Abstract
Background
Several lines of evidence suggest that autism may be associated with abnormalities in white matter development. However, inconsistencies remain in the literature regarding the nature and extent of these abnormalities, partly due to the limited types of measurements that have been used. Here, we used Magnetization Transfer Imaging (MTI) to provide insight into the myelination of the corpus callosum in children with autism.
Methods
MTI scans were obtained in 101 children with autism and 35 typically developing children who did not significantly differ with regard to gender or age. The midsagittal area of the corpus callosum was manually traced and the magnetization transfer ratio (MTR) was calculated for each voxel within the corpus callosum. Mean MTR and height and location of the MTR histogram peak were analyzed.
Results
Mean MTR and MTR histogram peak height and location were significantly higher in children with autism than typically developing children, suggesting abnormal myelination of the corpus callosum in autism.
Conclusions
The differences in callosal myelination suggested by these results may reflect an alteration in the normally well-regulated process of myelination of the brain, with broad implications for neuropathology, diagnosis, and treatment of autism.
Introduction
Autism is a complex neurodevelopmental disorder characterized by impairments in social interaction and communication, along with the presence of repetitive behaviors and restricted interests (1). While autism is an urgent public health concern (2–4), the neuropathology of the disorder remains widely unknown (5, 6).
A number of studies have suggested that autism is a disorder of functional and anatomical connectivity (7–9), with alterations found in both short- and long-distance white matter fiber tracts (10, 11). Evidence from diffusion tensor imaging (DTI) supports the hypothesis that white matter microstructure is abnormal in autism (12). The magnitude and direction of the results, however, varies greatly across studies, with both decreased (13–18) and increased (19, 20) values of fractional anisotropy (FA). Findings in the corpus callosum, the major interhemispheric white matter tract, have been mixed, with reductions in the total area of the corpus callosum and its subregions varying significantly across studies [see (21) for a recent review]. Given the critical role that altered development of the corpus callosum may play in the social, emotional and communication deficits of autism (21), additional complimentary information is needed to clarify the nature of corpus callosum abnormalities in autism.
Magnetization Transfer Imaging (MTI) is a magnetic resonance imaging (MRI) technique that is considered to be sensitive to the presence of myelin in the brain (22). MTI is based on the exchange of magnetization between protons that are bound to large, poorly mobile macromolecules such as myelin and mobile protons in free water (23). In the white matter of the pediatric brain, age-related changes in the amount of magnetization transfer quantified by calculation of the magnetization transfer ratio (MTR) are consistent with expected myelination patterns (24).
It has been demonstrated that MTR values vary among different anatomical structures in the normal brain, presumably reflecting differences in the degree of myelination. Higher MTR values are found in the white matter as compared with the gray matter, and within the white matter, the highest MTR values are found in the corpus callosum (25, 26). High MTR values in the corpus callosum are consistent with the histological appearance of a large number of heavily myelinated white matter fibers in this structure (25, 26).
In comparison to DTI, MTI has been interpreted to represent a better indicator of myelin (27–29). Although DTI was initially thought to provide a measure of myelin status (30), subsequent studies found similar degrees of diffusion anisotropy in myelinated and unmyelinated axons (31, 32), and it is now clear that DTI does not measure a single characteristic of white matter development. Rather, DTI reflects a number of changes in addition to myelination (30). MTI, on the other hand, provides a more specific measure of myelin status, as demonstrated by studies showing that the primary source of magnetic transfer in the white matter is the lipids in myelin, especially galactocerebroside (24, 33). Postmortem data from multiple sclerosis brains also confirm that the primary magnetic resonance correlate of myelination is MTR (34). A recent study showing different heritability patterns for FA and MTR, and a low correlation between the two, adds to the evidence that FA and MTR measurements provide different types of information (27).
To date, changes in MTR have been reported in disorders known to affect myelin, such as multiple sclerosis (34–36), progressive multifocal leucoencephalopathy (37) and central pontine myelinolysis (38). MTI has also been used to detect previously unknown alterations in individuals with schizophrenia (29, 39) and Tourette syndrome (40). Given that autism has been repeatedly associated with abnormal white matter development (9, 20, 41), there are a priori reasons to believe that MTR may be abnormal in children with autism as compared with typically developing children.
Here, we report the first study using MTI in autism. We used MTI to investigate the myelination of the corpus callosum in a group of young children with autism compared with typically developing children. The relationships between MTI measures and potentially relevant variables such as gender and age were also explored.
Methods and Materials
Participants
The study included a total of 136 subjects with MTI scans, divided into 101 children with autism (82 males, 19 females; mean age = 4.5, SD = 1.4) and 35 typically developing children (23 males, 12 females; mean age = 4.0, SD = 1.7). These and additional demographic data are reported in Table 1. Potential participants were recruited through a variety of methods, including flyers and advertisements posted in the local community and communication with autism organizations. Interested and potentially eligible participants completed an on-site screening visit to determine study eligibility.
