Abstract
Alcohol and vagal activity may be important triggers for paroxysmal atrial fibrillation (PAF), but it remains unknown if these associations occur more often than would be expected by chance alone due to the lack of a comparator group in previous studies. We compared the self-reported frequency of these triggers in PAF patients to those with other supraventricular tachycardias (SVT). Consecutive consenting patients presenting for electrophysiology procedures at a single University Medical Center underwent a structured interview regarding arrhythmia triggers. Two hundred and twenty three patients with a documented arrhythmia (133 with PAF and 90 with SVT) completed the survey. After multivariable adjustment, PAF patients had a 4.42 greater odds (95% CI 1.35–14.44) of reporting alcohol consumption (p=0.014) and a 2.02 greater odds (95% CI 1.02–4.00) of reporting vagal activity (p=0.044) as an arrhythmia trigger compared to SVT patients. Among PAF patients, drinking primarily beer was associated with alcohol as a trigger (odds ratio [OR] 4.49, 95% CI 1.41–14.28, p=0.011), while younger age (OR 0.68, 95% CI 0.49–0.95, p=0.022) and a family history of atrial fibrillation (AF) (OR 5.73, 95% CI 1.21–27.23, p=0.028) were each independently associated with having vagal activity provoke an episode. PAF patients with alcohol triggers were more likely to have vagal triggers (OR 10.32, 95% CI 1.05–101.42, p=0.045). In conclusion, alcohol consumption and vagal activity elicit PAF significantly more often than SVT. Alcohol and vagal triggers often were found in the same PAF patients, raising the possibility that alcohol may precipitate AF via vagal mechanisms.
Keywords: paroxysmal atrial fibrillation, alcohol, vagal, supraventricular tachycardia
Most studies describing the association between alcohol and atrial fibrillation (AF) have focused on alcohol as a risk factor for incident AF, with conflicting results.1–5 Less studied, but no less relevant, is the importance of alcohol as a trigger for AF in those who already have the disease. While up to 34% of paroxysmal atrial fibrillation (PAF) patients report that alcohol consumption precedes their episodes,6,7 all previous studies on this subject have been descriptive—a lack of a comparator group has precluded a quantitative study to determine if alcohol as a trigger for AF occurs more often than would be expected by chance alone. For example, since PAF may occur randomly and because alcohol intake is so common, it remains possible that alcohol appears to provoke AF episodes when in fact a causal association is not present. Similarly, activation of the parasympathetic nervous system has been implicated in the pathogenesis of PAF,8,9 but again studies examining activities that increase vagal tone as triggers for AF in humans have been descriptive and not comparative.6,7 We sought to determine if alcohol consumption or vagal activation precipitated PAF episodes more often than would be expected by chance alone by comparing the self-reported frequencies of these potential triggers between patients with paroxysmal supraventricular tachycardia (SVT) and those with PAF.
Methods
From September 2004 to March 2011, consecutive consenting patients presenting for procedures to the UCSF Electrophysiology (EP) laboratory were surveyed. We assigned a primary arrhythmia type to each subject based on documented arrhythmias from chart review, 12 lead electrocardiograms, and invasive EP procedures. We identified consenting patients with a primary diagnosis of either PAF or SVT (n=520). PAF was defined as at least 2 episodes of AF that terminate without intervention in less than 7 days.10 A primary diagnosis of SVT included the diagnoses atrioventricular nodal reentrant tachycardia (AVNRT), atrioventricular reentrant tachycardia (AVRT), atrial tachycardia (AT), and junctional tachycardia. We excluded SVT patients with any history of AF or atrial flutter, as well as PAF patients with any current or past SVT (remaining n=438). The analyses were limited to patients who completed survey questions regarding alcohol habits and other arrhythmia triggers (n=223). “Lone AF” was defined as AF occurring in patients under the age of 60 years and without hypertension, diabetes, coronary artery disease, significant valve disease, or congestive heart failure.11 A family history of AF was defined as having any first- or second-degree relative with a diagnosis of AF determined by patient interview.
