Abstract
While most organs undergo development in utero, the mouse mammary gland orchestrates five major developmental stages following birth: pre-puberty, puberty, pregnancy, lactation, and involution. Induced by both local and systemic factors, these five developmental stages transpire with dramatic alterations in glandular morphology and cellular function. As an experimental system, the mammary gland provides remarkable accessibility to processes regulating stem cell function, hormone response, and epithelial-stromal-extracellular matrix interactions. This review will provide a historical perspective of the unique in vitro and in vivo techniques used to study the mammary gland and how these methods have provided valuable insight into the biology of this organ.
I. Introduction
Beginning with its origins in the embryo and throughout adulthood, the growth and differentiation of the mouse mammary gland is regulated by diverse molecular, cellular and hormonal pathways [1–2]. These processes maximize the surface area of mammary epithelium, and establish a glandular network poised for milk production. Importantly, the gland only becomes fully differentiated during a defined and transient period in the adult mammal. Rather than persisting in a functionally differentiated, milk-producing state, which would be energetically disadvantageous, mammary glands synchronize their differentiation with the onset of pregnancy (Figure 1A). Thus, the morphogenesis and systematized differentiation of the mammary gland are unique characteristics of this organ.
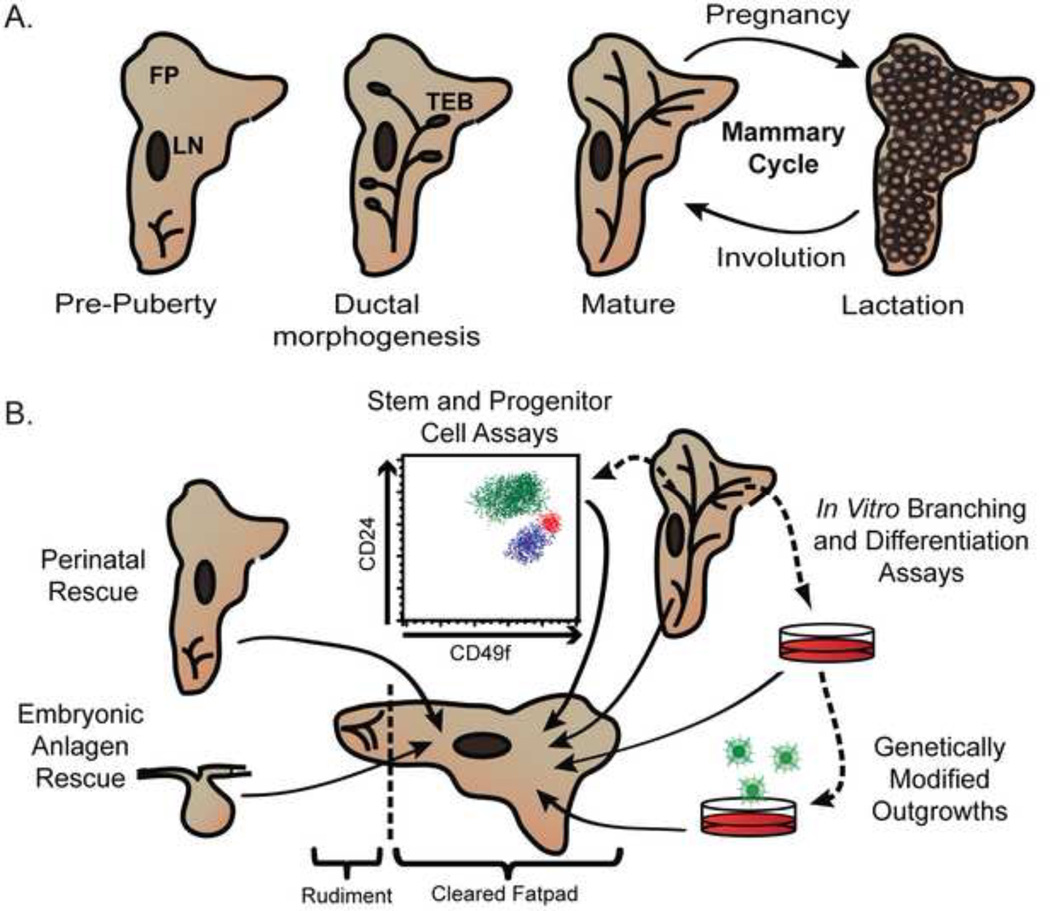
Figure 1. Schematic representation of postnatal mammary gland development and sources for transplantable tissue.
A) Four stages of postnatal mammary gland development in the mouse are depicted. In pre-puberty, the ducts are small and located proximal to the nipple. At three weeks of age puberty commences, marking the beginning of ductal morphogenesis. Highly proliferative terminal end buds, which invade the mammary fat pad, form at the distal tip of ducts. Puberty completes when the ductal network reaches the end of the fat pad. During late pregnancy and lactation, lobulo-alveoli develop along ducts and fill the interductal space of the fat pad. After weaning, the gland remodels back to the mature state during involution. The “mammary cycle“ occurs with each round of pregnancy and involution. FP= fat pad, LN= lymph node, TEB= terminal end bud
B) Prior to puberty, the rudimentary gland can be surgically removed and mammary tissue or dissociated cells can be transplanted into the cleared fat pad. A variety of different cells and tissues can be transplanted into the mammary gland or grown in complex culture systems.
The mammary gland is a tissue with specialized cell biology. The primary structure of the mammary duct is an epithelial bilayer consisting of luminal and myoepithelial cells. At first glance, its cellular organization appears simple in comparison to other epithelial organs such as skin and intestine. However, its simple organizational facade masks its true cellular complexity. Each epithelial layer consists of several functionally distinct cell populations, including stem, progenitor, and differentiated cells; it is only through their closely orchestrated interactions that mammary development proceeds [3].