Table 1.
Comparison of children with autism (n = 101) and typically developing children (n = 35)
| Characteristic | TYP | AUT | p Value |
|---|---|---|---|
| Male/Female, n/n | 23/12 | 82/19 | .06 |
| Nonverbal DQ, Mean ± SD | 108.2 ± 13.0 | 59.1 ± 18.3 | <.001* |
| Age, Mean ± SD | 4.0 ± 1.7 | 4.5 ± 1.4 | .14 |
| Total CC Midsagittal Area, Mean ± SD | 380 ± 72 | 368 ± 70 | .40 |
| ADOS Total (SA+RRB), Mean ± SD | 1.4 ± 1.5 | 19.5 ± 3.5 | <.001* |
TYP, typically developing children; AUT, autism; DQ, developmental quotient; CC, corpus callosum; ADOS, Autism Diagnostic Observation Schedule; SA, social affect; RRB, restrictive repetitive behaviors. ADOS scores were obtained on 128 of the 136 children, 8 children were tested using the toddler version of the ADOS.
p < 0.05
Children with autism were included after an evaluation with research-reliable administrations of the Autism Diagnosis Interview-Revised (42), the Autism Diagnostic Observation Schedule (43), and clinical observation by qualified clinicians at the NIH. All children fulfilled the DSM-IV diagnostic criteria for autism.
Screening for typically developing children was done through clinical assessment, including cognitive testing, administration of the ADOS, and parent-report questionnaires that included the Social Communication Questionnaire (44) and the Child Behavior Checklist (45). We administered the ADOS to both groups to collect comparable information from typically developing children and children with autism. Typically developing children were included if they had no cognitive impairments or signs of an autism spectrum disorder (ASD). Children in this group were also excluded if they had a history of a substantial medical, neurological, or developmental disorder.
Nonverbal developmental quotients (DQ) were estimated on the basis of age- and developmentally-appropriate cognitive/developmental tests [Mullen Scales of Early Learning (MSEL; 46) or Differential Ability Scales (DAS; 47)] depending on age and skill level. Children were administered the MSEL even if they were out of the normed age range when they could not achieve a basal score on the majority of the DAS subtests.
In all cases, written informed consent was obtained from the participants’ parent(s). The study was approved by the Institutional Review Board at the National Institutes of Health.
Image acquisition and analysis
The study was conducted at the NIH Clinical Center in Bethesda, Maryland, using a GE Signa 1.5 Tesla scanner (General Electric, Milwaukee, Wisconsin) equipped with an eight-channel receiver head coil. Head motion was restricted using foam pads placed around the participant’s head. Given the challenges of gathering high-quality neuroimaging data in young children, and particularly in those with autism, controls were scanned without sedation during normal sleep and autistic subjects were scanned under sedation using propofol. Sedation was performed by board-certified anesthesiologists following a strict clinical protocol.
All subjects were scanned using the same protocol, which included MTI images (T1 spin echo sequence with and without saturation pulse, TR = 500 ms, TE = 12 ms, FA = 90, slice thickness = 3 mm, gap = 0 mm, acquisition matrix = 256×192, and FOV = 24 cm) and T2-weighted images (2D Fast spin echo, TR = 6000 ms, effective TE = 12-14 ms, FA = 90, slice thickness = 3 mm, gap = 0 mm, acquisition matrix = 256 × 192, and FOV = 24 cm).