Patients were interviewed by research assistants using a structured questionnaire designed by the study authors. Surveyed areas were history of cardiovascular illness, a detailed arrhythmia history, family history, and health-related behaviors. Patients reported alcohol consumption on a 6-point Likert scale ranging from “Never” to “>2 drinks per day.“ “Binge Drinking” was defined as ever consuming more than 5 drinks in a 24-hour period. The type of alcoholic beverage consumed the most (wine, beer, or spirits) was ascertained. Patients reported whether their arrhythmia symptoms initiated or terminated with various activities or behaviors, answering either on a 5-point Likert scale from “Never” to “Always” or “yes/no”. Vagal activation was considered a trigger if resting or eating provoked arrhythmia symptoms “often” or “always”, or if symptoms terminated with exercise.12,13 Sympathetic activation was considered a trigger if patients answered “yes” to exertion, caffeine intake, or stress bringing about their symptoms.
Continuous variables are expressed as means and standard deviation (SD). Analyses were performed using the Student’s T-test or analysis of variance for continuous variables and the Chi-Squared test for categorical variables. Unadjusted and multivariable adjusted odds ratios (OR) with 95% confidence intervals (CI) were obtained using logistic regression for binary outcome variables. Ordered logistic regression was used to obtain ORs for the frequency of alcohol as a trigger, which was grouped into 3 levels (“Never”, “Rarely/Sometimes”, or “Often/Always”) to assure that the models satisfied the assumption of proportional odds.14 Missing data were excluded from regression analyses.
Covariates were included in multivariable models based on a priori beliefs regarding potential confounding (such as age, sex, and race) or if they were associated with both the predictor and the outcome with a p value < 0.1. In all analyses related to alcohol consumption, multivariable models also included the amount of alcohol consumed as well as the presence or absence of binge drinking. Data analysis was performed using Stata version 12 (College Station, TX).
Results
The baseline characteristics are shown in Table 1. Among the SVT group, the primary diagnosis was AVNRT in 50 patients (56%), AVRT in 24 (27%), AVNRT and AVRT in 1 (1%), and atrial tachycardia (AT) in 14 (16%); one individual with SVT had no identifiable SVT mechanism after invasive electrophysiologic testing.
Table 1.
Baseline Characteristics of Patients with Paroxysmal Atrial Fibrillation and Supraventricular Tachycardia
| Variable | Paroxysmal Atrial Fibrillation (n=133) | Supraventricular Tachycardia (n=90) | P Value |
|---|---|---|---|
| Age (years) | 59 ± 12 | 47±16 | <0.001 |
| Male | 94 (71%) | 32 (36%) | <0.001 |
| Race | 0.06 | ||
| White | 106 (80%) | 59 (66%) | |
| Black | 0 (0%) | 3 (3%) | |
| Asian | 15 (11%) | 14 (16%) | |
| Latino | 6 (5%) | 4 (4%) | |
| Other | 6 (5%) | 9 (10%) | |
| Prior Myocardial Infarct | 2 (2%) | 0 (0%) | 0.24 |
| Coronary Artery Disease* | 16 (12%) | 3 (3%) | 0.022 |
| Heart Failure | 7 (5%) | 1 (1%) | 0.10 |
| Diabetes Mellitus | 15 (11%) | 4 (4%) | 0.07 |
| Hypertension | 43 (32%) | 17 (19%) | 0.026 |
| Average Alcohol Consumption | 0.30 | ||
| Never (or none for >3 months) | 39 (31%) | 25 (30%) | |
| Rare (<1–2 drinks/month) | 14 (11%) | 15 (18%) | |
| Monthly (>1–2 drinks/month) | 14 (11%) | 12 (14%) | |
| Weekly (>1–2 drinks/week) | 29 (23%) | 19 (23%) | |
| Daily (1–2 drinks/day) | 26 (20%) | 8 (10%) | |
| >2 drinks/day | 5 (4%) | 4 (5%) |
Continuous variables are presented as mean ± SD; categorical variables are presented as numbers (percentages).
Defined as present if there was any mention of coronary artery disease in the medical record.
One hundred and ten patients (83%) with PAF and 68 (76%) with SVT answered questions regarding how frequently alcohol consumption provoked their episodes (Table 2). Prior to multivariable adjustment, subjects with PAF were more likely to report alcohol as a trigger compared to those with SVT, but this association did not reach statistical significance (Table 3). After multivariable adjustment, PAF patients were significantly more likely to report that alcohol provoked their arrhythmia (Table 3).
Table 2.
Frequency of Alcohol Consumption as a Trigger for Arrhythmia Episodes in Patients with Paroxysmal Atrial Fibrillation and Supraventricular Tachycardia
| Frequency | Paroxysmal Atrial Fibrillation (n=110) | Supraventricular Tachycardia (n=68) |
|---|---|---|
| Never | 86 (78%) | 59 (87%) |
| Rarely/Sometimes | 16 (15%) | 8 (12%) |
| Often/Always | 8 (7%) | 1 (1%) |
Table 3.