The purpose of this review is to discuss the unique techniques available to mammary gland researchers, and how these methods have led to significant discoveries in mammary gland biology. We have purposely left out a discussion of important but broadly used methodology, such as genetic engineering, to focus on non-standard experimental approaches that have provided considerable insight into mammary development and differentiation. First, we will cover mammary transplantation and the role it has played in our knowledge of epithelial stem cell biology and stromal-epithelial cell interactions. Next, our focus will turn to how novel cell culturing methods have provided insight to the function of the extracellular matrix in differentiation and branching morphogenesis. Finally, we will discuss the use of virus transduction and mammary reconstitution to investigate gene function during mammary development.
II. Transplantation Techniques Define a Cellular Hierarchy in the Mammary Gland
The seminal observation that mammary tissue could reconstitute itself upon transplantation into cleared mammary fat pads of syngeneic hosts led to a transformation in mammary gland research. Transplantation techniques have been the experimental foundation of some of the most significant discoveries in mammary gland biology. The success of this technique is imparted by several distinct developmental characteristics of the organ. First, the mammary gland develops postnatally in a tissue that is highly accessible to surgical intervention. Second, the pre-pubertal gland is confined within a small portion of the mammary fat pad that is easily removed to establish a “cleared fat pad”, which readily accepts exogenous tissue. Finally, the mammary gland contains a highly regenerative adult stem cell population that can reconstitute the gland upon transplantation. DeOme and colleagues exploited the deferred maturation of the mammary gland by surgically removing the rudimentary ducts prior to puberty and placing a fragment of donor mouse mammary epithelium into the cleared fat pad of a recipient mouse (Figure 1). In 1959, they published the first successful mammary gland reconstitution experiment in mice [4]. Variations on their technique remain one of the most important experimental methods for studying mammary development (Figure 1B).
Initially, the transplantation method was used as a way to demonstrate that neoplastic tissues were the origin of tumors. However, the early transplantation method also allowed researchers to study the regenerative capacity of the gland, and to eventually hypothesize the existence of mammary stem cells. To test the regenerative potential of mammary tissue, Daniel and colleagues performed up to seven serial transplantations over the course of two years. Published in 1968, this study demonstrated that while mammary epithelial cells have substantial regenerative potential, as discerned by their ability to reconstitute the gland following serial transplantations, the robustness of outgrowth decreased with each passage. The authors concluded that normal mammary epithelial cells have a finite lifespan. They also observed that individual outgrowths exhibited considerable variation in overall lifespan, suggesting that “epithelial cells of mammary gland may be heterogeneous with respect to their proliferative potential.” This was perhaps the first inclination that the mouse mammary gland exhibits a cellular hierarchy with regard to regenerative potential [5].
In follow up studies published in 1971, factors influencing the regenerative capacity of mammary epithelial cells were assessed. These studies again utilized the DeOme transplantation method and revealed that mammary reconstitution capacity was independent of the donor’s age, reproductive history, and region within the gland from which the tissue was removed [6–7]. In 1988, Smith and Medina performed an eloquent extension to this study. They dissected specific ductal fragments, including primary and tertiary branches, alveoli, and terminal end buds (TEBs), and demonstrated that each structure was capable of establishing mammary outgrowths [8]. These experiments provided the first clues for the distribution of stem cells within the gland, demonstrating they resided throughout the ductal network. This work also showed that mammary stem cells were resilient to age or the influences of mammary development, and exhibited a finite proliferation potential.
An important modification to the transplantation technique was published by DeOme et al. in 1978. The authors assessed whether normal-appearing mammary epithelial cells derived from glands known to be prone to pre-neoplastic progression would establish normal or pre-neoplastic outgrowths. To perform this study, they modified the transplantation technique by developing a method to transplant dissociated mammary cells rather than tissue fragments. Their new method not only revealed that apparently non-transformed cells could establish pre-neoplastic outgrowths, but also demonstrated that preparations of dissociated mammary cells contained stem cell activity [9]. The latter observation was critical for the subsequent enrichment of mammary stem cells that occurred over two decades later.
In 1996, Smith took advantage of dissociation techniques and performed limiting dilution transplantation in order to determine the minimal number of cells required to establish outgrowths. The results of the study were surprising. Smith observed that limiting dilution transplantation resulted in three morphologically distinct outgrowths in lactating hosts: ductal, lobular, and mixed. This study offered evidence for the presence of distinct mammary progenitor populations with limited developmental potential, and also provided an estimate of the frequency of multipotent stem cells in the mammary gland [10]. This limiting dilution transplantation technique has now become the “gold standard” for defining stem cell activity in enriched mammary cell populations.
An important question, inspired by the limiting dilution experiments, was whether a single mammary stem cell could generate an entire mammary outgrowth. Kordon and Smith performed the first experiments to address this question. Their study used endogenous mouse mammary tumor virus (MMTV) as a tool to assess the heterogeneity of DNA within a mammary outgrowth. The concept was both novel and simple: MMTV DNA integrates randomly into the genome of infected cells, creating a unique DNA restriction-site fragmentation pattern detectable by Southern blot hybridization. They transplanted random fragments of MMTV-infected mammary tissue into cleared mammary fat pads of uninfected mice, and examined the MMTV integration pattern in serially transplanted outgrowths. Both the pattern and intensity of DNA fragmentation suggested that the majority of epithelial tissue in the outgrowths was derived from a single infected mammary cell. The authors also provided a compelling discussion with regard to the proliferative capacity of mammary stem cells and estimated that a single stem cell may possess the capacity to generate 1012–1013 clonal descendants before undergoing proliferative senescence [11]. Taken together, these studies provided an early basis for our current understanding that the mammary gland is composed of a cellular hierarchy comprising progenitor and differentiated cells as well as post-embryonic and organ-specific stem cells.