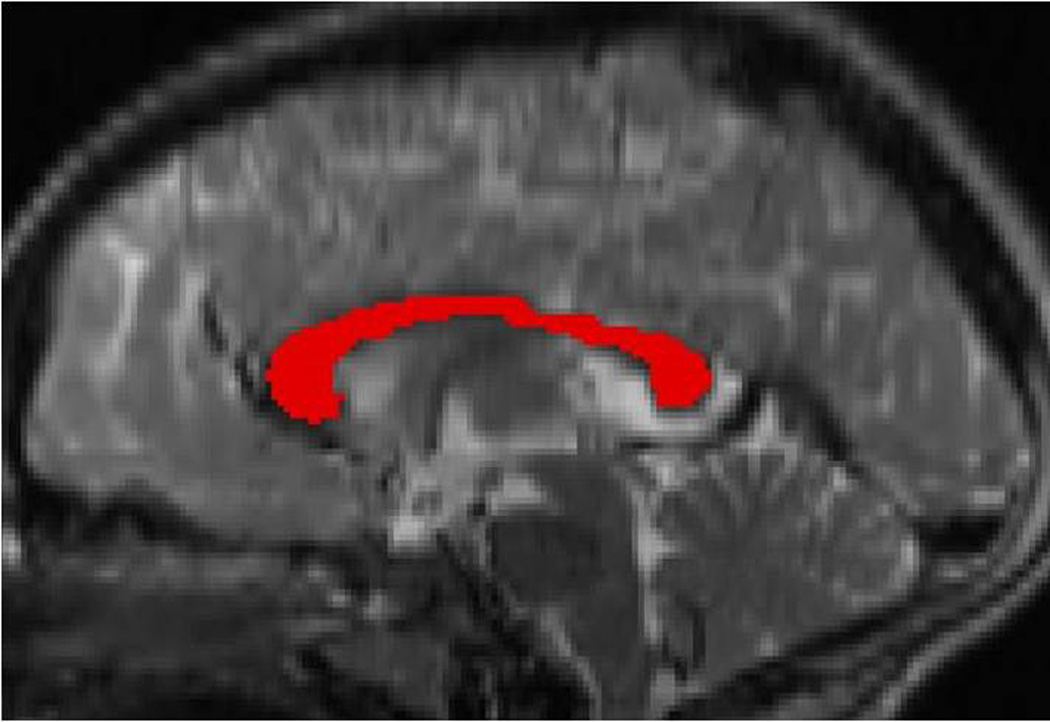
Using Medical Image Processing, Analysis, and Visualization software [MIPAV; (48)], we applied the midsagittal line alignment algorithm for the automatic detection of the midsagittal line in the T2-weighted image, which was then placed in standard Talairach alignment. A mask of the corpus callosum was manually traced by an experienced tracer (blind to diagnosis) on the midsagittal slice of the T2-weighted image (see Fig.1). The adjacent slices were checked to ensure that the midsagittal slice contained the entirety of the corpus callosum. Using the intraclass correlation coefficient, the intra-rater reliability for corpus callosum measurement was 0.87. The MTI scans were then co-registered to the T2-weighted image, and the corpus callosum mask was used to select the region of interest in the MTI image.
Figure 1.
Corpus callosum tracing. The corpus callosum was manually traced on the midsagittal T2-weighted image.
Using software developed in-house (49), the MTR values were calculated on a voxel-by-voxel basis within the corpus callosum using the equation MTR = [1- (MTon/MToff)] × 100%, with MTon and MToff representing the signal intensity of the voxels with the saturation on and off, respectively (22). MTR values were assembled into MTR histograms, which were smoothed to make them monotonic. Voxels were included in the histograms if (i) MTon > 0; (ii) MToff > 0; (iii) MTR ≥ 0; and (iv) MTR < 1. If a voxel did not meet all of these qualifications it was ignored.
Based on previous studies (22, 50), the following parameters were computed from the MTR histogram of each subject: (a) the mean MTR, which indicates the average MTR value; (b) the peak location of the MTR histogram, which corresponds to the mode, or most common MTR value; and (c) the peak height of the histogram, which represents the number of voxels having the most common MTR value and can be considered as a measure of the uniformity of brain tissue in terms of MTR values. As the number of voxels having a given MTR value is affected by the size of the corpus callosum, the peak height was normalized by dividing the number of voxels having the most common MTR value by the number of voxels in the corpus callosum.
Statistical Analysis
Between-group differences in age, gender, and nonverbal DQ were determined using t-test or chi-square analyses. The MTI measures were compared between children with autism and typically developing children using t-tests. Bivariate correlations were used to examine relationships between MTI measures and potential confounding factors. Additional analyses (such as inclusion of covariates, use of an individually age- and gender-matched sample, and repetition of the analyses with and without females) were performed to explore the effects of potential confounding factors such as age, gender, IQ, and total brain volume (TBV). All statistical analyses were carried out using SPSS 16.0 (SPSS Inc., Chicago, IL). The significance level was set at p < 0.05, two-tailed.
Results
Characteristics of the samples
The scanning success rate was > 95% for the children with autism and > 70% for the typically developing children (after multiple attempts). As shown in Table 1, the autism and control groups did not differ significantly on age (p = 0.14) or gender (p = 0.06). Nevertheless, we conducted additional analyses to explore whether these variables affected the results. In addition, the groups differed in nonverbal DQ (p < 0.001) with the control group performing better than the autism group. The autism and control groups did not differ significantly on total corpus callosum area (p = 0.40).
