Abstract
The regular extract of Ginkgo biloba has been shown to possess neuroprotective properties in disorders like hypoxia, ischemia, seizure activity and peripheral nerve damage. Also, G. biloba has received attention as a potential cognitive enhancer for the treatment of Alzheimer's disease, but there is not any documentation about the effect of an extract of G. biloba on astrocytes. Therefore, the aim of this study was examined the effects of G. biloba extract on the rat's hippocampal astrocytes after scopolamine based amnesia. In this study, 36 adult male Wistar rats were used. Rats were randomly distributed into control, sham, protective and treatment groups. The rats in the sham group only received scopolamine hydrobromide (3 mg/kg) intraperitoneally. The rats in the protective and treatment groups received G. biloba extract (40, 80 mg/kg) for 7 days intraperitoneally before and after scopolamine injection. Forty eight hours after the last injection, the brains of the rats were withdrawn and fixed with paraformaldehide, and then after histological processing, the slices were stained with phosphotungstic acid-haematoxylin for astrocytes. Data were analyzed by the analysis of variance (ANOVA) post hoc Tukey test; P<0.05 was considered significant. Results showed that scopolamine can reduce the number of astrocytes in all areas of hippocampal formation compared with the control. However, G. biloba extract can compensate for the reduction in the number of astrocytes in the hippocampus before or after the encounter with scopolamine. We concluded that a pretreatment and treatment injection of G. biloba extract can have a protective effect for astrocytes in all areas of hippocampal formation.
Keywords: Ginkgo biloba extract, Hippocampus, Astrocytes, Rat
Introduction
Failure of memory may result from damage to parts of the brain critical for memory storage, processing, or recall such as the hippocampus of the limbic system. Amnesia or forgetfulness refers to the loss of memory, and can also be a symptom of neurodegenerative diseases. Alzheimer's disease is one form of dementia that gradually gets worse over time. It affects memory, thinking, and behavior. Similarities in the memory deficits between Alzheimer patients and scopolamine treated animals have been reported. Scopolamine as a muscarinic cholinergic receptor antagonist that impairs memory performance has been proposed as an animal model of dementia [1].
Scopolamine induces severe losses of hippocampal cholinergic neurons, as indicated by decreased choline acetyltransferase immunoreactivity and increased acetylcholinesterase reactivity. Daily administration of some wild plants such as ginseng reduced the loss of cholinergic immunoreactivity in the hippocampus. The reduced expression of brain-derived neurotrophic factor mRNA due to the scopola mine injection was recovered to normal levels by the administration of wild ginseng [2].
The other plant, Ginkgo biloba, is one of the widely cultured Chinese plants [3] has antioxidant and free radical-scavenger properties which could contribute to its neuroprotective/anti-apoptotic activity. It has also been shown to reverse age-related decline of neurotransmitter systems [4].
The dried leaves of the G. biloba tree provide the rudimentary drug from which the standardized G. biloba extract (EGb 761) is obtained. The defined but complex product consists of two major groups of substances, the flavone glycosides (flavonoid fraction, 24%) and the terpene lactones (terpenoid fraction, 6%) [5].
The standardized extract of G. biloba has been shown to possess neuroprotective properties under conditions like hypoxia, ischemia, seizure activity and peripheral nerve damage. Recently, G. biloba has received attention as a potential cognitive enhancer for the treatment of Alzheimer's disease [3]. Other studies indicated that EGb761 upregulated the expression of several genes, such as AMPA and chloride channel proteins, and the synaptic plasticity in hippocampal slices of aged mice was improved by EGb 761 [6].
Preclinical and clinical studies have demonstrated that G. biloba extract was effective in mild to moderate dementia in Alzheimer's disease. This suggests that G. biloba extract does have modest therapeutic potential as a memory and cognition enhancing drug and its action may target the hippocampus [7].
Astrocytes have privileged access to synapses and we are beginning to learn that as a consequence they are able to regulate both the pre- and postsynaptic terminals of excitatory and inhibitory synapses. Because of the reciprocal signaling that can occur between astrocytes and synaptic terminals, these structures have been termed the 'Tripartite Synapse' [8].