III. Flow-Cytometry and Transplantation: The Methods that Identified Mammary Stem Cells
The concepts and functional assays necessary for identifying mammary stem cells converged in the late-1990s and early 2000s. At this time, the existence of mammary stem cells was well supported by forty years of transplantation studies, but prospective identification of this cell population remained elusive. Capitalizing on successful identification of distinguishing cell surface markers for hematopoietic stem cells [12–14], several groups performed fluorescence activated cell sorting (FACS) to isolate mammary epithelial cell populations and utilized limiting-dilution transplantation as a functional assay for stem cell activity [15–17]. However, there were significant technical hurdles to using flow cytometry to sort cells derived from the mammary gland. Mammary epithelial cells form tight interactions, forcing the use of vigorous mechanical and enzymatic methods to dissociate the tissue. In addition, mammary epithelial cells undergo anoikis upon detachment from extracellular matrix (ECM). It was not known if such harsh treatments would alter expression of cell surface markers or affect the viability of stem cells. In 2006, two studies were published that demonstrated the effectiveness of using FACS and limiting-dilution transplantation to functionally identify mammary stem cells. Using these techniques, the authors showed that stem cell function (as assessed by outgrowth capacity) was highly enriched in flow-sorted CD29hi/CD24low and CD49Fhi/CD24low cells, and further demonstrated these sorted cell populations could give rise to all mammary cell types [18]. These experiments also answered an important question that remained following Kordon and Smith’s publication: could a single sorted cell and its progeny generate an entire mammary outgrowth? Indeed, this was true. Shackleton and colleagues purified CD29hi/CD24low cells from the mammary glands of β-galactosidase (LacZ)-expressing mice and transplanted single, labeled cells into cleared mammary fat pads of wild type mice. They found that a single CD29hi/CD24low cell possessed the ability to generate an entire LacZ positive outgrowth, and demonstrated self-renewal capacity by doing serial transplantations [19]. Thus, the merging of two major techniques, FACS and mammary reconstitution, were critical for the prospective isolation of mammary stem cells. These studies were the crux of nearly fifty years of both speculation and experimental evidence on the existence of mammary stem cells, and have provided the basis for many subsequent studies on the biology of this unique cell population.
While the existence of a multi-potent mammary stem cell is now well acknowledged, many questions remain about how stem and progenitor cells contribute to mammary growth and tissue homeostasis. Recently, Van Keymeulen and Rocha et al. [20] published studies of lineage tracing and mammary reconstitution to delineate antecedents of the luminal, myoepithelial, and alveolar cellular compartments during ductal morphogenesis and lactation. Using a Cre-reporter strategy to permanently mark and trace cells within the luminal and myoepithelial lineages, they identified that each tissue compartment was derived from a lineage-committed progenitor, rather than a stem cell during normal development of the mammary gland. In contrast, when cells were transplanted at limiting-dilution only a mammary stem cell, derived from the basal lineage, was capable of generating both the luminal and myoepithelial cell compartments [20]. These findings provide new insight to the biology of the cellular hierarchy in the mammary gland, and support the idea that lineage-specific progenitors are the major contributors to growth and maintenance of the postnatal gland. Taken together, the original findings by DeOme and others not only seeded the concept of mammary stem cells, but also provided the technical advances essential to both identify and elucidate the biological function of the gland’s cellular hierarchy.
IV. Defining Epithelial and Stromal Interactions Through Tissue Recombination
In 1953, a series of papers published by Clifford Grobstein implicated epithelial-mesenchymal interaction as a key effector of organ morphogenesis [21]. Since mammary reconstitution separates stromal and epithelial components between host and recipient, this method can be used to investigate tissue-tissue interactions during mammary development. Two different techniques are typically employed in recombination experiments. The first method uses the standard mammary reconstitution technique where mammary tissue fragments or dissociated cells are transplanted into cleared fat pads of recipient mice. The second method involves dissecting epithelium from one animal, recombining it with stroma from another, and placing the tissue recombinant under the kidney capsule of recipient mice. Early studies by Kratochwil using tissue recombination demonstrated the importance of mammary stroma on morphogenesis of the mammary gland. His experiments showed that mammary tissue, dissected from the embryonic anlagen and recombined with submandibular mesenchyme, developed salivary morphology in organ culture [22]. Subsequent studies by Sakakura and Nishizuka using kidney capsule transplants supported this observation, but further demonstrated that functional differentiation of mammary epithelium was not entirely lost; recombinants of mammary epithelium and submandibular mesenchyme were capable of producing milk in pregnant recipients [23]. These studies presented some of the first evidence that epithelial-mesenchymal interactions were important for dictating glandular morphology.
When used in conjunction with genetically engineered mouse models, tissue recombination studies provide a powerful method to investigate specific genetic modulators of stromal-epithelial interactions during mammary development. An additional benefit of mammary reconstitution is the ability to rescue mammary tissue from genetically engineered mice exhibiting late embryonic or perinatal lethality. Both of these applications have contributed to understanding the role of many signaling pathways in mammary development [24–25]; we will use epidermal growth factor receptor (EGFR) signaling as an example to illustrate this point.
The EGFR ligand, epidermal growth factor (EGF), had long been known to influence the growth of mammary glands [26–27], but it was unknown whether EGF elicited a direct or indirect effect on epithelium. In 1999, a paper by Wiesen et al. provided insight on the stromal requirement of this signaling pathway on mammary development. Since most EGFR−/− mice die soon after birth as a result of intestinal and pulmonary defects, the authors performed mammary gland rescue experiments by recombining immature EGFR−/− or EGFR+/+ mammary tissue with EGFR−/− or EGFR+/+ fat pads. While neither the EGFR−/− nor EGFR+/+ epithelial cells could generate outgrowths when recombined with EGFR−/− stroma, either EGFR+/+ or EGFR−/− cells established outgrowths when recombined with EGFR+/+ stroma [28]. This study demonstrated the importance of EGFR signaling in the stroma, and not in the epithelium, for ductal morphogenesis.