Group differences in MTI measures
The mean MTR value was significantly higher in children with autism than typically developing children (mean ± SD; autism group: 23.86 ± 2.17%; control group: 22.85 ± 1.85%; p = 0.015; d = 0.43, 95% CI [.04, .82]). When mean MTR signal was corrected for callosal area, the results remained significant (p = 0.008), suggesting that area did not influence the results. We also looked at the median, which is less affected by outliers or extreme values. Again, we found that children with autism had higher median MTR value (mean ± SD; autism group: 24.52 ± 2.20%; control group: 23.49 ± 1.77%; p = 0.014; d = 0.43, 95% CI [.04, .82]) than typically developing children.
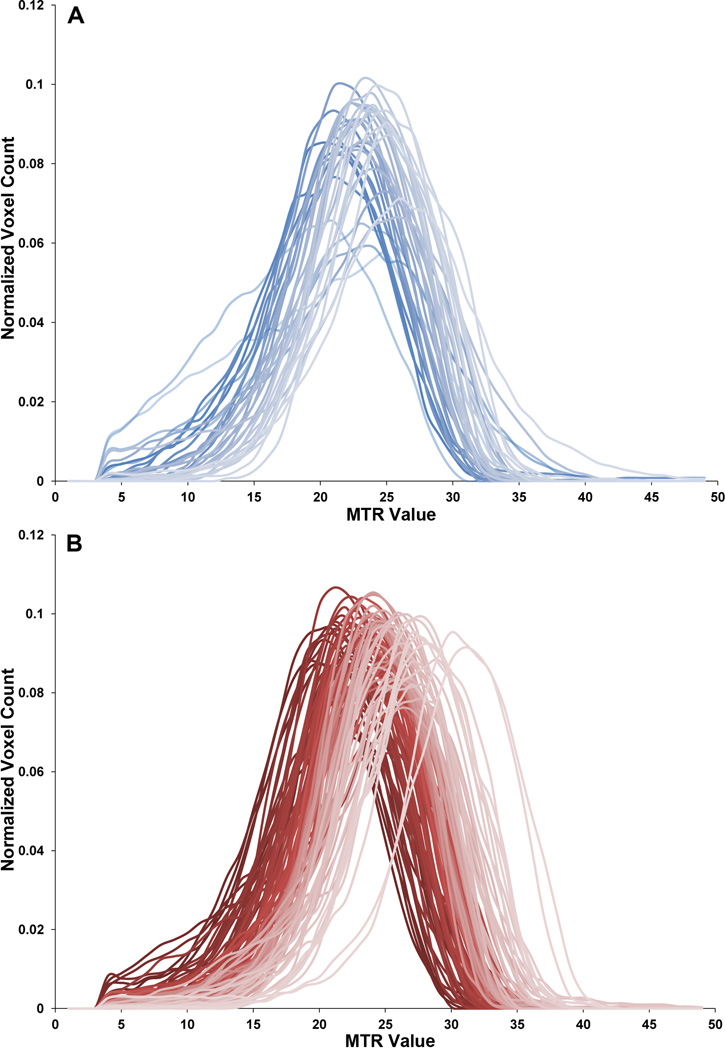
Compared with typically developing children, children with autism also showed higher MTR histogram peak location (mean ± SD; autism group: 24.074 ± 2.252; control group: 23.186 ± 1.549; p = 0.032; d = 0.37, 95% CI [.02, .76]) and higher MTR histogram peak height (mean ± SD; autism group: 0.091 ± 0.008; control group: 0.085 ± 0.012; p = 0.007; d = 0.83, 95% CI [.43, 1.23]; see Fig.2).
Figure 2.
Magnetization Transfer Ratio (MTR) histograms. Normalized MTR histograms are shown for (A) each subject in the control group and (B) each subject in the autism group.
The results did not change substantially when outliers (i.e., values exceeding +/− 2.5 standard deviations from the mean) were excluded from the analyses, ruling out the possibility that the results were driven by outliers.
Given the group differences in nonverbal DQ, we tested whether it correlated with mean MTR or MTR histogram peak height and location. The correlation was not significant for either the children with autism (mean MTR: r = 0.01, p = 0.90; peak height: r = − 0.03, p = 0.76; peak location: r = 0.02, p = 0.86) or the controls (mean MTR: r = − 0.19, p = 0.26; peak height: r = − 0.26, p = 0.13; peak location: r = 0.13, p = 0.47). When co-varying for nonverbal DQ, we found no significant main effect of DQ and no significant interaction between DQ and group for mean MTR value (DQ: p = 0.39; DQ×group: p = 0.33), MTR histogram peak height (DQ: p = 0.06; DQ×group: p = 0.10), and MTR histogram peak location (DQ: p = 0.57; DQ×group: p = 0.67). This suggests that DQ does not influence potential group differences.