Since so far the protective effects of G. biloba extract on the cellular structure of the hippocampus have not been studied, the aim of this study was to examine the effects of G. biloba extract on the rat hippocampus structure after exposure with scopolamine.
Materials and Methods
Thirty-six adult male Wistar rats, weighing 200-250 g, were used in the study. They were given free access to normal laboratory chow and water. The temperature of the animal house was 22±3℃. This research was conducted under the Ethics Committee of Golestan University of Medical Sciences, Gorgan, Iran.
The drugs included scopolamine hydrobromide (Tocris, Bristol, UK) and G. biloba extract (NIAC Pharmaceutical Factory, Gorgan, Iran). All drugs were dissolved in sterile saline and were injected into the peritoneal cavity.
The rats were randomly divided into six groups (n=6) as follows:
- Control: Without receiving G. biloba extract and scopolamine
- Sham: Scopolamine injection on the first day, 3 mg/kg, IP
- Protective (two groups): G. biloba extract injection (40 and 80 mg/kg, IP) for a week, and then scopolamine injection on the eight day (3 mg/kg, IP)
- Treatment (two groups): Scopolamine injection on the first day (3 mg/kg, IP), and then G. biloba extract injection (40 and 80 mg/kg, IP) for a week.
Forty eight hours after the last injection, the animals were anesthetized with chloroform and their brains were withdrawn and then the brains were fixed for two weeks in 10% formaldehyde. Different degrees of alcohol were used for dehydration followed by clarification with xylol. After histological processing, the tissue was impregnated and then embedded in paraffin wax.
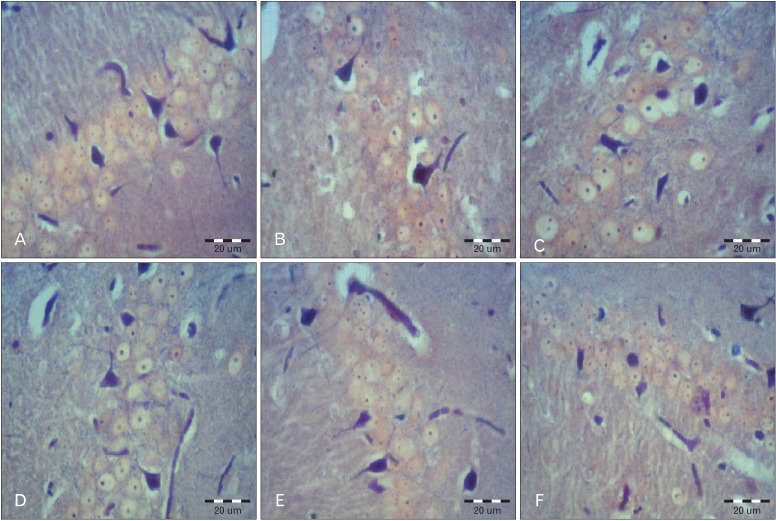
The 8 µm coronal sections were serially collected from Bregma -3.30 mm to -6.04 mm of the hippocampal formation [9]. An interval of 20 µm was placed between each two consecutive sections. The sections were stained with phosphotungstic acid-haema toxylin staining (specific staining for astrocytes) (Fig. 1) [10].
Fig. 1.
Astrocytes in the CA1 area of the hippocampus in all groups. (A) Control. (B) Sham (scopolamine). (C) Treatment 40. (D) Treatment 80. (E) Pretreatment 40. (F) Pretreatment 80 (PTAH staining). Scale bars=20 µm.
A photograph of each section was produced using an Olympus BX 51 microscope (Olympus, Tokyo, Japan) and a DP 12 digital camera under a magnification of 400. An area of 22,500 µm2 was selected randomly in all areas of hippocampal formation (CA1, CA3 and dentate gyrus) in all sections. To measure the area density of the astrocytes, the images were transferred onto a computer. Using OLYSIA Autobioreport soft ware (Olympus), the appropriate grids were superimposed on the pictures and the cells were counted manually [11, 12].