Odds Ratios for Triggers of Arrhythmia Episodes in Patients with Paroxysmal Atrial Fibrillation compared to Patients with Supraventricular Tachychardia
| Trigger | Unadjusted | Multivariable Adjustment | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | P Value | 95% Confidence Interval | Odds Ratio | P Value | 95% Confidence Interval | |
| Alcohol Consumption | 1.90 | 0.13 | 0.83 – 4.36 | 4.42* † | 0.014 | 1.35 – 14.44 |
| Vagal Activation | 1.14 | 0.47 | 0.66 – 1.95 | 2.02* | 0.044 | 1.02 – 4.00 |
| Sympathetic Activation | 0.29 | 0.009 | 0.11 – 0.73 | 0.68* ‡ | 0.52 | 0.21 – 2.18 |
Adjusted for age, sex, and race
Adjusted for binge drinking and average amount of alcohol consumption
Adjusted for presence of hypertension and congestive heart failure
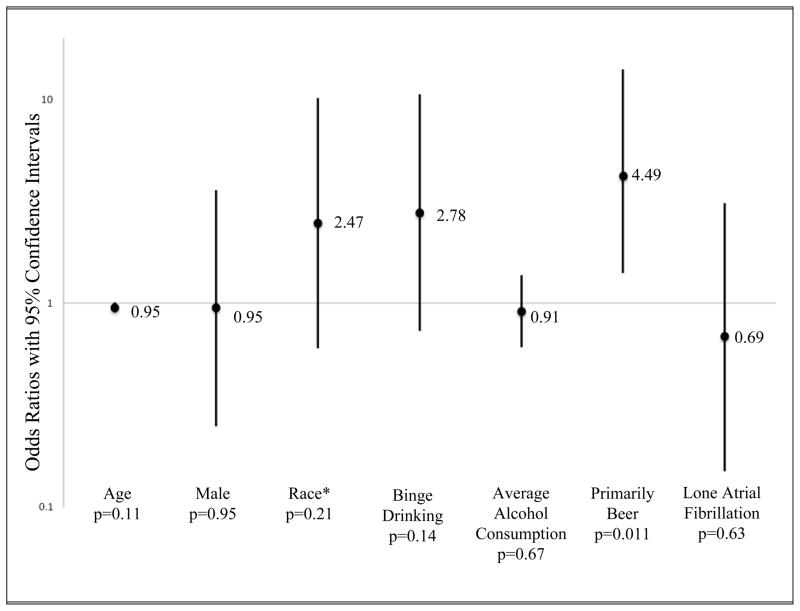
In a multivariate ordered logistic regression model including age, gender, race, and all covariates associated with alcohol as a trigger with a p value < 0.1, only drinking primarily beer was independently associated with alcohol as a trigger among those with PAF (Figure 1).
Figure 1.
Multivariate Adjusted Predictors of Alcohol as a Trigger for Arrhythmia Symptoms in Paroxysmal Atrial Fibrillation Patients. *Race was analyzed as white versus non-white in order to satisfy the assumption of proportional odds for this ordered logistic regression model. Error bars denote 95% confidence intervals.
Fifteen of the 28 patients that reported drinking primarily beer also reported drinking primarily wine or spirits. When those 15 were excluded, the analysis regarding drinking primarily beer as a predictor of alcohol triggering PAF was no longer statistically significant. However, when patients reporting more than one type of alcoholic beverage as their primary drink were grouped with either wine or spirits, no significant associations (either unadjusted or adjusted) with alcohol as a trigger were observed.
One hundred and thirty-two (99%) patients with PAF and 89 (99%) with SVT answered questions regarding activities that increased vagal tone. Overall, 111 (50%) reported that vagal activation precipitated their arrhythmia symptoms. Behaviors reported were resting/sleeping (n=105; 95%), eating (n=14; 13%), and symptoms that terminated with exercise (n=17;15%). After multivariable adjustment, PAF patients were significantly more likely to report vagal activation as a trigger compared to those with SVT (Table 3).