Because there was a trend towards between-group differences in gender distribution, additional analyses were performed using gender as a covariate. These analyses revealed no significant main effect of gender and no significant interaction between gender and group for mean MTR value (gender: p = 0.88; gender×group: p = 0.69), MTR histogram peak height (gender: p = 0.95; gender×group: p = 0.64), and MTR histogram peak location (gender: p = 0.53; gender×group: p = 0.58). Analogously, when performing further analyses using age as a covariate, we found no significant main effect of age and no significant interaction between age and group for mean MTR value (age: p = 0.15; age×group: p = 0.18), MTR histogram peak height (age: p = 0.09; age×group: p = 0.68), and MTR histogram peak location (age: p = 0.68; age×group: p = 0.43).
As a further confirmation that the findings were not the result of differences in gender or age distribution between the groups, 35 children in the autism group were individually age- and gender-matched to 35 typically developing children based on the following criteria: same gender and closest age within one year. As a result, there were 12 females and 23 males in each group; 80% of our matches were within 4 months of age. Mean MTR and MTR histogram peak height and location were compared between groups, and the results remained significant (mean MTR value: p = 0.002, d = 1.17, 95% CI [.76, 1.58]; median MTR: p = 0.001, d = 1.19, 95% CI [.78, 1.60]; MTR histogram peak location: p = 0.006; d = 1.01, 95% CI [.61, 1.41]; MTR histogram peak height: p = 0.032, d = 0.77, 95% CI [.37, 1.17]). We also repeated the analyses excluding females, and once again the results remained significant.
When performing an additional analysis co-varying for TBV, we found no significant main effect of TBV and no significant interaction between TBV and group for mean MTR value (TBV: p = 0.32; TBV×group: p = 0.87), MTR histogram peak height (TBV: p = 0.11; TBV×group: p = 0.87), and MTR histogram peak location (TBV: p = 0.73; TBV×group: p = 0.93). This suggests that TBV does not influence potential group differences.
In line with a previous study of myelination in children younger than seven (24), we found a positive correlation between age and mean MTR in typically developing children younger than seven (N = 31, r = 0.464, p = 0.009). When including children over 84 months old (N = 4), the significance decreased to a p value of 0.05 (r = 0.333). The children with autism did not show any significant correlations between age and mean MTR (r = 0.009, p = 0.929). When including gender, nonverbal DQ, and TBV in a linear regression model, with mean MTR as the dependent variable, we found that none of these variables significantly contributed to the model. It is important to note that age effects in our study should be interpreted with caution because of the modest sample size (especially of the control group), the paucity of subjects in the upper end of the age spectrum, and the cross-sectional design. A longitudinal study would be required to establish meaningful correlations between MTR and age.
Using the Hofer scheme (51), the corpus callosum was automatically divided into five discrete partitions. Area, mean MTR, and median MTR were computed for each of the five subregions (“Hofer I,” “II,” “III,” “IV,” and “V”) and compared between children with autism and typically developing children using t-tests. We found that area did not significantly differ between the groups for any of the five subregions (Hofer I: i = 0.377; Hofer II: p = 0.546; Hofer III: p = 0.216; Hofer IV: p = 0.751; Hofer V: p = 0.756). When considering mean MTR, we found significant differences between the groups for Hofer II and Hofer III (Hofer I: p = 0.073; Hofer II: p = 0.016; Hofer III: p = 0.005; Hofer IV: p = 0.076; Hofer V: p = 0.065), and when looking at median MTR, we found significant differences between the groups for Hofer II, Hofer III, and Hofer V (Hofer I: p = 0.119; Hofer II: p = 0.019; Hofer III: p = 0.004; Hofer IV: p = 0.060; Hofer V: p = 0.026) with higher values in children with autism than controls. Overall, these results suggest that subregions of the corpus callosum may be driving the observed differences, but establishing the role of specific subregions was behind the scope of this study.
Discussion
Here, we investigated corpus callosum abnormalities in autism using MTI, which provides a relatively specific measure of myelination. In comparison with a group of 35 typically developing children, a group of 101 children with autism showed increased mean MTR and increased MTR histogram peak height and location in the midsagittal corpus callosum, suggesting abnormal myelination of the corpus callosum in autism.
In the developing brain, myelination occurs following an orderly and predetermined pattern (52). Previous MTI studies have shown that during typical brain development, there is a shift from a homogeneously unmyelinated brain to in-homogeneous myelination as different regions of the brain are myelinated at different paces (22, 24). Since MTR values are heavily dependent on specific pulse sequence characteristics, MTR results are difficult to compare across studies (30); however, our results suggest that this process of maturation may be disturbed in young children with autism, at least in the corpus callosum.
