Abstract
The arcuate fasciculus (AF) is believed to be fundamental to the neural circuitry behind many important cognitive processes. Connecting Wernicke’s and Broca’s area, these fibers are thought to be especially important for repetition. In this case study we present evidence from a patient that set doubt on these assumptions. We present structural imaging, diffusion tensor imaging, and language data on a patient with a large left-sided stroke and severely damaged left AF who showed intact word repetition and relatively intact sentence repetition performance. Specifically, his sentence repetition is more fluent and grammatical, with less hesitation than spontaneous speech, and with rare omissions only during the longest sentences. These results challenge classical theories that maintain the left AF is the dominant language processing pathway or mechanism for repetition.
Keywords: Arcuate fasciculus, Repetition, DTI, Aphasia, Case-study
The exact architecture of the neural processing of language in humans is at present unresolved. Studies of these complex constructions have begun to elucidate an interwoven network of fibers, pathways, and structures that mediate the many cognitive processes humans undertake. Stroke has the startling tendency to disrupt these processes leading to debilitating clinical syndromes such as aphasia, ataxia, and aprosodia. The task of repetition exemplifies the complexity to be found in neural networks, comprised of many cognitive processes governed by various regions of the brain. The traditional view of this process, and language circuitry in general, holds that the caudal temporal region (critical for hearing and understanding) and the anterior language region in the inferior frontal cortex (critical for speech articulation) are connected by the arcuate fasciculus (AF). Disruption in this fibrous connection, such as by stroke, would therefore lead to impairment in repetition performance. However, the clinico-pathological studies available do not present a clear picture. Some studies have found transient repetition impairments after small AF lesions (Arnett, Rao, Hussain, Swanson, & Hammeke 1996; Tanabe et al., 1987), but others documented no repetition problems after AF lesions (Shuren et al., 1995; Whittle & Fraser, 1991). More importantly, a recent study using in vivo imaging techniques of diffusion tensor imaging (DTI) and fiber tracking documented intact repetition performance with AF damage (Selnes, van Zijl, Barker, Hillis, & Mori, 2002). Nevertheless, these previous reports describe patients with damage restricted to only a part of the AF, whereas the present report discusses total absence of AF.
The role of the AF in repetition becomes more complicated given recent evidence from tractography studies in the human (Frey, Cambell, Pike, & Petrides, 2008; Jiang, van Zijl, Kim, Pearlson, & Mori, 2006; Mori, Crain, Chacko, & Van Zijl, 1999) and the monkey (Petrides & Pandya, 1988, 2009) where the very existence of AF as a single tract uniting posterior and anterior language areas has been set to doubt. These studies suggest the existence of other neural pathways in language processing which comprise a richer and more complex system of connections. Specifically, they suggest that the connection between Broca’s area and the posterior temporal region, also described as Wernicke’s area, is subserved by two distinct tracts: the superior longitudinal fasciculus III (SLF III), which connects Broca’s area to the inferior parietal lobule, and the caudal part of the inferior longitudinal fasciculus (ILF), which connects the inferior parietal lobule to the caudal superior temporal region. Here we report MRI, DTI and behavioral testing data from a middle-aged male stroke patient with an extensive left hemisphere lesion including the entire SLF III (what was traditionally considered the AF) and part of the ILF. We show that the patient’s repetition performance is nearly normal indicating that the SLF III is not necessary for recovery of repetition. Although a partially spared left ILF was present, there was no intact connection between Wernicke’s area (mostly infarcted) and the inferior parietal lobule to Broca’s area (also mostly infarcted), making it unlikely that this isolated tract supports recovery of repetition.
CLINICAL BACKGROUND
Our patient, a 62-year-old right-handed man, presented with a large left-sided stroke 3 years before testing. Initial MRI revealed a large left middle cerebral artery stroke and an incidental finding of a completely occluded right carotid artery. Additionally, MRI revealed ischemia in the corpus callosum. Initially, the patient was globally aphasic, but had improved through neurorehabilitation. Three years post-stroke, his aphasia type was unclassifiable; he was classified as anomic by the Western Aphasia Battery (WAB), but he had ‘asyntactic’ auditory comprehension on sentence comprehension tasks. In detail, his scores in a sentence–picture matching task were at 85% correct for active sentences, 80% for passive, 65% for cleft subject (e.g., ‘It was the man that kicked the girl.’), 55% for cleft object (e.g., ‘It was the niece that the father kicked.’), 48% for semantically reversible (e.g., ‘The dog chased the boy.’, and 95% for irreversible sentence sentences, ‘The girl kicked the ball.’). On an enactment task (in which he had to enact the sentence with paper dolls), he was 100% correct with active sentences, 35% with passive, 95% with cleft subject, 50% with cleft object, 55% with reversible, and 90% with irreversible sentences. Notable sub-scores on the WAB are: 90% correct for sentence repetition, 100% correct for object naming, 100% correct sentence completion, and 100% correct responsive speech (naming to definition). His reading was close to normal in both accuracy and speed, but he remained unable to spell words. DTI and functional magnetic resonance imaging (fMRI) were both performed 3.5 years post-stroke. See Table 1 for remaining behavioral data.
