Abstract
Fatigue, which is one of the most commonly reported symptoms in cancer, can negatively impact the functional status and the health-related quality of life of individuals. This paper systematically reviews 34 studies to determine patterns of associations between immunogenomic markers and levels of cancer-related fatigue (CRF). Findings from the longitudinal studies revealed that elevated fatigue symptoms especially of women with early stages of breast cancer were associated with high levels of neutrophil/monocyte, IL-1ra, and IL-6 during radiation therapy; high levels of CD4+, IL-1β, and IL-6 with stressing stimuli; high levels of IL-1β during chemotherapy; low NK cell levels after chemotherapy; and presence of homozygous IL-6 and TNF alleles. In the cross-sectional studies, associations between levels of fatigue and immune/inflammatory markers were not consistently found, especially when covariates such as BMI, ethnicity, menopausal status, and educational level were controlled in the statistical analyses. However, a number of genomic markers were observed to be elevated mostly in fatigued breast cancer survivors in the cross-sectional studies. Gaps in knowledge and recommendations for future research are discussed.
Advances in cancer treatment have led to high survival rates and prolonged the natural history of the disease. However, improved survival rates are mitigated by symptoms associated with treatments that lower the health-related quality of life (HRQOL) for survivors. Fatigue is one of the most commonly reported symptoms in cancer with a prevalence rate of 59% to 100% depending on the clinical status of the disease (Weis, 2011).
Cancer-related fatigue (CRF) is defined as a “distressing, persistent subjective sense of tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and that interferes with usual functioning” (Berger et al., 2010, p. 906). CRF considerably impacts the functional status and HRQOL of individuals by imposing physical limitations and psychological impairments (Curt et al., 2000). Although studies have been conducted to identify associations between immune and inflammatory markers with the severity and intensity of CRF, no causation between a specific biomarker and CRF has been established, contributing to its inadequate clinical management.
The diversity of factors that predispose to CRF suggests that it is a multidimensional symptom that has common related molecular pathways with multiple contributory mechanisms (Miaskowski, 2002). The current understanding about the etiology of CRF is based on limited evidence that environmental, genetic, psychological, and physiological factors play important roles in the impairment of oxygen supply, neuromuscular signaling, and the hypothalamus-pituitary-adrenal axis functioning. The goal of this literature review was to systematically review studies that evaluated immunogenomic markers and CRF in order to identify patterns of associations between these variables.
Methods
An initial generic search in PubMed (any date) using the following key words, “Fatigue AND cancer”[title] yielded 867 articles. A basic search query based on the terms from the 867 articles was developed. These key words/phrases include: (“Neoplasms”[Medical Subject Heading (Mesh)]) AND (“Fatigue/blood”[Mesh] OR “Fatigue/cerebrospinal fluid”[Mesh] OR “Fatigue/enzymology”[Mesh] OR “Fatigue/etiology”[Mesh] OR “Fatigue/genetics”[Mesh] OR “Fatigue/immunology”[Mesh] OR “Fatigue/pathology”[Mesh] OR “Fatigue/physiology”[Mesh] OR “Fatigue/physiopathology”[Mesh] OR “Fatigue/urine”[Mesh]. This basic search query yielded 1992 articles. The search was further refined, limiting the search using specific terms to include, (“Cytokines”[Mesh] OR “Receptors, Cytokine”[Mesh]) OR (“Antigens, Surface”[Mesh] OR “Receptors, Immunologic”[Mesh] OR “Immunologic Factors”[Mesh])) OR “Antigens”[Mesh]) OR “Lymphocytes”[Mesh]) OR “Inflammation”[Mesh]) OR “Interleukins”[Mesh]) OR “Leukocytes”[Mesh]) OR “Biological Markers”[Mesh]) OR (“Gene Expression/genetics”[Mesh] OR “Gene Expression/immunology”[Mesh] OR “Gene Expression/physiology”[Mesh])) OR “Genotype”[Mesh]) OR (“Immunity, Cellular/genetics”[Mesh] OR “Immunity, Cellular/immunology”[Mesh] OR “Immunity, Cellular/physiology”[Mesh]). This refined search yielded 348 articles. Review, editorial, case studies, and meta-analysis articles were excluded from the list, which reduced the number of indexed articles to 63. To retrieve recent, unindexed articles in PubMed, the following key words were used, Fatigue AND (Cancer OR neoplasms) AND protein* OR biomarker* OR receptor* OR genes OR gene OR genet* OR immunolog* (*truncated words). The search for unindexed articles was limited to articles published in the last 90 days. This search yielded 35 articles. The abstracts of these 98 articles (indexed=63, unindexed=35) were visually reviewed to determine if they met the following inclusion criteria: (a) tested a relationship between specific immune agents and CRF or (b) mentioned the function, concentration, and/or activity of an immune marker and its association with the increasing level, duration, or worsening in intensity of CRF.
Results
The 34 articles that met the eligibility criteria were reviewed. The earliest article appeared in 2001 and 73.5% (n = 25) of the articles were published from 2006 to the present. Eleven (32%) of the studies used longitudinal designs, while 23 (68%) were cross-sectional. Eighteen (53%) studies enrolled only women subjects, most with breast cancer (94%). Of these, 24% studied women in early stages of breast cancer and 65% studied breast cancer survivors (BCS). About 39% of studies (N = 7/18) investigating BCS were written by one research team who screened participants from the same subject data pool. Sixteen (47%) studies enrolled both men and women participants, with eight (50%) investigating participants with various types of cancer and seven (44%) focusing on terminal cases. One study enrolled pediatric patients (Vallance et al., 2010) and one study enrolled only male participants (Orre et al., 2009). Findings on the relationships between immunogenomic markers and fatigue are presented for the longitudinal and cross-sectional studies separately, according to five categories: systemic inflammatory markers, signals of immune response, concentrations of cytokines, markers of cytokine activity, and genomic markers.
Longitudinal studies
There were 10 studies that serially collected biologic samples from study subjects and explored the associations between levels of immunogenomic markers and fatigue at different time points. Seven of these studies showed significant associations between fatigue and an immunogenomic marker. Eleven different fatigue measures were used in the 10 longitudinal studies, with one study using multiple fatigue scales (Panju et al., 2009) and another using an interview to measure fatigue (Olson et al., 2002). Three of these fatigue questionnaires were administered and validated in non-English languages (Ahlberg et al., 2004; Geinitz et al., 2001; Reinertsen et al., 2011). No other questionnaire, except for the Functional Assessment of Cancer Therapy – Fatigue (FACT-F) was used twice, by two longitudinal studies (Panju et al., 2009; Wratten et al., 2004). The FACT-F is the most preferred instrument to measure CRF because it has been used extensively in large studies, has been shown to be sensitive to clinically significant changes in fatigue, and has robust psychometric properties (Minton et al., 2009). No other fatigue measure was used by more than one study.
In four of the ten longitudinal studies, subjects were followed during their radiation therapy (Ahlberg et al., 2004; Bower et al., 2009; Geinitz et al., 2001; Wratten et al., 2004), three studies followed subjects during chemotherapy (Mills et al., 2005; Panju et al., 2009; Vallance et al., 2010), and in one study, subjects were followed while they were receiving chemotherapy with or without radiation as adjuvant treatment (Olson et al., 2002). One study followed BCS pre and post stress testing (Bower et al., 2007), and another followed patients after completing their adjuvant therapy and 2–3 years after that initial assessment (Reinertsen et al., 2011). A number of studies controlled for covariates in data analyses. Body mass index (BMI) was the most commonly controlled covariate in the longitudinal studies, followed by depressed mood and age.
Systematic Inflammatory Markers
Four longitudinal studies evaluated associations of systemic inflammatory markers with levels of fatigue (Bower et al., 2007; Olson et al., 2002; Reinertsen et al., 2011; Wratten et al., 2004) and all used whole blood samples. Two of the longitudinal studies followed patients with early breast cancer stages during their radiation therapy (RT). Of these, one demonstrated a significant association between high levels of C-reactive protein (CRP) and increased fatigue duration (Bower et al., 2009) and the other study showed a significant association between high levels of CRP and fatigue at baseline but not during RT (Wratten et al., 2004). Significant association was also noted between higher fatigue levels and elevated neutrophil count prior to cancer treatment in patients with terminal cases (Olson et al., 2002) and during RT in women with early breast cancer (Wratten et al., 2004). An elevated monocyte count also was significantly correlated with higher fatigue levels at baseline and during RT after controlling for BMI (Wratten et al., 2004). Lymphocyte count did not show an association with fatigue levels (Wratten et al., 2004).
Signal of Immune Response
CD4+ lymphocyte was the only signal of immune response measured using a longitudinal design in patients with early stage breast cancer, and it showed a significant association with fatigue level (Bower et al., 2007). These systemic inflammatory markers and the signal of immune response are measures of cumulative activity of pro-inflammatory cytokines such as IL-6 (Bower et al., 2009). The positive associations between these systemic inflammatory markers, signal of immune response, and fatigue may reflect subclinical inflammatory changes related to the disease and/or treatment. Another possible explanation for these associations may be related to chance effect from the multiple analyses brought about by the use of several covariates.
Concentrations of Cytokines
Eleven cytokines were measured in eight longitudinal studies, with IL-6, TNF-α, and IL-1β being the most measured cytokines. These three pro-inflammatory cytokines showed inconsistent associations with fatigue levels; however, IL-6 levels from serum and blood cell supernatant samples of breast cancer patients with early disease showed positive significant associations with fatigue levels during RT (Wratten et al., 2004) and with induction of stress (Bower et al., 2007). However, plasma levels of IL-6 and fatigue symptoms were negatively associated in women with early stages of uterine cancer before and during their RT (Ahlberg et al., 2004). This significant association with fatigue levels was not observed when serum IL-6 levels were measured from cancer patients with terminal disease (Olson et al., 2002) nor in older individuals (>50 years) with acute myeloid leukemia (Panju et al., 2009), suggesting that the association of IL-6 and fatigue in these longitudinal studies is influenced by the stage and type of cancer and not the specific type of sample used. TNF-α was not associated with fatigue in three longitudinal studies that followed patients during cancer treatment (Ahlberg et al., 2004; Olson et al., 2002; Panju et al., 2009). Higher levels of IL-1β were associated with higher levels of fatigue in women with early stage breast cancer when stress was induced (Bower et al., 2007). However, a significant association between IL-1β and fatigue was not observed in a German study of women with breast cancer receiving RT (Geinitz et al., 2001), which might be related to the inability to find a change in fatigue score during RT using the German-version of the Fatigue Assessment Questionnaire (Geinitz et al., 2001). The negative associations found in the above mentioned studies may be related to the variability in laboratory methodologies used, limited biological half-life of cytokines, or insufficient diffusion of cytokines from peripheral sites into the blood stream (Fuchs et al., 1988).
