 | 5 | 0.2 |
| 1000 | 1.3 |
Determination of the Combination Index (CI) for p-XSC and rapamycin
The combinatory efficacy of p-XSC and rapamycin was evaluated further in C4-2 cells, as these cells were the most sensitive of the prostate cancer cells examined. The above experiments clearly show that the combination of p-XSC and rapamycin have inhibitory effects on C4-2 cell viability after 48 hr which are significantly greater than with use of each agent individually. Our approach to evaluate synergistic or additive effects for the combination of p-XSC and rapamycin involved the method of Chou and Talalay (29-31). We chose a constant molar ratio of p-XSC to rapamycin (5000:1) and treated C4-2 cells with a range of doses at that ratio for viability. Dm values for p-XSC alone and in combination with rapamycin were determined to be 7.13 and 5.06 μM, respectively, while those for rapamycin, alone and in combination with p-XSC were 107.17 and 0.001 μM, respectively. The individual median effect plots for p-XSC and rapamycin were not parallel and therefore exclusivity could not be determined with certainty. Thus, we calculated CIs assuming that both compounds exhibited mutual and non-mutual exclusivity (α = 0 and 1, respectively). All CI values were similar when calculated using both values for α except for the two lowest effect levels (0.2 and 0.3) (See Table 2). At effect levels ranging from 0.2 to 0.9, the CI values indicated varying degrees of synergy, with the degree of synergy increasing as the fraction affected increased (Table 2).
Table 2.
Calculated values for the combination index (CI) as a function of fractional inhibition (Fa) of C4-2 cell viability for a mixture of p-XSC and rapamycin (molar ratio 5000:1) after 48 hr of treatment.
| Fractional Inhibition (Fa) | Combination Index (CI) | Description of combined effect |
|---|---|---|
| 0.2 | 0.808 | Moderate Synergism |
| 0.3 | 0.724 | Moderate Synergism |
| 0.4 | 0.715 | Moderate Synergism |
| 0.5 | 0.710 | Moderate Synergism |
| 0.6 | 0.705 | Moderate Synergism |
| 0.7 | 0.700 | Synergism |
| 0.8 | 0.694 | Synergism |
| 0.9 | 0.684 | Synergism |
The effects of combining p-XSC and rapamycin on mTOR target proteins
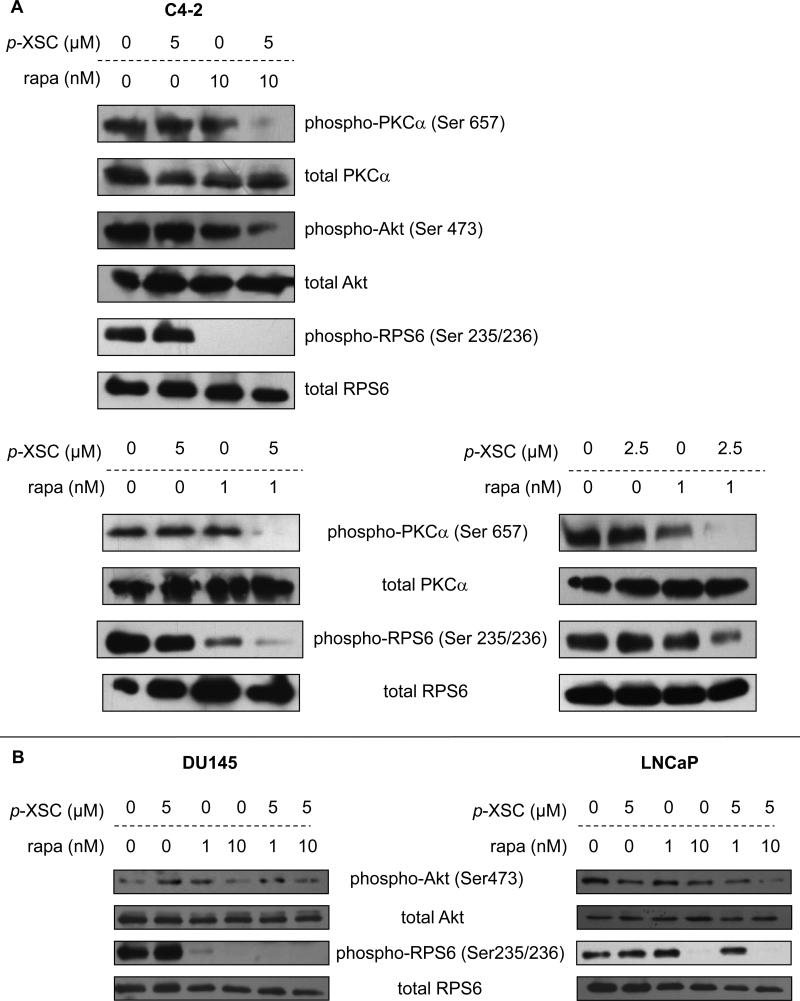
We investigated whether combining p-XSC and rapamycin at a set ratio (2.5 or 5 μM p-XSC and 1 or 10 nM rapamycin) for 90 min decreased phosphorylation of their early mTOR molecular targets in C4-2 cells. Treated cells were analyzed by immunoblotting and phosphorylations of downstream targets of the mTOR pathway were determined using combinations of p-XSC and rapamycin. Results showed that the combination had greater efficacy to inhibit phosphorylation of the mTORC2 downstream targets, Akt and PKCα, than either agent alone at the same dose (Figure 3A). By contrast, treatment