Peptidoglycan is a continuous covalent macromolecular structure found on the outside of the cytoplasmic membrane of almost all bacteria and exclusively in these organisms. As the main structural component of the bacterial cell wall, its function is to preserve cell integrity by withstanding the internal osmotic pressure. It is also responsible for the maintenance of a defined cell shape, and it is intimately involved in the cell division process (1). The two basic structural features of this giant macromolecule are linear glycan chains interlinked with short peptide bridges. The glycan chains are composed of alternating units of N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc). The carboxyl group of each N-acetylmuramic acid residue is substituted by a short stem peptide subunit. The formation of the three-dimensional network structure of peptidoglycan is ensured by cross-linking between the peptide subunit of one chain with that of a neighboring chain (2).
Its biosynthesis is a complex two-stage process. The first one concerns the assembly of the disaccharide-peptide monomer unit via a series of UDP precursors and lipid intermediates (3). Six cytoplasmic steps (MurA to MurF) lead to the formation of the UDP-MurNAc-pentapeptide precursor from UDP-GlcNAc. Thereafter, the phospho-MurNAc-pentapeptide moiety of UDP-MurNAc-pentapeptide is transferred to the membrane acceptor undecaprenyl phosphate, yielding lipid I. The addition of GlcNAc to lipid I leads to lipid II, which carries the complete disaccharide peptide monomer unit: GlcNAc-MurNAc-l-Ala-γ-d-Glu-l-Lys (or A2pm)-d-Ala-d-Ala. In the second stage of peptidoglycan synthesis, lipid II is transferred, by a yet unknown mechanism, to the outside surface of the cytoplasmic membrane, where it is used as substrate in the polymerization reactions. Glycosyltransferases catalyze the formation of the linear glycan chains containing the repeating disaccharide peptide units, whereas transpeptidases catalyze the formation of the peptide cross-bridges between glycan chains and the concomitant binding of nascent peptidoglycan to the preexisting cell wall. These transpeptidases are penicillin-binding proteins (PBP) because they are specifically inhibited by the covalent binding of β-lactams antibiotics to their active site (2, 4). Modification of their structure, either by mutations near or directly in some of their conserved motives, or by formation of mosaic structures after recombination of heterologous pbp genes, results in PBPs with low affinities for β-lactams and explains the acquired resistance to these antibiotics, in particular that of Gram-positive organisms (4). Such modifications, therefore, lead to the modulation of their β-lactam affinities and, presumably, to that of their transpeptidase specificities. Thus, it is not surprising that over the past years a number of papers have shown that the structure of the monomer peptide subunit, the specificity of the transpeptidases, and the susceptibility toward β-lactams are closely related features. The work presented in this issue of PNAS by Filipe and Tomasz (5) is an illustration of such a correlation in pneumococci.
In principle, the most simple structure for bacterial peptidoglycan is that of a heteropolymer in which the linear glycan chains have repeating GlcNAc-β,1→4-MurNAc-peptide units and in which the peptide cross-bridges d-Ala→ l-Lys (or → A2pm) are directly established between two monomer units. However, in most bacteria, if not all, a great variety of additional structural features are encountered in their peptidoglycan (2, 3, 6). These modifications vary from one organism to another. Moreover, the peptidoglycan of a given bacteria can undergo important structural modifications under a variety of circumstances (growth conditions, antibiotic treatments, mutations). In many Gram-positive organisms, the presence of interpeptide cross-bridges between two peptide subunits is one of the most important observed structural variation, as revealed by detailed analyses of the muropeptide composition of their peptidoglycan after muramidase digestion (2, 6). Such analyses are now greatly facilitated by use of the HPLC procedure designed by Glauner (7). For instance, it has been established that in Enterococcus faecium, there are bridges with d-Asp or d-Asn (8, 9);
in Enterococcus faecalis, there is a direct linkage and a bridge with one or two l-Ala (10, 11);
in staphylococci, there are pentapeptides with Gly, l-Ala, and l-Ser (12, 13);
in pneumococci, A. Tomasz and his group have shown that there is a also a complex situation with three types of cross-bridges: a direct one, d-Ala→l-Lys, and interpeptides l-Ala-l-Ala and l-Ala-l-Ser (14, 15).