Marker of Cytokine Activity
IL-1ra was the only marker of cytokine activity measured in the study by Bower and colleagues (2009), where it showed a positive significant association with fatigue levels. Pro-inflammatory cytokines such as IL-6 and IL-1β are known as key mediators of neuroimmune interactions, and are thought to play a role in the development of fatigue (Bower et al., 2009). These pro-inflammatory cytokines, especially the IL-1 family of cytokines (IL-1α, IL-1β) have also been suggested to play an important role in breast cancer progression (Miller et al., 2000; Pantschenko et al, 2003). Animal data strongly suggest that IL-1 is the crucial factor in determining the balance between immunity and inflammation in tumor progression (Voronov et al., 2010). The elevation of IL-1ra in the reviewed articles may be related to its role as a natural inhibitor of IL-1 and is necessary to counter the pro-metastatic activities of IL-1 and IL-6 (Apte et al., 2006).
Genomic Markers
Although there were two longitudinal studies that measured genomic markers, neither found a significant association between a genomic marker and fatigue (Reinertsen et al., 2011; Vallance et al., 2010). DNA extracted from peripheral blood cells of children with acute lymphoblastic leukemia treated with dexamethasone explored polymorphisms from 3 genes (AHSG, IL6, POLDIP3), which were found to be related to sleep disturbance but not to fatigue (Vallance et al., 2010). Single nucleotide polymorphisms (SNPs) of inflammation-related genes including, IL1β (rs16944), IL6 (rs1800795), IL6 receptor (rs4129267, rs4845617, rs2228145), and CRP (rs2794521) did not show significant correlations with persistent fatigue in BCS; however, the CRP gene SNP rs3091244 was associated with serum hsCRP level among persistent fatigued BCS (Reinertsen et al., 2011). The study by Vallance and colleagues (2010) study was the first to explore associations between genomic markers and fatigue in pediatric cancer population, therefore more investigation is necessary to validate the results. Reinertsen and colleagues (2011) grouped subjects into persistent fatigued, defined as having fatigue for more than 6 months in duration, much different than the proposed definition CRF (Cella et al., 2001). This study also collected samples at 2 time points that were two to three years apart, which should be considered in interpreting their results.
Summary
Findings from the longitudinal studies reveal that elevated fatigue symptoms especially of women with early stages of breast cancer were associated with high levels of neutrophil/monocyte, IL-1ra, and IL-6 during RT; as well as high levels of CD4+, IL-1β, and IL-6 with stressing stimuli. There were too few longitudinal studies that focused on terminal (Olson et al., 2002) and pediatric cases (Vallance et al., 2010) to identify a trend in association between CRF and immunogenomic markers. In addition, no associations were found between fatigue levels and most genomic markers (Reinertsen et al., 2011; Vallance et al., 2010). Findings from these longitudinal studies suggest that the relationship between fatigue levels and inflammation is complex and not easily discernible.
Cross-sectional studies
Twenty four studies collected biologic samples from subjects at one study time point and explored associations between the levels of immunogenomic markers and fatigue. Twenty two studies showed significant associations between fatigue and an immunogenomic marker. Of these 24 studies, 13 enrolled women with breast cancer and 46% of these 13 studies were conducted by one research team using participants from the same data pool of BCS (Bower et al., 2002; Bower et al., 2003; Bower et al., 2011a; Bower et al., 2011b; Collado-Hidalgo et al., 2006; Collado-Hidalgo et al., 2008). Five studies enrolled patients with terminal disease (Kwak et al., 2012; Inagaki et al., 2008; Minton et al., 2012; Rausch et al., 2010; Scheede-Bergdahl et al., 2012).
Fourteen different fatigue questionnaires were used in the cross-sectional studies and six of these were administered and validated in languages other than English. One non-English fatigue measure was author-developed (Inagaki et al., 2008) and reliability and validity had been established for it in a previous study (Okuyama et al., 2000). The vitality scale of the Medical Outcomes Study Short Form (SF)-36 was the most frequently used to categorize fatigued from non-fatigued subjects (Bower et al., 2002; Bower et al., 2003; Bower et al., 2011a; Collado-Hidalgo et al., 2006; Rausch et al., 2010). Although the SF-36 vitality scale has been found to have good internal consistency (0.85 – 0.87) using large samples, and a high test-retest reliability (0.80) over a two-week period, one concern is that this four-item scale with two items asking about energy and two asking about fatigue might be an inadequate representation of fatigue (O’Connor, 2004). Age was the most commonly controlled covariate in these cross-sectional studies. Other covariates controlled during statistical analyses included gender, body mass index (BMI), type of cancer treatment received, time since completion of cancer treatment, depression, and behavioral status such as smoking, caffeine, and alcohol uses.
Systemic Inflammatory Markers
Of the 24 cross-sectional studies, 13 evaluated associations of systemic inflammatory markers with levels of fatigue. White blood cells (Alexander et al., 2009; Bower et al., 2003; Kwak et al., 2012; Landmark-Høyvik et al., 2009; Orre et al., 2011; Paddison et al., 2009), CRP (Alexander et al., 2009; Booker et al., 2009; Bower et al., 2011b; Kwak et al., 2012; Minton et al., 2012; Orre et al., 2009; Orre et al., 2011; Schroecksnadel, 2007; Scott et al., 2002), and lymphocytes (Alexander et al., 2009; Bower et al., 2003; Collado-Hidalgo et al., 2006; Landmark-Høyvik et al., 2009; Paddison et al., 2009) were the most commonly measured. Results were inconsistent in regard to significant associations between levels of these systemic inflammatory markers and fatigue regardless of type and stage of cancer. WBC levels were significantly elevated in fatigued BCS (Alexander et al., 2009; Landmark-Høyvik et al., 2009; Orre et al., 2011) and in lung cancer patients with stage IIIb-IV disease (Paddison et al., 2009). However, this significant association was not observed in other studies that enrolled BCS (Bower et al., 2003) nor in a study of patients with various types of terminal cancers (Kwak et al., 2012). Although CRP levels were significantly elevated in patients with high fatigue symptoms in five studies (Alexander et al., 2009; Booker et al., 2009; Kwak et al., 2012; Orre et al., 2009; Schroecksnadel, 2007), two other studies did not find empirical support for this association (Bower et al., 2011b; Minton et al., 2012). Three studies also showed an association between high levels of lymphocytes and fatigue in BCS (Bower et al., 2003; Collado-Hidalgo et al., 2006; Landmark-Høyvik et al., 2009), but two other studies did not show similar significant associations between the two variables (Alexander et al., 2009; Paddison et al., 2009).
A common observation that may explain these inconsistent associations between levels of fatigue and systemic inflammatory markers was the type of covariates used in the analyses. Positive, significant associations between levels of fatigue and the systemic inflammatory markers (WBC, CRP, and lymphocytes) were generally found after controlling for covariates such age, gender, and time since completion of cancer treatment. However, significant associations, especially of fatigue with WBC and CRP, were not observed when BMI was added as a covariate to the statistical analyses (Bower et al., 2003, Bower et al., 2011; Kwak et al., 2012; Minton et al., 2012). Low tryptophan concentrations and high kynurenine/tryptophan ratio showed significant associations with high fatigue levels in patients with malignant disease (Schroecksnadel et al., 2007). Further investigation is necessary to determine the role of tryptophan, a precursor of serotonin in CRF, which can be valuable information for CRF management.
Signals of Immune Response
Four cross-sectional studies explored associations between levels of fatigue and signals of immune response, all in breast cancer survivors (Bower et al., 2002; Bower et al., 2003; Collado-Hidalgo et al., 2006; Von Ah et al., 2008). All these studies showed significant associations between levels of fatigue and signals of immune response. Although NK cell activities were noted to be lower in fatigued BCS (Bower et al., 2002; Von Ah et al., 2008), CD3+ and CD4+ T lymphocytes were higher in the same population in one study (Bower et al., 2003).
Concentrations of Cytokines
Seven cross-sectional studies explored the relationship between levels of fatigue and cytokines (Collado-Hidalgo et al., 2006; Kwak et al., 2012; Inagaki et al., 2008; Orre et al., 2009; Orre et al., 2011; Scheede-Bergdahl et al., 2012; Von Ah et al., 2008). The pro-inflammatory cytokine, IL-6, was the most common cytokine investigated. Higher plasma levels of IL-6 were significantly associated with fatigue symptoms in cancer patients with terminal disease (Inagaki et al., 2008). IL-6 production was also elevated in an ex-vivo experiment of stimulated monocytes from fatigued BCS; however, plasma IL-6 levels from the same subjects were not significantly different from non-fatigued BCS samples (Collado-Hidalgo et al., 2006). Four other cross-sectional studies did not find significant associations between levels of fatigue and IL-6 (Kwak et al., 2012; Orre et al., 2009; Orre et al., 2011; Scheede-Bergdahl et al., 2012).
The inconsistency in association between the levels of IL-6 in the blood and fatigue might be related to the fatigue measure used. Although an author-developed questionnaire showed significant association between these two variables, the rest of the cross-sectional studies using more psychometrically sound scales failed to document an association. TNF-α was the other cytokine that was most measured among the cross-sectional studies and its blood level did not show an association with fatigue in three studies (Kwak et al., 2012; Scheede-Bergdahl et al., 2012; Von Ah et al., 2008).
Markers of Cytokine Activity
Four cross-sectional studies investigated the association between levels of fatigue and markers of cytokine activity. Three were written by one research team who screened participants from the same data pool of BCS (Bower et al., 2002; Bower et al., 2011; Collado-Hidalgo et al., 2006). IL-1ra and sTNF-RII were the two signals of immune response that were most measured in these eight cross-sectional studies. Inconsistent results in the association between levels of fatigue and these two signals of immune response might be related to confounders adjusted during analysis. The positive, significant association between levels of fatigue and IL-1ra persisted even after controlling for age, BMI, depressive symptom scores, time since completion of cancer treatment (Collado-Hidalgo et al., 2006) and behavioral factors such as smoking, caffeine, and alcohol use (Bower et al., 2002). However, these positive, significant associations between fatigue scores with IL-1ra and sTNF-RII were not observed when ethnicity, menopausal status (Collado-Hidalgo et al., 2008), and educational level (Orre et al., 2011) were added as covariates. One study showed a positive association between level of fatigue and sTNF-RII in breast cancer survivors who received chemotherapy as their primary cancer treatment or initial therapy to treat their cancer, but not in those who received other cancer treatments as primary therapy (Bower et al., 2011b).
