Abstract
Use of individually ventilated caging (IVC) systems for mouse-based laboratory investigation has dramatically increased. We found that without mice present, intra-cage oxygen concentration was comparable (21%) between IVC housing and ambient environment caging (AEC) that used wire top lids. However, when mice were housed 4-to-a-cage for 1 week, intra-cage oxygen dropped to 20.5% in IVC housing as compared to 21% for AEC housing. IVC intra-cage humidity was also elevated relative to AEC housing. Mice raised in IVC housing as compared to mice raised in AEC housing had higher RBC mass, hematocrit and hemoglobin concentrations. They also had elevated platelet counts but lower white blood cell counts. IVC mice relative to AEC mice had increased saccharin preference and increased fluid consumption but similar locomotion, food intake, social exploration and novel object recognition when tested in an AEC environment. Taken together, these data indicate that ventilated caging systems can have a 0.5% reduction from ambient oxygen concentration that is coupled to mouse red blood cell indices indicative of chronic exposure to a hypoxia. Importantly, IVC housing can impact behavioral testing for depressive-like behavior.
Keywords: Hypoxia, blood, housing, altitude, depression, anhedonia, locomotor, social exploration, novel object
Introduction
An important trend in laboratory rodent housing is the use of individually ventilated caging (IVC) systems. Purported advantages of IVC systems over conventional, ambient environment caging (AEC) (i.e. wire top, open air caging) are allergen and volatile organic compound (VOCs) reduction and the ability to increase animal population densities (Höglund and Renström, 2001; Mineu and Crusio, 2009; Silveman et al., 2008). In animal care/research personnel, allergy to laboratory animals can be as high as 44%, with a median time to allergy onset of less than 2 years (Fisher et al., 1998; Hunskaar and Fosse, 1990). Allergen exposure can originate from sources such as urine, fur/pelt, saliva and serum proteins (Gordon, 1997), and these allergens can contaminate the animal facility in both airborne particulate and fomite forms (Gordon and Preece, 2003; Kaliste et al., 2004). VOCs such as ammonia have been identified as causative agents of “sick building syndrome”, with animal care/research personnel reporting headache, nausea and fatigue (Kacergis et al., 1996). Educational training programs focused on personal hygiene and the use of personal protective equipment have reduced the incidence of laboratory animal-associated allergies, but with AEC the impact of such interventions has been modest (up to 22% of staff still developing allergies) (Fisher et al., 1998). On the other hand, IVC housing has been shown to significantly reduce the important mouse-derived human allergen, murine urinary protein (Gordon et al., 2001).
As we (York et al., 2011) and others (Jennings et al., 1998) have reviewed, pre-experimental conditions are critical to rodent-based behavioral testing outcomes. The methodology by which mice are fed, handled and housed can dramatically impact a host of behaviors and like-behaviors including those reliant on locomotion, food intake, learning/memory, social interaction, anxiety and depression (Hrabé de Angelis et al., 2004). Most rodent behavioral testing is conducted outside the home cage and in macro-environments removed from where the animal was reared. This is especially true of mice bred and raised with commercial suppliers. A critical assumption made is that once rodents of a particular strain are acclimatized to their new surroundings whether it is a new cage, intra/inter-facility room or institution that they behave equivalently. This notion has encompassed micro-environmental concerns as well because sophisticated behavioral testing requires specialized equipment and tasks that are unable to fit inside a standard shoebox sized cage. Thus, rodent behavioral testing is almost exclusively performed in an open air environment.
Little is known concerning how pre-experimental IVC housing affects mouse behaviors when compared to AEC housing. The single study published to date showed no effects of IVC housing in mice during plus maze, open field, radial arm maze, acoustic startle or resident intruder tests (Mineu and Crusio, 2009). While others have investigated the impact of IVC housing on mouse behavior, these studies either used IVC system cages outside of the ventilation unit (Kallnik et al., 2007) or were comparing different IVC systems to one another (Höglund and Renström, 2001). Importantly, no clear differences in IVC versus AEC housing were seen or mechanism for behavioral change presented (Höglund and Renström, 2001; Kallnik et al., 2007; Mineu and Crusio, 2009). In contrast, a cornucopia of data exists on the intra-cage microenvironmental differences between IVC and AEC housing with special attention paid to carbon dioxide, ammonia vapor and relative humidity (Höglund and Renström, 2001; Kacergis et al., 1996; Krohn and Hansen, 2002; Rosenbaum et al., 2010; Silverman et al., 2008). Surprisingly, intra-cage oxygen concentration in IVC housing has been ignored, although it is well known that in confined spaces with sealed ventilation systems like commercial airplanes (Rushkin et al., 2008), submarines (Luria and Morris, 1988) and space stations (Stewart et al., 2007), oxygen concentrations can easily fall below 21%. In turn, hypoxia impacts a variety of physiologic functions and bioactives including behavior, as we have shown (Johnson et al., 2007) and reviewed (Johnson et al., 2008). In sum, no studies have reported on intra-cage oxygen concentration in IVC housing. Therefore, we examined intra-cage oxygen in IVC housing to determine its potential relevance to pre-experimental mouse physiology.
Materials and Methods
Materials
All reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except as noted.
Animals and housing
Animal use was conducted in accordance with institutional guidelines for the care and use of laboratory mice. All experimental procedures were approved by the University of Illinois at Urbana-Champaign IACUC. All animals were housed in an AAALAC accredited laboratory facility as outlined in the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Research, 2011). Male C57BL/6J mice, 3 wks of age, were obtained from The Jackson Laboratory (Bar Harbor, MN). Since IVC systems/care varies (pressure, air exchange, animal density, bed changes), 3-wk old mice were used to maximize time spent within our IVC housing system. Prior to shipping, mice had been housed in either IVC or AEC conditions by The Jackson Laboratory. Mice were group housed (4 per cage except for oxygen and humidity studies where 2 and 3 mice per cage were also examined) in standard shoebox cages (28 cm × 17 cm × 12.5 cm) with wire top cage lids that were either open to the ambient environment (AEC) or attached to a positive-pressure Micro-VENT Mouse individually ventilated caging (IVC) system (Allentown, Inc., Allentown, NJ). All mice were allowed water and standard rodent chow (NIH-31 7013, Harlan Laboratories, Inc., Indianapolis IN) ad libitum. Regardless of housing method used, the room in which the mice resided was environmentally controlled on a 12:12 h dark:light cycle (2000 h-0800 h) at a temperature of 72° F, relative humidity of 26-48% and 10-15 hourly air changes. The total number of mice used was 250.
Oxygen, carbon dioxide, ammonia and humidity
Room and intra-cage air oxygen and carbon dioxide were measured using ProOx oxygen and ProCO2 carbon dioxide sensors, respectively (Biospherix, Lacona NY). Humidity was measured using a digital hygrometer (Cat No. 11-661-18, Fisher Scientific, Pittsburgh PA). Ammonia was measured using a Kwik-Draw Sampling Pump (Cat. No. 488543, MSA, Pittsburgh PA) with 5-700 ppm Ammonia-specific sampling tubes (Cat. No. CH20501, Dräger Germany). Room and intra-cage air and humidity measurements were performed at 1400 h daily for 3 consecutive days. For intra-cage measurements, the sensors were located in the food hopper. Measurements were randomized in regard to sensor placement within the room and to cage location within the cage racks.
