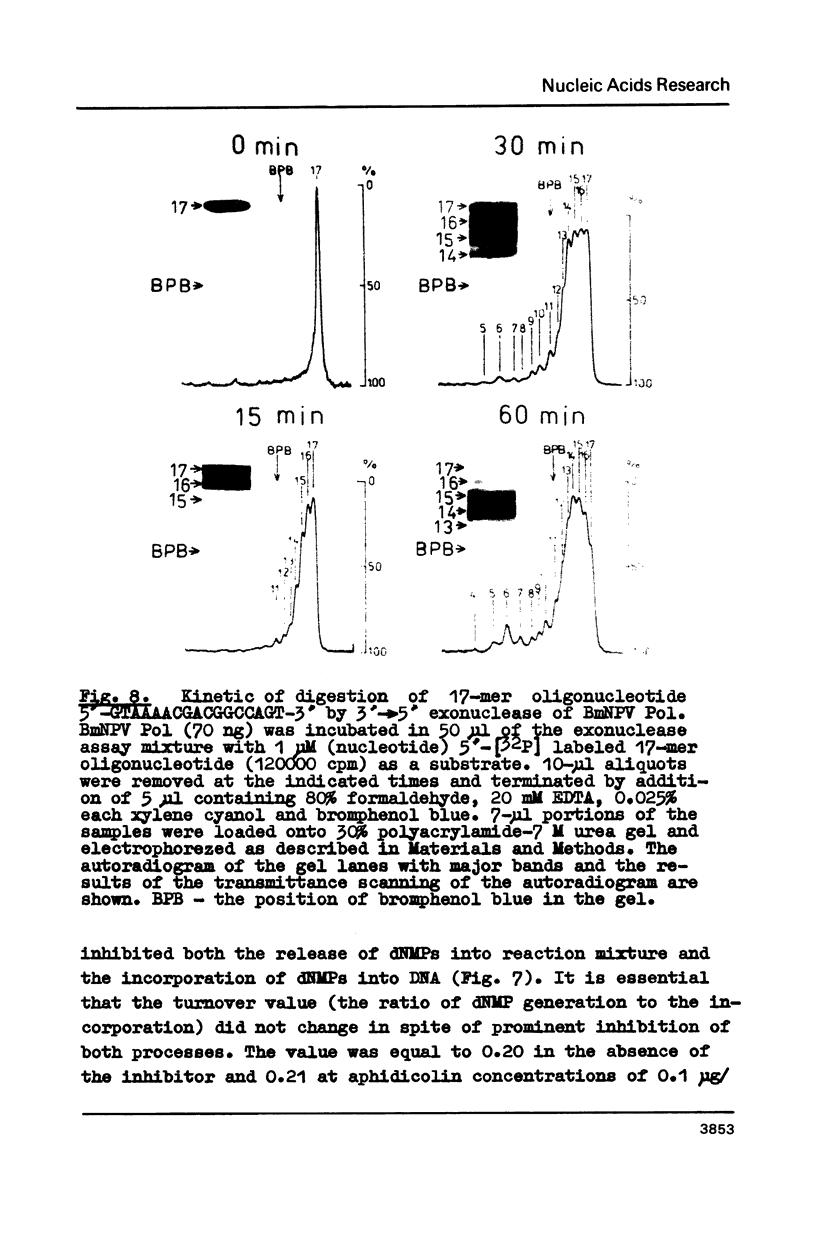
Abstract
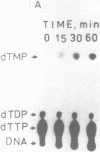
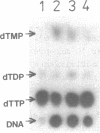
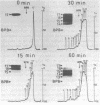
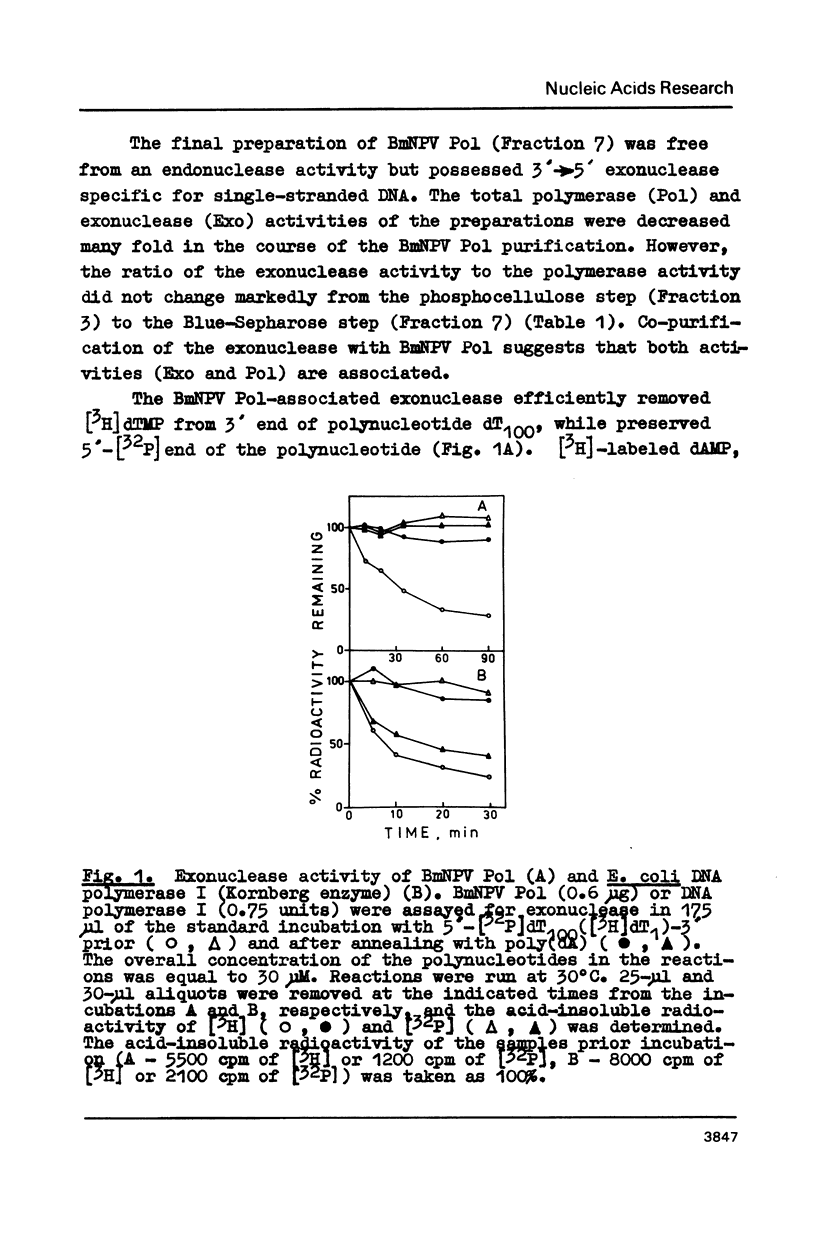
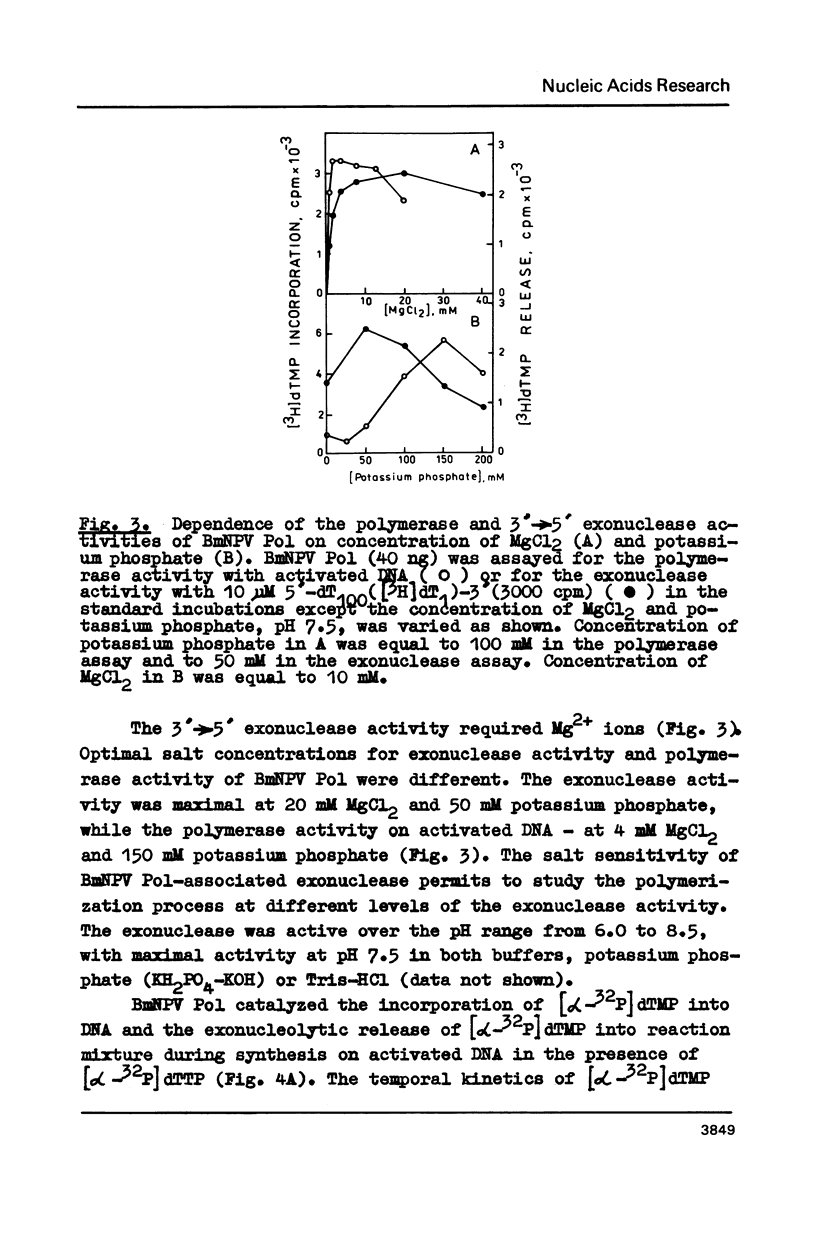
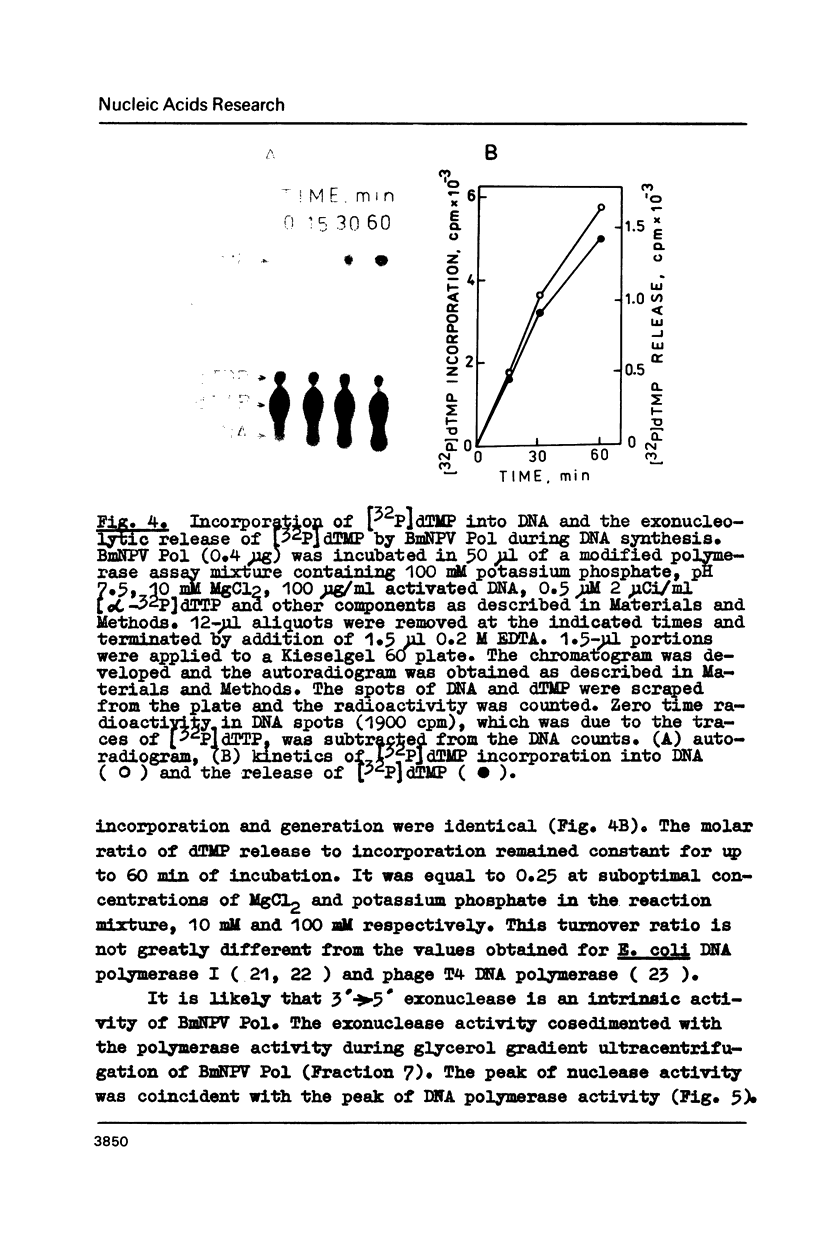
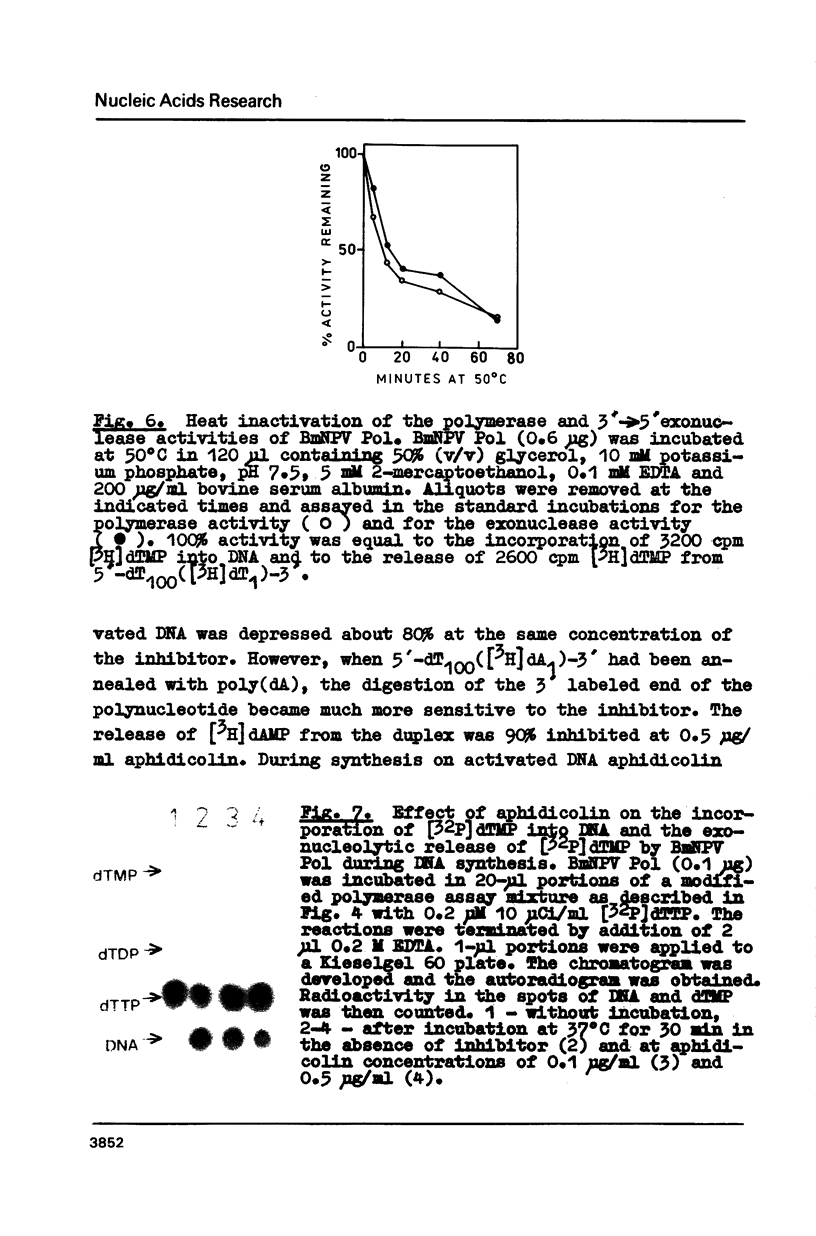
3'----5' Exonuclease specific for single-stranded DNA copurified with DNA polymerase of nuclear polyhedrosis virus of silkworm Bombyx mori (BmNPV Pol). BmNPV Pol has no detectable 5'----3' exonuclease activity on single-stranded or duplex DNA. Analysis of the products of 3'----5' exonucleolytic reaction showed that deoxynucleoside monophosphates were released during the hydrolysis of single-stranded DNA. The exonuclease activity cosedimented with the polymerase activity during ultracentrifugation of BmNPV Pol in glycerol gradient. The polymerase and the exonuclease activities of BmNPV Pol were inactivated by heat with nearly identical kinetics. The mode of the hydrolysis of single-stranded DNA by BmNPV Pol-associated exonuclease was strictly distributive. The enzyme dissociated from single-stranded DNA after the release of a single dNMP and then reassociated with a next polynucleotide being degradated.