with 10 nM rapamycin alone was sufficient to eradicate phosphorylation of the mTORC1 downstream target, RPS6, and therefore we were unable to conclude whether p-XSC caused enhancement of the activity of rapamycin at this dose and time point. As a result of this finding, the dose of rapamycin was decreased to 1 nM in combination with p-XSC. This combination was sufficient to inhibit phosphorylation of PKCα at Ser 657, an mTORC2 specific site (8). Treatment with 1 nM rapamycin alone still decreased phospho-RPS6 (Ser 235/236), however, the combination of p-XSC and rapamycin was considerable more efficacious in decreasing phosphorylation. Results similar to these were observed in C4-2 cells treated with 2.5 μM p-XSC and 1 nM rapamycin, reinforcing the concept that combination of these agents are effective at low and clinically achievable doses. Immunoblotting analysis also showed that the responses of the LNCaP and DU145 cells paralleled those of the C4-2 cells in that the combination of p-XSC (5 μM) with rapamycin (1 or 10 nM) inhibited phosphorylation of both the mTORC1 downstream target, RPS6, and the mTORC2 target, Akt (Figure 3B).
Figure 3.
The effects of combining p-XSC and rapamycin on mTOR pathway molecules. Immunoblot analysis of phosphorylated downstream targets of mTOR in A. C4-2 cells treated with 5 μM p-XSC and 10 nM or 1 nM rapamycin (rapa) or 2.5 μM p-XSC and 1 nM rapa. B. DU145 and LNCaP cells treated with 5 μM p-XSC and 1 or 10 nM rapa.
DISCUSSION
This study determined that the synthetic organoselenium compound, p-XSC, inhibits mTORC2 signaling in human AI prostate cancer cells. A previous report showed that high doses (0.125–2 mM) of inorganic sodium selenate inhibit IGF-1-stimulated phosphorylation of Akt and mTOR in HT-29 human colon cancer cells (34). This study suggested that selenate inhibits mTORC1 by both an Akt-dependent and an Akt-independent mechanism. In other studies, 24 hr treatment of prostate cancer cells with an extract of selenium-enriched broccoli, which accumulates two active anticancer agents: sulforaphane and Se-methylselenocysteine, reduced phosphorylation of mTOR (35). Our data show that p-XSC exhibits specificity and selectivity in that the mTORC2 is preferentially targeted. p-XSC inhibited phosphorylation of Akt and PKCα both downstream targets of mTORC2 whereas it had no effect on phosphorylation of downstream targets of mTORC1. In addition, p-XSC decreased the levels of mTORC2 protein, rictor, associated with mTOR but had no effect on the levels of raptor, the protein bound to mTORC1. The present study was specifically designed to examine early changes (t = 90 min) mediated by p-XSC. Subsequent studies will evaluate whether extended exposures to p-XSC in AI and AR prostate cancer cells would affect changes in mTORC1 signaling.