TABLE 1.
Performance of patient WCR in the Western Aphasia Battery (WAB)
| Overall aphasia quotient: | 85 |
| Aphasia type: | aAnomic but asyntactic |
| Spontaneous speech total: | 15/20, 75% Correct |
| – Information content: | 10/10, 100% Correct |
| – Fluency, grammatical competence: | 5/10, 50% Correct |
| Auditory verbal comprehension | |
| – Yes/no questions: | 54/60, 90% Correct |
| – Auditory word recognition: | 55/60, 92% correct |
| – Sequential commands: | 60/80, 75% correct |
| Repetition total: | 90/100, 90% correct |
| Naming and word finding | |
| – Object naming: | 60/60, 100% correct |
| – Word fluency: | 8/20, 40% correct |
| – Sentence completion: | 10/10, 100% correct |
| Responsive speech: | 10/10, 100% correct |
METHODS AND RESULTS
DTI methods
Images were acquired using an 8-channel SENSE head coil on a Philips 3-T MRI scanner. For DTI acquisitions, a single-shot spin echo-echo planar imaging (EPI) was used, with diffusion gradients applied in 16 non-collinear directions and b = 700 s/mm2. One reference image with least diffusion weighting (b = 33 s/mm2) was also acquired (here called the B0). Thirty axial slices were acquired, parallel to the AC–PC line. The field of view (FOV), the size of the acquisition matrix, and the slice thickness were 230 × 230 mm/96 × 96/4 mm. The 140 axial MPRAGE had FOV, size of matrix, and slice thickness of 212 × 212 mm/ 192 × 192/1.1 mm. All images were zero-filled to the final reconstruction matrix of 256 × 256.
Raw diffusion-weighted images (DWIs) were first co-registered to the B0 image and corrected for subject motion using a 12-mode affine transformation of Automated Image Registration (AIR) (Woods, Grafton, Holmes, Cherry, & Mazziotta, 1998). Warping was applied to all raw DWIs; 6 elements of the diffusion tensor were calculated for each voxel with multivariate linear fitting (Basser, Mattiello, & LeBihan, 1994; Jiang et al., 2006). After diagonalization, 3 eigenvalues and eigenvectors were obtained. For the anisotropy map, FA was used (Pierpaoli & Basser, 1996). The eigenvector associated with the largest eigenvalue (v1) was used as an indicator of fiber orientation. All data processing was performed using DTIStudio (H. Jiang and S. Mori, Johns Hopkins University, Kennedy Krieger Institute) (Jiang et al., 2006). Fiber tract maps were reconstructed using the ‘FACT’ algorithm8 and the protocols described by Zhang et al. (2010).
DTI Results
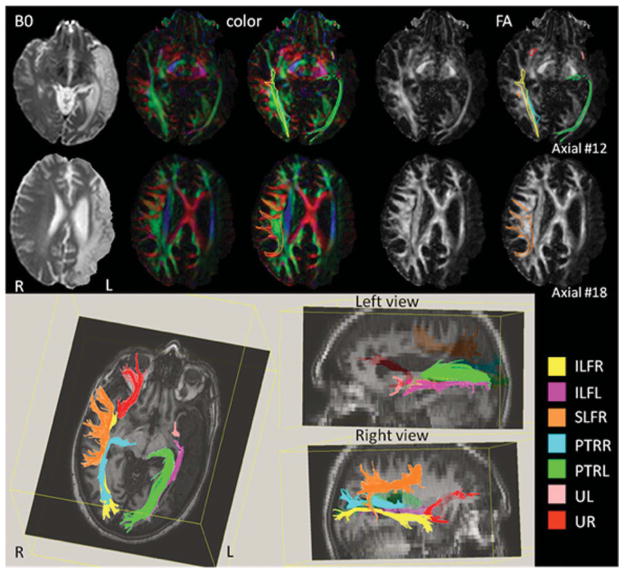
Figure 1 shows B0, color map and FA at two different axial slices. A large infarction can be seen in the left hemisphere (hyperintense in B0). By comparison with the right hemisphere, the uncinate fasciculus in the left hemisphere is much less prominent as well as the ILF, particularly the caudal part. The superior longitudinal fasciculus cannot be identified in the left hemisphere.
Figure 1.