Given previous studies on the relationship between MTR and myelination, we suggest that abnormal MTR values in the corpus callosum may indicate abnormal myelin development, which could possibly be associated with previously described altered connectivity among individuals with autism. Future studies are needed to further determine the type of abnormality of myelination, for example by combining MTI with other types of noninvasive imaging techniques sensitive to different aspects of myelination, such as T1- or T2-weighted imaging methods. Integration of imaging results with postmortem findings or indices of myelination in animal models would also serve to illuminate physiological bases for differences in MTR. We should also note that MTR measurements represent an indirect measure of myelination (53), and other macromolecules may contribute to the MTR signal (27).
The results of this investigation are consistent with recently reported differences in axons and their insulation, demonstrated via postmortem examinations of the brains of five individuals with autism and four controls (54). Even though the corpus callosum was not specifically examined, the group with autism had atypical myelin thickness below the orbitofrontal cortex (area 11). In the discussion, the authors combined data from nonhuman and human primates to propose a model in which orderly myelin development in the typical brain is controlled by gene-development interactions. Of particular importance would be the growth-associated protein 43 kDa (GAP-43), which stimulates axon branching, inhibits myelin growth, and drops after myelination begins. In the autistic brain, there may be a disturbance that affects the onset and perhaps the duration of expression of GAP-43 and its interaction with myelin. Another postmortem study (55) analyzed brains from 19 individuals with autism and 17 controls. The authors found that regional patterns of gene expression that typically distinguish frontal and temporal cortex are attenuated in the autistic brain, suggesting abnormalities in cortical patterning. Typical gene-expression patterns are disrupted by genetic vulnerabilities and environmental influences.
Our assessment of MTR was confined to the corpus callosum because of its major role in interhemispheric communication and previous reports of neuropathological abnormalities of the corpus callosum associated with autism. Numerous investigations have focused on the size of the corpus callosum and its subregions, reporting abnormalities in the midsagittal area (56) and volume (41). Recent studies using functional MRI and positron emission tomography (PET) have shown aberrant functional connectivity during rest (57) and during a variety of tasks such as sentence comprehension (58), working memory (59, 60), and attribution of mental states (61). It has been suggested that abnormalities in white matter myelination may contribute to this connectivity dysfunction, yet there is a lack of data specifically examining myelination of the corpus callosum. DTI investigations have reported differences in the corpus callosum of children and young adults with autism (12, 13). However, MTI provides a more specific measure of myelination than DTI. Since this is the first MTI study in autism, focusing on the corpus callosum represents a good starting point, but future research should examine other white matter tracts, including intrahemispheric fibers such as the arcuate fasciculus.
Notably, the results of the MTI analyses in this sample of young children cannot be attributed to variables such as gender, age, DQ or TBV. Sample size for the typically developing control group was limited by age and gender matching at the time of recruitment. Furthermore, since children with autism were scanned under sedation while controls were scanned without sedation during normal sleep, there were many more scans excluded from the control group because the MRI was not complete or unusable due to excessive motion artifacts, leaving a sample size of N = 101 children with autism and 35 typically developing children. There are currently no studies addressing the possible effect of sedation on MTR, but the short duration of exposure to propofol is unlikely to have had an effect on MTR. It is possible that differential use of sedation across groups could impact image quality, but this would have been mitigated by the exclusion of scans with excessive motion artifacts. Another potential limitation of the study is that the intra-rater reliability for total corpus callosum measurement was 0.87, which may be considered as a relatively low reliability in light of the usually clear boundaries of this structure. This may be due to the decreased sharpness of borders when converting the images from axial into sagittal orientation and magnifying them to trace the corpus callosum (see Fig. 1).
Degenerative and inflammatory disorders, such as multiple sclerosis, progressive multifocal leucoencephalopathy, and central pontine myelinolysis, are generally associated with decreased MTR values due to destruction of myelin by the disease processes (34–38). Our finding of increased MTR values may reflect atypicalities of neurodevelopment in children with autism.
Currently, we cannot make claims about the specificity of our findings to autism. Future studies including individuals with other neuropsychiatric disorders will be needed to determine whether the callosal abnormalities reported here are specific to autism, or are a more generalized marker of abnormal brain development.
In conclusion, in this first study using MTI in autism, we found that corpus callosum myelination may be abnormal in young children with autism. If replicated, these findings may provide important clues to understanding the role of myelination in the etiology and pathogenesis of autism. If abnormalities in myelination are confirmed, this could lead to the identification of a diagnostic biomarker and influence the development of diagnostic criteria based on specific neural mechanisms.
Acknowledgments
We thank the staff of the Pediatrics and Developmental Neuroscience Branch and the technologists in the NIH NMR Center for their help in acquiring data for this study, Nancy Richert for providing the methods, Michael Stockman for help with the analysis of callosal subregions, B. Hassenplug for valuable contributions, and all the children and their families for participating in our study. This research was supported by the Intramural Research Program of the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer:
The views expressed in this article do not necessarily represent the official views of the NIMH, the NIH, the US Department of Health and Human Services, or any other agency of the US Government.
