Abstract
Increasing evidence suggests that West Nile virus (WNV) induces a persistent infection in some humans and animals. Here, we characterized infection of mouse macrophage and kidney epithelial cell lines with a strain of WNV (H8912), cultured from urine of a persistently-infected hamster. WNV H8912 had a reduced replication rate, concurrent with a lower interferon (IFN)-β gene expression in both cell types compared to its parent strain-WNV NY99. In WNV H8912-infected macrophages, we observed higher interleukin (IL)-6 and tumor necrosis factor (TNF)-α expression and more nuclear factor kappa B (NF-κB)activation than in cells infected with WNV NY99. In contrast, there were reduced levels of TNF-β and IL-6 expression, as well as less NF-κB activation following WNV H8912 infection in the kidney epithelial cells compared to WNV NY99. Overall, our results demonstrate that the WNV isolate obtained from hamster urine is an attenuated virus and induces a differential proinflammatory cytokine response in mouse macrophage and kidney epithelial cell lines.
Keywords: West Nile virus, Proinflammatory cytokines, Persistence, Pathogenesis
1. Introduction
West Nile virus (WNV), a mosquito-borne, positive-sense, single-stranded flavivirus is now the most widely distributed arbovirus in the world, occurring on all continents except Antarctica (Kramer et al., 2008). The virus was originally isolated from a febrile woman in Uganda in 1937 and later was recognized as a cause of febrile illness and occasionally encephalitis in humans in Europe, Africa, the Middle East, and parts of Asia. In 1999, WNV was detected in New York City and rapidly spread throughout the continental United States, southern Canada, Mexico, Central America, the Caribbean and to several countries in South America (Campbell et al., 2002; Pletnev et al., 2006). It is now endemic in North America. Human infection results primarily from the bite of an infected mosquito, and outcomes can range from an asymptomatic acute infection, or a brief febrile illness (WN fever) to meningitis, encephalitis, flaccid paralysis, and death. Severity can be influenced by the age, health or the immune status of the subject (Gubler, 2007; Kramer et al., 2008). Currently, there is no specific therapeutic agent for the treatment of the infection or an approved vaccine for its prevention. Animal models, including mice, hamsters, monkeys and horses have been used to study host immunity and viral pathogenesis during acute WNV infection (Davis et al., 2001; Kramer and Bernard, 2001; Ratterree et al., 2004; Xiao et al., 2001).
In addition to acute WNV infection, follow-up clinical studies of patients who have had symptomatic WNV infection (WN fever and WNV neuroinvasive disease) indicate that many of them have significant long-term sequelae, such as weakness, fatigue, memory loss and ataxia following their acute illness (Carson et al., 2006; Cook et al., 2010; Ou and Ratard, 2005; Ravindra et al., 2004; Sadek et al., 2010). Some convalescent patients were reported to have detectable serum levels of WNV specific IgM antibodies for more than 6 months and up to several years after their initial infection (Busch et al., 2008; Papa et al., 2011). Recently, Murray et al. (Murray et al., 2010) reported the presence of WNV RNA in the urine of patients convalescent from WNV neuroinvasive disease for up to 6½ years after their initial illness, which indicates that persistent WNV infection occurs in some humans. In fact, evidence of WNV persistence has been well documented in several animal models, including monkeys, hamsters and mice (Appler et al., 2010; Pogodina et al., 1984; Tesh et al., 2005). The first demonstration of WNV persistence was reported by Pogodina et al., who recovered virus from the brain, lymph nodes, and kidneys of rheusus monkeys for up to 5½ months post-infection (Pogodina et al., 1984). Hamsters infected with a New York 99 strain (NY385-99) of WNV developed chronic renal infection and persistent viruria for up to 8 months post-infection with moderate histopathologic changes in the kidneys (Tesh et al., 2005). A WNV isolate obtained from the urine of a persistently–infected hamster was passaged for consecutive three times through the hamster kidneys and the progeny viruses all induced a persistent renal infection with significantly reduced neuroinvasiveness when infecting hamsters systemically (Ding et al., 2005; Tesh et al., 2005; Wu et al., 2008). To examine WNV persistence in the murine model, here, we characterized and compared infection and induction of innate immune responses by a WNV isolate obtained from the urine of a persistently-infected hamster-WNV H8912 in two murine cell lines: mouse macrophage and kidney epithelial cell lines.