Genomic Markers
Seven cross-sectional studies exploring associations between levels of fatigue and genomic markers showed positive, significant associations between these markers and fatigue (Aouizerat et al., 2009; Bower et al., 2011a; Collado-Hidalgo et al., 2008; Fernandez-de-las-Penas et al., 2011; Landmark-Høyvik H et al., 2009; Miaskowki et al., 2010; Rausch et al., 2010). TNF and IL-6 alleles extracted from DNA of archived buffy coat samples were significantly associated with fatigue levels. Both studies used the same sample population. Common, homozygous (AA) alleles of IL-6 were associated with higher levels of evening and morning fatigue symptoms among oncology patients and their family caregivers (Miaskowski et al., 2010). Higher morning fatigue, but not evening fatigue was also noted with homozygous (GG) alleles of the TNF-α gene in the same subjects (Aouizerat et al., 2009). SNPs of several cytokines including IL-1β (rs1143633, rs2853550), IL-1RN (rs397211, rs4252041), and IL-10 (rs1878672, rs3021094) showed significant associations with fatigue levels in lung cancer survivors (Rausch et al., 2010). PLOD1 (procollagen-lysine 1, 2-oxoglutarate 5-dioxygenase 1), a gene involved in glycoprotein metabolism, and the NPCDR1 gene (nasopharyngeal carcinoma, downregulated 1) were differentially expressed in fatigued BCS (Landmark-Høyvik H et al., 2009). Overrepresentation of CC alleles in the IL1B-511 (C/T) polymorphism and elevated occurrence of homozygosity for both the variant C allele and the wild type G allele of the IL6 – 174 (G/C) polymorphism were found to be independent predictors of CRF among breast cancer survivors (Collado-Hidalgo et al., 2008). Genes associated with activation of inflammatory cytokines, chemokine signaling, activation of transcriptions and vascular growth factor, as well as NF-κB response were differentially expressed in fatigued BCS (Bower et al., 2011a). Furthermore, genotypic characterization of BCS showed that specific catechol-O-methyltransferase (COMT) genotypes (Valine (Val)/Methionine (Met) and Met/Met) were significantly correlated with higher fatigue scores compared to survivors with Val/Val genotype (Fernandez-de-las-Penas et al., 2011).
Summary
Findings from the cross-sectional studies demonstrate that elevated fatigue symptoms had positive, significant associations with systemic inflammatory markers such as WBC, CRP, and lymphocytes, however, these significant associations failed to persist after including BMI as a covariate in the analyses (Bower et al., 2003, Bower et al., 2011; Kwak et al., 2012; Minton et al., 2012). Moreover, associations between levels of fatigue and signals for immune response (IL-1ra, sTNF-RII) also failed to persist when ethnicity, menopausal status, and educational level were added as covariates to the statistical analyses (Collado-Hidalgo et al., 2008, Orre et al., 2011), and when these signals were measured in BCS who received primary cancer treatments other than chemotherapy (Bower et al., 2011b). A number of studies did not find significant associations between levels of fatigue and the pro-inflammatory cytokine, IL-6 (Kwak et al., 2012; Orre et al., 2009; Orre et al., 2011; Scheede-Bergdahl et al., 2012). A marker of IL-6 activity (IL-6R) was found to be elevated in stimulated cells from fatigued BCS (Bower et al., 2002).
All genomic markers explored by the seven cross-sectional studies showed positive, significant associations with fatigue (Aouizerat et al., 2009; Bower et al., 2011a; Collado-Hidalgo et al., 2008; Fernandez-de-las-Penas et al., 2011; Landmark-Høyvik H et al., 2009; Miaskowki et al., 2010; Rausch et al., 2010). Results from cross-sectional immunogenomic studies provide a pattern that subclinical inflammation and immune dysregulation are observed in patients who had a tendency to get fatigued. However, because of the research design used, these findings fell short in identifying whether the experience of fatigue was related to cancer and/or its treatment. Table 1 summarizes the studies demonstrating significant associations between CRF and immunogenomic markers and Table 2 reports on the studies that did not find empirical support for such an association.
Table 1.
Studies With Significant Associations Between Immunogenomic Markers And CRF
| Longitudinal Studies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Cancer | Sample | Exclusion | Control | Intervention | Measure | Data Collection | Marker | p value | Association | Covariates | Direction of Association |
| Ahlberg et al., 2004 | Uterine, 86% stage I, post hysterectomy | n = 15 | Dementia, history of psychiatric disorder | None mentioned | Radiation Therapy (RT) | Multidimensional Fatigue Inventory (MFI) (Swedish) | Plasma, pre RT, post 30 Gy (+3 wks), post RT (46 Gy, or +5–6 wks), 1 week post RT | Interleukin (IL)-6 | 0.006 | r = −0.65 | Positive, significant correlation between IL-6 and fatigue from baseline to 3 weeks into RT | |
| 0.04 | r = −0.54 | Positive, significant correlation between IL-6 and fatigue from baseline to end of RT | ||||||||||
| Bower et al., 2007 | Breast, stages 0, I, or II | n = 10 (fatigued) | Cancer recurrence, diagnosis with other cancer, history of immunologic or hormonal disease, current medical illness, heavy alcohol use | n = 15 (non-fatigued) | Survivors | Short Form (SF)36– vitality scale | Lymphocytes extracted at baseline, post stress, after 30 minutes of recovery. | CD4+ | 0.024 | F (2,46) = 4.0 | Time between blood draw, age, marital status, cancer treatment, body mass index (BMI), and depressed mood score | Higher level and greater increase in number during stress test in fatigued group |
| Blood supernatant at baseline, post stress, after 30 minutes of recovery. | IL-6 | 0.006 | F (1,20) = 9.3 | Increase in lipopolysaccharide (LPS)-stimulated production during stress test in fatigued group | ||||||||
| IL-1β | 0.02 | F(1,20) = 6.1 | Increased from baseline to recovery from stress test in fatigued group. | |||||||||
| Bower et al., 2009 | Breast (stage 0, I, II); prostate (T1-Tumor 3, Node 0, Mets 0) | n = 28 (breast); n = 20 (prostate) | Tobacco use, recurrent cancer, previous/planned chemo, immunosuppressant use, active illness/infection | Baseline values for each patient | RT | Fatigue Symptom Inventory (FSI) | Serum drawn at baseline, 4 time points during RT, 2 time points post RT | C-reactive protein (CRP) | 0.022 | β = 0.32, SE = 0.14 | Sleep disturbance, depressive symptoms, age, body mass index, hormone therapy | Higher with increased fatigue duration |
| IL-1ra | 0.016 | β = 0.63, SE = 0.26 | Higher with increased fatigue severity | |||||||||
| Mills et al., 2005 | Breast, stages 1–IIIA | n = 29 (Same subjects used by Aouizerat et al., 2009) | Undergoing bone marrow transplants, metastatic breast cancer, confounding illness (e.g. renal failure, pre-existing anemia) | none | Chemo (adjuvant or neoadjuvant) | Multidimensional FSI (MFSI) | Plasma before chemo cycles 1 and 4; 2.5 months between plasma collection | Vascular Growth factor (VEGF) | < 0.03 | beta = 0.468 | Depression, stage of disease | Higher levels predicted high fatigue in the 4th cycle of chemo, but no association with fatigue when depression was added. |
| Olson et al., 2002 | Colon, rectal, Small cell lung cancer (SCLC), NSCLC (stages IIIb–IV) | n = 18 | None mentioned | none | Chemo and/or RT (for rectal and SCLC patients) | Interview | Serum before treatment, midtreatment, 3 and 6 months post treatment | Absolute neutrophil | 0.01 | r = 0.737 | None mentioned | Higher levels associated with high fatigue pre-chemo in colon cancer patients |
| Reinerts en et al., 2011 | Breast, stages II and III | Timepoint 1 (T1)=302, Timepoint 2 (T2)=236 (175 eliminated for reporting fatigue only in 1 time point). Fatigued, n=55 | Breast cancer recurrence, other cancer (except melanoma, ovarian cancer in situ), depression in T1 | None fatigue d, n =120, | None | Fatigue Question naire (FQ - Norwegian) | Deoxyribonucleic acid (DNA) from peripheral blood, blood drawn 2–3 years apart for 2 timepoints | hsCRP | 0.03 | OR = 1.11, 95% CI (1.01 – 1.21) | Treatment strategies; subset of subjects: BMI, treatment-area related fibrosis | Higher level in patients with non-depressed, chronic fatigue at T1 and subset analysis (higher with persistent fatigue after controlling for covariates) |
| Wratten et al., 2004 | Breast, early stage | n = 52 | Severe current illness, history of major breast surgery, history of breast irradiation treatment, metastatic disease | None mentioned | Adjuvant RT post surgery | Functional Assessment of Cancer Therapy –Fatigue (FACT-F) | Blood before RT, weekly during RT, then 2 & 6 weeks post RT | Neutrophil | Baseline (p=0.03,), week 5 (p=0.01) | Baseline (r=− 0.315), week 5 (r=−0.381) | BMI | Fatigue correlated with higher neutrophil level at baseline & week 5 of RT |
| Monocyte | baseline (p=0.05), week 5 (p=0.01) | baseline (r= −0.289), wk5 (r=−0.394) | Fatigue correlated with higher monocyte level at baseline & week 5 of RT | |||||||||
| CRP | p<0.01 | r=−0.456 | Fatigue correlated with higher CRP at baseline | |||||||||
| Serum before RT, weekly during RT, then 2 & 6 weeks post RT | Fibroblast growth factor β | 0.04 | Not given | Decreased in non-fatigued subjects at week 5 of RT | ||||||||
| IL6 | baseline (p=0.05), wk5(p=0.03) | baseline (r= −0.322), wk5(r= −0.367) | Fatigue correlated with higher IL6 at baseline and week 5 of RT | |||||||||
| Intercellular adhesion molecule -1 (ICAM-1) | p=0.04 | r= −0.311 | Fatigue correlated with higher ICAM-1 at baseline | |||||||||
| Cross-sectional Studies | ||||||||||||
| Authors | Cancer | Sample | Exclusion | Control | Intervention | Measure | Design | Marker | p value | Association | Covariates | Direction of Association |
| Aouizerat et al., 2009 | Various, non-metastatic | n = 185 | Metastatic disease, more than one cancer diagnosed, diagnosed sleep disorder | n = 103 (family caregivers) | RT | Lee Fatigue Scale (LFS) | Archived buffy coat DNA | Homozygous allele of TNFA genotype (i.e., GG) | 0.02 | t = −2.22 | age, TNFA genotype | Higher in patients with morning fatigue |