Statistical analysis
Experimental results concerning this study were evaluated using SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA) and expressed as the mean±SD. To compare the means of the measured parameters in the three groups by the analysis of variance (ANOVA) test, after confirmation of normality, the means were compared by the ANOVA post hoc Tukey test; P<0.05 was considered significant.
Results
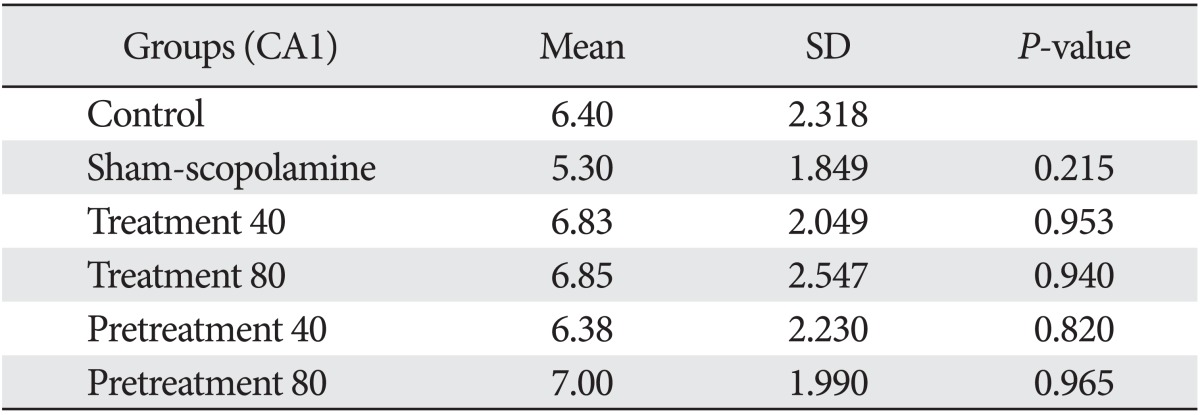
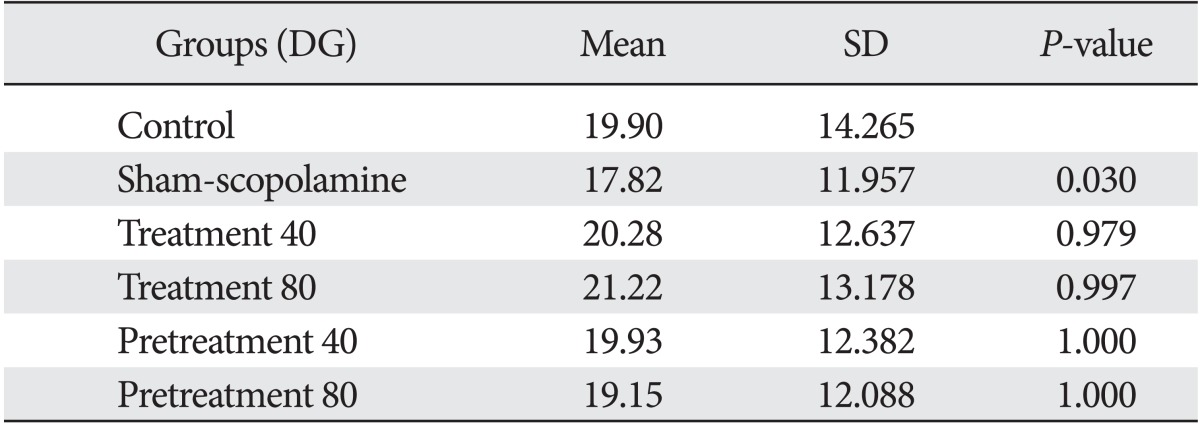
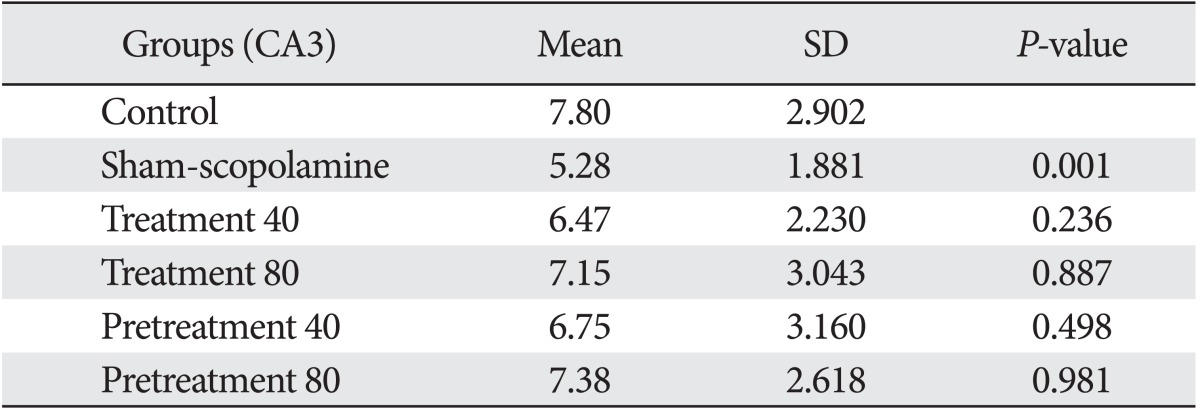
Our results showed that injection of scopolamine in the sham group reduced the number of astrocytes in all areas of the hippocampal formation compared with the control (Tables 1-3). This reduction in the CA3 (P=0.001) and in the dentate gyrus (P=0.03) was statistically significant, and the reduction in the CA1 area was not significant (P=0.21). The differences of cell density are shown in Fig. 1.
Table 1.
Mean and SD of astrocytes number in CA1 area of hippocampus
Table 3.
Mean and SD of astrocytes number in DG area of hippocampus
DG, dentate gyrus.
We found that G. biloba extract could compensate for the reduction of astrocytes in the hippocampus within 7 days after injection of scopolamine (as treatment groups 40 and 80 mg/kg). On the other hand, the number of astrocytes was reduced after injection of scopolamine and then increased with G. biloba.
Also, our data showed similar results when we injected G. biloba extract within 7 days before injection of scopolamine (as protective groups 40 and 80 mg/kg). Statistical analysis showed differences between the sham-scopolamine group and the other groups were significant in the CA3 and dendrate gyrus areas.
It seems in all areas of the hippocampus, an 80 mg/kg dose of G. biloba extract had a greater effect than 40 mg/kg dose of Ginkgo. There are no significant differences between the treatment and protective effects of G. biloba extract.