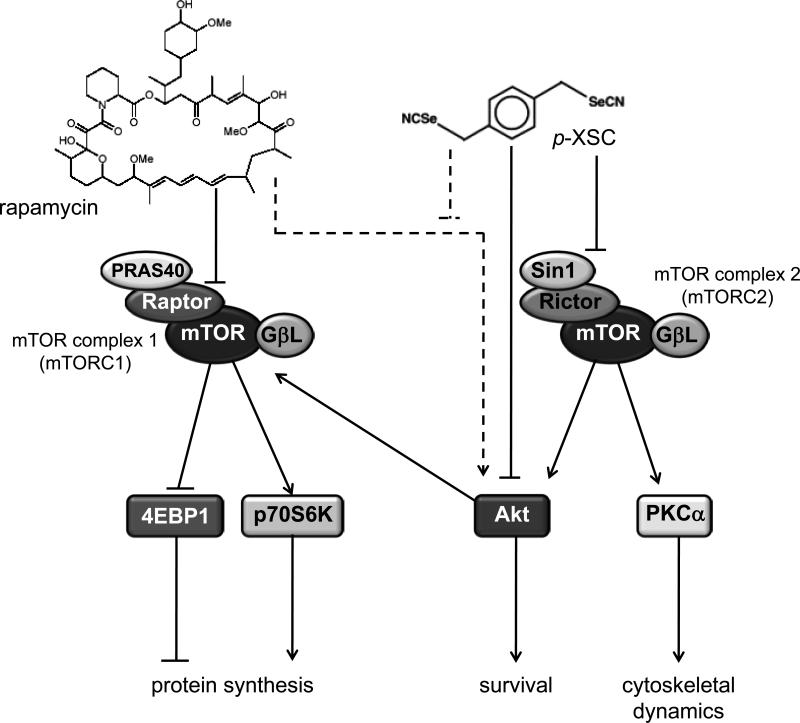
Our findings markedly expand our current understanding of p-XSC-mediated inhibition of prostate cancer cell growth (20,26,27). Inhibition of mTORC2 by p-XSC, subsequently results in inhibition of Akt as well as its other downstream targets and thus greatly expands the impact that organoselenium compounds have on growth of androgen independent prostate cancer cells (Figure 4). A recent study in PTEN null mice showed that mTORC2 was required for the development of prostate cancer caused by the loss of Pten but was non-essential in normal prostate, highlighting mTORC2 as a significant target for prostate cancer treatment (36) especially apropos to hormone refractory disease. This further supports the potential of p-XSC for the management of advanced prostate cancer, which is frequently characterized by deletion of Pten (37,38) and significant “crosstalk” between androgen receptors and mTOR signaling to evoke a survival advantage of prostate cancer cells during androgen deprivation and under conditions of low nutrient intake (1).
Figure 4.
Proposed scheme for the inhibition of both arms of the mTOR pathway by p-XSC and rapamycin in C4-2 cells. Rapamycin inhibits mTORC1 signaling while p-XSC inhibits mTORC2 signaling. p-XSC treatment may offset feedback activation of Akt by rapamycin (represented by broken lines).