Treatments and testing
Mice examined were between 8-12 wks of age and had spent 5-9 wks in AEC or IVC conditions. AEC mice were housed in AEC conditions (10-15 air changes/h) and then treated and/or tested in AEC conditions. IVC mice were housed in IVC conditions (60 air changes/h) and then treated and/or tested in AEC conditions.
Hematology
After being housed in either AEC or IVC housing, mice were euthanized using carbon dioxide. Blood was drawn using post-mortem intracardiac puncture. A total approximate volume of 0.6-0.8 mL of blood was obtained from each mouse and placed into two separate (0.3-0.5 mL) pediatric EDTA anticoagulation microtainer tubes (Cat No. 365974, Becton Dickinson, Franklin Lakes NJ). Complete blood counts (CBC) and differentials were performed at University of Illinois Veterinary Diagnostic Laboratory (Urbana IL) on an Abbott Diagnostics Cell Dyn 3700 automated hematology cell counter (Abbott Park IL).
Body mass and food and water consumption
Immediately prior to testing, mice were individually housed in AEC. Body mass and food and water consumption were measured daily at 1000 h by weight. Food and water consumption were determined from the weight of the water bottle plus water and the weight of the food container plus food before and after each 24 h data collection period by methods we have previously described (Sherry et al., 2010). Briefly, the daily mass of the food or water in their respective containers were subtracted from the previous days mass, to determine amount consumed. Cage floors and bedding were carefully checked to account for food spillage and potential hoarding.
Group housed water consumption
Similar to the above, water consumption was determined from the weight of the water bottle plus water from AEC and IVC group housed mice. After 1 week, water loss was recorded. Briefly, final mass was subtracted from initial mass to determine amount consumed. Grams of water consumed per gram of mouse (total water consumed/total cage mouse weight) was calculated.
Movement
Movement was assessed by biotelemetry (Mini Mitter, Bend OR) as we have previously described (Sherry et al., 2010). In brief, the surgical area (bench top) was cleaned with 70% ethanol. Mice were anesthetized via intraperitoneal injection of (80 mg/mL:12 mg/mL) ketamine:xylazine (Butler Schein Animal Health, Dublin OH) at 1.5 mL/kg body weight. Immediately preceding surgery, mice were injected intraperitoneally with buprenorphine at a dose of 0.05 mg/kg. Fur was shaved and the surgical site was aseptically cleaned and prepared using 10% povidone iondine and 70% ethanol in three alternating wipes. Mice were kept on a heating pad during surgery and recovery. The abdomen was opened and a sterilized G2 e-mitter (Cat. No. 870-0010-01; Mini Mitter, Bend OR) was placed in the abdominal cavity along the saggital plane. 3-0 Vicryl violet braided dissolvable suture material (Cat. No. J393; Ethicon, Inc., Cornelia GA) was then passed through the silastic tubing attached to the outer wall of the e-mitter, and the e-mitter sutured to the body wall. The skin was then closed with 18/8 acid resistant, antimagnetic stainless steel 7.5 mm × 1.75 mm Michel suture clips (Cat. No. 12040-01; Fine Science Tools, Foster City CA) using forceps-style application. Immediately post-recovery, mice were individually housed in AEC and movement was recorded every hr for 5 days via under-cage ER-4000 receiver pads (Mini Mitter, Bend OR). Movement was quantified using Vital View data acquisition software (Mini Mitter, Bend OR).
Saccharin preference testing
Three days prior to saccharin preference testing (adaptation phase) mice were singly housed in AEC conditions adapted for two bottle water access. Both bottles contained water. After the adaptation phase, fluid bottles (randomized to right verses left) contained either water or a 0.4% sodium saccharin solution (Sigma-Aldrich, CN 4-7839) as we have previously described (Lavin et al., 2011). Fluid consumption was recorded after 24 h. Water and saccharin consumption were recorded at 1000 h and determined by weight of the bottle and fluids before and after the 24 h data collection period.
Social exploration
24 h prior to testing, AEC and IVC mice were individually housed in AEC and IVC conditions, respectively. After 24 h of new home cage acclimation, social exploration testing was conducted in AEC conditions, as we have previously described (Johnson et al., 2007). At the time points of 0, 2, 4 and 6 h, a 3-4 week-old novel, conspecific juvenile mouse of the same sex (challenge mouse) was introduced into the home cage of the subject mouse. The challenge mouse was placed in a 7.62 cm × 7.62 cm × 7.62 cm square metal mesh enclosure. Testing duration was 5 min and a new challenge mouse was used to test each subject mouse at every time point examined. Investigation/exploration was evaluated from the video record by a trained observer blinded to the pre-experimental housing conditions. Social exploration was considered as nose-to-enclosure contact. Video recording of animal behavior was performed under red light using a Sony HDR-XR500V Night Shot capable video camera (San Diego CA). Social exploration testing was initiated at 1000 h.
Novel object recognition
One hour prior to testing, mice were individually removed from their home cage and placed for 5 minutes in a novel arena (home cage-sized with light bedding) containing two identical objects (large stainless steel bolts) positioned 10 cm apart at the short-side wall end 5 cm from the short side wall and 3.5 cm from the long-side wall. After training, mice were returned to their home cage for 1 hr. Testing was initiated by returning mice to the testing arena where one of the identical objects (familiar object) was replaced (randomized to right or left) by an unfamiliar object (novel object, a 5 mL microfuge tube). Investigative behavior was video recorded for 5 min and evaluated from the video record using EthoVision XT 7 (Noldus Information Technology, Leesburg VA). Percent investigation was calculated by dividing the time spent examining each object by the total time spent investigating both objects. Testing occurred 4.5 h after the beginning of the dark cycle (1200 h). Video recording of animal behavior was performed under red light using a Night Shot capable video camera.
Spontaneous locomotor activity
Immediately prior to testing, mice were individually removed from their home cage and placed in a novel arena (home cage-sized with light bedding). Spontaneous locomotor activity (sLMA) was video recorded immediately following relocation to the novel arena and at the time points indicated and total distance moved (cm), velocity of movement (cm/s) and duration of movement (s) over 5 min were evaluated from the video record using EthoVision XT 7.
Forced swim test
The forced swim was performed as previously described (Lavin et al., 2011). Testing was initiated by transferring group housed AEC or IVC mice individually to a clean novel white cylindrical polyvinyl chloride container (diameter 16 cm; height 31 cm) containing 15 cm of water maintained at 25 ± 1 °C. Total swim duration was 6 min and immobility was evaluated from the video record using Ethovision XT 7.
Sickness behavior
Mice were injected IP with 100 μg/kg ultra-pure lipopolysaccharide (LPS) (Escherichia coli, O127:B8; CN L5024) or 250 μL of sterile saline. At the times indicated, mouse sLMA was video recorded within the home cage. Total distance moved (cm) was evaluated from the video record using Ethovision XT. To control for mouse-to-mouse variability in baseline sLMA and allow comparison of relative changes in sLMA, the pre-LPS exposure (baseline) was used as an internal control for each mouse. Testing was initiated 1 h after the beginning of the dark cycle (1000 h).