Effects of amnesia caused by scopolamine were previously known, but this study demonstrated that scopolamine can affect the hippocampal structure.
Discussion
Our main findings suggest that scopolamine could reduce the number of astrocytes in the hippocampus, while both the protective and therapeutic injection of G. biloba has been able to compensate for the reduced number of astrocytes. It seems this effect was dose-dependent. Our data did not show any significant differences between the protective and therapeutic effects of G. biloba extract.
Animal studies have revealed that G. biloba is able to facilitate acquisition and retention of memory, with one of the major protective actions taking place in the hippocampus, which is related to the acquisition of new memories [13].
Wang et al. [6] showed that repeated administration of G. biloba (50 and 100 mg/kg/day) induced anxiolytic-like activity; however, the performance of the passive avoidance task and the animals' response to electric shock were not affected in aged animals.
Rocher et al.[14] demonstrated that chronic treatment with G. biloba after ischemia were compensated pyramidal cell damage in the CA1 region of the hippocampus. Also in all sham operated animals, the astrocytes defined by glial fibrillary acidic protein (GFAP) immunoreactivity appeared to be completely quiescent. On the other hand, GFAP immunoreactivity increased markedly in the CA1 area of gerbils that underwent an ischemic insult. This characteristic microglial activation was attenuated in animals treated with EGb 761. Their immunohistological findings show that neuroprotection by EGb 761 is accompanied by a reduction in astrogliosis and a decrease in GFAP immunoreactivity [14].