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. IX. The polymerase formed after T2 bacteriophage infection of Escherichia coli: a new enzyme. J Biol Chem. 1962 Feb;237:519–525. [PubMed] [Google Scholar]
- Blanco L., Salas M. Characterization of a 3'----5' exonuclease activity in the phage phi 29-encoded DNA polymerase. Nucleic Acids Res. 1985 Feb 25;13(4):1239–1249. doi: 10.1093/nar/13.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutlag D., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3' leads to 5' exonuclease activity in deoxyribonucleic acid polymerases. J Biol Chem. 1972 Jan 10;247(1):241–248. [PubMed] [Google Scholar]
- Challberg M. D., Englund P. T. Purification and properties of the deoxyribonucleic acid polymerase induced by vaccinia virus. J Biol Chem. 1979 Aug 25;254(16):7812–7819. [PubMed] [Google Scholar]
- Englund P. T. The initial step of in vitro synthesis of deoxyribonucleic acid by the T4 deoxyribonucleic acid polymerase. J Biol Chem. 1971 Sep 25;246(18):5684–5687. [PubMed] [Google Scholar]
- Field J., Gronostajski R. M., Hurwitz J. Properties of the adenovirus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9487–9495. [PubMed] [Google Scholar]
- Grossberger D., Clough W. Characterization of purified Epstein--Barr virus induced deoxyribonucleic acid polymerase: nucleotide turnover, processiveness, and phosphonoacetic acid sensitivity. Biochemistry. 1981 Jul 7;20(14):4049–4055. doi: 10.1021/bi00517a016. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Nossal N. G. Hydrolysis of template and newly synthesized deoxyribonucleic acid by the 3' to 5' exonuclease activity of the T4 deoxyribonucleic acid polymerase. J Biol Chem. 1972 Jun 10;247(11):3393–3404. [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- Knopf K. W. Properties of herpes simplex virus DNA polymerase and characterization of its associated exonuclease activity. Eur J Biochem. 1979 Jul;98(1):231–244. doi: 10.1111/j.1432-1033.1979.tb13181.x. [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Tan C. K., Downey K. M., So A. G. Further studies on calf thymus DNA polymerase delta purified to homogeneity by a new procedure. Biochemistry. 