Statistics
All data are presented as mean ± SEM. Data were analyzed using SAS 9.2 (SAS Institute, Inc., Cary NC). To test for statistical differences, a one-way or two-way ANOVA was used with or without repeated measurements where needed. Tukey’s test was used for post-hoc pair-wise multiple comparison procedures. All statistical analysis included testing for time point × housing type interactions, where appropriate. Relevant pair-wise differences are included in tables and figure legends, where appropriate. Statistical significance was denoted at p<0.05.
Results
Air oxygen concentration is reduced in IVC housing when compared to AEC housing
Table 1 demonstrates that intra-cage air oxygen in IVC housing was 2.5% less than in AEC housing when mice were housed 4 per cage. In addition, percent change in oxygen reduction between AEC and IVC housing was greater when mice were housed 4 per cage versus 3 and 2 per cage. Table 2 shows that intra-cage relative humidity (RH) was increased 44% in IVC housing compared to AEC housing when mice were housed 4 per cage. In addition, % change in RH between AEC and IVC housing was greater when mice were housed 4 per cage versus 2 per cage. Carbon dioxide and ammonia concentrations were comparable between IVC and AEC housing (data not shown).
Table 1.
Oxygen (O2) percentages in ambient room air and within AEC or IVC housing.
| Mice per cage: | 4 | 3 | 2 | |||
|---|---|---|---|---|---|---|
| AEC | IVC | AEC | IVC | AEC | IVC | |
| Ambient (room) O2 (%): | 20.98 ± 0.03a | 20.97 ± 0.05a | 20.98 ± 0.03a | 20.95 ± 0.02a | 20.95 ± 0.03a | 20.97 ± 0.02a |
| Cage O2 (%): | 20.98 ± 0.03a | 20.43 ± 0.05b | 20.98 ± 0.03a | 20.53 ± 0.05b | 20.95 ± 0.03a | 20.62 ± 0.03b |
|
| ||||||
| O2 percentage change (%): | 0.00 ± 0.00a | −2.54 ± 0.10c | 0.00 ± 0.00a | −1.99 ± 0.21b | 0.00 ± 0.00a | −1.69 ± 0.18b |
Results are expressed as mean ± s.e.m., n=4-6/housing condition. Main effects of O2 percentages: housing type (F(1,48) = 165.16, P < 0.01), mice per cage (F(2,48) = 1.26, P = 0.29); housing type x mice per cage interaction (F(2,48) = 3.90, P = 0.03). Results for Ambient (room) O2 (%) and Cage O2 (%) within Mice per cage columns without a common superscript letter are significantly different (P < 0.05). Main effects of O2 percentage change: housing type (F(1,24) = 336.64, P < 0.01), mice per cage (F(2,24) = 5.14, P = 0.01); housing type x mice per cage interaction (F(2,24) = 5.14). Results within the O2 percentage change (%) row without a common superscript are significantly different (P < 0.05).
Table 2.
Relative humidity (RH) percentages in ambient room air and within AEC or IVC housing.
| Mice per cage: | 4 | 3 | 2 | |||
|---|---|---|---|---|---|---|
| AEC | IVC | AEC | IVC | AEC | IVC | |
| Ambient (room) RH (%): | 26.67 ± 0.11a | 32.65 ± 0.75b | 47.90 ± 0.06a | 46.12 ± 0.09a | 47.95 ± 0.08a | 46.38 ± 0.15a |
| Cage RH (%): | 26.87 ± 0.09a | 47.28 ± 1.32c | 47.97 ± 0.06a | 64.42 ± 0.63b | 47.98 ± 0.10a | 54.15 ± 0.36b |
|
| ||||||
| RH percentage change (%): | 00.75 ± 0.37a | 44.87 ± 4.18c | 00.14 ± 0.05a | 39.69 ± 1.47c | 00.07 ± 0.05a | 16.75 ± 1.01b |
Results are expressed as mean±s.e.m., n=4-6/housing condition. Main effects of RH percentages: housing type (F(1,60) = 1021.83, P < 0.01), mice per cage (F(2,60) = 2299.72, P < 0.01); housing type x mice per cage interaction (F(2,60) = 174.98, P <0.01). Results for ambient (room) RH (%) and cage RH (%) within mice per cage columns without a common superscript letter are significantly different (P < 0.05). Main effects of RH percentage change: housing type (F(1,30) = 580.99, P < 0.01), mice per cage (F(2, 30) = 40.17, P < 0.01); housing type x mice per cage interaction (F(2,30) = 37.39, P < 0.01). Results within the RH percentage change (%) row without a common superscript are significantly different (P < 0.05).
IVC mice show hematologic evidence of chronic exposure to low-grade hypoxia
Table 3 shows that IVC mice when compared to AEC mice had a 9.7%, 8.6% and 8.8% increase in RBC number, hemoglobin and hematocrit, respectively. Table 4 demonstrates that leukocytes and platelets were decreased 37.5% and increased 16.7%, respectively in IVC mice when compared to AEC mice.
Table 3.
Red blood cell (RBC) count and related RBC parameters from group-housed (4/group) AEC and IVC mice.
| AEC | IVC | F & P values | |
|---|---|---|---|
| Red blood cells (M/μL): | 8.46 ± 0.09a | 9.28 ± (0.07)b | F(1,8) = 58.71, P < 0.01 |
| Hemoglobin (g/dL): | 12.78 ± 0.13a | 13.88 ± (0.06)b | F(1,8) = 52.50, P < 0.01 |
| Hematocrit (%): | 41.57 ± 0.39a | 45.23 ± (0.31)b | F(1,8) = 54.66, P < 0.01 |
| Mean cell volume (fL): | 49.02 ± 0.15 | 48.80 ± (0.31) | F(1,8) = 0.63, P = 0.45 |
| Mean cell hemoglobin (pg): | 15.08 ± 0.11 | 14.95 ± (0.10) | F(1,8) = 0.86, P = 0.38 |
| Mean cell hemoglobin concentration (g/dL): | 30.75 ± 0.20 | 30.68 ± (0.10) | F(1,8) = 0.10, P = 0.76 |
| RBC distribution width (%): | 18.55 ± 0.56 | 18.58 ± (0.26) | F(1,8) = 0.00, P = 0.97 |
Results are expressed as mean ± s.e.m., n=4-6/housing condition. Results within rows without a common superscript letter are significantly different (P < 0.05).
Table 4.
Leukocyte count, differential and platelet count from group-housed (4/group) AEC and IVC mice.
| AEC | IVC | F & P values | |
|---|---|---|---|
| Leuckocytes (K/μL): | 2.99 ± 0.34a | 1.87 ± 0.17b | F(1,8) = 7.78, P = 0.02 |
| Neutrophils (%): | 7.14 ± 1.02 | 10.63 ± 1.64 | F(1,8) = 4.66, P = 0.06 |
| Lymphocytes (%): | 80.82 ± 6.12 | 81.18 ± 1.99 | F(1,8) = 0.00, P = 0.96 |
| Monocytes (%): | 8.43 ± 4.91 | 5.17 ± 1.96 | F(1,8) = 0.32, P = 0.59 |
| Eosinophils (%): | 0.96 ± 0.25 | 0.97 ± 0.25 | F(1,8) = 0.00, P = 0.98 |
| Basophils (%): | 2.64 ± 0.90 | 2.07 ± 0.48 | F(1,8) = 0.28, P = 0.61 |
| Platelets (K/μL): | 890.83 ± 37.30a | 1039.30 ± 27.35b | F(1,8) = 10.19, P = 0.01 |
| Mean platelet volume (fL): | 6.47 ± 0.14 | 6.26 ± 0.09 | F(1,8) = 1.47, P = 0.26 |
Results are expressed as mean ± s.e.m., n=4-6/housing condition. Results within rows without a common superscript letter are significantly different (P < 0.05).