Most histological studies evaluated the effect of G. biloba on neurons; therefore, some related studies about the effects of G. biloba on nerve tissue are as follows.
The neuroprotective effect of G. biloba has been demonstrated in several in vitro and in vivo models. Ahlemeyer and Krieglstein [5] showed some in vitro and in vivo effects of G. biloba. They reported that 10-100 µg/ml of Ginkgo protected cultured neurons against death induced by hypoxia, hydrogen peroxide, glutamate, verapamil, amyloid β (Aβ), nitric oxide (NO), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and cyanide. In vivo, reduction of neuronal damage by Ginkgo [10-100 mg/kg, PO (per os), or IP] has been observed after transient middle cerebral artery occlusion in rats and gerbils, focal cerebral ischemia in mice and rats, hypoxia, heat stress, subchronic cold stress, amphetamine-induced behavioral sensitization and in a transgenic mouse model of amyotrophic lateral sclerosis [5].
Takuma et al. [15] examined the effect of G. biloba extract (EGb 761) on cognitive dysfunction and neuromorphological change in ovariectomised (OVX)/stress subjected rats. Their results showed that treatment with EGb 761 improved memory impairment and neuronal loss of the hippocampus in the OVX/stress-subjected group in the same ways as 17-β-estradiol. Microscopic images of the Nissl stained hippocampal CA3 region showed that chronic treatment with EGb 761 (50 mg/kg) over 2 months improved the chronic stress-induced neuronal cell loss in OVX rats to the levels of the sham-operated controls. Chronic treatment with EGb 761 specifically protected against neuronal cell loss induced by the combination of repeated restraint stress and OVX in the CA3 region, and did not affect Nissl-positive cell numbers in the CA1 region and the dentate gyrus of the hippocampus, in which the combination of stress and OVX did not cause neuronal cell loss [15].
Mallet et al. [16] characterized the neural effects of an extract of G. biloba by using c-Fos immunoreactivity (Fos-IR) in rats to quantify functional activity in several brain regions known to be involved in learning and memory. Their results showed that oral administration of EGb761 (150 mg/kg) increased Fos-IR moderately, but significantly, in the insular cortex and amygdala. The intraperitoneal administration of EGb761 also significantly increased Fos-IR in several brain regions including the prelimbic and insular cortices, nucleus accumbens (shell region), lateral septal nucleus (ventral part), thalamus (paraventricular nucleus), amygdala (central nucleus) and dentate gyrus (EGb761, 5 mg/kg), bed nucleus of the stria terminalis (BNST) lateral division (dorsal) (EGb761, 2/5 and 5 mg/kg) [16].
Another report, by Rocher et al. [14], provided evidence that G. biloba extract improves memory processes and normalizes cognitive deficits in experimental paradigms, including the Morris water maze in a transgenic mouse model of Alzheimer's disease or in aged rats, scopolamine- or stress-induced memory deficits in rats and operant conditioning protocols in mice [14].
Therefore, we concluded that pretreatment and treatment injection of G. biloba extract can have a protective effect for astrocytes in all areas of the hippocampal formation. On the other hand, G. biloba extract can compensate for the reduction in number of astrocytes in the hippocampus before or after encounter with scopolamine.
Table 2.
Mean and SD of astrocytes number in CA3 area of hippocampus
Acknowledgements
The authors would like to thank of the NIAC Pharmaceutical Factory, Gorgan, Iran and Dr. Homan Bayat for donating the Gingko extract to the Neuroscience Research Center. We are also thankful for the financial support of Research Affairs of Golestan University of Medical Sciences.