A major highlight of our study is the finding that mixtures of p-XSC and rapamycin are more effective than either agent alone at low dose to inhibit prostate cancer cell viability. After 48 hr of treatment, C4-2 cell viability was significantly inhibited. The most promising combinations were those comprised of p-XSC with low-dose rapamycin (0.1, 1, and 10 nM). The efficacy of these compounds was markedly increased by 38% when p-XSC and rapamycin were mixed rather than used as single agent therapies thus providing dose ranges which are physiologically achievable and potentially clinically safe. The physiological level of rapamycin achieved in patients is on the order of 1 nM, although higher trough levels (up to 20 nM) have been reached in some clinical studies with marginal side effects (11,28,39). When added to rapamycin, p-XSC decreased its IC50 from the micromolar range to the low-nanomolar range (Table 1). In a similar fashion, addition of 1 nM rapamycin to p-XSC decreases its IC50 more than two-fold (Table 1). It is of vital importance to clinical management of prostate cancer to be able to achieve inhibition of growth and proliferation of both AR and AI prostate cancer cells using doses of these agents that are sustainable in humans.
An ideal objective in creating a strategy for combination therapy is to achieve synergy between drugs. In this study, we used median effect analysis described by Chou and Talalay (29) to determine the combination index (CI) for p-XSC and rapamycin at a constant molar ratio of 5000:1. The median effect plots generated for p-XSC and rapamycin alone and in combination had correlation coefficients greater than 0.9, confirming the applicability of this means of analysis for our study. We could not determine the exclusivity of the mechanisms of action of p-XSC and rapamycin because the slopes of their median effect plots were not parallel. Our analyses clearly indicate that the combination of p-XSC with rapamycin at a constant molar ratio of 5000:1 does exhibit synergistic inhibition of C4-2 cell viability. The calculated CIs indicate a moderate but highly significant degree of synergism that can be achieved by combining p-XSC with rapamycin. The strength of synergy of this combination increased as the effect level increased, reaching a maximal degree (CI = 0.684) at 90% inhibition.
Our data also show that treatment of C4-2 cells with combinations of p-XSC and rapamycin achieve early target inhibition (at least by 90 min) at concentrations that have little or no effect when administered individually. Mixtures of 2.5 or 5 μM p-XSC with 1 nM rapamycin significantly inhibit phosphorylation of downstream targets of both mTORC1 and mTORC2, likely contributing to their detrimental effects on cell viability. This study is of clinical significance as it identifies a combination regimen that fits the medical paradigm of a highly effective two-agent therapy. Future studies are needed to provide exact mechanisms by which rapamycin sensitizes AI prostate cancer cells to the actions of p-XSC.
The combination of p-XSC and rapamycin was also effective in AR LNCaP cells as well as in prostate cells with mutated p53 and absence of androgen receptors, namely, DU-145 cells. Although less responsive to p-XSC alone, AI DU-145 cells respond well to the combination of p-XSC with rapamycin. RPS6 phosphorylation was dramatically inhibited in DU-145 cells treated with mixtures of p-XSC and rapamycin compared to cells treated with the individual agents. Mixtures of p-XSC and rapamycin at doses higher than 5 μM and 1 nM, respectively, significantly inhibited DU-145 cell viability to extents greater than either agent alone (data not shown). Interestingly, the combination was effective in DU145 cells despite this line being known to have less of a dependence on the PI3K pathway than the other lines used. p-XSC has been shown to inhibit multiple targets and it may be unknown mechanisms of action of this compound and its synergy with rapamycin that drive inhibition in the DU145 line.
Often multiple survival pathways are activated in cancer cells. The use of combinations of agents that inhibit different pathways is an attractive alternative to treatments with a single chemotherapeutic agent. Our studies show that p-XSC and rapamycin inhibit prostate cancer cell viability more effectively than either agent alone, in part, by targeting two distinct arms of the mTOR signaling cascade (Figure 4). Other studies have shown that inhibition of mTORC1 can activate Akt (40). Rapamycin treatment, while suppressing mTORC1 signaling, may be regulating Akt signaling in prostate cancer cells. Thus simultaneous treatment of prostate cancer cells with p-XSC, which specifically inhibits mTORC2 and subsequently Akt, along with rapamycin, may be acting to compensate for feedback activation of this regulatory cell survival pathway.