Building on this research, Sternlicht and colleagues published a study that further elaborated on the EGFR crosstalk between epithelial and stromal cells in mammary development. Knowing that ductal development was impaired in mice carrying knockout alleles of an EGFR ligand, amphiregulin (Areg) [29], the authors investigated whether proteolytic shedding of Areg by ADAM metallopeptidase domain 17 (Adam17) was also required for mammary development. Similar to EGFR knockout mice, Adam17−/− mice exhibited perinatal lethality, requiring rescue of mammary epithelium by transplantation. Using tissue recombination and mammary reconstitution, the authors demonstrated a requirement for Adam17 expression in mammary epithelium, but not stroma [30]. Put together, the studies by Wiesen and Sternlicht support a model where Areg is shed from epithelial cells by ADAM17, which activates EGFR on stromal cells. EGFR activation then induces stromally-derived effectors of branching morphogenesis that then act on the epithelium (reviewed in Sternlicht 2008 [31]). These data not only established a model for EGFR signaling in epithelial-stromal crosstalk, but also illustrated the effectiveness of tissue transplantation as an investigative tool in mammary gland biology.
V. Complex Cell Culture Systems for Studying Mammary Growth and Differentiation
Cell culture on plastic is a highly artificial and foreign environment to mammary epithelial cells. When cultured on plastic or glass substratums, primary mammary epithelial cells rapidly lose differentiation markers and respond only partially to stimulation with lactogenic hormones. As a result, early attempts to study the induction of mammary differentiation in culture were largely unsuccessful. In contrast, studies by Elias and others in the late 1950s and 1960s showed that whole organ culture of mammary glands derived from pregnant mice and maintained in media containing lactogenic hormones were able to undergo alveolar differentiation[32–33]. These early studies suggested that mammary epithelium did not have an intrinsic capacity to undergo differentiation when cultured directly on dishes, but stromal or matrix components were required for lactation.
In the late 1970s, studies by Emerman, Pitelka, and others attempted to isolate the extrinsic tissue constituents necessary for differentiation of mammary epithelial cells. Their studies demonstrated that culturing methods had a significant influence on morphological and functional differentiation of mammary epithelial cells. They showed that primary mammary epithelial cells grown on different substrates, including plastic, glass and collagen I gels, were not able to differentiate in the presence of lactogenic hormones. However, when cells were cultured on collagen I gels that were detached from plates and floating in medium, the cells exhibited dramatic changes: they showed secretory morphology, exited the cell cycle, and secreted casein into the media [34–35]. Interestingly, later studies would show that this effect was not observed when floating gels were made rigid by glutaraldehyde fixation [36]. Thus, mammary epithelial cells were able to functionally differentiate when grown on a flexible (floating) substratum of collagen I. The question remained, however, whether the critical factor(s) for differentiation was direct epithelial interactions with collagen I, an interstitial stromal protein that is not abundant around mammary epithelial cells, or an effect of the reduced rigidity of the floating gel. Answers to these questions would come in the subsequent decade.
Directly surrounding the myoepithelium is a complex proteinaceous matrix called the basement membrane (BM), which contains collagen IV, laminin proteins, heparin sulfate proetoglycans, nidogens and perlecan [37]. Interactions between the epithelium and BM are dynamic during different stages of mammary growth and differentiation. In non-pregnant mice, luminal epithelial cells are nearly completely sheathed by myoepithelial cells, resulting in sparse interactions between luminal cells and BM. In contrast, during lactation, alveolar cells have significantly more exposure to BM [38]. The importance of BM interactions on alveolar differentiation was not fully realized until the 1980s when Wicha and colleagues directly linked the presence of BM with milk production. They developed an acellular biomatrix consisting of extracellular matrix derived from rat mammary glands, and assessed alveolar differentiation of rat mammary epithelial cells cultured on this matrix. Their study showed that mammary epithelial cells cultured on floating biomatrix in the presence of insulin, prolactin, and hydrocortisone produced 10-times more α-lactalbumin after ten days in culture than cells cultured on floating collagen gels. Moreover, cells cultured on biomatrix that was attached to the plate produced 50-times more α-lactalbumin than cells cultured directly on plastic [39–40]. These data demonstrated that ECM derived from the mammary gland was superior to collagen I for alveolar differentiation.
Engelbreth-Holm-Swarm tumor (EHS) is a transplantable mouse tumor that produces copious amounts of BM protein - an attribute that has been widely exploited and has contributed to important discoveries in mammary growth and differentiation. EHS tumors became a valuable resource for isolating BM proteins and investigating their macromolecular complexes. In the late 1970s, Timpl and colleagues isolated a non-collagenous protein constituent of the BM from EHS tumors and named the protein laminin [41]. This protein would later become known as a critical effector of alveolar differentiation. In 1986, a paper by Kleinman et al. showed that protein fractions of EHS matrix formed a resilient gel containing a defined proportion of laminin, collagen IV, heparin sulfate proteoglycan, nidogen and entactin. They further demonstrated the biological activity of the gel by showing it affected the morphology and pigmentation of B16C3 melanoma cells cultured on the gel [42]. An important technical advance attributed to this study was the development of a convenient, durable gel to assess the effects of BM on cell growth and differentiation. This gel, which would later be commercialized as Matrigel™, was instrumental for studies that would identify the molecular association between BM and lactation.