The interpeptide amino acids are added to the peptide subunit before polymerization at the level of UDP-MurNAc-pentapeptide or of the lipid intermediates. Although yet studied to a limited extent, two different mechanisms have been described for the formation of the branched peptide units. For instance, in staphylococci, depending on the species, several glycine, alanine, and serine residues are added from an aminoacyl tRNA intermediate to lipids I and II (16, 17). In enterococci, it was found that an enzyme preparation could catalyze the in vitro addition of Asp to UDP-MurNAc-pentapeptide in the presence of ATP (18).
The structural variations observed in peptidoglycan imply differences in the specificities of the enzymes of the pathway or the presence of additional specific activities. Both factors determine the extent of flexibility of peptidoglycan synthesis and thus the extent of structural variability. In a given bacteria, the specificity of the various synthetases differ from one another. Because the assembly of the monomer unit is essentially a unique sequential process, any variation at a given step must be accepted by the following ones. Therefore, the cumulative effect of the different specificities along the pathway limits the structural variability of the complete monomer unit and also that of peptidoglycan. The enzymatic specificities of the different steps of peptidoglycan synthesis have been studied to various extents with isolated activities or by following the incorporation of unusual constituents (2, 3).
The cytoplasmic steps appear to be highly specific, and only a limited number of structural variations are encountered before the formation of UDP-MurNAc-pentapeptide. The two most noteworthy ones concern the presence of either A2pm or l-lysine in position 3 of the peptide subunit (2, 3) and that of d-alanine, d-lactate, or d-serine at its C-terminal end (19, 20). Both positions are involved in cross-linking by transpeptidation. On the other hand, the low specificity of the transferases catalyzing the formation of lipids I and II for the peptide subunit has been clearly exemplified in many circumstances (3, 21). The same is true for the transfer mechanism of lipid II through the membrane (whatever it might be) and for the polymerization by glycosyltransfer. In many cases, it has been shown both by in vitro and in vivo experiments that the glycosyltransferases accept shorter or modified peptide subunit. In a fem strain of Staphylococcus aureus with an altered lysine-adding enzyme UDP-MurNAc-Ala-d-Glu is the main nucleotide precursor, and l-Ala-d-Glu peptide subunits are predominant in peptidoglycan (22). In Escherichia coli, it has been established in vitro and in vivo that the glycosyltransfer reaction accepts tripeptide subunits (23, 24) and lysine-containing pentapeptide subunits (25). In Gaffkya homari and Bacillus licheniformis, glycan chain formation will accept in the peptide subunit dansyl-Lys (26) and acetyl-A2pm (27), respectively.
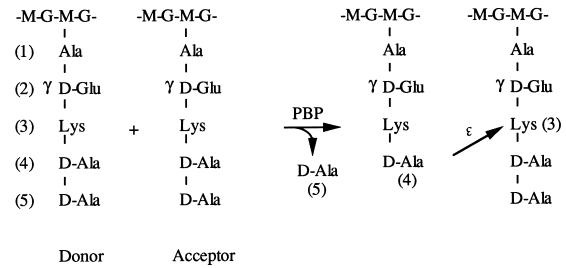
In contrast, the specificity of the transpeptidation reaction for the peptide subunit is far more stringent. Transpeptidation involves the formation of a peptide bond between the carboxyl group of the d-alanine in position 4 of a donor peptide subunit and the Nɛ amino group of l-Lys or A2pm (or the Nαamino group of a branching amino acid) of an acceptor peptide subunit, concomitantly with the release of the donor C-terminal d-Ala (Fig. 1). The specificities of transpeptidases for the donor and acceptor differ. For example, the high specificity for the acceptor is illustrated in E. coli where partial replacement of meso-A2pm by ll-A2pm or l-lysine results in peptide subunits that can only be used as donors (25, 28). However, the C-terminal d-Ala of the pentapeptide donor can be replaced without any effect on transpeptidation by d-lactate or d-serine, as observed in enterococci after the induction of either the natural or acquired additive metabolic pathway leading to vancomycin resistance (19, 20).
Figure 1.
Mechanism of peptide crosslinking by transpeptidation. G, GlcNAc; M, MurNAc; PBP, transpeptidase; →, CO-NH.