IVC mice consume less water
Table 5 shows that IVC mice during their first 24 h of individual housing in an AEC environment drink 15.1% less water compared to AEC mice transferred to individual housing in AEC conditions. Percent daily changes in body weight and food intake were not impacted by this housing switch. After 48 hr in an AEC environment, IVC mice water intake was comparable to AEC mice (Table 5). Table 6 shows that IVC mice consume 12 % less water compared to AEC mice when examined in their respective housing conditions.
Table 5.
Percent change in body weight, food and water consumption (g) following relocation from group-housed (4/group) IVC or AEC cages to novel AEC housing 24, 48 and 72 h following relocation.
| 24 h | 48 h | 72 h | ||||
|---|---|---|---|---|---|---|
| AEC | IVC | AEC | IVC | AEC | IVC | |
| Body weight: | 0.68 ± 0.33 | 0.51 ± 0.51 | -0.26 ± 0.55 | 0.16 ± 0.32 | 0.52 ± 0.28 | 0.11 ± 0.68 |
| Food intake: | 3.92 ± 0.29 | 3.90 ± 0.15 | 3.80 ± 0.24 | 3.98 ± 0.20 | 3.53 ± 0.23 | 3.53 ± 0.09 |
| Water intake: | 4.83 ± 0.15a | 4.10 ± 0.12b | 4.25 ± 0.17a,b | 4.03 ± 0.18b | 4.17 ± 0.18b | 3.83 ± 0.19b |
Results are expressed as mean ± s.e.m., n = 6-12 per group. Percent change in body weight main effects: time (F(2,30) = 1.15, P = 0.33), housing type (F(1,30) = 0.03, P = 0.87; time × housing type interaction (F(2,30) = 0.50, P = 0.61). Food intake main effects: time (F(2,30) = 2.45, P = 0.10), housing type (F(1,30) = 0.13, P = 0.72); time × housing type interaction (F(2,30) = 0.17, P = 0.85). Water intake main effects: time (F(2,66) = 4.62, P = 0.01), housing type (F(1,66) = 11.15, P < 0.01); time × housing type interaction (F(2,66) = 1.40, P = 0.25). Results within individual rows without a common superscript letter are significantly different (P < 0.05).
Table 6.
Water consumption of AEC and IVC housed mice.
| AEC: | 0.97 ± 0.04a |
| IVC: | 0.85 ± 0.03b |
Results are expressed as grams water consumed per gram mouse per week, means±s.e.m., n=6/housing condition. F(1,10) = 7.09, P = 0.0238 AEC v. IVC.
IVC mice have an increased preference for saccharin but no change in locomotion, social exploration or novel object recognition
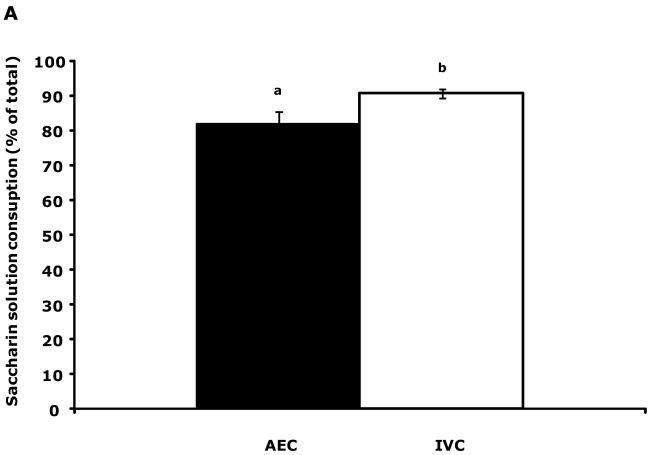
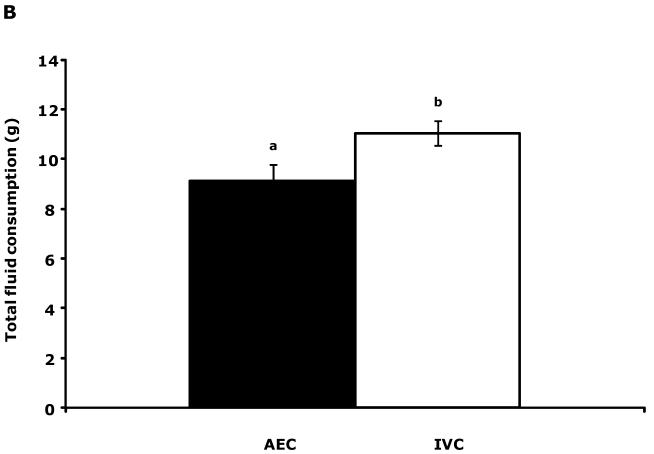
Fig.1A shows that IVC mice had a 10.9% increase in saccharin preference compared to AEC mice (81.9 ± 3.6 v. 90.8 ± 1.2). This elevation was coupled to a 21.5% increase in total fluid consumption (water + saccharin solution) (9.1 ± 0.7 v. 11.1 ± 0.5) (Fig.1B). Table 7 demonstrates that, after implanting intraperitoneal biotelemetry probes, mouse movement for both AEC and IVC mice took 48 h to stabilize. Social exploration (Table S1), novel object recognition (Table S1), spontaneous locomotor activity (Table S2) and forced swim time (Table S2) were comparable between AEC and IVC mice.
Figure 1.
IVC mice show an increased preference for saccharin and consume more total fluid than AEC mice. (A) Results are expressed as percent saccharin consumed, means ± s.e.m., n = 12. Bars without a common superscript are significantly different (81.89 ± 3.60 v. 90.80 ± 1.24, AEC v. IVC; F(1,22) = 5.98, P = 0.02). (B) Results are expressed as total fluid consumed in grams, means ± SEM: n = 12. Bars without a common superscript are significantly different (9.11 ± 0.68 v. 11.07 ± 0.49, AEC v. IVC; F(1,22) = 6.02, P = 0.02).
Table 7.
Continuous locomotor activity (cLMA) following e-mitter implantation surgery and relocation from group-housed (4/group) IVC or AEC cages to novel AEC housing 24, 48, 72, 96 and 120 h following relocation.
| AEC | IVC | |
|---|---|---|
| 24 h: | 8475.33 ± 1457.10a | 9885.50 ± 1782.18a |
| 48 h: | 11595.17 ± 2257.04a,b | 14466.13 ± 4145.89a,b |
| 72 h: | 14911.33 ± 2738.22a,b | 17247.25 ± 3216.67a,b |
| 96 h: | 18354.17 ± 3142.76a,b | 22431.25 ± 3757.63b |
| 120 h: | 15831.83 ± 2886.55a,b | 20510.13 ± 2371.41a,b |
Results are expressed as number of activity counts, mean ± s.e.m., n=6-8/housing condition. Main effects: time (F(4,60) = 4.83, P < 0.01), housing type (F(1,60) = 2.95, P = 0.09); time × housing type interaction (F(4,60) = 0.11, P = 0.98). Results without a common superscript are significantly different (P < 0.05).