| Alexander et al., 2009 | Breast, stages 1–IIb | n = 60 (fatigue) | Pregnancy, other cancer, recurrent disease, confusion, dementia | n = 104 (non-fatigued) | Survivors, 3 months -2 year post primary therapy | Brief Fatigue Scale | Peripheral Blood | White blood cell (WBC) | 0.021 | Not given | None mentioned | Higher in fatigued group |
| CRP | 0.015 | Not given | Higher in fatigued group | |||||||||
| Basophil | 0.04 | Not given | Higher in fatigued group | |||||||||
| Booker et al., 2009 | Multiple myeloma, stages I, II, III | n = 56 | Other plasma cell dyscrasia | None | None | FACT-F | Peripheral blood | CRP | 0.034 | β = −0.350 | None mentioned | Significant predictor of fatigue using FACT-F |
| Quality of Life Questionnaire (QLQ)-C30 | 0.003 | β = 0.514 | Significant predictor of fatigue using QLQ-C30 | |||||||||
| Bower et al., 2002 | Breast, recruited from a pool of 332 survivors who met eligibility criteria used by Bower et al., 2006 | n = 20 (fatigued) | Change in energy level between 2 assessments, cancer recurrence, other cancer, comorbid medical problem, immune disease, on immunosuppressant, psychiatric hospitalization in past 6 months, heavy alcohol use | n = 20 (non-fatigued) | Survivors recruited from 1994–1997; 5 years from diagnosis | RAND36, FSI | Serum taken between 8–10 am, fasting/no alcohol/caffeine/smoking 12 hours before draw | IL-1ra | 0.006 | 95% CI = 2% to 89% | caffeine, alcohol use, smoking | 46% more in fatigued group |
| Soluble tumor necrosis factor (TNF)-RII | 0.005 | 95% CI = 6% to 59% | 18% more in fatigued group | |||||||||
| Neopterin | 0.018 | 95% CI = 1% to 34% | 33% more in fatigued group | |||||||||
| Natural killer (NK) cells | < 0.05 | F(1,33) = 4.33 | Smoking | Lower percentage in fatigued group | ||||||||
| CD45RO:CD45DA | 0.05 | F(1,33) = 4.01 | Higher ratio in fatigued group | |||||||||
| Bower et al., 2003 | Breast, recruited from a pool of 332 survivors who met eligibility criteria used by Bower et al., 2002 | n = 19 (fatigued) | Recurrence of breast cancer, history of immune disease | n = 18 (non-fatigued) | Survivors recruited from 1994–1997 | RAND SF36 fatigue scale grouped subjects to fatigued and non-fatigued during 2 assessments | Lymphocytes from fasting blood | Lymphocytes | 0.011 | 95% CI = 7% to 49%, | age, income, ethnicity, BMI, depressed mood, cancer treatment | 28% more in fatigued group |
| CD3+ | 0.015 | 95% CI = 6% to 56% | 31% more in fatigued group | |||||||||
| CD4+ | 0.003 | 95% CI = 15% to 68% | 41% more in fatigued group | |||||||||
| CD3+/CD56+ | 0.027 | 95% CI = 4% to 99% | 52% more in fatigued group | |||||||||
| Bower et al., 2011a | Breast (stage 0,I, II); selected from same pool of subjects identified by Collado-Hidalgo et al., 2006. | n = 11 (fatigue d), < 40 score with SF-36 fatigue scale for 2–3 assessments | Cancer recurrence, immune disease, immunosuppressant use | n = 10 (non-fatigued), >70 in SF-36 fatigue scale for 2–3 assessments | Survivors, 1–5 years post diagnosis, 21– 65 years of age | SF-36 vitality scale | Peripheral blood mononuclear cells | IL1A, IL1B, IL6, OSM, GZMH | <0.001 | Not given | Age, time since diagnosis and cancer treatment prior to gene analyses | Upregulated in fatigued group (>30% difference) |
| CXCL2, CXCR5, CCL20, CMKLR1 | <0.001 | Not given | Upregulated in fatigued group (>30% difference) | |||||||||
| IER3, ZNF331, NR4A2, NR4A3 | <0.001 | Not given | Upregulated in fatigued group (>30% difference) | |||||||||
| VEGFA, TRGC2, TIGIT, CX3CR1 | <0.001 | Not given | Upregulated in fatigued group (>30% difference) | |||||||||
| NLRC4, HP, CROP, MGAM | <0.001 | Not given | Upregulated in fatigued group (>30% difference) | |||||||||
| NF-κB response elements in promoters of upregulated genes | <0.0001 | 2.28-fold difference ±0.09 | Promoter upregulated in fatigued group (across nine combinations of promoter length) | |||||||||
| Glucocorticoid response elements in promoters of upregulated genes | <0.007 | 0.45-fold difference ±0.07 | Promoter under represented in fatigued group (across nine combinations of promoter length) | |||||||||
| Proinflammatory transcription factor-binding motifs in the promoters of upregulated genes | 0.041 | 2.72 fold increase | Upregulated in fatigued group | |||||||||
| Bower et al., 2011b | Breast, stages 0–IIIA, selected from same cohort as Bower et al., 02; Bower et al., 2003; Bower et al., 2011a; Collado-Hidalgo et al., 2006 | n = 103 | Neurologic or immune disease, smokers | None mentioned | Completed primary treatment within 3 months and not started on endocrine treatmen | FSI | Plasma | sTNF-RII | 0.036 | Not given | Exposure to RT, age, time since treatment completion, BMI | Higher levels associated with higher fatigue scores with subjects treated with chemotherapy only. |
| Collado-Hidalgo et al., 2006 | Breast (stages 0,I, II); recruited from same pool of subjects used by Bower et al., 2002 and Bower et al., 2003 | n = 32 (fatigued), < 50 score with SF-36 fatigue scale | Cancer recurrence, immune disease, immunosuppressant use, psychiatric disease, smokers, alcohol use, SF-36 fatigue score 50–70, advanced cancer stage, >5 years post treatment | n = 18 (non-fatigued), score >70 in SF-36 fatigue scale | Survivors, 1–5 years post diagnosis | SF36–vitality scale grouped subjects to fatigued and non-fatigued during 2–3 assessments | Plasma | IL-1ra | 0.05 | t (48)= −1.53 | Age, BMI, time since treatment, treatment mode, depressive symptom scores | Higher level in fatigued group |
| sIL-6R | < 0.001 | t (44) = −4.07 | Higher level in fatigued group | |||||||||
| Monocytes | IL-6 (ex vivo) | 0.049 | t (45) = −1.813 t = −1.983 t (29) = 2.195 | Increased ex-vivo monocyte production in fatigued group after LPS exposure | ||||||||
| TNF-α (ex vivo) | 0.03 | Increased ex-vivo monocyte production in fatigued group after LPS exposure | ||||||||||
| IL-6R (in vivo) | 0.03 | Lower levels on CD14+ cells in fatigued group after exposure of monocyte to toll-like receptor (TLR) 4 ligand LPS | ||||||||||
| Peripheral blood mononuclear cells (PBMCs) | Lymphocytes | Not given | Increased % as fraction of total leukocyte in fatigued group with selective increase in frequency of CD4 T lymphocytes. | |||||||||
| Myeloid dendritic cells (HLA-DR+/CD11c+/CD14 dim) | 0.04 | t (29) = 2.047 | Decreased frequency in circulating dendritic cells from fatigued group | |||||||||
| Activated T lymphocytes (CD3+/CD69+) | 0.04 | t (29) = 1.077 | Decreased frequency in circulating activated T lymphocytes from fatigued group | |||||||||
| Healthy blood | IL-6R (in vitro) | <0.01 for IL6 and IL1β; 0.23 for TNFα; 0.0006 for IL6, IL1β, TNFα | t (4) = 4.12 for IL6; t (4) = 4.51 for IL1β; t (4) = 1.36 for TNFα; t (4) = 5.18 for all 3 cytokines | Lower IL6R from cell surface of PBMCs of fatigued group after exposure to 3 cytokines | ||||||||
| Collado-Hidalgo et al., 2008 | Breast (stage 0,I, II); same pool of subjects used by Bower et al., 2002; Bower et al., 2009; subset used by Collado-Hidalgo et al., 2006 | n = 33 (fatigue d), SF36 fatigue score <55 | Cancer recurrence, immune disease, on immunosuppressant | n = 14 (non-fatigued), SF36 fatigue score >70 | Survivors, 1–5 years post diagnosis | MFSI | Leukocytes from peripheral blood | IL1B-511– CC alleles | 0.008 | None given | Age, ethnicity, menopausal status, BMI, depressive symptoms, cancer treatment | Overrepresentation in fatigued group. |
| IL1B-511 – TT alleles | 0.008 | None given | Underrepresentation in fatigued group. | |||||||||
| IL1B-511 – cytosine | 0.052 | 95% CI = 0.91 to 16.6 | Higher prevalence in fatigue group | |||||||||
| IL6 – 174 (G/C) | 0.024 | 95% CI = 1.12 to 17.9 | Elevated homozygosity in variant C and wildtype G alleles noted in fatigued group except when covariants were controlled. | |||||||||
| Plasma | sIL-6R | 0.028 | None given | Higher levels in fatigued group | ||||||||
| Fernandez-de-las-Penas et al., 2011 | Breast, stage I–IIIA | n = 128 (34 Val/Val, 64 Val/Met, 30 Met/Met) | Active cancer, receiving chemo/RT, breast surgery for cosmetic purpose, inflammatory disease, recurrent cancer, fibromyalgia | Val/Val versus Val/Met versus Met/Met genotypes | Survivors treated with RT and chemo (from 6/2009 to 3/2011), 36–65 years old | Piper Fatigue Scale (PFS-Spanish | Genomic DNA extracted from saliva cell sediments | COMT genotypes Val/Met and Met/Met: | < 0.01 | Not given | Pain intensity | Significantly correlated with higher fatigue scores than those with Val/Val genotype. |
| Inagaki et al., 2008 | Various, terminal cases recruited from 10/1997–11/1999 | n = 27 (fatigued) | Receiving curative cancer treatment, too ill to answer, cognitive impairment, non-Japanese speakers, only included those that died 6 months after 1st assessment, taking NSAIDS and steroids | n = 19 (non-fatigued) | None | Cancer Fatigue Scale (CFS - Japanese – author developed) | Plasma | IL-6 | 0.01 | β = 0.38 | Gender, weight, survival time | Higher levels in fatigued group (correlated w/physical subscale score, but not with total, affective & cognitive scores). |
| Kwak et al., 2011 | Various, terminal cases recruited from 6/2009–7/2010; survival time is less than 6 months | n = 90 (mild= 23, modera te = 30, severe =37) | Cognitive impairment, chemo/RT to treat active cancer, hematologic malignancies, use of psychostimulant, h/o psychiatric disease, fever, use of antibiotics and antiepileptics, high TSH | Mild versus moderate versus severe categories of fatigue | None | Brief Fatigue Inventory (BFI -Korean) | Peripheral blood collected within 24 hours from enrolment between 9–11 am | CRP | 0.005 | r = 0.29 | Age, gender, BMI, cancer site, previous cancer treatment, comorbid disease, pain score, sleep disorder, dyspnea | Higher levels in more severe categories of fatigue |
| Landmark-Høyvik H et al., 2009 | Breast (stage II, III, unclassifiable) | n = 403 | Cancer recurrence, >75 years old, other cancer except basal cell cancer or cancer in situ, prior surgery for contralateral breast cancer stage I with no adjuvant therapy | Low fatigue | Survivors, treated from 1998–2002 with adjuvant RT | FQ (Norwegian), responses categorized into high and low fatigue | Peripheral blood drawn in 2004 | Leukocytes | 0.0016 | Not given | None mentioned | Higher in fatigued, non-depressed group |