Financial Disclosures
None of the authors reported any relevant biomedical financial interests or potential conflict of interest.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Flanders SC, Engelhart L, Pandina GJ, McCracken JT. Direct health care costs for children with pervasive developmental disorders: 1996–2002. Adm Policy Ment Health. 2007;34:213–220. doi: 10.1007/s10488-006-0098-3. [DOI] [PubMed] [Google Scholar]
- 3.Newschaffer CJ, Curran LK. Autism: an emerging public health problem. Public Health Rep. 2003;118:393–399. doi: 10.1093/phr/118.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimabukuro TT, Grosse SD, Rice C. Medical expenditures for children with an autism spectrum disorder in a privately insured population. J Autism Dev Disord. 2008;38:546–552. doi: 10.1007/s10803-007-0424-y. [DOI] [PubMed] [Google Scholar]
- 5.Casanova MF. The neuropathology of autism. Brain Pathol. 2007;17:422–433. doi: 10.1111/j.1750-3639.2007.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz C, Rezaie P. The neuropathology of autism: where do we stand? Neuropathol Appl Neurobiol. 2008;34:4–11. doi: 10.1111/j.1365-2990.2007.00872.x. [DOI] [PubMed] [Google Scholar]
- 7.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13:85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, et al. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Shukla DK, Keehn B, Smylie DM, Muller RA. Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia. 2011;49:1378–1382. doi: 10.1016/j.neuropsychologia.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groen WB, Buitelaar JK, van der Gaag RJ, Zwiers MP. Pervasive microstructural abnormalities in autism: a DTI study. J Psychiatry Neurosci. 2011;36:32–40. doi: 10.1503/jpn.090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 14.Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Ke X, Tang T, Hong S, Hang Y, Zou B, Li H, et al. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain Res. 2009;1265:171–177. doi: 10.1016/j.brainres.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- 17.Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 18.Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Y, Chou KH, Chen IY, Fan YT, Decety J, Lin CP. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage. 2010;50:873–882. doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Frazier TW, Hardan AY. A meta-analysis of the corpus callosum in autism. Biol Psychiatry. 2009;66:935–941. doi: 10.1016/j.biopsych.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Buchem MA, Steens SC, Vrooman HA, Zwinderman AH, McGowan JC, Rassek M, et al. Global estimation of myelination in the developing brain on the basis of magnetization transfer imaging: a preliminary study. AJNR Am J Neuroradiol. 2001;22:762–766. [PMC free article] [PubMed] [Google Scholar]
- 23.Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10:135–144. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- 24.Engelbrecht V, Rassek M, Preiss S, Wald C, Modder U. Age-dependent changes in magnetization transfer contrast of white matter in the pediatric brain. AJNR Am J Neuroradiol. 1998;19:1923–1929. [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta RC, Pike GB, Enzmann DR. Magnetization transfer MR of the normal adult brain. AJNR Am J Neuroradiol. 1995;16:2085–2091. [PMC free article] [PubMed] [Google Scholar]
- 26.Silver NC, Barker GJ, MacManus DG, Tofts PS, Miller DH. Magnetisation transfer ratio of normal brain white matter: a normative database spanning four decades of life. J Neurol Neurosurg Psychiatry. 1997;62:223–228. doi: 10.1136/jnnp.62.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brouwer RM, Mandl RC, Peper JS, van Baal GC, Kahn RS, Boomsma DI, et al. Heritability of DTI and MTR in nine-year-old children. Neuroimage. 2010;53:1085–1092. doi: 10.1016/j.neuroimage.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Madler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging. 2008;26:874–888. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 29.Mandl RC, Schnack HG, Luigjes J, van den Heuvel MP, Cahn W, Kahn RS, et al. Tract-based analysis of magnetization transfer ratio and diffusion tensor imaging of the frontal and frontotemporal connections in schizophrenia. Schizophr Bull. 2010;36:778–787. doi: 10.1093/schbul/sbn161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wozniak JR, Lim KO. Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci Biobehav Rev. 2006;30:762–774. doi: 10.1016/j.neubiorev.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beaulieu C, Allen PS. Water diffusion in the giant axon of the squid: implications for diffusion-weighted MRI of the nervous system. Magnetic Resonance in Medicine. 1994;32:579–583. doi: 10.1002/mrm.1910320506. [DOI] [PubMed] [Google Scholar]