EHS matrix provided an appropriate substratum to characterize the effects of BM on differentiation of mammary epithelial cells. In 1986, a publication by Li et al. demonstrated that mammary epithelial cells cultured on EHS matrix exhibited several morphological features consistent with alveolar differentiation, including apical located microvilli, extensive rough endoplasmic reticulum, and evidence of secretory activity. Using β-casein as a measure of functional differentiation, they observed that cells cultured on attached or floating EHS gels produced 6-fold and 20-fold more protein, respectively, than cells cultured on floating collagen gels. They also demonstrated that over 90% of mammary epithelial cells grown on EHS gels produced β-casein, while only 30–40% of cells grown on released collagen I gels were immune-reactive for milk protein [43]. These studies demonstrated that functional differences in mammary differentiation could arise due to the influence of various extracellular matrix components (collagen I versus EHS) and/or distinct culturing methods (floating versus attached gels). A series of papers published by Streuli et al. in the early 1990s provided a mechanism that unified these observations. The first paper, published in 1990, revealed that floating collagen I cultures were not inherently permissive for mammary differentiation. Rather, floating gels enabled cells to deposit BM, which was necessary for their differentiation. The authors demonstrated that cells grown on plastic or attached collagen I gels failed to produce substantial laminin or collagen IV, and subsequently produced only a limited quantity of milk protein. In contrast, cells cultured on floating collagen I gels generated an extensive BM substrata and produced an abundance of caseins [44]. A follow up study further defined the cell-cell and cell-BM interactions critical for mammary differentiation. Streuli and colleagues demonstrated that β-casein expression occurred independent of both cell-cell contact and cell polarity. They further showed that β1-integrin, a co-receptor for laminin, was required for differentiation by demonstrating that culturing cells with a pan-specific β1-integrin antibody effectively blocked β-casein expression [45]. This was the first of many studies that established the importance of integrin-mediated interactions between mammary epithelial cells and BM proteins for functional differentiation [46–47].
In addition to its application in differentiation studies, EHS matrix has been used as an in vitro means to investigate the molecular mechanisms of branching morphogenesis. While several key effectors of mammary ductal development have been identified, many of the molecular pathways regulating ductal morphogenesis are poorly understood. An “organoid” system, originally described by Simian et al. and later modified by Fata et al., provides a method to study branching and lateral budding in vitro [48–49]. Organoids are defined as large, multi-cellular fragments of mammary ducts that maintain the cellular composition and organization of the ductal epithelium. When embedded in EHS matrix and cultured in defined media with growth factors, organoids undergo morphological changes that replicate the nascent budding of ducts in vivo, including maintenance of ductal polarity (Figure 2 A–J). Using this 3-dimensional (3D) culturing method in combination with time-lapse imaging, Ewald and colleagues imaged organoids to investigate the cellular processes of branching morphogenesis. They defined several distinct phases of growth in the 3D cultures, including complex cyst formation, and pre-invasive and invasive stages of branching, each of which exhibited a unique cellular organization. Using soluble kinase inhibitors, they identified Rac and myosin light-chain kinase as key regulators of branching morphogenesis, and further proposed a model that luminal epithelial cells use collective migration to invade through EHS matrix [50]. Taken together, these studies reveal the utility of EHS matrix and complex 3D culture systems to investigate the molecular mechanisms of mammary differentiation and branching morphogenesis in vitro.
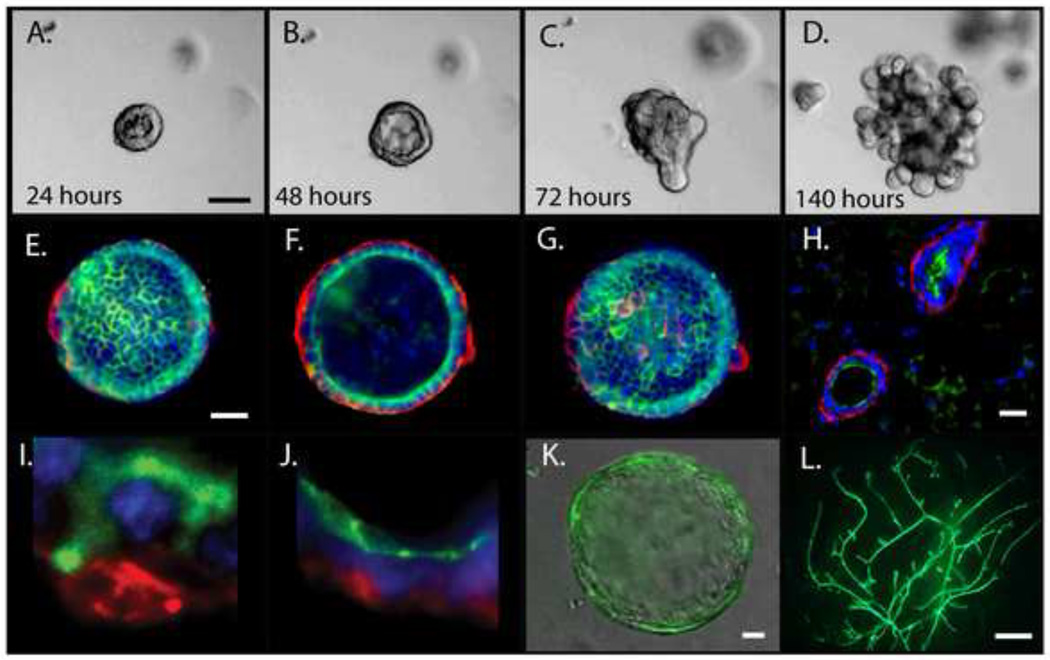
Figure 2. Organoid 3D culture and mammary reconstitution.