| Neutrophils | 0.0059 | Not given | Higher in fatigued, non-depressed group | |||||||||
| Lymphocytes | 0.0046 | Not given | Higher in fatigued, non-depressed group | |||||||||
| Ribonucleic acid (RNA) from blood collected using PAX tube | PLOD1 | False Discovery Rate (FDR) < 0.20 | Not given | Downregulated in fatigued group, regardless of depression status | ||||||||
| NPCDR1 | FDR < 0.20 | Not given | Upregulated in fatigued, non-depressed group | |||||||||
| Meyers et al., 2005 | Acute myelogenous leukemia or myelodys plastic syndrome | n = 54 (baseline), n= 26 (1 month of treatment) | None mentioned | Normal control (no N given) | Chemo | BFI | Serum drawn at baseline, fatigue measure at baseline and 1 month of treatment | IL-6 | Not given | r = 0.62 | None given | Higher levels associated with higher fatigue scores |
| IL-IRA | Not given | r = 0.52 | Higher levels associated with higher fatigue scores | |||||||||
| TNF-α | Not given | r = 0.41 | Higher levels associated with higher fatigue scores | |||||||||
| Miaskowski et al., 2010 | Various, non-metastatic | n = 185 | Metastatic disease, more than one cancer diagnosed, diagnosed sleep disorder | n = 103 (family caregivers) | RT | LFS | Archived buffy coat DNA | IL-6 AA genotype | 0.001 | 3.8% | Age, gender, IL-6 genotype | Associated with higher mean evening fatigue scores |
| Orre et al., 2009 | Testicular | n = 92 (chronic fatigue) | Mental retardation, extragonadal germ cell malignancy (except skin), removal of non-affected testicle related to benign condition, concurrent infection or inflammation | n = 191 (without chronic fatigue) | Survivors, recruited from 1980–1998 database, 18–75 years old | FQ (Norwegian) | Plasma drawn from 0800-12 noon; subjects allowed light breakfast | IL-1ra | < 0.01 | r = 0.18 | Age, BMI, smoking, anxiety, depression, neuroticism | Higher in fatigued group, but lost significance when adjusted for BMI |
| CRP | < 0.05 | r = 0.16 | Higher in fatigued group but lost significance when adjusted for behavior (smoking) | |||||||||
| Orre et al., 2011 | Breast (stage II, III, unclassifiable). | n = 299 | Cancer recurrence, >75 years old, other cancer except basal cell cancer or cancer in situ, prior surgery for contralateral breast cancer stage I with no adjuvant therapy | None | Survivors, treated from 1998–2002 with adjuvant RT; subsample of Reinersten et al., 2011 & Landmark-Høyvik H et al., 2009 | FQ (Norwegian) | Blood drawn from 0900-12 noon; subjects allowed light breakfast | Leukocytes | <0.018 | Beta = 0.014 | Age, educational level | Higher levels associated with higher fatigue scores but lost significance when adjusted for covariates (p = 0.78) |
| Serum | High sensitivity CRP | < 0.001 | Beta = 0.26 | Associated with total fatigue even after controlling for age and education (p = 0.02) | ||||||||
| Paddison et al., 2009 | NSCLC, Stages IIIb and IV | n = 44 | None mentioned | None | None | Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) – 2 items, 1 item from Hamilton Depression scale | Retrospective extraction of clinical results (peripheral blood) | WBC | 0.01 | R2 = 0.27, β = 0.41 | Age, gender, time since treatment completion, hemoglobin | Increased with higher fatigue |
| Neutrophil | 0.01 | R2 = 0.28, β = 0.43 | Increased with higher fatigue | |||||||||
| Monocyte | 0.05 | R2 = 0.20, β = 0.31, | Increased with higher fatigue | |||||||||
| Rausch et al., 2010 | Lung, stages I–IV | n = 1149 | None mentioned | None mentioned | None | Lung Cancer Symptom Scale, (LCSS) | DNA from peripheral blood | IL-1B rs1143633 | Not given | OR estimate = 1.00 – 1.02 | Age at diagnosis, sex, smoking status, disease stage, treatment modality | Increased with higher fatigue |
| IL-1B rs2853550 | Not given | OR estimate = 1.01 – 1.06 | Increased with higher fatigue | |||||||||
| IL-1RN rs397211 | Not given | OR estimate = 0.97 – 1.00 | Increased with higher fatigue | |||||||||
| SF-8 | IL-10 rs3021094 | Not given | OR estimate = 1.02 – 1.18 | Increased with higher fatigue | ||||||||
| IL-10 rs1878672 | Not given | OR estimate = 0.91 – 0.94 | Increased with higher fatigue | |||||||||
| IL-1RN rs4252041 | Not given | OR estimate = 0.80 – 0.97 | Increased with higher fatigue | |||||||||
| Schroecks nadel et al., 2007 | Various | n = 146 (stable/remitting = 60; progressing = 86) | None mentioned | None mentioned | None | Fatigue scale | Serum | Neopterin | <0.01 | rs = 0.274 | Increased with high fatigue | |
| Tryptophan | <0.05 | rs = −0.179 | Lower concentration associated with increase fatigue | |||||||||
| kyn/trp | <0.01 | rs = 0.276 | High ratio with high fatigue | |||||||||
| CRP | <0.001 | rs = 0.375 | Increased with high fatigue | |||||||||
| Erythrocyte sedimentation rate (ESR)-1 | <0.01 | rs = 0.234 | Increased with high fatigue | |||||||||
| ESR-2 | <0.01 | rs = 0.241 | Increased with high fatigue | |||||||||
| Scott et al., 2002 | Inoperable NSCLC, Stages III and IV | n = 106 | Infection | None | None | QLQ-C30 | Peripheral blood collected from 1/1995–11/1998 | CRP | 0.011 | Not given | None mentioned | High levels associated with high fatigue scores |
| Von Ah et al., 2008 | Breast, stage 0–IIIa | n = 44 | Psychiatric disorder, dementia, history or current substance abuse, thyroid issues, immune disorders, immunosuppression, other cancer except noninvasive type | None mentioned | Adjuvant chemo + RT | Revised Piper Fatigue Scale (rPFS) | Blood collected 9–33 days post surgery (lumpectomy or mastectomy) before adjuvant therapy | IL-1β | ≤ 0.01 | r = 0.32 | Type of adjuvant therapy, mood, network support, satisfaction, cortisol, perceived stress, optimism | Higher levels associated with high fatigue scores |
| Mononuclear cells collected 9-33 days post surgery (lumpectomy or mastectomy) before adjuvant therapy | NK cell activity | ≤ 0.01 | r ≥ 0.20 | Lower levels associated with high fatigue scores | ||||||||
OR = odds ratio, kyn/trp = kynurenine to tryptophan ratio
Table 2.
Studies With Non-Significant Associations Between Immunogenomic Markers And CRF
| Longitudinal Studies | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Cancer | Sample | Exclusion | Control | Intervention | Measure | Data Collection | Marker | p value | Association | Covariates |
| Ahlberg et al., 2004 | Uterine, 86% stage I, post hysterectomy | n = 15 | Dementia, history of psychiatric disorder | None mentioned | Radiation Therapy (RT) | Multidimensional Fatigue Inventory (MFI) (Swedish) | Plasma, pre RT, post 30 Gy (+3 wks), post RT (46 Gy, or +5–6 wks), 1 week post RT | Tumor necrosis factor (TNF)-α | 0.51 (baseline - 30 Gray), 0.50, (baseline to 46 Gray) | r= 0.20 (baseline - 30 Gy), r = −0.19 (baseline to 46 Gy) | None mentioned |
| Interleukin (IL)-1 | Not given | Low concentration, cannot estimate | |||||||||
| Geinitz et al., 2001 | Breast, no stage specified | n = 41 | Metastatic disease, chemo with RT, 2nd cancer, inflammatory disease, thyroid disease, history of depression, use of tranquilizers, streroids, non-steroidal | None | Adjuvant RT (27–120 days post surgery) | Fatigue Assessment Questionnaires (FAQ), German version | Serum pre RT, end of weeks 1–5 during RT, 2 months post RT | IL-1β | Not given | No association with fatigue | None mentioned |
| IL-6 | |||||||||||
| TNF-α | |||||||||||
| Olson et al., 2002 | Colon, rectal, Small cell lung cancer (SCLC), NSCLC (stages IIIb–IV) | n = 18 | None mentioned | None | Chemo and/or RT (for rectal and SCLC patients) | Interview | Serum before treatment, midtreatment, 3 and 6 months post treatment | IL-6 | 0.12–0.62 | r = 0.19–0.61 | None mentioned |
| TNF-α | Not given | Not given | |||||||||
| Panju et al., 2009 | Acute myeloid leukemia, within 1 year of diagnosis | Timepoint (T)1=34 (23 Male, 11 Female), T2=28 | Other cancer, hematopoietic stem-cell transplantation, taking growth factors | None | Chemo or supportive care | Functional Assessment of Cancer Therapy –Fatigue (FACT-F) and Edmonton Symptom Assessment System (ESAS) fatigue severity | Serum T1 (pre treatment, between treatment, upon completion of full treatment); T2 (4–6 weeks post T1) | Interferon (IFN)-γ | Not given | No association with fatigue level and severity | None mentioned |
| IL-2 | Not given | ||||||||||
| IL-8 | Not given | ||||||||||
| TNF-α | Not given | ||||||||||
| Monocyte chemotactic protein-1 | Not given | ||||||||||
| FACT-F and Quality of Life Questionnaire (QLQ)-C30 and ESAS | IL-5 | 0.08 (FACT-F), 0.07 (QLQ-C30) | r=0.33 (FACT-F), r=0.34(QLQ-C30) | ||||||||
| IL-6 | 0.059 | r=0.332 | |||||||||
| IL-10 | 0.09 | r=0.33 | |||||||||
| ESAS fatigue severity | Monokine induced by IFN-γ | Not given | Strongest trends of change across changes in fatigue severity | ||||||||
| IL-4 | Not given | ||||||||||
| Reinerts en et al., 2011 | Breast, stages II and III | Timepoint 1 (T1)=302, Timepoint 2 (T2)=236 (175 eliminated for reporting fatigue only in 1 time point). Fatigued, n=55 | Breast cancer recurrence, other cancer (except melanoma, ovarian cancer in situ), depression in T1 | None fatigue d, n =120, | None | Fatigue Questionnaire (FQ - Norwegian) | Deoxyribonucleic acid (DNA) from peripheral blood, blood drawn 2–3 years apart for 2 timepoints | IL6R mRNA | 0.59–0.92, subset = 0.57–0.7, T2= 0.6–0.64 | No association with fatigue | Treatment strategies; subset of subjects: BMI, treatment-area related fibrosis |
| IL1β mRNA | 0.59–0.92, subset = 0.57–0.8 | ||||||||||
| IL1Brs1694 4 (A/G) | Not given | ||||||||||
| IL6Rrs4129 267 (C/T) | |||||||||||
| IL6Rrs4845 617 (A/G) | |||||||||||
| IL6Rrs2228 145 (A/C) | |||||||||||
| IL6Rrs1800 795 (G/C) | |||||||||||
| CRPrs2794 521 (C/T) | |||||||||||
| CRPrs3091 244 (A/G/T) | |||||||||||
| Vallance et al., 2010 | Acute Lymphoblast ic Leukemia (ALL), low or standard risk using St Jude & Children’s Oncology Group (COG) | n = 72 | Do not meet COG/St Jude low and standard risk criteria | None | Chemo + dexamethaso ne | Fatigue Symptom Inventory (FSI), pediatric and parent versions | DNA from blood pre dexamethasone, 1, 2, 4, 8 hours post oral dexamethasone dose | AHSG | None given | Expression not associated with fatigue | Ethnicity |
| IL6 G17AC | |||||||||||
| IL6 C634G | |||||||||||
| POLDIP3 | |||||||||||