References
- 1.Collerton D. Cholinergic function and intellectual decline in Alzheimer's disease. Neuroscience. 1986;19:1–28. doi: 10.1016/0306-4522(86)90002-3. [DOI] [PubMed] [Google Scholar]
- 2.Lee B, Park J, Kwon S, Park MW, Oh SM, Yeom MJ, Shim I, Lee HJ, Hahm DH. Effect of wild ginseng on scopolamine-induced acetylcholine depletion in the rat hippocampus. J Pharm Pharmacol. 2010;62:263–271. doi: 10.1211/jpp.62.02.0015. [DOI] [PubMed] [Google Scholar]
- 3.Das A, Shanker G, Nath C, Pal R, Singh S, Singh H. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: anticholinesterase and cognitive enhancing activities. Pharmacol Biochem Behav. 2002;73:893–900. doi: 10.1016/s0091-3057(02)00940-1. [DOI] [PubMed] [Google Scholar]
- 4.Williams B, Watanabe CM, Schultz PG, Rimbach G, Krucker T. Age-related effects of Ginkgo biloba extract on synaptic plasticity and excitability. Neurobiol Aging. 2004;25:955–962. doi: 10.1016/j.neurobiolaging.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Ahlemeyer B, Krieglstein J. Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci. 2003;60:1779–1792. doi: 10.1007/s00018-003-3080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Wang L, Wu J, Cai J. The in vivo synaptic plasticity mechanism of EGb 761-induced enhancement of spatial learning and memory in aged rats. Br J Pharmacol. 2006;148:147–153. doi: 10.1038/sj.bjp.0706720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mak YT, Chan WY, Lam WP, Yew DT. Immunohistological evidences of Ginkgo biloba extract altering Bax to Bcl-2 expression ratio in the hippocampus and motor cortex of sene scence accelerated mice. Microsc Res Tech. 2006;69:601–605. doi: 10.1002/jemt.20322. [DOI] [PubMed] [Google Scholar]
- 8.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 9.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press; 1998. [Google Scholar]
- 10.Bancroft JD, Gamble M. Theory and practice of histological techniques. 5th ed. Edinburgh: Churchill Livingstone; 2004. [Google Scholar]
- 11.Jahanshahi M, Sadeghi Y, Hosseini A, Naghdi N, Marjani A. The effect of spatial learning on the number of astrocytes in the CA3 subfield of the rat hippocampus. Singapore Med J. 2008;49:388–391. [PubMed] [Google Scholar]
- 12.Jahanshahi M, Golalipour MJ, Afshar M. The effect of Urtica dioica extract on the number of astrocytes in the dentate gyrus of diabetic rats. Folia Morphol (Warsz) 2009;68:93–97. [PubMed] [Google Scholar]
- 13.Smith JV, Luo Y. Studies on molecular mechanisms of Ginkgo biloba extract. Appl Microbiol Biotechnol. 2004;64:465–472. doi: 10.1007/s00253-003-1527-9. [DOI] [PubMed] [Google Scholar]
- 14.Rocher MN, Carré D, Spinnewyn B, Schulz J, Delaflotte S, Pignol B, Chabrier PE, Auguet M. Long-term treatment with standardized Ginkgo biloba extract (EGb 761) attenuates cognitive deficits and hippocampal neuron loss in a gerbil model of vascular dementia. Fitoterapia. 2011;82:1075–1080. doi: 10.1016/j.fitote.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Takuma K, Hoshina Y, Arai S, Himeno Y, Matsuo A, Funatsu Y, Kitahara Y, Ibi D, Hayase M, Kamei H, Mizoguchi H, Nagai T, Koike K, Inoue M, Yamada K. Ginkgo biloba extract EGb 761 attenuates hippocampal neuronal loss and cognitive dysfunction resulting from chronic restraint stress in ovariectomized rats. Neuroscience. 2007;149:256–262. doi: 10.1016/j.neuroscience.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 16.Mallet PE, Moore CA, Collie MT, Satvat E. Regional distribution of Ginkgo biloba-induced c-Fos immunoreactivity. Phytomedicine. 2009;16:361–368. doi: 10.1016/j.phymed.2008.07.007. [DOI] [PubMed] [Google Scholar]