B0, color map and FA images show a chronic infarct in the left hemisphere and the identified tracts surrounded. Tractography results (bottom) projected in axial and saggital views show reduction of uncinate (U) and inferior longitudinal fasciculus (ILF) at left hemisphere as well as the absence of superior longitudinal fasciculus (SLF). PTR, posterior thalamic radiation; L, left; R, right. [To view this figure in color, please visit the online version of this Journal.]
Language testing
He was administered the WAB and supplemental tests of sentence comprehension and working memory (see Table 1). With regard to repetition, he was administered a set of 50 five-word sentences with the same syntactic structure (SV0). We used only active sentences his documented syntactic deficit would not confound his repetition performance. Here we present two examples of these sentences: ‘the boy carried the water’, ‘the lady fed the chicken’. He repeated correctly 45/50 sentences while he omitted the article ‘the’ in agent position in all nouns of all the sentences. The percentage of correct sentences he repeated was not significantly different from what it would have if he had repeated all sentences correctly, η2(1, N =50) =0.50, p> .1. The 5 errors he made were perseveration errors from the previous sentences. The sentences were recorded by a male native speaker of English and presented to the patient through earphones.
DISCUSSION
In this report we documented a patient with intact repetition performance despite severe disruption of the left AF (including complete absence of SLF III as well as other important fibers) using DTI. There have been other cases in the literature documenting patients with intact repetition performance after partial damage in the AF due to surgical removal (Shuren et al., 1995; Whittle & Fraser, 1991) or stroke (Selnes et al., 2002). However, this is the first case study of a patient with complete lack of left SLF III component of the AF after an extensive stroke. This study comes to clarify the question whether the AF is a necessary structure for repetition and adds to the growing evidence that counters the traditional view of AF. This view, established by Geschwind (1965; Benson et al., 1973) holds the AF to be paramount for processing in language functions such as repetition. Although there is still a vivid debate about the role of the AF in language repetition (Bernal & Ardila, 2009), the present case demonstrates that repetition might be accomplished by the right AF or by right cortical structures (IPL). In fact, at stroke onset the patient had a global aphasia after a massive left FTP infarction with abnormal repetition. The patient regained a nearly normal repetition performance (90% in the repetition subtest of the WAB) and naming 3 years later thus suggesting compensation of these functions by the intact right hemisphere. Reorganization of repetition (e.g., Bando, Ugawa, & Sugishita, 1986; Berthier et al., 1991; Pulvermüller & Schönle, 1993) and naming (Berthier et al., 1991; Heilman, Tucker, & Valenstein, 1976, Heilman, Rothi, McFarling, & Rottmann, 1981) in the right hemisphere has been demonstrated using various ancillary techniques (PET, amytal testing) and this may be the likely mechanism underpinning recovery of repetition in the present case. In our patient, in addition to behavioral and MRI evidence showing severe disruption of tracts connecting anterior and posterior language areas in the brain, we presented detailed white matter fiber tracking evidence showing the exact tracts that are missing (Mori et al., 1999). Therefore, this study represents the most direct evidence that the left AF is not a necessary pathway subserving recovery of repetition, even though it might have been engaged in repetition prior to stroke.
Previous tractography work in the monkey (Petrides & Pandya, 1988, 2009) and the human brain (Frey et al., 2008; Mori et al., 1999) sets doubt as to the very existence of AF, at least as the main white matter fiber tract connecting posterior superior temporal areas (Wernicke’s area, well-documented to subserve language comprehension in the human) with inferior frontal areas (Broca’s area, well-documented to subserve language production in the human). In particular, the previous studies showed that what was considered to be the AF is actually comprised by two fiber tracts: the superior longitudinal fasciculus III, connecting the inferior frontal area (Broca’s area and in particular Brodmann’s area 44) to the inferior parietal lobule and the ILF, connecting the inferior parietal lobule to the posterior superior temporal area (Wernicke’s area). As for the AF, when it can be reliably detected, it comprises the white matter tracts that connect posterior superior temporal areas to frontal areas Brodmann’s area 6 and 8 but not to frontal language production areas (Broca’s area). Therefore, the emerging picture for fiber tracts connecting anterior and posterior language areas in the human brain is far more complex than previously thought.
CONCLUSION
In the present study, the total absence of the superior longitudinal fasciculus III and partial absence of the ILF in the left hemisphere did not disrupt the patient’s recovery of repetition of words and sentences. There are various explanations for this result, including possible recovery of repetition function incorporating portions of the right hemisphere with connections to intact areas of the left hemisphere, or complete shift of repetition function to homologous regions in the right hemisphere. We plan to address this issue in functional MRI studies of language. Irrespective of the pattern of reorganization, his excellent word repetition and relatively spared sentence repetition despite the absence of any clear connection between Wernicke’s area and Broca’s area indicates that left SLF III does not appear to be essential for recovery of repetition function. However, the left inferior parietal lobule, partially spared in our patient, itself might be crucial for repetition and other working memory functions (Fridriksson et al., 2010; Wagner, Shannon, Kahn, & Buckner, 2005).