| Wratten et al., 2004 | Breast, early stage | n = 52 | Severe current illness, history of major breast surgery, history of breast irradiation treatment, no metastatic disease | None mentioned | Adjuvant RT post surgery | FACT-F | Blood before RT, weekly during RT, then 2 & 6 weeks post RT | Lymphocyte | Baseline and week 5 (p<0.01) | Decreased in both fatigued and nonfatigued groups from baseline to week 5 | Body mass index (BMI) |
| Cross-sectional Studies | |||||||||||
| Authors | Cancer | Sample | Exclusion | Control | Intervention | Measure | Data Collection | Marker | p value | Association | Covariates |
| Alexand er et al., 2009 | Breast, stages 1–IIb | n = 60 (fatigue) | Pregnancy, other cancer, recurrent disease, confusion, dementia | n = 104 (non-fatigue d) | Survivors, 3 months - 2 year post primary therapy | Brief Fatigue Scale (BFS) | Peripheral Blood | Neutrophils | 0.51 | No association with fatigue | None mentioned |
| Lymphocyte | 0.25 | ||||||||||
| Monocyte | 0.052 | Trended towards significant association with fatigue | |||||||||
| Eosinophil | 0.051 | ||||||||||
| Bower et al., 2003 | Breast | n = 19 (fatigued) | Recurrence of breast cancer, history of immune disease | n = 18 (non-fatigued) | Survivors recruited from 1994–1997 | RAND SF36 fatigue scale grouped subjects to fatigued and non-fatigued during 2 assessments | Peripheral blood, fasting | White blood cells (WBC) | Not given | No association with fatigue | Age, income, ethnicity, BMI, depressed mood, cancer treatment |
| Granulocyte | |||||||||||
| Monocyte | |||||||||||
| Lymphocytes extracted from fasting blood | CD8+T lymphocyte | 0.124 | 31% more in fatigued group | ||||||||
| CD38 T lymphocyte | Not given | No association with fatigue | |||||||||
| HLA-DR T lymphocyte | |||||||||||
| Serum from fasting blood | IL-1ra | 0.253 | |||||||||
| Bower et al., 2011b | Breast, stages 0–IIIA | n = 103 | Neurologic or immune disease, smokers | None mentioned | Completed primary treatment within 3 months and not started on endocrine treatment | FSI | Plasma | IL1ra | >0.70 | No association with fatigue | Exposure to RT, age, time since treatment completion, BMI |
| C-reactive Protein (CRP) | |||||||||||
| Soluble TNF-RII | Especially with patients receiving therapy other than chemo | ||||||||||
| Collado-Hidalgo et al., 2006 | Breast (stages 0,I, II) | n = 32 (fatigued), < 50 score with SF-36 fatigue scale | Cancer recurrence, immune disease, immunosuppr essant use, psychiatric disease, smokers, alcohol use, SF-36 fatigue score 50–70, advanced cancer stage, >5 years post treatment | n = 18 (non-fatigue d), score >70 in SF-36 fatigue scale | Survivors, 1–5 years post diagnosis | SF36 – vitality scale grouped subjects to fatigued and non-fatigued during 2–3 assessments | Lymphocytes taken in the morning | CD8+T lymphocyte | >0.10 | No difference between fatigued and non-fatigued groups | Age, BMI, time since treatment, treatment mode, depressive symptom scores |
| CD18 B cells | |||||||||||
| CD3−/CD16+/C D56+ natural killer (NK) cells | |||||||||||
| Plasma taken in the morning | IL-6 | Not given | Plasma levels did not reach significance | ||||||||
| TNF-rII | |||||||||||
| Collado-Hidalgo et al., 2008 | Breast (stage 0,I, II) | n = 33 (fatigued), SF36 fatigue score <55 | Cancer recurrence, immune disease, on immunosuppressant | n = 14 (non-fatigue d), SF36 fatigue score >70 | Survivors, 1–5 years post diagnosis | Multidimensional FSI | DNA from leukocytes extracted from peripheral blood | IL1B511cytosine | 0.052 | Not significantly associated with fatigue after controlling for depression | Age, ethnicity, menopausal status, BMI, depressive symptoms, cancer treatment |
| Plasma | IL1ra | 0.074 | Marginally higher in fatigued group | ||||||||
| Minton et al., 2011 | Various, incurable metastatic or locally advanced cancer (multicenter, international study, 16 centers) | n = 741 (324 with severe fatigue) | Physical/cognitive impairment, language problems | n = 417 with no severe fatigue | May be undergoing palliative anticancer treatment | QLQ-C30 (multi-language version): 3-item fatigue subscale | Whole blood drawn within 72 hours after obtaining questionnaire responses. | CRP | Not given | No association with fatigue | Age, BMI, disease stage |
| Orre et al., 2009 | Testicular | n = 92 (chronic fatigue) | Mental retardation, extragonadal germ cell malignancy (except skin), removal of non-affected testicle related to benign condition, concurrent infection or inflammation | n = 191 (without chronic fatigue) | Survivors, recruited from 1980–1998 database, 18–75 years old | FQ (Norwegian) | Plasma drawn from 0800-12 noon; subjects allowed light breakfast | IL-6 | 0.835 | No association with fatigue | Age |
| sTNF-RI | 0.321 | ||||||||||
| Neopterin | 0.390 | ||||||||||
| Orre et al., 2011 | Breast (stage II, III, unclassifiable). | n = 299 | Cancer recurrence, >75 years old, other cancer except basal cell cancer or cancer in situ, prior surgery for contralateral breast cancer stage I with no adjuvant therapy | None | Survivors, treated from 1998–2002 with adjuvant RT; subsample of Reinersten et al., 2011 & Landmark-Høyvik H et al., 2009 | FQ (Norwegian) | Blood drawn from 0900-12 noon; subjects allowed light breakfast | IL-6 | 0.76 | Beta = −0.015 | Age, educational level |
| sTNF-RI | 0.713, | Beta = 0.019 | |||||||||
| Neopterin | 0.85 | Beta = 0.009 | |||||||||
| IL-1ra | 0.183 | Beta = 0.71 | |||||||||
| Paddison et al., 2009 | NSCLC, Stages IIIb and IV | n = 44 | None mentioned | None | None | Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) – 2 items, 1 item from Hamilton Depression scale | Retrospective extraction of clinical results (peripheral blood) | Lymphocytes | 0.78 | R2 = 0.11, β = 0.04 | Age, gender, time since treatment completion, hemoglobin |
| Scheede-Bergdahl et al., 2011 | Inoperable III & IV GI or NSCLC, from 3–11/2007 | n = 83 | Less than 18 years of age | None mentioned | None | Brief Fatigue Inventory (BFI) | Plasma processed within 4 hours of collection | IL1β | >0.05 | β = 8.8 | Sex, age, diagnosis, cancer treatment, Charlson comorbidity index, and concurrent pharmcological treatment |
| IL-6 | β = 10.78 | ||||||||||
| IL-8 | β = 5.37 | ||||||||||
| TNF-α | β = 4.6 | ||||||||||
| Von Ah et al., 2008 | Breast, stage 0–IIIa | n = 44 | Psychiatric disorder, dementia, history or current substance abuse, thyroid issues, immune disorders, immunosuppr ession, other cancer except noninvasive type | None mentioned | Adjuvant chemo + RT | Revised Piper Fatigue Scale (rPFS) | Blood collected 9–33 days post surgery (lumpectomy or mastectomy) before adjuvant therapy | TNF-α | Not significant | r = 0.07– 0.14 | Type of adjuvant therapy, mood, network support, satisfaction, cortisol, perceived stress, optimism |
| Kwak et al., 2011 | Various, terminal cases recruited from 6/2009–7/2010; survival time is less than 6 months | n = 90 (mild=23, moderate = 30, severe =37) | Cognitive impairment, chemo/RT to treat active cancer, hematologic malignancies, use of psychostimula nt, h/o psychiatric disease, fever, use of antibiotics and antiepileptics, high TSH | Mild versus moderate versus severe categories of fatigue | None | BFI – Korean version | Peripheral blood collected within 24 hours from enrolment between 9–11 am | IL-6 | 0.29 | r = 0.12 | Age, gender, BMI, cancer site, previous cancer treatment, comorbid disease, pain score, sleep disorder, dyspnea |
| TNF-α | 0.67 | r = 0.05 | |||||||||
| WBC | 0.75 | Not given | |||||||||
Discussion
The goal of this review was to determine patterns of associations between immunogenomic markers and CRF. In the longitudinal studies, there were trends of associations between levels of CRF and markers of inflammation and immune response, especially in women with early stage of breast cancer. It is premature to specify potential biomarkers for CRF based on the findings because all results were based on associations and therefore do not prove causation. However, this review provides empirical support for the association between high levels of CRF and elevated systemic inflammatory markers (CRP, neutrophils, monocytes, lymphocytes); increased signal of immune response (CD4+); high cytokine (IL-6, IL-1β) concentrations; and increased markers of cytokine activities (IL-1ra, sTNF-RII).
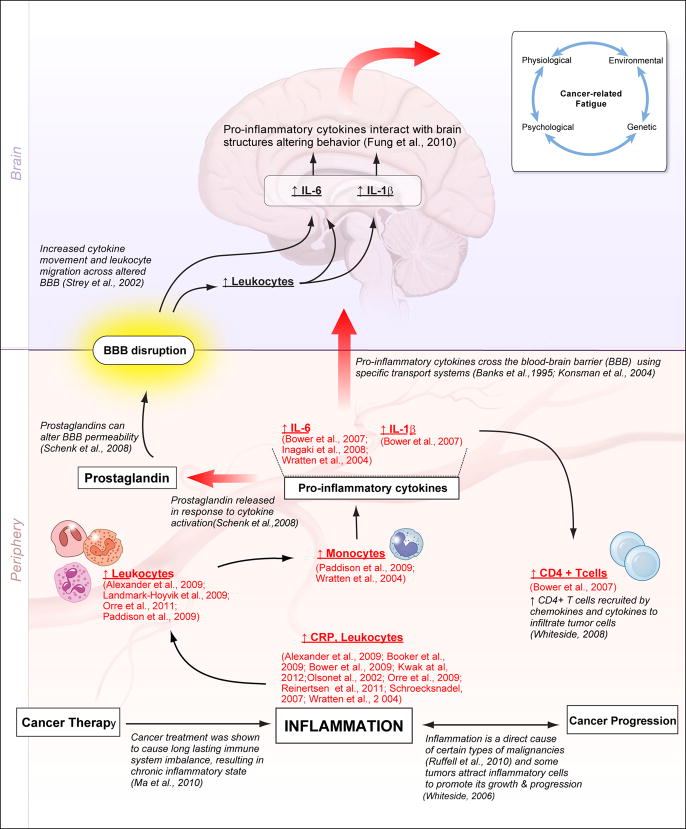
Elevation of inflammatory/immune markers has been observed with cancer progression and cancer treatment. Inflammation is considered a direct cause of certain types of malignancies (Ruffell et al., 2010) and some tumors attract inflammatory cells to promote its growth and progression (Whiteside, 2006). Tumor cells are infiltrated by immune cells, predominantly by CD4+ T cells, which are recruited by chemokines and cytokines (Whiteside, 2008). These tumor cells have been shown to induce immune cell dysfunction by interfering with signal transduction, cytokine production and proliferation, and cell migration (Whiteside, 2010). On the other hand, cancer treatment has been shown to cause long-lasting imbalance of the immune system, resulting in a chronic inflammatory state (Ma et al., 2010), as seen by the elevated levels of pro-inflammatory cytokines even during survivorship (Bower et al., 2007; Reinertsen et al., 2011). These pro-inflammatory cytokines are produced in large amounts by monocytes (Fieren, 2012), which were found to be elevated in the studies included in this review (Paddison et al., 2009; Wratten et al., 2004). These pro-inflammatory cytokines are known to act on brain structures to alter behavior; and variations in their concentrations, especially IL-6, IL-1β, and TNF-α can lead to sickness behavior including the symptom of fatigue (Fung et al., 2012). Cytokines cross the blood-brain barrier (BBB) using specific transport systems as demonstrated in previous radio-imaging studies (Banks et al., 1995; Konsman et al., 2004). Prostaglandins released in response to cytokine activation can alter BBB permeability (Schenk et al., 2008) leading to increased cytokine movement and leukocyte migration across the BBB (Strey et al., 2002). The pathway involved in the interaction of cytokines and brain structures may help explain pathways behind CRF as displayed in Figure 1.