2. Materials and Methods
2.1. Cell lines and WNV infection
The mouse macrophage cell line (H36.12j) and the mouse kidney epithelial cell line (Renca) were purchased from the American Type Culture Collection (Manassas, VA). Two WNV strains were used: the parental strain WNV NY385-99 (WNV NY99, (Xiao et al., 2001)), which had been passaged once in African Green Monkey kidney (Vero) cells and twice in Aedes albopictus (C6/36) cells; and WNV H8912, which was recovered from hamster urine 274 days post-infection after three consecutive passages of a WNV isolate obtained from the urine of a persistently infected hamster (Wu et al., 2008). WNV H8912 was passaged once more in Vero cells to prepare virus stocks for cell culture studies. Cells were infected with WNV NY99 or WNV H8912 at a multiplicity of infection (MOI) of 1. At various times post-infection, cells and supernatant were collected for measurement of viral load and cytokine production.
2.2. Quantitative PCR (Q-PCR) for v2iral load and cytokine production
RNA was extracted from WNV-infected cells using RNeasy extraction kit (Qiagen, Valencia, CA). RNA was used to synthesize complementary (c)DNA using qScript cDNA synthesis kit (Quanta Biosciences, Gaithersburg, MD). The sequences of the primer sets for the WNV envelope gene (WNVE) and cytokine gene cDNA and the PCR reaction conditions were described previously (Lanciotti et al., 2000; Wang et al., 2004). The assay was performed in an iCycler (Bio-Rad, Hercules, CA). To normalize the samples, the same amount of cDNA was used in a Q-PCR for β-actin. The ratio of the amount of amplified gene compared with the amount of β-actin cDNA represented the relative levels in each sample.
2.3. Plaque assay
Vero cells were seeded in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Sigma, St. Louis, MO) and incubated with serial dilutions of culture supernatant collected from WNV-infected mouse macrophages and kidney epithelial cells for 1 h. 2X DMEM containing 1% low-melting-point agarose was added and incubated for 3 days. A second overlay of 1% agarose-medium containing 0.01% neutral red was added to visualize plaques. Virus concentrations were calculated as plaque forming unit (PFU)/ml.
2.4. Indirect immunofluorescence microscopy
For the detection of WNV antigen, cells were harvested at indicated time points post-infection. About 80,000 cells were fixed by submerging them in acetone at −20°C for 30 min. Cells were subsequently stained with the flavivirus E protein-specific monoclonal antibody 4G2 ((Henchal et al., 1982) 1:200 dilution) for 1.5 h at 37°C followed by FITC labeled goat anti-mouse antibody (Sigma; 1:200) for 30 min at 37°C. Hoechst dye (Sigma) was used for nuclear counterstaining. Images were acquired on an Olympus IX71 Inverted Microscope at 40 X magnification. To detect nuclear translocation of nuclear factor kappa light chain enhancer of activated B cells (NF-κB), cells were washed once with phosphate buffered saline (PBS) and fixed using chilled acetone for 30 min. Cells were then treated with PBS containing 2% bovine serum albumin, 5% normal goat serum and 0.1% Triton-X-100 for 30 min at RT, followed by sequential one-hour incubations with rabbit polyclonal anti-NFκB-p65 (1:200, Millipore, Billerica, MA), and FITC labeled goat anti-rabbit antibody (Sigma; 1:100). After staining, cells were washed twice with PBS and mounted with mounting media containing Hoechst dye (Sigma) for nuclear counterstaining. Images were acquired using a 1.0 Zeiss LSM 510 UV META Laser Scanning Confocal Microscope at UTMB Infectious Disease and Toxicology Optical Imaging Core Facility.