It is likely that, among the PBPs of a given bacterial species, more than one can catalyze in vivo a transpeptidation reaction with specificities differing for both the acceptor and donor peptide subunit. Furthermore, these specificities as well as the affinities for β-lactam antibiotics can vary from PBP to PBP and also from one organism to another. This opens the way to different correlations between the structure of the peptide subunit, the specificity of the functionally active transpeptidases, and the susceptibility to β-lactams. In particular, the recognition by different transpeptidases of structurally modified donors or acceptors may lead to changes in the susceptibility to β-lactam antibiotics. For instance, this is exemplified in S. aureus, where the homogenous expression of methicillin resistance is associated with the acquisition of new PBP 2A. Inactivation of the auxilliary fem genes can result in various modifications of the peptide subunit, in particular shortening of the interpeptide bridges. This is associated with a decreased antibiotic resistance that becomes expressed in a heterogeneous fashion (29, 30). It is likely that transpeptidation is no longer carried out efficiently by PBP2A but is taken over by other PBPs, which are methicillin-sensitive; in E. faecalis, where the presence or absence of NaCl in the medium was accompanied by the concomitant modification of the number of the l-Ala residues present in the interpeptide bridge and that of the susceptibility to a third generation cephalosporin: cefotaxime (11). NaCl presumably interferes with the synthesis of the peptide subunit containing the branching l-Ala residues, which here too is likely to result in a change in which PBPs are catalyzing transpeptidation.
Conversely, it is possible that modifications in the PBPs could not only lead to altered susceptibility to β-lactams but also to variations in the cross-bridges. Such a situation has been investigated in pneumococci by Severin and Tomasz (15). In a penicillin-sensitive strain of Streptococcus pneumoniae, the direct cross-linking accounts for 25% of the dimers. When a penicillin-resistant clinical isolate containing mosaic pbp genes encoding PBPs with reduced penicillin affinities was considered, the direct cross-bridges accounted for only ≈3% of the dimers whereas interpeptide cross-bridges Ala-Ala and Ser-Ala were predominant. Thus, altered affinity of mosaic PBPs for penicillin was paralleled with an apparent altered specificity of their transpeptidase activity showing a substrate preference for branched subunits. However, further genetic experiments carried out by the same group (31) indicated that genetic elements encoding the low affinity of PBPs and the penicillin resistance of the bacteria are separable from determinants that are responsible for the unusual cross-linking accompanying penicillin resistance. Now, in the paper presented in this issue by Filipe and Tomasz (5), it is shown that non-PBP determinants, required together with low affinity PBPs for the expression of penicillin resistance in pneumococci, are intact genes coding presumably for or interfering with the transferases catalyzing the formation of the branched peptide subunit.
The situation in pneumococci is thus similar to that of the fem genes responsible for the synthesis of the interpeptide bridge in S. aureus and that of the salt effect in enterococci. For a defined species, in vitro and in the clinical setting, natural as well as acquired variations in the structure of the peptide subunit may well influence the efficacy of the transpeptidase activity of different PBPs. Presumably, in all of these examples, in which low and high affinity PBPs are present simultaneously, the decrease in penicillin resistance is attributable to the preferential use of the peptide subunit by high affinity PBPs rather than by the low affinity ones. Provided that these variations are compatible with the survival of the bacteria and that one or more of these transpeptidases are also essential targets, their susceptibility versus resistance behavior toward β-lactam antibiotics will be modified.
Footnotes
See companion article on page 4891 in issue 9 of volume 97.