1984 Apr 24;23(9):1906–1913. doi: 10.1021/bi00304a003. [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Tan C. K., So A. G., Downey K. M. Purification of deoxyribonucleic acid polymerase delta from calf thymus: partial characterization of physical properties. Biochemistry. 1980 May 13;19(10):2096–2101. doi: 10.1021/bi00551a015. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Dube D. K., Beckman R. A., Koplitz M., Gopinathan K. P. On the fidelity of DNA replication. Nucleoside monophosphate generation during polymerization. J Biol Chem. 1981 Apr 25;256(8):3978–3987. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mikhailov V. S., Gulyamov D. B. Changes in DNA polymerase alpha, beta, gamma activities during early development of the teleost fish Misgurnus fossilis (loach). Eur J Biochem. 1983 Sep 15;135(2):303–306. doi: 10.1111/j.1432-1033.1983.tb07653.x. [DOI] [PubMed] [Google Scholar]
- Miller L. K., Jewell J. E., Browne D. Baculovirus induction of a DNA polymerase. J Virol. 1981 Oct;40(1):305–308. doi: 10.1128/jvi.40.1.305-308.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y., Maeno K., Yoshida S. Characterization of human cytomegalovirus-induced DNA polymerase and the associated 3'-to-5', exonuclease. Virology. 