The ability of p-XSC to inhibit mTORC2 signaling, a potentially critical target in cancer treatment, is a significant finding in support of its use as a chemotherapeutic tool against advanced prostate cancer. These data together with the finding that rapamycin can enhance the anti-cancer effects of p-XSC in C4-2 cells, as well as LNCaP and DU145 cells, provide a rationale for continuing exploration of this and other organoselenium derivatives, alone or in combination, as means for improving patient survivorship especially for those with hormone refractory prostate cancer. Further characterization of the anti-cancer mechanisms of p-XSC in prostate cancer cells will be useful for understanding and subsequently improving cancer treatments using targeted approaches with organoselenium. Investigations of the effects of p-XSC and rapamycin individually and in combination on tumorigenesis in animal models of prostate cancer are a necessary next step in the evaluation of the potential applicability of these agents to a clinical setting. Lastly, our studies provide a framework for future investigations to treat hormone refractory disease using other organoselenium compounds in combination with analogs of rapamycin or other signal pathway inhibitors.
ACKNOWLEDGEMENTS
Authors thank Dr. Yuan-Wan Sun, Department of Biochemistry and Molecular Biology, Penn State College of Medicine for synthesizing p-XSC for this study. The study was supported in part by NCI CA111842 (JTP and RS), George L. Laverty Foundation (RS), NCI CA 127729 (KEB) and Penn State Hershey Cancer Institute Funds.
ABBREVIATIONS USED
- AI
androgen independent
- AR
androgen responsive
- CI
combination index
- DMSO
dimethylsulfoxide
- FBS
fetal bovine serum
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex 1
- mTORC2
mTOR complex 2
- MTT
(3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide)
- PBS
phosphate buffered saline
- p-XSC
1,4-phenylenebis(methylene) selenocyanate
References
- 1.Wu Y, Chhipa RR, Cheng J, Zhang H, Mohler JL, Ip C. Androgen receptor-mTOR crosstalk is regulated by testosterone availability: implication for prostate cancer cell survival. Anticancer Res. 2010;30:3895–3901. [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiol. 2006;21:362–9. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons JJ, Abraham RT, Yu K. Mammalian target of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Seminars in Oncol. 2009;36:S3–S17. doi: 10.1053/j.seminoncol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Kremer CL, Klein RR, Mendelson J, Browne W, Samadzedeh LK, Vanpatten K, Highstrom L, Pestano GA, Nagle RB. Expression of mTOR signaling pathway markers in prostate cancer progression. Prostate. 2006;66:1203–12. doi: 10.1002/pros.20410. [DOI] [PubMed] [Google Scholar]
- 5.Brown RE, Zotalis G, Zhang PL, Zhao B. Morphometric confirmation of a constitutively activated mTOR pathway in high grade prostatic intraepithelial neoplasia and prostate cancer. Int J Clinical Exp Path. 2008;1:333–42. [PMC free article] [PubMed] [Google Scholar]
- 6.Dai B, Kong YY, Ye DW, Ma CG, Zhou X, Yao XD. Activation of the mammalian target of rapamycin signaling pathway in prostate cancer and its association with patient clinicopathological characteristics. BJU Int. 2009;104:1009–16. doi: 10.1111/j.1464-410X.2009.08538.x. [DOI] [PubMed] [Google Scholar]
- 7.Sarbassov DD, Guertin DA, Ali AM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 8.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Jozwiak J, Jozwiak S, Oldak M. Molecular activity of sirolimus and its possible application in tuberous sclerosis treatment. Med Res Rev. 2005;26:160–80. doi: 10.1002/med.20049. [DOI] [PubMed] [Google Scholar]