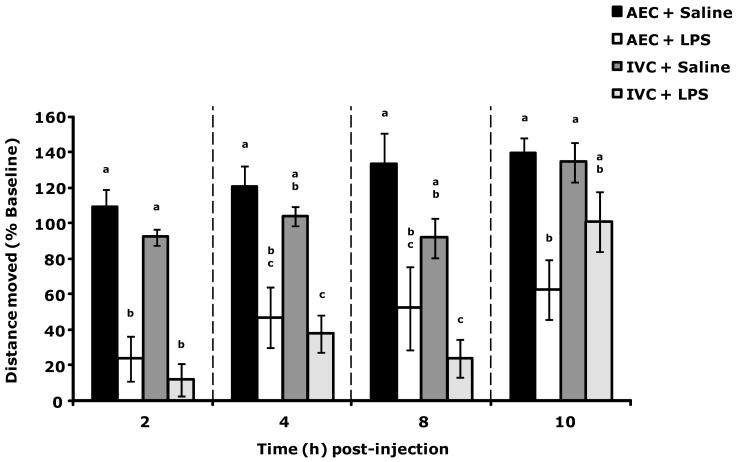
IVC mice recover more rapidly from LPS than AEC mice
Fig. 2 shows that LPS-induced reduction in sLMA was not significantly different between AEC and IVC mice. At 10 h post LPS administration, IVC mice had sLMA comparable to saline administered IVC mice while AEC mice sLMA was reduced compared to saline administered AEC mice.
Figure 2.
IVC mice recover more rapidly than AEC mice from LPS-induced loss of locomotor activity (LMA). Results are expressed as percent baseline (pre-LPS) LMA, means ± s.e.m., n = 6. Main effects: time (F(3,77) = 13.08, P < 0.01), housing type (F(1,77) = 3.69, P = 0.06); time × housing type interaction (F(3,77) = 3.31, P = 0.02). Bars within individual time points without common superscript letters are significantly different (P < 0.05). 2 h time point: P < 0.05, AEC + Saline v. AEC + LPS, IVC + LPS (108.712 ± 10.100 v. 23.911 ± 12.702, 12.088 ± 9.093, respectively); IVC + Saline v. AEC + LPS, IVC + LPS (92.242 ± 4.401 v. 23.911 ± 12.702, 12.088 ± 9.093, respectively). 4 h time point: P < 0.05, AEC + Saline v. AEC + LPS, IVC + LPS (120.219 ± 11.883 v. 47.083 ± 17.265, 37.702 ± 10.455, respectively); IVC + Saline v. IVC + LPS (103.703 ± 5.407 v. 37.702 ± 10.455). 8 h time point: P < 0.05, AEC + Saline v. AEC + LPS, IVC + LPS (132.792 ± 17.631 v. 52.404 ± 23.524, 24.057, respectively); IVC + Saline v. IVC + LPS (91.945 ± 11.038 v. 24.057 ± 10.648, respectively). 10 h time point: P < 0.05, AEC + Saline v. AEC + LPS (139.290 ± 9.015 v. 62.530 ± 16.733); IVC + Saline v. AEC + LPS (134.529 ± 11.157 v. 62.530 ± 16.733).
Discussion
To our knowledge, this is the first study to report a significant reduction in oxygen within IVC housing when compared to AEC housing (Table 1). In addition, the mean RH was higher in IVC as compared to AEC housing consistent with previous reports (Table 2) (Rosenbaum et al., 2010). IVC cages housing 2-4 mice were examined to determine if the number of mice housed within a cage impacted intra-cage oxygen and RH. Table 1 showed that oxygen reduction occurred in all IVC cages and that mouse number correlated with oxygen reduction. Mouse number also increased IVC intra-cage RH and intra-cage RH correlated with mouse numbers. Singly housed mice were not studied because general (long-term) housing of mice individually is a model of social isolation that causes adverse behaviors (Guo et al., 2004). Not unexpectedly, a difference in the RH of the AEC and IVC housing rooms was observed and was especially pronounced in the 4 mouse per cage group. The reason for this occurrence appears related to atmospheric RH on the day of measurement reported as 31.8% (Illinois State Water Survey, 2011) and room differences with the animal facility. Despite daily differences in atmospheric and room RH the percent change in RH within IVC housing scaled with increased mouse number. RH within AEC housing was no different than room RH. Finally, laboratory rodents are also housed in isolator top cages. Oxygen and relative humidity within isolator top cages (4 mice per cage) was comparable to IVC cages demonstrating reduced oxygen and increased RH (data not shown). This was not surprising in that IVC housing was developed to provide the protective benefits (allergen and VOC reduction) of isolator top cages plus an ability to increase animal population densities.
An expected physiologic response to reduced oxygen is increased red blood cell (RBC) mass and blood hemoglobin (Bennett and Plum, 1996; Casas et al., 2000). Table 3 reflects such changes because IVC mice, compared to AEC mice, showed an elevation in hematocrit as well as RBC and hemaglobin concentrations. Studies examining RBC physiology in mice at altitude have been performed with increases in hematocrit and RBC/hemoglobin concentrations reported (Sawin, 1970). It is important to note, that IVC housing is a low-grade hypoxia equivalent to an altitude of 90 m. IVC housing is also normobaric (760 mm Hg) (Peacock, 1998). At an altitude of 1760 m, the oxygen concentration is 84% that of sea level and the atmospheric condition is hypobaric (614 mm Hg) (Peacock, 1998; Portland State Aerospace Society, 2004). In general, atmospheric pressure and inspired oxygen percentage decrease in a near linear fashion from 100% at sea level, with 50% of sea level oxygen available at 5500 m and 30% of sea level oxygen available at 8900 m (the peak of Mt Everest) (Peacock, 1998). The likely mechanism for the increase in blood oxygen carrying capacity seen in mice and men exposed to altitude is hypoxia-dependent up-regulation of erythropoietin which has been demonstrated in naturally occurring (living at altitude (Casas et al., 2000)) and experimentally-induced hypoxia (Knaupp et al.,1992).
Interestingly, total leukocytes were lower in IVC mice compared to AEC mice (Table 4). This drop in white cell count was not associated with a shift in leukocyte relative percentages. The reason for a lower white cell count in the face of a higher RBC mass is not clear. In humans, leukocyte numbers measured at sea level do not change significantly when re-measured after 8 months at an altitude of 3550 m (Siqués et al., 2007). Unfortunately, data for leukocyte counts in mice housed at altitude is lacking. A possible cause of the lower WBC counts seen in IVC mice is the relative lack of ammonia in the IVC microenvironment (Höglund and Renström, 2001). Von Borell et al.(2007) has shown that pigs exposed to 35-50 ppm atmospheric ammonia for 19 days have increased absolute monocyte, lymphocyte and neutrophils counts. We, however, did not observe measurable ammonia in either IVC or AEC housing.