Primary MECs grown in EHS matrix develop into organoids that retain the cellular organization, polarity and differentiation capacity of the mammary gland. A-D) Aggregated primary MECs were embedded in EHS matrix and cultured for 140 hours. A and B) Organoids organize into hollow cysts by 24-48 hours. C) Cysts collapse and begin branching around 72 hours in culture. D) By 140 hours the cyst is fully branched. E-G) Immunofluorescence of organoids that show the cellular organization of myoepithelial cells (Keratin 14, red) and luminal cells (Keratin 18, green). E) Top F) middle and G) bottom of cyst. H) Mouse mammary gland immunofluorescence of myoepithelial cells (smooth-muscle α-actin, red) and luminal cells (mucin, green). I) High magnification of Keratin 14 (red) and Keratin 18 (green) cells in a cyst growing in EHS matrix showing the distinct myoepithelial and luminal cell layers. J) Magnified image of a cyst growing in EHS matrix demonstrating polarity of luminal cells with smooth-muscle α-actin in red and the apical marker ZO1 in green. K-L) Viral-mediated genetic modification of MEC's in culture and a mammary outgrowth. K) A cyst grown in EHS matrix derived from a MEC transduced with a GFP expressing lentivirus. L) Mammary outgrowth derived from MECs transduced with a GFP expressing lentivirus. A-D) Scale bar=50um E-G) Scale bar=20um H) Scale bar=25um K-L) Scale bar=1mm
VI. Advances in Viral-Based Systems for Establishing Genetically-Modified Mammary Outgrowths
The ability to maintain primary mammary cells in short-term culture without loss of stem cell activity permits viral-mediated transduction prior to transplantation, in order to establish transgenic mammary outgrowths. Early studies that took advantage of this method were primarily focused on oncogenic transformation of the gland [51–53]. In 1991, Smith and colleagues introduced a non-oncogenic gene, LacZ, into primary mammary epithelial cultures by retroviral-transduction, and used flow cytometry to select LacZ expressing cells prior to transplantation. The resulting outgrowths exhibited LacZ expression in ducts and lobulo-alveoli, demonstrating the feasibility of using retroviruses combined with mammary reconstitution to establish genetically-modified glands [54]. However, the technique was inefficient, particularly with early generation retroviruses and, until recently, few studies used viral-mediated methods to investigate normal mammary development.
In the 1990s, the explosion of gene therapy research spawned the development of potent vectors for gene delivery. Emerging during this period were replication-incompetent adenovirus-based vectors that enabled efficient, transient expression of exogenous genes in targeted cells. At the same time, Cre/loxP recombination became a major technical advance for establishing conditional genetic knockouts. In 2001, Rijnkels and Rosen et al. combined these methodologies to establish mammary outgrowths with conditional knockout of a gene flanked by loxP sites (floxed). They infected primary mammary cells isolated from ROSA 26 LacZ reporter mice (which express LacZ only following Cre-mediated recombination) with an adenovirus containing Cre recominase, and transplanted the cells into cleared mammary fat pads. Primary and secondary outgrowths derived from infected cells exhibited LacZ expression, indicating that adenovirus could effectively target mammary stem or progenitor cells and could therefore be used to genetically modify the mammary gland [55].
Depending on the efficiency of viral transduction, transplantation of infected populations of cells can lead to a mosaic outgrowth. While often an undesirable outcome, the mosaic nature of virally-modified outgrowths can be advantageous for some studies. Experimental somatic mosaicism is commonly employed in model organisms such as Drosophila, in order to investigate both the cell autonomy of genetic events and mutations that would otherwise be embryonic lethal in a germline null background [56]. Taking advantage of this experimental approach, Lu and colleagues used genetic mosaicism to study the competitive advantage of fibroblast growth factor receptor 2 (FGFR2) expression during ductal morphogenesis. They relied on conditional FGFR2 knockout experiments for their study due to the embryonic lethality of FGFR2 null mice. Mammary epithelial cells derived from floxed FGFR2 mice, which also contained the ROSA 26 LacZ reporter allele, were infected with Cre-expressing adenovirus, resulting in infection of 50–70% of cells. They transplanted the mixed pool of infected and uninfected cells and stained outgrowths with X-gal to detect LacZ activity as a marker for FGFR2 null cells. Outgrowths exhibited FGFR2 deletion only in ducts proximal to the injection site, while more distal ductal outgrowths were almost exclusively composed of wild type (WT) cells. In a complementary experiment, whereby mice carrying the floxed FGFR2 gene were crossed to MMTV-Cre transgenic mice to allow for conditional deletion in intact mammary glands, FGFR2 loss was again observed predominantly in ducts located proximal to the nipple, while distal ducts were largely FGFR2 WT. The authors further determined that FGFR2 deletion was associated with reduced proliferation specifically within FGFR2-null cells located in TEBs, but not FGFR2-null cells in mature ducts [57]. These data demonstrated the cell-autonomous effects of FGFR2 loss specifically on proliferating cells during ductal morphogenesis, and highlight the utility of using experimental somatic mosaicism for studying mechanisms of mammary development.
Competition between cells during ductal morphogenesis and mammary outgrowth poses a significant challenge when using viral transduction and transplantation to study genetic inhibitors of mammary development. Unless the vast majority of stem and progenitor cells contain the genetic modification, outgrowths will preferentially develop from uninfected WT cells. Significant enhancements to viral vector systems and recent optimization of infection methods have improved the effectiveness of this technique (Figure 2 K–L). Several groups have now demonstrated the effectiveness of viral-mediated transduction and mammary reconstitution to study signaling pathways important for mammary development and alveolar differentiation [58–59]. Recently, Bouras and Pal et al. used retrovirus-mediated shRNA knockdown of Cbf1, a transcriptional co-regulator of Notch signaling, to investigate effectors of mammary stem cell differentiation. They showed that mammary outgrowths transduced with retroviruses expressing an shRNA against Cbf1 exhibited an increase in stem cell activity, as measured by both limiting dilution transplantation and FACS analysis of CD29hi/CD24+ cells, and an expansion of the myoepithelial cell compartment within TEBs [60]. This study demonstrated that Notch signaling imparts a negative effect on stem cells and promotes the expansion of luminal cells during ductal development. Virus-mediated shRNA knockdown has also been used to investigate transcriptional effectors of alveolar differentiation. Vafaizadeh et al. reported a modified infection method and used this technique to infect mammary epithelial cells with a lentivirus expressing an shRNA against Stat5a/b. They showed that knockdown of Stat5 did not affect ductal development, but impaired both side branching and alveolar differentiation, and reduced the CD24+/CD29low/CD61+ luminal cell population [61]. These studies demonstrate the utility of combining mammary transplantation with viral transduction to establish genetically modified mammary outgrowths for functionally dissecting molecular pathways of growth and differentiation.