1983 Jan 30;124(2):221–231. doi: 10.1016/0042-6822(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Nossal N. G., Hershfield M. S. Nuclease activity in a fragment of bacteriophage T4 deoxyribonucleic acid polymerase induced by the amber mutant am B22. J Biol Chem. 1971 Sep 10;246(17):5414–5426. [PubMed] [Google Scholar]
- Ostrander M., Cheng Y. C. Properties of herpes simplex virus type 1 and type 2 DNA polymerase. Biochim Biophys Acta. 1980 Sep 19;609(2):232–245. doi: 10.1016/0005-2787(80)90234-8. [DOI] [PubMed] [Google Scholar]
- Radman M., Villani G., Boiteux S., Kinsella A. R., Glickman B. W., Spadari S. Replicational fidelity: mechanisms of mutation avoidance and mutation fixation. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):937–946. doi: 10.1101/sqb.1979.043.01.103. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Olivera B. M. Processivity of DNA exonucleases. J Biol Chem. 1978 Jan 25;253(2):424–429. [PubMed] [Google Scholar]
- Weissbach A., Hong S. C., Aucker J., Muller R. Characterization of herpes simplex virus-induced deoxyribonucleic acid polymerase. J Biol Chem. 1973 Sep 25;248(18):6270–6277. [PubMed] [Google Scholar]