Platelet counts were higher in IVC mice relative to AEC mice. Previous work has demonstrated that humans exposed to12.8% oxygen (normobaric) for 3 h had increased platelet counts that persisted for 24 h post-treatment (Hodkinson et al., 2003). Mice exposed to 5.5-6.5% oxygen (normobaric) for 1-7 days have elevated platelet counts for the first 3 d of exposure before returning to normoxic control levels at day 4 and 5 of hypoxia exposure. Day 6 and 7 of hypoxia exposure show significantly lower platelet counts compared to normoxic control mice (McDonald et al., 1978). Mechanisms of hypoxia-dependent thrombocytosis may potentially be gleaned from obesity research in that the obese state is associated with both elevated platelet counts in human females (Charles et al., 2007) and tissue hypoxia in animals (Ye, 2009). Bioactives implicated in thrombocytosis include leptin, IL-6 and IL-1 which have all been shown to be increased in the obese state (Vandanmagsar et al., 2011; Yudkin et al., 2000) and the hypoxic state (Johnson et al., 2007; Klausen et al., 1997; Sherry et al., 2009).
Given the leukocyte count differences between AEC and IVC mice, an altered innate immune response was anticipated. While AEC and IVC mice have a comparable initial response to LPS, IVC mice recovered more quickly from LPS than AEC mice (Fig.2). A possible explanation for this result lies in the IVC mice having a reduced white blood cell count. Neutrophils and monocytes both possess Toll-like receptor-4 (TLR4) which recognizes LPS and initiates most of the sickness symptoms seen after LPS administration (Halfhide et al., 2009; Muzio et al., 2000). A lower white blood cell count in IVC mice may lead to a diminished peripheral immune response. However, if this were the mechanism, it would be expected that the initial (2 h) response would have been impacted. While these data support the concept that IVC systems improve overall rodent colony health, it is likely that a mechanism distinct from a lower leukocyte count is responsible for accelerated behavioral recovery from LPS.
We have previously shown that acute hypoxia leads to sickness behaviors in mice via a mechanism reliant on IL-1 (Johnson et al., 2007; Sherry et al., 2009). The behavioral tests in the present study, similar to our previous acute hypoxia work, tested mice exposed to either normoxic (AEC) or slightly hypoxic (IVC) environments in AEC/normoxic conditions. Behavioral testing was initiated immediately following relocation from AEC or IVC housing to novel AEC housing when possible, but was not always possible as some tests require acclimatization to single housing, a separate testing room, etc... to minimize the impact of novelty. AEC testing conditions also broaden the applicability and impact of our findings to other researchers who may conduct behavioral studies in AEC conditions while their mice are normally housed in IVCs. Because all behavioral tests were conducted in AEC conditions, it is difficult to determine if significant differences between AEC and IVC mice would occur in IVC (20.5% O2/47% RH) conditions. Unlike mice exposed to 5% oxygen for 2 h, IVC mice had comparable locomotor activity (Chiu et al., 2011), both immediately after relocation to a novel AEC arena for 5 min (Table S2) and continuously over 5 days following e-mitter implantation surgery (Table 7). Although hypoxia can cause lethargy (Johnson et al., 2008), IVC-dependent low-grade hypoxia does not. Telemetric sLMA data was analyzed hourly to determine if there were disruptions in circadian rhythm and no differences were observed (data not shown). In addition, IVC mice demonstrated had comparable food intake (Table 5), social exploration (Table S1) and novel object recognition (Table S1) as AEC mice. These findings are consistent with those of Mineu et al. (2009), who showed that aggression, learning/memory and anxiety-like behaviors were unaffected by IVC housing. Water consumption and saccharin preference were significantly different between IVC and AEC mice (Table 5 and Fig.1). To determine if relocation to a new caging system caused reduced water intake in IVC mice, water consumption was examined during IVC housing. A 1 wk group housing method was used to mimic 4-cage housing conditions and to prevent daily opening of the IVC housing system. Cage changes/bedding replacement is performed weekly in our facility. Table 6 shows that IVC mice drank less water than AEC mice. This was likely due to the increased humidity of IVC housing compared to AEC housing. IVC mice had an enhanced preference for saccharin suggesting increased hedonism. Neither housing condition induced anhedonic behavior in mice, which would be evident in a 50/50 saccharin:water consumption ratio. Since IVC mice drink more saccharin solution, however, preference testing results following experimental treatment may differ from those of AEC mice. This finding was unexpected due to human data showing an association between living at altitude and a higher rate of suicide (Cheng et al., 2002; Cheng and Brenner, 2010). In humans, studies examining mood and cognitive function at altitude have been performed in locations between 3,000 m and 6,000 m above sea level (Barhke and Shukitt-Hale, 1993; Shukitt-Hale, et al., 1998). Acute mood disturbances and reduced performance of tasks have been reported in some individuals at altitudes as low as 3,000 m (Shukitt-Hale et al., 1998). Most persons will report impaired function at 5,000 m which has an oxygen concentration of 58% that of sea level (Peacock, 1998; Shukitt-Hale et al., 1998). With ascent to altitude, a euphoric phase often occurs prior to the onset of mood depression (Barhke and Shukitt-Hale, 1993; Shukitt-Hale et al., 1998). Additional evidence for the subjective feeling of euphoria can be found in reports dealing with “the choking game,” which is a potentially life-threatening ritual used among adolescents around the world, which essentially consists of compression of the carotid arteries while holding one’s breath (Ullrich et al., 2008). Acute hypoxia is known to cause reductions in body temperature (Tb) and food intake in rodents (Gordon and Fogelson, 1991; Ettinger and Staddon, 1982). While not directly measured, the Tb of AEC and IVC mouse would likely not differ based on the work of Gordon and Fogelson (1991) which showed that exposure to an oxygen concentration of 7.3 % or below for 60 min was required to cause significant reduction. However, in order to assess whether the results of the saccharin preference test might be due to an IVC-induced change in metabolic state (i.e. reduced saccharin consumption), the forced swim test was performed in AEC and IVC mice 3 days after relocation to AEC group (4 mice per cage) housing. Although no significant differences in time swam were observed, IVC mice swam for a greater amount of time than AEC mice (Table 9). This evidence, though non-significant, supports the saccharin preference results indicating that IVC mice may have a slightly more anti-depressive phenotype than AEC mice. Since our mice were chronically exposed to low-grade hypoxia and then tested in a normoxic environment, the physiology modeled may be more akin to hyperoxygenation, as the reduced oxygen environment is eliminated for IVC mice following relocation to AEC conditions. Hyperoxygenation has been shown to increase memory and learning in rodent models of traumatic brain injury (Harch et al., 2007). However, the role of hyperoxygenation in memory and learning remains clouded, as studies in rodent models of Alzheimer’s Disease show it leads to cognitive impairment (Arendash et al., 2009). In humans, fatigue is a reported complication of oxygen therapy, especially, hyperbaric oxygen therapy (Leach et al., 1998). The impact of increased oxygen on persons without hypercapnic respiratory problems are not known. Hyperventilation is associated with anxietal symptoms in humans (Wollburg et al., 2011) which are thought to be related to the impact of respiratory alkalosis on the CNS (Judd et al., 1985). Increasing the concentration of inhaled carbon dioxide by breathing into a paper bag is an effective way of decreasing anxiety associated with hyperventation while raising blood pCO2 and, hence blood pH (Callaham, 1989). Unfortunately, no work has been performed on depressive-like behaviors in mice associated with hyperoxygenation.