VII. Conclusion
The impact of the mouse mammary gland as an experimental tool for investigating diverse mechanisms of organ development has been argued to parallel the Drosophila eye for invertebrate genetics [62]. This analogy may be debatable but it is clear that, through the pioneering efforts of many creative investigators over the last fifty years, we have a deep understanding of the highly coordinated cellular and molecular mechanisms that regulate mammary development, differentiation, and transformation in vivo and in vitro. Future studies on mammary gland biology will surely benefit from the innovative technical advances that are achievable in the context of this unique organ.
Highlights.
Diverse molecular, cellular and hormonal pathways regulate the growth and differentiation of the mouse mammary gland.
These developmental processes are uniquely accessible to investigation through a variety of powerful experimental techniques that developed from over fifty years of research.
The experimental methods discussed here include mammary gland transplantation, tissue reconstitution, complex cell culture systems, and viral-mediated establishment of genetically-modified mammary outgrowths.
Acknowledgements
We would like to thank Drs. Dawne Shelton and Collin Kieffer for contributing images. Funding from the National Institute of Health (R01CA143815 and R01CA140296) supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Developmental cell. 2001;1(4):467–475. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- 2.Sternlicht MD. The cues that regulate ductal branching and morphogenesis. Breast Cancer Res. 2006;8(1):201. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes & development. 2009;23(22):2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeOme K, Faulkin L, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19(5):515. [PubMed] [Google Scholar]
- 5.Daniel CW, De Ome KB, Young J, Blair PB, Faulkin L., Jr The in vivo life span of normal and preneoplastic mouse mammary glands: a serial transplantation study. Proceedings of the National Academy of Sciences of the United States of America. 1968;61(1):53. doi: 10.1073/pnas.61.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young L, Medina D, DeOme K, Daniel C. The influence of host and tissue age on life span and growth rate of serially transplanted mouse mammary gland. Experimental Gerontology. 1971;6(1):49–56. doi: 10.1016/0531-5565(71)90048-9. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino K, Gardner WU. Transplantability and life span of mammary gland during serial transplantation in mice. Nature. 1967;213:193–194. doi: 10.1038/213193a0. [DOI] [PubMed] [Google Scholar]
- 8.Smith G, Medina D. A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. Journal of cell science. 1988;90(1):173. doi: 10.1242/jcs.90.1.173. [DOI] [PubMed] [Google Scholar]
- 9.DeOme K, Miyamoto MJ, Osborn RC, Guzman RC, Lum K. Detection of inapparent nodule-transformed cells in the mammary gland tissues of virgin female BALB/cfC3H mice. Cancer Res. 1978;38(7):2103. [PubMed] [Google Scholar]
- 10.Smith GH. Experimental mammary epithelial morphogenesis in an in vivo model: Evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast cancer research and treatment. 1996;39(1):21–31. doi: 10.1007/BF01806075. [DOI] [PubMed] [Google Scholar]
- 11.Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125(10):1921. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 12.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241(4861):58. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 13.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. In vivo and in vitro stem cell function of c-kit-and Sca-1-positive murine hematopoietic cells. Blood. 1992;80(12):3044. [PubMed] [Google Scholar]
- 14.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1(8):661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 15.Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA. Sca-1pos cells in the mouse mammary gland represent an enriched progenitor cell population. Developmental Biology. 2002;245(1):42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 16.Smalley MJ, Titley J, O’Hare MJ. Clonal characterization of mouse mammary luminal epithelial and myoepithelial cells separated by fluorescence-activated cell sorting. In Vitro Cellular & Developmental Biology-Animal. 1998;34(9):711–721. doi: 10.1007/s11626-998-0067-0. [DOI] [PubMed] [Google Scholar]
- 17.Sleeman K, Kendrick H, Ashworth A, Isacke C, Smalley M. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Research. 2005;8(1):R7. doi: 10.1186/bcr1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439(7079):993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 19.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. NATURE-LONDON- 2006;439(7072):84. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 20.Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–195. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- 21.Grobstein C. Inductive epitheliomesenchymal interaction in cultured organ rudiments of the mouse. Science (New York, NY) 1953;118(3054):52. doi: 10.1126/science.118.3054.52. [DOI] [PubMed] [Google Scholar]
- 22.Kratochwil K. Organ specificity in mesenchymal induction demonstrated in the embryonic development of the mammary gland of the mouse* 1. Developmental Biology. 1969;20(1):46–71. doi: 10.1016/0012-1606(69)90004-9. [DOI] [PubMed] [Google Scholar]
- 23.Sakakura T, Nishizuka Y, Dawe CJ. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976;194(4272):1439. doi: 10.1126/science.827022. [DOI] [PubMed] [Google Scholar]
- 24.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor a is required for proliferation and morphogenesis in the mammary gland. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2196. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proceedings of the National Academy of Sciences. 1998;95(9):5076. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonelli QJ, Sorof S. Epidermal growth factor requirement for development of cultured mammary gland. 1980 doi: 10.1038/285250a0. [DOI] [PubMed] [Google Scholar]
- 27.Vonderhaar BK. Local effects of EGF, TGF, and EGF like growth factors on lobuloalveolar development of the mouse mammary gland in vivo. Journal of cellular physiology. 1987;132(3):581–584. doi: 10.1002/jcp.1041320324. [DOI] [PubMed] [Google Scholar]
- 28.Wiesen JF, Young P, Werb Z, Cunha GR. Signaling through the stromal epidermal growth factor receptor is necessary for mammary ductal development. Development. 1999;126(2):335. doi: 10.1242/dev.126.2.335. [DOI] [PubMed] [Google Scholar]