- 32.Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magnetic Resonance in Medicine. 1994;31:394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- 33.Kucharczyk W, Macdonald PM, Stanisz GJ, Henkelman RM. Relaxivity and magnetization transfer of white matter lipids at MR imaging: importance of cerebrosides and pH. Radiology. 1994;192:521–529. doi: 10.1148/radiology.192.2.8029426. [DOI] [PubMed] [Google Scholar]
- 34.Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol. 2004;56:407–415. doi: 10.1002/ana.20202. [DOI] [PubMed] [Google Scholar]
- 35.Bellmann-Strobl J, Stiepani H, Wuerfel J, Bohner G, Paul F, Warmuth C, et al. MR spectroscopy (MRS) and magnetisation transfer imaging (MTI), lesion load and clinical scores in early relapsing remitting multiple sclerosis: a combined cross-sectional and longitudinal study. Eur Radiol. 2009;19:2066–2074. doi: 10.1007/s00330-009-1364-z. [DOI] [PubMed] [Google Scholar]
- 36.Horsfield MA. Magnetization transfer imaging in multiple sclerosis. J Neuroimaging. 2005;15:58S–67S. doi: 10.1177/1051228405282242. [DOI] [PubMed] [Google Scholar]
- 37.Dousset V, Armand JP, Lacoste D, Mieze S, Letenneur L, Dartigues JF, et al. Magnetization transfer study of HIV encephalitis and progressive multifocal leukoencephalopathy. Groupe d'Epidemiologie Clinique du SIDA en Aquitaine. AJNR Am J Neuroradiol. 1997;18:895–901. [PMC free article] [PubMed] [Google Scholar]
- 38.Silver NC, Barker GJ, MacManus DG, Miller DH, Thorpe JW, Howard RS. Decreased magnetisation transfer ratio due to demyelination: a case of central pontine myelinolysis. J Neurol Neurosurg Psychiatry. 1996;61:208–209. doi: 10.1136/jnnp.61.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foong J, Symms MR, Barker GJ, Maier M, Woermann FG, Miller DH, et al. Neuropathological abnormalities in schizophrenia: evidence from magnetization transfer imaging. Brain. 2001;124:882–892. doi: 10.1093/brain/124.5.882. [DOI] [PubMed] [Google Scholar]
- 40.Muller-Vahl KR, Kaufmann J, Grosskreutz J, Dengler R, Emrich HM, Peschel T. Prefrontal and anterior cingulate cortex abnormalities in Tourette Syndrome: evidence from voxel-based morphometry and magnetization transfer imaging. BMC Neurosci. 2009;10:47. doi: 10.1186/1471-2202-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardan AY, Pabalan M, Gupta N, Bansal R, Melhem NM, Fedorov S, et al. Corpus callosum volume in children with autism. Psychiatry Res. 2009;174:57–61. doi: 10.1016/j.pscychresns.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 43.Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule—Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- 44.Rutter M, Bailey A, Lord C. Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 45.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for children, Youth, and Families; 2000. [Google Scholar]
- 46.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 47.Elliott CD. Manual for the Differential Ability Scales (2nd Ed.) San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- 48.McAuliffe M, Lalonde FM, McGarry D, Gandler W, Csaky K, Trus BL. Medical Image Processing, Analysis & Visualization In Clinical Research. IEEE COMPUTER-BASED MEDICAL SYSTEMS (CBMS) 2001:381–386. [Google Scholar]
- 49.Ostuni JL, Richert N, Wisniewski R, Lewis BK, Howard T, Patel J, et al. Comparison of methods for obtaining longitudinal whole-brain magnetization transfer measurements. J Magn Reson Imaging. 2002;15:8–15. doi: 10.1002/jmri.10040. [DOI] [PubMed] [Google Scholar]
- 50.Steens SC, Bosma GP, Steup-Beekman GM, le Cessie S, Huizinga TW, van Buchem MA. Association between microscopic brain damage as indicated by magnetization transfer imaging and anticardiolipin antibodies in neuropsychiatric lupus. Arthritis Res Ther. 2006;8:R38. doi: 10.1186/ar1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 52.Barkovich AJ, Lyon G, Evrard P. Formation, maturation, and disorders of white matter. AJNR Am J Neuroradiol. 1992;13:447–461. [PMC free article] [PubMed] [Google Scholar]
- 53.Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, et al. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zikopoulos B, Barbas H. Changes in prefrontal axons may disrupt the network in autism. J Neurosci. 2010;30:14595–14609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piven J, Bailey J, Ranson BJ, Arndt S. An MRI study of the corpus callosum in autism. Am J Psychiatry. 1997;154:1051–1056. doi: 10.1176/ajp.154.8.1051. [DOI] [PubMed] [Google Scholar]
- 57.Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- 58.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 59.Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 60.Luna B, Minshew NJ, Garver KE, Lazar NA, Thulborn KR, Eddy WF, et al. Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology. 2002;59:834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- 61.Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]