In sum, our findings indicate that IVC-housed mice could be used to model chronic low-grade hypoxia. Use of IVC-housing without a diligent examination of its impact on RBC mass and oxygen carrying capacity, is unadvised for those researchers investigating diseases tied to the hematopoietic system. How changes in oxygen effect mood is not understood but given the findings here warrants further study.
Supplementary Material
Acknowledgments
Support: This research was supported by the National Institutes of Health (DK064862, NS058525 and AA019357 to G.G.F.) and by the NSBRI CARR grant funded through NASA NCC 9-58
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arendash GW, Cox AA, Mori T, Cracchiolo JR, Hensley KL, Roberts LJ., II. Oxygen treatment triggers cognitive impairment in Alzheimer’s transgenic mice. NeuroReport. 2009;20:1087–1092. doi: 10.1097/WNR.0b013e32832e6459. [DOI] [PubMed] [Google Scholar]
- Barhke MS, Shukitt-Hale B. Effects of altitude on mood, behavior and cognitive functioning: a review. Sports Medicine. 1993;16:97–125. doi: 10.2165/00007256-199316020-00003. [DOI] [PubMed] [Google Scholar]
- Bennett JC, Plum F. Cecil textbook of medicine. W.B. Saunders Company; Philadelphia (PA): 1996. [Google Scholar]
- Callaham M. Hypoxic hazards of traditional paper bag rebreathing in hyperventilating patients. Ann Emer Med. 1989;18:622–628. doi: 10.1016/s0196-0644(89)80515-3. [DOI] [PubMed] [Google Scholar]
- Casas H, Casas M, Ricart A, Rama R, Ibáñez J, Palacios L, Rodríguez FA, Ventura JL, Viscor G, Pagés T. Effectiveness of three short term intermittent hypobaric hypoxia protocols: hematological responses. J Excerc Physiol Online. 2000;3:38–45. [Google Scholar]
- Charles LE, Fekedulegn D, McCall T, Burchfiel CM, Andrew ME, Violanti JM. Obesity, white blood cell counts, and platelet counts among police officers. Obesity. 2007;15:2846–2854. doi: 10.1038/oby.2007.338. [DOI] [PubMed] [Google Scholar]
- Cheng D, Brenner B. Altitude suicide death rate hypothesis since 2002. Med Hypotheses. 2010;74:618–619. doi: 10.1016/j.mehy.2009.10.049. [DOI] [PubMed] [Google Scholar]
- Cheng D, Yakobi R, Dobbins WN, Neuman K, Brenner BE. Moderate altitude increases suicide deaths. Ann Emer Med. 2002;40:S55. [Google Scholar]
- Chiu GS, Chatterjee D, Meling DD, Johnson RW, Freund GG. Acute hypoxia causes memory deficits due to activation of the inflammasome; 2011 Neuroscience Meeting Planner; Washington, DC: Society for Neuroscience. 2011; Program No. 398.15. Online. [Google Scholar]
- Ettinger RH, Staddon JER. Decreased feeding associated with acute hypoxia in rats. Physiol Behav. 1982;29:455–458. doi: 10.1016/0031-9384(82)90266-9. [DOI] [PubMed] [Google Scholar]
- Fisher R, Saunders W,B, Marray SJ, Stave GM. Prevention of laboratory animal allergy. J Occup Environ Med. 1998;40:609–613. doi: 10.1097/00043764-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Fogelson L. COmparitive effects of hypoxia on behavioral thermoregulation in rats, hamsters and mice. Am J Physiol. 1991;260:R120–R125. doi: 10.1152/ajpregu.1991.260.1.R120. [DOI] [PubMed] [Google Scholar]
- Gordon S. Allergy to furred animals. Clin Exp Allergy. 1997;27:470–481. [PubMed] [Google Scholar]
- Gordon S, Preece R. Prevention of laboratory animal allergy. Clin Exp Allergy. 2003;53:371–377. doi: 10.1093/occmed/kqg117. [DOI] [PubMed] [Google Scholar]
- Guo M, Wu CF, Liu W, Yang JY, Chen D. Sex difference in psychological behavior changes induced by long-term social isolation in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:115–121. doi: 10.1016/j.pnpbp.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Halfhide CP, Creary SP, Flanagan BF, Hunt JA, Howarth D, Cummerson J, Edwards S, Hart CA, Smyth RL. Neutrophil TLR4 expression is reduced in the airways of infants with severe bronchitis. Thorax. 2009;64:798–805. doi: 10.1136/thx.2008.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harch PG, Kriedt C, Van Meter KW, Sutherland RJ. Hyperbaric oxygen therapy improves spatial learning and memory in a rat model of chronic traumatic brain injury. Brain Res. 2007;1174:120–129. doi: 10.1016/j.brainres.2007.06.105. [DOI] [PubMed] [Google Scholar]
- Hodkinson PD, Hunt BJ, Parmar K, Ernsting J. Is mild normobaric hypoxia a risk factor for venous thromboembolism? J Thromb Haemostasis. 2003;1:2131–2133. doi: 10.1046/j.1538-7836.2003.00407.x. [DOI] [PubMed] [Google Scholar]
- Höglund AU, Renström A. Evaluation of individually ventilated cage systems for laboratory rodents: cage environment and animal health aspects. Lab Anim. 2001;35:51–57. doi: 10.1258/0023677011911372. [DOI] [PubMed] [Google Scholar]
- Hrabé de Angelis M, Adler A, Beckers J, Soewarto D, Wagner S, Gailus-Durner V, Imai K. Mouse Genetics. In: Hedrich H, editor. The Laboratory Mouse. Elsevier Academic Press; London (UK): 2004. pp. 47–84. [Google Scholar]
- Hunskaar S, Fosse RT. Allergy to laboratory mice and rats: a review of the pathophysiology, epidemiology and clinical aspects. Lab Anim. 1990;24:358–374. doi: 10.1258/002367790780865877. [DOI] [PubMed] [Google Scholar]
- Illinois State Water Survey Water and Atmospheric Resources Monitoring Program (WARM) – Illinois Climate Network Daily Data (~1989-2011) 2011 Retrieved from: http://www.isws.illinois.edu/warm/data/cdfs/cmiday.txt.