- 29.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, et al. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126(12):2739–2750. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 30.Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132(17):3923. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sternlicht MD, Sunnarborg SW. The ADAM17–amphiregulin–EGFR axis in mammary development and cancer. Journal of mammary gland biology and neoplasia. 2008;13(2):181–194. doi: 10.1007/s10911-008-9084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elias JJ. Cultivation of adult mouse mammary gland in hormone-enriched synthetic medium. Science. 1957;126(3278):842. doi: 10.1126/science.126.3278.842-a. [DOI] [PubMed] [Google Scholar]
- 33.Juergens WG, Stockdale FE, Topper YJ, Elias JJ. Hormone-dependent differentiation of mammary gland in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1965;54(2):629. doi: 10.1073/pnas.54.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro Cellular & Developmental Biology-Plant. 1977;13(5):316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Richards J, Bowman P, Guzman R, Enami J, McCormick K, et al. Sustained growth and three-dimensional organization of primary mammary tumor epithelial cells embedded in collagen gels. Proceedings of the National Academy of Sciences. 1979;76(7):3401. doi: 10.1073/pnas.76.7.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee E, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. The Journal of cell biology. 1984;98(1):146. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Timpl R. Structure and biological activity of basement membrane proteins. European journal of biochemistry/FEBS. 1989;180(3):487. doi: 10.1111/j.1432-1033.1989.tb14673.x. [DOI] [PubMed] [Google Scholar]
- 38.Pitelka DR, Hamamoto ST, Duafala JG, Nemanic MK. Cell contacts in the mouse mammary gland. The Journal of cell biology. 1973;56(3):797–818. doi: 10.1083/jcb.56.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wicha M, Liotta L, Vonderhaar B, Kidwell W. Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Developmental Biology. 1980;80(2):253–266. doi: 10.1016/0012-1606(80)90402-9. [DOI] [PubMed] [Google Scholar]
- 40.Wicha MS, Lowrie G, Kohn E, Bagavandoss P, Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proceedings of the National Academy of Sciences. 1982;79(10):3213. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin--a glycoprotein from basement membranes. Journal of Biological Chemistry. 1979;254(19):9933. [PubMed] [Google Scholar]
- 42.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, et al. Basement membrane complexes with biological activity. Biochemistry. 1986;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 43.Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proceedings of the National Academy of Sciences. 1987;84(1):136. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Streuli CH, Bissell MJ. Expression of extracellular matrix components is regulated by substratum. The Journal of cell biology. 1990;110(4):1405. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. The Journal of cell biology. 1991;115(5):1383. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, et al. Ablation of β1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. The Journal of cell biology. 2005;171(4):717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katz E, Streuli CH. The extracellular matrix as an adhesion checkpoint for mammary epithelial function. The international journal of biochemistry & cell biology. 2007;39(4):715–726. doi: 10.1016/j.biocel.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001;128(16):3117. doi: 10.1242/dev.128.16.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Research. 2003;6(1):1. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Developmental cell. 2008;14(4):570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edwards PAW, Abram CL, Bradbury JM. Genetic manipulation of mammary epithelium by transplantation. Journal of mammary gland biology and neoplasia. 1996;1(1):75–89. doi: 10.1007/BF02096304. [DOI] [PubMed] [Google Scholar]
- 52.Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54(1):105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 53.Thompson TC, Southgate J, Kitchener G, Land H. Multistage carcinogenesis induced by ras and myc oncogenes in a reconstituted organ. Cell. 1989;56(6):917–930. doi: 10.1016/0092-8674(89)90625-9. [DOI] [PubMed] [Google Scholar]
- 54.Smith G, Gallahan D, Zwiebel J, Freeman S, Bassin R, Callahan R. Long-term in vivo expression of genes introduced by retrovirus-mediated transfer into mammary epithelial cells. Journal of virology. 1991;65(11):6365. doi: 10.1128/jvi.65.11.6365-6370.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rijnkels M, Rosen JM. Adenovirus-Cre-mediated recombination in mammary epithelial early progenitor cells. Journal of cell science. 2001;114(17):3147. doi: 10.1242/jcs.114.17.3147. [DOI] [PubMed] [Google Scholar]
- 56.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117(4):1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 57.Lu P, Ewald AJ, Martin GR, Werb Z. Genetic mosaic analysis reveals FGF receptor 2 function in terminal end buds during mammary gland branching morphogenesis. Developmental Biology. 2008;321(1):77–87. doi: 10.1016/j.ydbio.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welm BE, Dijkgraaf GJP, Bledau AS, Welm AL, Werb Z. Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell Stem Cell. 2008;2(1):90–102. doi: 10.1016/j.stem.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Welm AL, Kim S, Welm BE, Bishop JM. MET and MYC cooperate in mammary tumorigenesis. Proc Natl Acad Sci U S A. 2005;102:4324–4329. doi: 10.1073/pnas.0500470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3(4):429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Vafaizadeh V, Klemmt P, Brendel C, Weber K, Doebele C, Britt K, et al. Mammary Epithelial Reconstitution with Gene-Modified Stem Cells Assigns Roles to Stat5 in Luminal Alveolar Cell Fate Decisions, Differentiation, Involution, and Mammary Tumor Formation. Stem Cells. 2010;28(5):928–938. doi: 10.1002/stem.407. [DOI] [PubMed] [Google Scholar]
- 62.McGee SF, Lanigan F, Gilligan E, Groner B. Mammary gland biology and breast cancer. EMBO reports. 2006;7(11):1084–1088. doi: 10.1038/sj.embor.7400839. [DOI] [PMC free article] [PubMed] [Google Scholar]