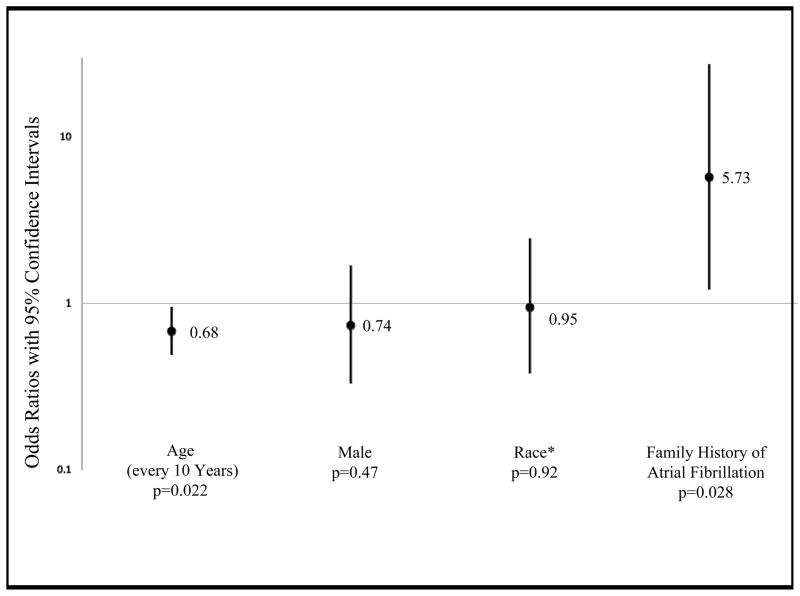
In a multivariate model designed to identify independent predictors of PAF patients with vagal triggers, both younger age and a family history of AF were significantly associated with having vagal triggers (Figure 2).
Figure 2.
Multivariate Adjusted Predictors of Vagal Activation as a Trigger for Arrhythmia Symptoms in Paroxysmal Atrial Fibrillation Patients. *Race was analyzed as white versus non-white. Error bars denote 95% confidence intervals.
To validate the association with vagal activation, we assessed whether sympathetic activation was more often associated with SVT. Eighty-nine patients reported some sympathetic behavior as a trigger for their arrhythmia symptoms, including exertion in 79 (89%), stress in 16 (18%), and caffeine consumption in 5 (6%). In an unadjusted model, individuals with SVT were more likely to report sympathetic behaviors as a trigger than those with PAF, but this association was no longer significant after multivariable adjustment (Table 3).
Among all patients, those who reported that alcohol precipitated their symptoms “often” or “always” were more likely to report vagal activation as a trigger, both prior to (OR 8.82, 95% CI 1.08–72.39, p=0.043) and after multivariable adjustment (OR 13.17, 95% CI 1.37–126.76, p=0.026). In an analysis restricted to PAF patients, the unadjusted point estimate favored an association between alcohol and vagal triggers (OR 7.51, 95% CI 0.89–63.72, p=0.065). After adjusting for age, sex, race, average amount of alcohol consumption, and binge drinking, those who reported alcohol triggered their symptoms “often” or “always” were more likely to describe vagal activation as a trigger for their PAF symptoms (OR 10.32, 95% CI 1.05–101.42, p=0.045). A similar association was not observed when restricting the analysis to those with SVT.
Within the SVT group, there was no significant association between having a particular type of SVT (AVNRT, AVRT, or AT) and reporting alcohol, vagal activation, or sympathetic activation as a trigger for their arrhythmia symptoms, both prior to and after multivariable adjustment.
Discussion
Self-reported alcohol consumption and behaviors that activate the parasympathetic nervous system trigger episodes of PAF significantly more often than SVT. Those who drink primarily beer are more likely to have alcohol consumption provoke PAF. Vagal activation is more likely to initiate PAF episodes in younger patients and in those with a family history of AF. PAF patients who report alcohol as a trigger are also more likely to report vagal tone as a trigger.
Previous studies on alcohol consumption and AF largely focused on chronic consumption and the risk of developing AF,1–5 while binge drinking has been associated with new-onset AF without a comparison group.15,16 Two studies specifically surveying subjects with PAF found that 22–34% reported alcohol consumption preceded their episodes,6,7 suggesting that alcohol may be an important trigger for PAF episodes. It remains possible, however, that this perceived association is due to chance given the common nature of both PAF episodes and alcohol consumption. Our finding that alcohol consumption is more frequently a trigger for PAF compared to other SVTs provides evidence that the association between alcohol consumption and PAF episodes occurs more often than would be expected by chance alone.