- 10.Lane HA, Breuleux M. Optimal targeting of the mTORC1 kinase in human cancer. Curr Opin Cell Biol. 2009;21:219–29. doi: 10.1016/j.ceb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Mikhailova M, Bose S, Pan CX, deVere White RW, Ghosh PM. Regulation of androgen receptor transcriptional activity by rapamycin in prostate cancer cell proliferation and survival. Oncogene. 2008;27:7106–17. doi: 10.1038/onc.2008.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C, Abate-Shen C. Targeting Akt/mTOR and ERK MAPK signaling inhibits hormone refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–64. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schayowitz A, Sabnis G, Njar VC, Brodie AM. Synergistic effect of a novel antiandrogen, VN/124-1, and signal transduction inhibitors in prostate cancer progression to hormone independence in vitro. Mol Cancer Ther. 2008;7:121–32. doi: 10.1158/1535-7163.MCT-07-0581. [DOI] [PubMed] [Google Scholar]
- 14.Liu QJ, Xu XH, Shang DH, Tian Y, Lü WC, Zhang YH. Rapamycin enhances the susceptibility of both androgen-dependent and -independent prostate carcinoma cells to docetaxel. Chin Med J. 2010;123:356–60. [PubMed] [Google Scholar]
- 15.Zhang W, Zhu J, Efferson CL, Ware C, Tammam J, Angagaw M, Bettano KA, Kasibhatla S, Reilly JF, Sur C, Majumder PK. Inhibition of tumor growth progression by antiandrogens and mTOR inhibitor in a Pten-deficient mouse model of prostate cancer. Cancer Res. 2009;69:7466–72. doi: 10.1158/0008-5472.CAN-08-4385. [DOI] [PubMed] [Google Scholar]
- 16.Facompre ND, El-Bayoumy K. Potential stages for prostate cancer prevention with selenium: Implications for cancer survivors. Cancer Res. 2009;69:2699–703. doi: 10.1158/0008-5472.CAN-08-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai D, Sinha I, Null K, Wolter W, Suckow MA, King T, Amin S, Sinha R. Synthesis and anti-tumor properties of selenocoxib-1 against rat prostate adenocarcinoma cells. Int J Cancer. 2010;127:230–8. doi: 10.1002/ijc.25033. [DOI] [PubMed] [Google Scholar]
- 18.Pinto JT, Lee J-I, Sinha R, MacEwan M, Cooper AJL. Chemopreventive mechanisms of α- keto acid metabolites of naturally occurring organoselenium compounds. Amino Acids. 2011;41:29–41. doi: 10.1007/s00726-010-0578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinha I, Null K, Walter W, Suckow MA, King T, Pinto JT, Sinha R. Methylseleninic acid down regulates hypoxia inducible factor-1α in invasive prostate cancer. Int J Cancer. 2011 Apr 15; doi: 10.1002/ijc.26141. doi: 10.1002/ijc.26141. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Facompre ND, El-Bayoumy K, Sun YW, Pinto J, Sinha R. 1,4-phenylenebis(methylene) selenocyanate (p-XSC) but not selenomethionine inhibits androgen receptor and Akt signaling in human prostate cancer cells. Cancer Prev Res. 2010;3:975–84. doi: 10.1158/1940-6207.CAPR-10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu H, Jiang C, Ip C, Rustum YM, Lü J. Methylseleninic acid potentiates apoptosis induced by chemotherapeutic drugs in androgen-independent prostate cancer cells. Clin Cancer Res. 2005;11:2379–88. doi: 10.1158/1078-0432.CCR-04-2084. [DOI] [PubMed] [Google Scholar]
- 22.Zhao R, Xiang N, Domann FE, Zhong W. Effects of selenite and genistein on G2/M cell cycle arrest and apoptosis in human prostate cancer cells. Nutr Cancer. 2009;613:397–407. doi: 10.1080/01635580802582751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vadgama JV, Wu Y, Shen D, Hsia S, Block J. Effect of selenium in combination with Adriamycin or Taxol on several different cancer cells. Anticancer Res. 2000;20:1391–414. [PubMed] [Google Scholar]