References
- 1.Nanninga N. Microbiol Mol Biol Rev. 1998;62:110–129. doi: 10.1128/mmbr.62.1.110-129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers H J, Perkins H R, Ward J B. Microbial Cell Walls and Membranes. London: Chapman & Hall; 1980. pp. 190–382. [Google Scholar]
- 3.van Heijenoort J. In: Bacterial Cell Wall. Ghuysen J M, Hakenbeck R, editors. Amsterdam: Elsevier Science; 1994. pp. 39–54. [Google Scholar]
- 4.Hakenbeck R, Coyette J. Cell Mol Life Sci. 1998;54:332–340. doi: 10.1007/s000180050160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filipe S R, Tomasz A. Proc Natl Acad Sci USA. 2000;97:4891–4896. doi: 10.1073/pnas.080067697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schleifer K H, Kandler O. Bacteriol Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glauner B. Anal Biochem. 1988;172:451–464. doi: 10.1016/0003-2697(88)90468-x. [DOI] [PubMed] [Google Scholar]
- 8.Billot-Klein D, Shlaes D, Bryant D, Bell D, van Heijenoort J, Gutmann L. Biochem J. 1996;313:711–715. doi: 10.1042/bj3130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jonge B L M, Gage D, Handwerger S. Microb Drug Resist. 1996;2:225–229. doi: 10.1089/mdr.1996.2.225. [DOI] [PubMed] [Google Scholar]
- 10.de Jonge B L M, Handwerger S, Gage D. Antimicrob Agents Chemother. 1996;40:863–869. doi: 10.1128/aac.40.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mainardi J-L, Billot-Klein D, Coutrot A, Legrand R, Schoot B, Gutmann L. Microbiology. 1998;144:2679–2685. doi: 10.1099/00221287-144-10-2679. [DOI] [PubMed] [Google Scholar]
- 12.de Jonge B L M, Sidow T, Chang Y-S, Labischinski H, Berger-Bachi B, Gage D A, Tomasz A. J Bacteriol. 1993;175:2779–2782. doi: 10.1128/jb.175.9.2779-2782.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billot-Klein D, Gutmann L, Bryant D, Bell D, van Heijenoort J, Grewal J, Shlaes D. J Bacteriol. 1996;178:4696–4703. doi: 10.1128/jb.178.15.4696-4703.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Bustos J F, Chait B T, Tomasz A. J Biol Chem. 1987;262:15400–15405. [PubMed] [Google Scholar]
- 15.Severin A, Tomasz A. J Bacteriol. 1996;178:168–174. doi: 10.1128/jb.178.1.168-174.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuhashi M, Dietrich C P, Strominger J L. Proc Natl Acad Sci USA. 1965;54:587–594. doi: 10.1073/pnas.54.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuhashi M, Dietrich C P, Strominger J L. J Biol Chem. 1967;242:3191–3206. [PubMed] [Google Scholar]
- 18.Staudenbauer W, Strominger J L. J Biol Chem. 1972;247:5095–5102. [PubMed] [Google Scholar]
- 19.Arthur M, Reynolds P, Courvalin P. Trends Microbiol. 1996;4:401–407. doi: 10.1016/0966-842X(96)10063-9. [DOI] [PubMed] [Google Scholar]
- 20.Billot-Klein D, Gutmann L, Sablé S, Guittet E, van Heijenoort J. J Bacteriol. 1994;176:2398–2405. doi: 10.1128/jb.176.8.2398-2405.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouhss A, Mengin-Lecreulx D, Lebeller D, van Heijenoort J. Mol Microbiol. 1999;34:576–585. doi: 10.1046/j.1365-2958.1999.01623.x. [DOI] [PubMed] [Google Scholar]
- 22.Ornelas-Soares A, de Lencastre H, de Jonge B L M, Tomasz A. J Biol Chem. 1994;269:27246–27250. [PubMed] [Google Scholar]
- 23.Pisabarro A G, Prats R, Vasquez D, Rodriguez-Tebar A. J Bacteriol. 1986;168:199–206. doi: 10.1128/jb.168.1.199-206.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Heijenoort Y, Gomez M, Derrien M, Ayala J, van Heijenoort J. J Bacteriol. 1992;174:3549–3557. doi: 10.1128/jb.174.11.3549-3557.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mengin-Lecreulx D, Falla T, Blanot D, van Heijenoort J, Adams D J, Chopra I. J Bacteriol. 1999;181:5909–5914. doi: 10.1128/jb.181.19.5909-5914.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weppner W A, Neuhaus F C. J Biol Chem. 1977;252:2296–2303. [PubMed] [Google Scholar]
- 27.Ward J B, Perkins H R. Biochem J. 1974;139:781–784. doi: 10.1042/bj1390781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mengin-Lecreulx D, Michaud C, Richaud C, Blanot D, van Heijenoort J. J Bacteriol. 1988;170:2031–2039. doi: 10.1128/jb.170.5.2031-2039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Lencastre H, Tomasz A. Antimicrob Agents Chemother. 1994;38:2590–2598. doi: 10.1128/aac.38.11.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp U, Roos M, Wecke J, Labischinski H. Microb Drug Resist. 1996;2:29–41. doi: 10.1089/mdr.1996.2.29. [DOI] [PubMed] [Google Scholar]
- 31.Severin A, Figueiredo A M S, Tomasz A. J Bacteriol. 1996;178:1788–1792. doi: 10.1128/jb.178.7.1788-1792.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]