- Institute for Laboratory Animal Research . Guide for the care and use of laboratory animals. National Academies Press; Washington (DC): 2011. [Google Scholar]
- Jennings M, Batchelor GR, Brain PF, Dick A, Elliot H, Francis RJ, Hubrecht RC, Hurst JL, Morton DB, Peters AG, Raymond R, Sales GD, Sherwin CM, West C. Refining rodent husbandry: the mouse. Report of the rodent refinement working party. Lab Anim. 1998;32:233–259. doi: 10.1258/002367798780559301. [DOI] [PubMed] [Google Scholar]
- Johnson DR, O’Connor JC, Hartman ME, Tapping RI, Freund GG. Acute hypoxia activates the neuroimmune system, which diabetes exacerbates. J Neurosci. 2007;27:1161–1166. doi: 10.1523/JNEUROSCI.4560-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DR, Sherry CL, York JM, Freund GG. Acute hypoxia, diabetes and neuroimmune dysregulation: converging mechanisms in the brain. Neuroscientist. 2008;14:235–239. doi: 10.1177/1073858407309544. [DOI] [PubMed] [Google Scholar]
- Judd FK, Burrows GD, Norman TR. The biological basis of anxiety: an overview. J Affective Disord. 1985;9:271–284. doi: 10.1016/0165-0327(85)90058-8. [DOI] [PubMed] [Google Scholar]
- Kacergis JB, Jones RB, Reeb CK, Turner WA, Ohman JL, Ardmann MR, Paigen B. Air quality in an animal facility: Particulates, ammonia, and volatile organic compounds. Am Ind Hyg Assoc J. 1996;57:634–640. doi: 10.1080/15428119691014693. [DOI] [PubMed] [Google Scholar]
- Kaliste E, Limmainmaa M, Meklin T, Torvinen E, Nevalainen A. The bedding of laboratory animals as a source of airborne contaminants. Lab Anim. 2004;38:25–37. doi: 10.1258/00236770460734362. [DOI] [PubMed] [Google Scholar]
- Kallnik M, Elvert R, Ehrhardt N, Kissling D, Mahabir E, Welzl G, Faus-Kessler T, Hrabe de Angelis M, Wurst W, Schmidt J, Holter SM. Impact of IVC housing on emotionality and fear learning in male C3HeB/FeJ and C57BL/6J mice. Mamm Genome. 2007;18:173–186. doi: 10.1007/s00335-007-9002-z. [DOI] [PubMed] [Google Scholar]
- Klausen T, Olsen NV, Poulsen TD, Richalet JP, Pedersen BK. Hypoxemia increases serum interleukin-6 in humans. Eur J Appl Physiol. 1997;76:480–482. doi: 10.1007/s004210050278. [DOI] [PubMed] [Google Scholar]
- Knaupp W, Khilnani S, Sherwood J, Scharf S, Steinberg H. Erythropoietin response to acute normobaric hypoxia in humans. J Appl Physiol. 1992;73:837–840. doi: 10.1152/jappl.1992.73.3.837. [DOI] [PubMed] [Google Scholar]
- Krohn TC, Hansen AC. Carbon dioxide concentrations in unventilated IVC cages. Lab Anim. 2002;36:209–212. doi: 10.1258/0023677021912361. [DOI] [PubMed] [Google Scholar]
- Lavin DN, Joesting JJ, Chiu GS, Moon ML, Meng J, Dilger RN, Freund GG. Fasting induces an anti-inflammatory effect on the neuroimmune system which a high-fat diet prevents. Obesity. 2011;19:1586–1594. doi: 10.1038/oby.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach RM, Rees PJ, Wilmshurst P. ABC of oxygen: Hyperbaric oxygen therapy. Br Med J. 1998;317:1140–1143. doi: 10.1136/bmj.317.7166.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria SM, Morris N. Report number 1108: Visual sensitivities under reduced oxygen. Naval Submarine Medical Research Laboratory. 1988:1–14. [Google Scholar]
- McDonald TP, Cottrell M, Clift R. Effects of short-term hypoxia on platelet counts of mice. Blood. 1978;51:165–175. [PubMed] [Google Scholar]
- Mineu YS, Crusio WE. Behavioral effects of ventilated micro-environment housing in three inbred mouse strains. Physiol Behav. 2009;97:334–340. doi: 10.1016/j.physbeh.2009.02.039. [DOI] [PubMed] [Google Scholar]
- Muzio M, Bosisio D, Polentarutti N, D’amico G, Stoppacciaro A, Mancinelli R, van’t Veer C, Penton-Rol G, Ruco LP, Allavana P, Mantovani A. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- Peacock AJ. ABC of Oxygen – Oxygen at High Altitude. Br Med J. 1998;317:1063–1066. doi: 10.1136/bmj.317.7165.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portland State Aerospace Society A quick derivation relating altitude to air pressure. Version 1.03. 2004 [Cited 15 November 2011]. Available at: http://www.psas.pdx.edu.
- Rosenbaum MD, VandeWoude S, Volckens J, Johnson TE. Disparities in ammonia, temperature, humidity and airborne particulate matter between the micro- and macroenvironments of mice in individually ventilated caging. J Am Assoc Lab Anim Sci. 2010;49:177–183. [PMC free article] [PubMed] [Google Scholar]
- Rushkin KJ, Hernandez KA, Barash PG. Management of in-flight medical emergencies. Anesthesiology. 2008;108:749–755. doi: 10.1097/ALN.0b013e31816725bc. [DOI] [PubMed] [Google Scholar]
- Sawin CF. Hematology of sea-level and high-altitude native Sonoran deer mice. Am J Physiol. 1970;218:1701–1704. doi: 10.1152/ajplegacy.1970.218.6.1701. [DOI] [PubMed] [Google Scholar]
- Sherry CL, Kim SS, Freund GG. Accelerated recovery from acute hypoxia is due to obesity-associated up-regulation of Interleukin-1 receptor antagonist. Endocrinology. 2009;150:2660–2667. doi: 10.1210/en.2008-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry CL, Kim SS, Dilger RN, Bauer LL, Moon ML, Tapping RI, Fahey GC, Jr, Tappenden KA, Freund GG. Sickness behavior induced by endotoxin can be mitigated by the dietary soluble fiber, pectin, through up-regulation of IL-4 and Th2 polarization. Brain Behav Immun. 2010;24:631–640. doi: 10.1016/j.bbi.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukitt-Hale B, Banderet LE, Lieberman HR. Elevation-dependent symptom, mood and performance changes produced by exposure to hypobaric hypoxia. Int. J. Aviat. Psychol. 1998;8:319–334. doi: 10.1207/s15327108ijap0804_1. [DOI] [PubMed] [Google Scholar]
- Silverman J, Bays DW, Cooper SF, Baker SP. Ammonia and carbon dioxide concentrations in disposable and reusable ventilated mouse cages. J Am Assoc Lab Anim Sci. 2008;47:57–62. [PMC free article] [PubMed] [Google Scholar]
- Siqués P, Brito J, León-Velarde F, Barrios L, De La Cruz JJ, López V, Herruzo R. Hematological and lipid profile changes in sea-level natives after exposure to 3550-m altitude for 8 months. High Alt Med Biol. 2007;8:286–295. doi: 10.1089/ham.2007.8405. [DOI] [PubMed] [Google Scholar]
- Stewart LH, Trunkey D, Rebagliati GS. Emergency medicine in space. J Emerg Med. 2007;32:45–54. doi: 10.1016/j.jemermed.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Ullrich NJ, Bergin AM, Goodkin HP. The choking game”: Self-induced hypoxia presenting as recurrent seizurelike events. Epilepsy Behav. 2008;12:486–488. doi: 10.1016/j.yebeh.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Borell E, Özpinar A, Eslinger KM, Schnitz AL, Zhao Y, Mitloehner FM. Acute and prolonged effects of ammonia on hematological variables, stress responses, performance, and behavior of nursery pigs. J Swine Health Prod. 2007;15:137–145. [Google Scholar]
- Wollburg E, Roth WT, Kim S. Hypo- and hyperventilation in patients with panic disorder and episodic anxiety. Appl Psychophysiol Biofeedback. 2011;36:81–91. doi: 10.1007/s10484-011-9150-5. [DOI] [PubMed] [Google Scholar]
- Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obesity. 2009;33:54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York JM, Blevins NA, Baynard T, Freund GG. Mouse testing methods in psychoneuroimmunology. Methods in molecular biology. 2011 doi: 10.1007/978-1-62703-071-7_13. In Press. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.