We found that drinking primarily beer was associated with alcohol triggering PAF episodes. One previous study suggested red wine is the most common type of alcohol to precipitate a PAF episode.6 It is possible that the association we found is due to beer drinkers consuming more alcohol on average than those who drink wine or spirits; however this association was still present after controlling for average alcohol consumption and binging. Of note, our questionnaire asked what type of beverage patients consumed the most rather than what type triggered their episodes the most.
“Vagal AF” has been described as a clinical syndrome in which activities that increase vagal tone, such as resting, sleeping, and eating, trigger episodes of PAF.8,12 Up to 38% of subjects with PAF have reported at least one type of vagal trigger prior to their episodes.6,7 However, without a comparator group, it is again difficult to determine whether a true association with PAF exists. Our finding that vagal activation precedes PAF more often than SVT supports such an association. Vagal AF patients often do not have cardiac comorbidities8,13 or structurally abnormal atria.13 Our patients with vagally-triggered PAF were younger and more likely to have a family history of AF, suggesting that these patients may have an inherited PAF phenotype generally unrelated to cardiac comorbidities.
The mechanisms underlying the association between alcohol consumption and PAF episodes remain unclear.17–20 Given the relatively acute timing between either alcohol consumption or vagal activation and arrhythmia episodes, these triggers presumably act via some dynamic mechanism that leads to functional (rather than structural) changes. Both vagal activation and acute alcohol exposure appear to have electrophysiologic effects that can promote AF, such as shortening of both the action potential duration21,22 and the refractory period.23,24 As patients with PAF triggered by alcohol were more likely to have vagal tone initiate their symptoms, our data raise the possibility that both triggers may work through similar mechanisms. In fact alcohol consumption may elicit PAF episodes through vagal mechanisms.
Our survey relied on patient reports, and we were therefore unable to objectively validate the associations described. However, while reports of triggers could be subject to recall bias, it does not appear that that this would differ significantly by arrhythmia type and therefore would unlikely explain our positive results. Fifty-one percent of the identified PAF and SVT patients completed survey questions related to arrhythmia triggers, which may have affected our descriptive statistics. While this could introduce response bias, it is unlikely that it would differ significantly by arrhythmia type and explain our positive results. It is possible that patients with PAF experience more frequent arrhythmia episodes than those with SVT, which may lead to an apparent association between PAF and alcohol or vagal activation; because we did not record the frequency of episodes in both groups, we were unable to adjust for this possible confounder. Our study population is healthier and more symptomatic than the general atrial fibrillation population, and thus may exhibit a frequency of triggers due to alcohol and vagal tone that differs from others. Participants were not well balanced in baseline characteristics; however, this is to be expected given the known demographics of patients with PAF and SVT, and all analyses included adjustment for potential confounders. Because this is an observational study, we cannot exclude the possibility that residual (or unmeasured) confounding is responsible for our results.
Acknowledgments
Funding Sources: This publication was supported by the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health, through UCSF-CTSI Grant Number TL1 RR024129. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Gronbaek M. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation. 2005;112:1736–1742. doi: 10.1161/CIRCULATIONAHA.105.547844. [DOI] [PubMed] [Google Scholar]
- 2.Mukamal KJ, Psaty BM, Rautaharju PM, Furberg CD, Kuller LJ, Mittleman MA, Gottdiener JS, Siscovick DS. Alcohol consumption and risk and prognosis of atrial fibrillation among older adults: the Cardiovascular Health Study. Am Heart J. 2007;153:260–266. doi: 10.1016/j.ahj.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 3.Djousse L, Levy D, Benjamin EJ, Blease SJ, Russ A, Larson MG, Massaro JM, D’Agostino RB, Wolf PA, Ellison RC. Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. Am J Cardiol. 2004;93:710–713. doi: 10.1016/j.amjcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Frost L, Vestergaard P. Alcohol and risk of atrial fibrillation or flutter: a cohort study. Arch Intern Med. 2004;164:1993–1998. doi: 10.1001/archinte.164.18.1993. [DOI] [PubMed] [Google Scholar]