Figure 1. Association of Inflammatory Markers and Cancer-Related Fatigue.
The link between inflammatory markers and cancer-related fatigue may be related to the inflammatory state generated by cancer progression and/or cancer therapy. Both conditions trigger an increase in pro-inflammatory cytokine production by white blood cells (especially monocytes). The systemic experience of CRF may be related to the interactions of pro-inflammatory cytokines and immune cells with brain structures that migrate through a disrupted blood-brain barrier altered by pro-inflammatory cytokine-related activities. CRF intensity is dependent on physiological, psychological, genetic, and environmental factors.
Gaps in knowledge were also found that limit the ability to draw conclusions related to the associations of immunogenomic markers and CRF. In this section results of the review are discussed in relation to: (a) gaps in knowledge, (b) genomic findings, (c) study limitations, and (d) recommendations for future research.
Gaps in Knowledge
The first gap identified is the lack of longitudinal studies exploring the associations of immunogenomic markers and fatigue. Only 29% of the studies used a longitudinal design. More longitudinal studies are necessary to prospectively explore the important roles of cancer progression and treatment in the experience of fatigue in this population. Another gap is the lack of a case-definition for CRF. Only 12 of the 34 studies conceptually defined fatigue. In three studies, CRF was defined as a multidimensional concept where increased levels of CRF were associated with elevated levels of cytokines (IL-6, IL-1β) (Inagaki et al., 2008; Panju et al., 2009; Von Ah et al., 2008). A similar association was observed in one study that used the ICD-10 criteria to define CRF as the presence of significant fatigue nearly every day for two weeks in the past month (Alexander et al., 2009). Three studies did not observe a similar association between CRF and cytokine levels, but higher levels of CRF were associated with higher concentrations of systemic inflammatory markers (CRP, neutrophils) when CRF was conceptualized as having both physical and attentional/mental components (Booker et al., 2009; Olson et al., 2002: Scott et al., 2002) or when it was defined as a sense of tiredness that lasted more than 6 months (Orre et al., 2009; Reinertsen et al., 2011). A non-significant or inverse relationship was observed between CRF and levels of cytokine (IL-6) or systemic inflammatory marker (CRP) when CRF was defined as persistent tiredness (Ahlberg et al., 2004; Kwak et al., 2012) or as part of a symptom cluster (Minton et al., 2012). Variations in the associations between CRF and inflammatory/immune markers are related to the differences in scope of the concept of fatigue being measured and the duration of symptom experience.
A third gap identified is the lack of standard CRF measures that can predict clinical significance. Only 2 of the 11 questionnaires used in the ten longitudinal studies namely, the Fatigue Symptom Inventory (FSI) and the Multidimensional Fatigue Symptom Inventory (MFSI), were reported to be sensitive to changes of disease progression or treatment (Whitehead, 2009). Thus, the other CRF measures used in the longitudinal studies had not been validated for longitudinal designs to be sensitive to cancer progression or effect of cancer therapy. Another important gap that emerged from this review relates to identifying whether fatigue symptoms experienced by cancer patients were related to disease progression, cancer treatments, or other covariates. Inconsistent or non-significant associations were found in studies that enrolled subjects with terminal cases or older individuals or when BMI, ethnicity, and menopausal status were included as covariates in the analyses. A final gap of knowledge is the lack of consistent associations between concentrations of pro-inflammatory cytokines (IL-6 and TNF-α) and levels of fatigue. These latter associations require further investigation using more sophisticated approaches.
After addressing the aforementioned knowledge gaps, the use of genomic and proteomic technologies in identifying the roles of genes, proteins, and environment in CRF may best describe pathways that might play a crucial role in the development of fatigue in cancer populations. These approaches have been proven successful in identifying mechanisms in other symptoms such as depression (Keers R, Uher R, 2011) and pain (Kaszas et al., 2012).
Genomic Findings
Genomic technology is a novel approach for providing information about possible pathways that may explain development of CRF and a closer attention to the findings reported by the reviewed articles is warranted. The findings related to the genetic association studies conducted by Collado-Hidalgo et al. (2008) and Bower et al. (2011a) must be considered with caution because of two critical limitations: both used small samples and both further stratified their samples during analysis. For example, the genetic association study investigated 33 fatigue patients and 14 non-fatigue patients and further subdivided the sample into two ethnic groups (whites and non-whites) to control for ethnicity. However, a closer investigation of the non-white subjects especially those who were fatigued, revealed that 40% were Asians. Furthermore, there were no Asian subjects in the non-fatigued group (Collado-Hidalgo et al., 2008), which made it more difficult to interpret the results.
Aouizerat et al. (2009) and Miaskowski et al. (2010) reported a genetic association between certain SNPs and fatigue. Both used the same population of cancer patients and caregivers. Even though these studies found significant associations, there were some limitations in their similar study design. The authors combined cancer patients and their family members for the analysis, which could have led to different types of fatigue being analyzed: cancer-related fatigue and fatigue not related to cancer. The population stratification is another limitation of these studies considering that categorizing different races into a single, non-white, ethnic category would provide additional complexity to genomic analysis.
The Norwegian research team reported significant associations between genomic markers and fatigue in breast cancer survivors (Landmark-Høyvik H et al., 2009; Reinertsen et al., 2011). Among the eight articles reporting on genomic marker association with fatigue, the study conducted by Landmark-Høyvik, H. et al. (2009) deserves a closer inspection because of its large sample size. An initial sample of 403 subjects was reduced to a subset of 137 with chronic fatigue assessed at two different time points. These 137 subjects were further stratified into fatigue and non-fatigue groups to control for differences in anxiety and depression levels. This study showed an association between a single gene expression using blood and fatigue levels using linear analysis. However, more sophisticated gene set enrichment analyses (GSEA) using pathways from the Molecular Signature Database (MSigDB) revealed a difference of gene expression in inflammatory process and immune system. The study conducted by Reinertsen et al. (2011) showed a weak association between a C-reactive protein encoding gene SNP (rs3091244) and fatigue. Because the uncorrected p-value of the reported association was only 0.02, it may be considered as a false positive result.
Findings from a study by Fernandez et al. (2011) also warrant further consideration. This study reported a positive association between high fatigue levels in breast cancer survivors with a Met/Met COMT 158 when these subjects also complained of more pain in the neck and shoulder areas. Considering high minor allele frequency of COMT 158, the sample size of this study (N = 128) seems to be appropriate. This COMT SNP has been studied extensively including its role in experimental and clinical symptoms such as opioid responses in cancer patients, but some inconsistencies have been reported (Kambur et al., 2010; Laugsand et al., 2011). The role of COMT SNP in CRF needs to be investigated further.
Recommendations for future research
More longitudinal studies that address the identified gaps are needed to fully advance the investigation of mechanisms related to CRF and to capture the dynamic changes that occur in these immunogenomic markers during cancer progression and treatment. Careful sample and study design selection, utilization of valid and reliable CRF measures that are sensitive to changes in fatigue overtime, and inclusion of relevant covariates in statistical analyses are important considerations in designing future studies. Establishing causation between biomarkers and CRF will not be fully realized without CRF being case defined, clearly measured, and clinically translated. Identifying a biomarker of CRF will be immensely beneficial not only in the clinical management of CRF but also in improving treatment outcomes of individuals with cancer.
Conclusion
This review identified some patterns of associations between specific immunogenomic markers and fatigue in survivors with early stages of cancer. Inconsistent associations between fatigue and immunogenomic markers were found in subjects with terminal cases of cancer and when other covariates where considered in the analysis. The most important findings of this review are the identification of the gaps of knowledge that must be addressed in order to advance the science of CRF research. Future efforts should focus on defining CRF, reassessing clinical significance of CRF measures especially using longitudinal approaches, and using biomarkers in predicting changes in CRF. Methodologically improved designs can pave the way in understanding etiologic mechanisms and therapeutic targets of CRF.
Highlights
This review identified patterns of associations between immunogenomic markers and CRF, and gaps in knowledge needed to advance the science of CRF research.