- 24.Yamaguchi K, Uzzo RG, Pimkina J, Makhov P, Golovine K, Crispen P, Kolenko VM. Methylseleninic acid sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Oncogene. 2005;24:5868–77. doi: 10.1038/sj.onc.1208742. [DOI] [PubMed] [Google Scholar]
- 25.El-Bayoumy K, Chae YH, Upadhyaya P, Meschter C, Cohen LA, Reddy BS. Inhibition of 7,12-dimethylbenz(a)anthracene-induced tumors and DNA adduct formation in the mammary glands of female Sprague-Dawley rats by the synthetic organoselenium compound 1,4-phenylenebis(methylene)selenocyanate. Cancer Res. 1992;52:2402–7. [PubMed] [Google Scholar]
- 26.Pinto JT, Sinha R, Papp K, Facompre ND, Desai D, El-Bayoumy K. Differential effects of naturally occurring and synthetic organoselenium compounds on biomarkers in androgen responsive and androgen independent human prostate carcinoma cells. Int J Cancer. 2007;120:1410–7. doi: 10.1002/ijc.22500. [DOI] [PubMed] [Google Scholar]
- 27.Sinha R, Pinto JT, Facompre N, Kilheffer J, Baatz JE, El-Bayoumy K. Effects of naturally occurring and synthetic organoselenium compounds on protein profiling in androgen responsive and androgen independent human prostate cancer cells. Nutr Cancer. 2008;60:267–75. doi: 10.1080/01635580701630479. [DOI] [PubMed] [Google Scholar]
- 28.Johnston LJ, Brown J, Shizuru JA, Stockerl-Goldstein KE, Stuart MJ, Blume KG, Negrin RS, Chao NJ. Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:47–55. doi: 10.1016/j.bbmt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 30.Chou TC. Preclinical versus clinical drug combination studies. Leukemia Lymphoma. 2008;49:2059–80. doi: 10.1080/10428190802353591. [DOI] [PubMed] [Google Scholar]
- 31.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 32.Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): Phoshpo-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–7. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soliman GA, Acosta-Jaquez, Dunlop EA, Ekim B, Maj NE, Tee AR, Fingar DC. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem. 2010;285:7866–79. doi: 10.1074/jbc.M109.096222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YK, Park SY, Kim YM, Kim DC, Lee WS, Surh YJ, Park OJ. Suppression of mTOR via Akt dependent and independent mechanisms in selenium treated colon cancer cells: involvement of AMPKα1. Carcinogenesis. 2010;31:1092–99. doi: 10.1093/carcin/bgq040. [DOI] [PubMed] [Google Scholar]
- 35.Abdulah R, Faried A, Kobayashi K, Yamazaki C, Suradji EW, Ito K, Suzuki K, Murakami M, Kuwano H, Koyama H. Selenium enrichment of broccoli sprout extract increases chemosensitivity and apoptosis of LNCaP prostate cancer cells. BMC Cancer. 2009;9:414. doi: 10.1186/1471-2407-9-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guertin DA, Stevens DM, Saitoh M, Kinkel S, Crosby K, Sheen JH, Mullholland DJ, Magnuson MA, Wu H, Sabatini DM. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009;15:148–150. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D. Frequent inactivation of PTEN/MMAC in primary prostate cancer. Cancer Res. 1997;59:4997–5000. [PubMed] [Google Scholar]
- 38.Whang YE, Wu X, Suzuki H, Reiter RE, Tran C, Vessella RL, Said JW, Isaacs WB, Sawyers CL. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci USA. 1998;95:5246–50. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee N, Woodrum CL, Nobil AM, Rauktys AE, Messina MP, Dabora SL. Rapamycin weekly maintenance dosing and the potential efficacy of combination sorafenib plus rapamycin but not atorvastatin or doxycycline in tuberous sclerosis preclinical models. BMC Pharmacol. 2009;9:8–22. doi: 10.1186/1471-2210-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]