- 5.Conen D, Tedrow UB, Cook NR, Moorthy MV, Buring JE, Albert CM. Alcohol consumption and risk of incident atrial fibrillation in women. JAMA. 2008;300:2489–2496. doi: 10.1001/jama.2008.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansson A, Madsen-Hardig B, Olsson SB. Arrhythmia-provoking factors and symptoms at the onset of paroxysmal atrial fibrillation: a study based on interviews with 100 patients seeking hospital assistance. BMC Cardiovasc Disord. 2004;4:13. doi: 10.1186/1471-2261-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maryniak A, Walczak F, Bodalski R, Szumowski L, Derejko P, Urbanek P, Orczykowski M, Szufladowicz E. Atrial fibrillation onset circumstances and their relation to patients’ quality of life. Kardiol Pol. 2006;64:1102–1108. [PubMed] [Google Scholar]
- 8.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J. 1994;15:9–16. doi: 10.1093/eurheartj/15.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 9.Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43:483–490. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 11.Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR, Jr, Ilstrup DM, Frye RL. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. 1987;317:669–674. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- 12.Rosso R, Sparks PB, Morton JB, Kistler PM, Vohra JK, Halloran K, Medi C, Kalman JM. Vagal paroxysmal atrial fibrillation: prevalence and ablation outcome in patients without structural heart disease. J Cardiovasc Electrophysiol. 2010;21:489–493. doi: 10.1111/j.1540-8167.2009.01658.x. [DOI] [PubMed] [Google Scholar]
- 13.Nemirovsky D, Hutter R, Gomes JA. The electrical substrate of vagal atrial fibrillation as assessed by the signal-averaged electrocardiogram of the P wave. Pacing Clin Electrophysiol. 2008;31:308–313. doi: 10.1111/j.1540-8159.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee J. Cumulative logit modelling for ordinal response variables: applications to biomedical research. Comput Appl Biosci. 1992;8:555–562. doi: 10.1093/bioinformatics/8.6.555. [DOI] [PubMed] [Google Scholar]
- 15.Thornton JR. Atrial fibrillation in healthy non-alcohol people after an alcoholic binge. Lancet. 1984;2:1013–1015. doi: 10.1016/s0140-6736(84)91109-7. [DOI] [PubMed] [Google Scholar]
- 16.Lowenstein SR, Gabow PA, Cramer J, Oliva PB, Ratner K. The role of alcohol in new-onset atrial fibrillation. Arch Intern Med. 1983;143:1882–1885. [PubMed] [Google Scholar]
- 17.Gould L, Reddy CV, Becker W, Oh KC, Kim SG. Electrophysiologic properties of alcohol in man. J Electrocardiol. 1978;11:219–226. doi: 10.1016/s0022-0736(78)80120-4. [DOI] [PubMed] [Google Scholar]
- 18.Greenspon AJ, Schaal SF. The “holiday heart”: electrophysiologic studies of alcohol effects in alcoholics. Ann Intern Med. 1983;98:135–139. doi: 10.7326/0003-4819-98-2-135. [DOI] [PubMed] [Google Scholar]
- 19.Carpentier RG, Gallardo-Carpentier A. Acute and chronic effects of ethanol on sinoatrial electrophysiology in the rat heart. J Cardiovasc Pharmacol. 1987;10:616–621. doi: 10.1097/00005344-198712000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Jain AK, Carpentier RG. Cardiac electrophysiological actions and interactions of ethanol, cocaine, and the metabolite ethylcocaine. J Electrocardiol. 1998;31:293–302. doi: 10.1016/s0022-0736(98)90014-0. [DOI] [PubMed] [Google Scholar]
- 21.Williams ES, Mirro MJ, Bailey JC. Electrophysiological effects of ethanol, acetaldehyde, and acetate on cardiac tissues from dog and guinea pig. Circ Res. 1980;47:473–478. doi: 10.1161/01.res.47.3.473. [DOI] [PubMed] [Google Scholar]
- 22.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 23.Marcus GM, Smith LM, Whiteman D, Tseng ZH, Badhwar N, Lee BK, Lee RJ, Scheinman MM, Olgin JE. Alcohol intake is significantly associated with atrial flutter in patients under 60 years of age and a shorter right atrial effective refractory period. Pacing Clin Electrophysiol. 2008;31:266–272. doi: 10.1111/j.1540-8159.2008.00985.x. [DOI] [PubMed] [Google Scholar]
- 24.Inoue H, Zipes DP. Changes in atrial and ventricular refractoriness and in atrioventricular nodal conduction produced by combinations of vagal and sympathetic stimulation that result in a constant spontaneous sinus cycle length. Circ Res. 1987;60:942–951. doi: 10.1161/01.res.60.6.942. [DOI] [PubMed] [Google Scholar]