Footnotes
Conflict of interest
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlberg K, Ekman T, Gaston-Johansson F. Levels of fatigue compared to levels of cytokines and hemoglobin during pelvic radiotherapy: a pilot study. Biol Res Nurs. 2004;5:203–210. doi: 10.1177/1099800403259500. [DOI] [PubMed] [Google Scholar]
- Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45:384–392. doi: 10.1016/j.ejca.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouizerat BE, Dodd M, Lee K, West C, Paul SM, Cooper BA, et al. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biol Res Nurs. 2009;11:27–41. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- Banks WA, Plotkin SR, Kastin AJ. Permeability of the blood-brain barrier to soluble cytokine receptors. Neuroimmunomodulation. 1995;2:161–165. doi: 10.1159/000096887. [DOI] [PubMed] [Google Scholar]
- Berger AM, Abernethy AP, Atkinson A, Barsevick AM, Breitbart WS, Cella D, et al. Cancer-related fatigue. J Natl Compr Canc Netw. 2010;8:904–931. doi: 10.6004/jnccn.2010.0067. [DOI] [PubMed] [Google Scholar]
- Booker R, Olson K, Pilarski LM, Noon JP, Bahlis NJ. The relationships among physiologic variables, quality of life, and fatigue in patients with multiple myeloma. Oncol Nurs Forum. 2009;36:209–216. doi: 10.1188/09.ONF.209-216. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL, Cole SW. T-cell homeostasis in breast cancer survivors with persistent fatigue. J Natl Cancer Inst. 2003;95:1165–1168. doi: 10.1093/jnci/djg0019. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav Immun. 2007;21:251–258. doi: 10.1016/j.bbi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15:5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-κB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immune. 2011a;25:147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PS, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011b;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Davis K, Breitbart W, Curt G, Coalition Fatigue. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19:3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: early findings. Brain Behav Immun. 2008;22:1197–1200. doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologists. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- Fernandez-de-las-Penas C, Fernandez-Lao C, Cantarero-Villanueva I, Ambite-Quesada S, Rivas-Martinez I, Del Moral-Avila R, et al. Catechol-O-methyltransferase genotype (Val158met) modulates cancer-related fatigue and pain sensitivity in breast cancer survivors. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1757-y. E-pub ahead of publication. [DOI] [PubMed] [Google Scholar]
- Fieren MW. The local inflammatory responses to infection of the peritoneal cavity in humans: their regulation by cytokines, macrophages, and other leukocytes. Mediators Inflamm. 2012:976241. doi: 10.1155/2012/976241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs D, Hausen A, Reibnegger G, Werner ER, Dierich MP, Wachter H. Neopterin as a marker for activated cell-mediated immunity: application in HIV infection. Immunol Today. 1988;9:150–155. doi: 10.1016/0167-5699(88)91203-0. [DOI] [PubMed] [Google Scholar]
- Fung A, Vizcaychipi M, Lloyd D, Wan Y, Ma D. Central nervous system inflammation in disease related conditions: Mechanistic prospects. Brain Res. 2012;1446:144–155. doi: 10.1016/j.brainres.2012.01.061. [DOI] [PubMed] [Google Scholar]
- Geinitz H, Zimmermann FB, Stoll P, Thamm R, Kaffenberger W, Ansorg K, et al. Fatigue, serum cytokine levels, and blood cell counts during radiotherapy of patients with breast cancer. Int J Radiat Oncol Biol Phys. 2001;51:691–698. doi: 10.1016/s0360-3016(01)01657-1. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Isono M, Okuyama T, Sugawara Y, Akechi T, Akizuki N, et al. Plasma interleukin-6 and fatigue in terminally ill cancer patients. J Pain Symptom Manage. 2008;35:153–161. doi: 10.1016/j.jpainsymman.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Kambur O, Männistö PT. Catechol-O-methyltransferase and pain. Int Rev Neurobiol. 2010;95:227–279. doi: 10.1016/B978-0-12-381326-8.00010-7. [DOI] [PubMed] [Google Scholar]
- Kaszas K, Keller JM, Coddou C, Mishra SK, Hoon MA, Stojilkovic S, et al. Small molecular positive allosteric modulation of TRPV1 activation by vanilloids and acidic pH. J Pharmacol Exp Ther. 2012;340:152–160. doi: 10.1124/jpet.111.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keers R, Uher R. Gene-environment interaction in major depression and antidepressant treatment response. Curr Psychiatry Rep. 2012;14:129–137. doi: 10.1007/s11920-011-0251-x. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Vigues S, Mackerlova L, Bristow A, Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472:113–129. doi: 10.1002/cne.20052. [DOI] [PubMed] [Google Scholar]
- Kwak SM, Choi YS, Yoon HM, Kim DG, Song SH, Lee YJ, et al. The relationship between interleukin-6, tumor necrosis factor-{alpha}, and fatigue in terminally ill cancer patients. Palliat Med. 2012;26:275–282. doi: 10.1177/0269216311406991. [DOI] [PubMed] [Google Scholar]
- Landmark-Høyvik H, Reinertsen KV, Loge JH, Fosså SD, Børresen-Dale AL, Dumeaux V. Alterations of gene expression in blood cells associated with chronic fatigue in breast cancer survivors. Pharmacogenomics J. 2009;9:333–340. doi: 10.1038/tpj.2009.27. [DOI] [PubMed] [Google Scholar]
- Laugsand EA, Fladvad T, Skorpen F, Maltoni M, Kaasa S, Fayers P, et al. Clinical and genetic factors associated with nausea and vomiting in cancer patients receiving opioids. Eur J Cancer. 2011;47:1682–1691. doi: 10.1016/j.ejca.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol. 2010;22:113–24. doi: 10.1016/j.smim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- Miakowski CA. Assessment and management of cancer-related fatigue. In: Berger AM, editor. Principles and Practice of Palliative Care and Supportive Oncology. Lippincott William & Wilkins; Philadelphia, PA: 2002. p. 143. [Google Scholar]
- Miaskowski C, Dodd M, Lee K, West C, Paul SM, Cooper BA, et al. Preliminary evidence of an association between a functional interleukin-6 polymorphism and fatigue and sleep disturbance in oncology patients and their family caregivers. J Pain Symptom Manage. 2010;40:531–544. doi: 10.1016/j.jpainsymman.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LJ, Kurtzman SH, Anderson K, Wang Y, Stankus M, Renna M, et al. Interleukin-1 family expression in human breast cancer: interleukin-1 receptor antagonist. Cancer Invest. 2000;18:293–302. doi: 10.3109/07357900009012171. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Parker B, Dimsdale JE, Sadler GR, Ancoli-Israel S. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol. 2005;69:85–96. doi: 10.1016/j.biopsycho.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF) Ann Oncol. 2009;20:17–25. doi: 10.1093/annonc/mdn537. [DOI] [PubMed] [Google Scholar]
- Minton O, Strasser F, Radbruch L, Stone P. Identification of Factors Associated with Fatigue in Advanced Cancer: A Subset Analysis of the European Palliative Care Research Collaborative Computerized Symptom Assessment Data Set. J Pain Symptom Manage. 2012;43:226–235. doi: 10.1016/j.jpainsymman.2011.03.025. [DOI] [PubMed] [Google Scholar]
- O’Connor PJ. Evaluation of four highly cited energy and fatigue mood measures. J Psychosom Res. 2004;57:435–441. doi: 10.1016/j.jpsychores.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Okuyama T, Akechi T, Kugaya A, Okamura H, Shima Y, Maruguchi M, et al. Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manage. 2000;19:5–14. doi: 10.1016/s0885-3924(99)00138-4. [DOI] [PubMed] [Google Scholar]
- Olson K, Tom B, Hewitt J, Whittingham J, Buchanan L, Ganton G. Evolving routines: preventing fatigue associated with lung and colorectal cancer. Qual Health Res. 2002;12:655–670. doi: 10.1177/104973202129120160. [DOI] [PubMed] [Google Scholar]
- Orre IJ, Reinertsen KV, Aukrust P, Dahl AA, Fosså SD, Ueland T, et al. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res. 2011;71:136–141. doi: 10.1016/j.jpsychores.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Orre IJ, Murison R, Dahl AA, Ueland T, Aukrust P, Fosså SD. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain, Behav Immun. 2009;23:868–874. doi: 10.1016/j.bbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Paddison JS, Temel JS, Fricchione GL, Pirl WF. Using the differential from complete blood counts as a biomarker of fatigue in advanced non-small-cell lung cancer: an exploratory analysis. Palliat Support Care. 2009;7:213–217. doi: 10.1017/S1478951509000273. [DOI] [PubMed] [Google Scholar]
- Panju AH, Danesh A, Minden MD, Kelvin DJ, Alibhai SM. Associations between quality of life, fatigue, and cytokine levels in patients aged 50+ with acute myeloid leukemia. Support Care Cancer. 2009;17:539–546. doi: 10.1007/s00520-008-0512-3. [DOI] [PubMed] [Google Scholar]
- Pantschenko AG, Pushkar I, Anderson KH, Wang Y, Miller LJ, Kurtzman LJ, et al. The interleukin-1 family of cytokines and receptors in human breast cancer: implications for tumour progression. Int J Oncol. 2003;23:269–284. [PubMed] [Google Scholar]
- Rausch SM, Clark MM, Patten C, Liu H, Felten S, Li Y, et al. Relationship between cytokine gene single nucleotide polymorphisms and symptom burden and quality of life in lung cancer survivors. Cancer. 2010;116:4103–4113. doi: 10.1002/cncr.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinertsen KV, Grenaker Alnæs GI, Landmark-Høyvik H, Loge JH, Wist E, Kristensen VN, et al. Fatigued breast cancer survivors and gene polymorphisms in the inflammatory pathway. Brain Behav Immun. 2011;25:1376–1383. doi: 10.1016/j.bbi.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;21:3–10. doi: 10.1016/j.cytogfr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheede-Bergdahl C, Watt HL, Trutschnigg B, Kilgour RD, Haggarty A, Lucar E, et al. Is IL-6 the best pro-inflammatory biomarker of clinical outcomes of cancer cachexia? E-pub ahead of publication. Clin Nutr. 2012;31:85–88. doi: 10.1016/j.clnu.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Investig. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroecksnadel K, Fiegl M, Prassl K, Winkler C, Denz HA, Fuchs D. Diminished quality of life in patients with cancer correlates with tryptophan degradation. J Cancer Res Clin Oncol. 2007;133:477–485. doi: 10.1007/s00432-007-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002;87:264–267. doi: 10.1038/sj.bjc.6600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strey A, Janning A, Barth H, Gerke V. Endothelial Rho signaling is required for monocyte transendothelial migration. FEBS Lett. 2002;517:261–266. doi: 10.1016/s0014-5793(02)02643-1. [DOI] [PubMed] [Google Scholar]
- Vallance K, Liu W, Mandrell BN, Panetta JC, Gattuso JS, Hockenberry M, et al. Mechanisms of dexamethasone-induced disturbed sleep and fatigue in paediatric patients receiving treatment for ALL. Eur J Cancer. 2010;46:1848–1855. doi: 10.1016/j.ejca.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Ah DM, Kang DH, Carpenter JS. Predictors of cancer-related fatigue in women with breast cancer before, during, and after adjuvant therapy. Cancer Nurs. 2008;31:134–144. doi: 10.1097/01.NCC.0000305704.84164.54. [DOI] [PubMed] [Google Scholar]
- Voronov E, Reich E, Dotan S, Dransh P, Cohen I, Huszar M, et al. Effects of IL-1 molecules on growth patterns of 3-MCA-induced cell lines: an interplay between immunogenicity and invasive potential. J Immunotoxicol. 2010;7:27–38. doi: 10.3109/15476910903405528. [DOI] [PubMed] [Google Scholar]
- Weis J. Cancer-related fatigue: prevalence, assessment, and treatment strategies. Expert Rev Pharmacoecon Outcomes Res. 2011;11:441–446. doi: 10.1586/erp.11.44. [DOI] [PubMed] [Google Scholar]
- Whitehead L. The measurement of fatigue in chronic illness: a systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manage. 2009;37:107–128. doi: 10.1016/j.jpainsymman.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. Immune responses to malignancies. J Allergy Clin Immunol. 2010;125:S272–S283. doi: 10.1016/j.jaci.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL. The role of immune cells in the tumor microenvironment. Cancer Treat Res. 2006;130:103–24. doi: 10.1007/0-387-26283-0_5. [DOI] [PubMed] [Google Scholar]
- Wratten C, Kilmurray J, Nash S, Seldon M, Hamilton CS, O’Brien PC, et al. Fatigue during breast radiotherapy and its relationship to biological factors. Int J Radiat Oncol Biol Phys. 2004;59:160–167. doi: 10.1016/j.ijrobp.2003.10.008. [DOI] [PubMed] [Google Scholar]