Acknowledgments
This study was supported by NIH RO1 DC 05375 and NIH/NIDCD R01 DC03681 to AH and RO1AG20012 and P41RR15241 to SM.
Footnotes
Disclosures: None of the authors reports any disclosures or conflicts of interest whatsoever.
References
- Arnett PA, Rao SM, Hussain M, Swanson SJ, Hammeke TA. Conduction aphasia in multiplesclerosis: A case report with MRI findings. Neurology. 1996;47:576–578. doi: 10.1212/wnl.47.2.576. [DOI] [PubMed] [Google Scholar]
- Bando M, Ugawa Y, Sugishita M. Mechanism of repetition in transcortical sensory aphasia. Journal of Neurology, Neurosurgery and Psychiatry. 1986;49:200–202. doi: 10.1136/jnnp.49.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. Journal of Magnetic Resonance. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Benson DF, Sheremata WA, Bouchard R, Segarra JM, Price D, Geschwind N. Conduction aphasia. Archives of Neurology. 1973;28:339–346. doi: 10.1001/archneur.1973.00490230075011. [DOI] [PubMed] [Google Scholar]
- Bernal B, Ardila A. The role of the arcuate fasciculus in conduction aphasia. Brain. 2009;132:2309–2316. doi: 10.1093/brain/awp206. Epub 2009 Aug 18. Review. [DOI] [PubMed] [Google Scholar]
- Berthier ML, Starkstein SE, Leiguarda R, Ruiz A, Mayberg HS, Wagner H, Price TR, Robinson RG. Transcortical aphasia. Importance of the nonspeech dominant hemisphere in language repetition. Brain. 1991;114:1409–1427. doi: 10.1093/brain/114.3.1409. [DOI] [PubMed] [Google Scholar]
- Frey S, Cambell SW, Pike B, Petrides M. Dissociating the human language pathways with high angular diffusion fiber tractography. The Journal of Neuroscience. 2008;28:11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Kjartansson O, Morgan P, Hjaltason H, Magnudottir S, Bonilha L, Rorden C. Impaired speech repetition and left parietal lobe damage. The Journal of Neuroscience. 2010;30(33):11057–11061. doi: 10.1523/JNEUROSCI.1120-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnection syndromes in animals and man. Brain. 1965;88:237–294. 585–644. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Tucker DM, Valenstein E. A case of mixed transcortical aphasia with intact naming. Brain. 1976;99:415–426. doi: 10.1093/brain/99.3.415. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Rothi L, McFarling D, Rottmann AL. Transcortical sensory aphasia with relatively spared spontaneous speech and naming. Archives of Neurology. 1981;38:236–239. doi: 10.1001/archneur.1981.00510040062010. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Computer Methods and Programs in Biomedicine. 2006;81(2):106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45:265–226. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association fiber pathways to the frontal corte from the superior temporal region in the rhesus monkey. The Journal of Comparative Neurology. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Distinct parietal and temporal pathways to the homologues of Broca’s area in the monkey. PLOS Biology. 2009;7(8):e1000170. doi: 10.1371/journal.pbio.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F, Schönle PW. Behavioral and neuronal changes during treatment of mixed transcortical aphasia: a case study. Cognition. 1993;48:139–161. doi: 10.1016/0010-0277(93)90028-t. [DOI] [PubMed] [Google Scholar]
- Selnes OA, van Zijl PCM, Barker PB, Hillis AE, Mori S. MR diffusion tensor imaging documented arcuate fasciculus lesion in a patient with normal repetition performance. Aphasiology. 2002;16:897–902. [Google Scholar]
- Shuren JE, Schefft BK, Yeh HS, Privitera MD, Cahill WT, Houston W. Repetition and the arcuate fasciculus. Journal of Neurology. 1995;242:596–598. doi: 10.1007/BF00868813. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Tanabe H, Sawada T, Inoue N, Ogawa M, Kuriyama Y, Shiraishi J. Conduction aphasia and arcuate fasciculus. Acta Neurologica Scandinavica. 1987;76:422–427. doi: 10.1111/j.1600-0404.1987.tb03597.x. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Whittle IR, Fraser DE. Resolution of fluent dysphasia following excision of metastatic carcinoma from the arcuate fasciculus. British Journal of Neurosurgery. 1991;5:647–649. doi: 10.3109/02688699109002891. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22(1):139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Oishi, Faria AV, Jiang H, Li X, Akhter K, Rosa-Neto P, Pike GB, Evans A, et al. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage. 2010;52(4):1289–1301. doi: 10.1016/j.neuroimage.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]