2.5. SiRNA knockdown for retinoic acid inducible gene (RIG)-I and Toll-like receptor (TLR)-3
Mouse macrophage cells were transfected with 100 nM TLR-3 specific siRNA (Dharmacon) or 50 nM RIG-I specific siRNA (SA Biosciences) by using Superfect (Qiagen) per the manufacturer’s instructions. Scrambled siRNA (SA Biosciences) were used as a negative control. Transfected cells were grown in RPMI medium containing 0.1% FBS for 11 h before being replaced with complete medium. Q-PCR analysis of the TLR3 and RIG-I mRNA to confirm the effects of the siRNA knockdown was performed. At 24 h post-transfection, cells were infected with WNV H8912 isolate (MOI =1). Cell supernatant was harvested at day 6 post-infection for analysis of interleukin (IL)-6 production.
2.6. Bioplex
Cell culture supernatant was collected for analysis of cytokine production by using a Bio-Plex Pro Mouse Cytokine Assay (Biorad).
2.7. Statistical analysis
Data analysis was performed using Prism software (Graph-Pad) statistical analysis. Values for viral burden, plaque assay, and cytokine production experiments were presented as means ± SEM. P values of these experiments were calculated with a non-paired Student’s t test. Statistical significance was accepted at P < 0.05.
3. Results
3.1. WNV H8912 replication in mouse macrophages and kidney epithelial cells
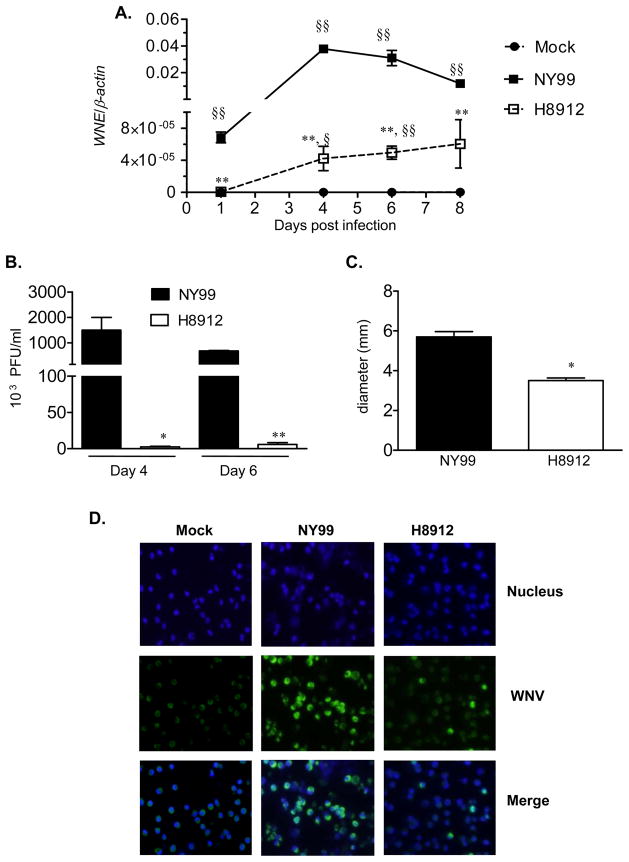
The murine model is an effective in vivo experimental animal model to investigate host immunity and viral pathogenesis of acute WNV infection (Beasley et al., 2002; Diamond et al., 2003b). WNV H8912 is a virus isolate from persistently-infected hamster urine. In this study, we characterized WNV H8912 infection in two mouse cells. Mouse macrophages are permissive to WNV infection in vitro (Cardosa et al., 1983). Initially, we infected a mouse peritoneal macrophage cell line with WNV H8912 and its parent WNV NY99 strains at an MOI of 1. Viral loads were determined by Q-PCR, plaque assay and immunofluorescence staining at different time points post-infection. Q-PCR analysis showed that both WNV strains reached the peak of viral infection at day 4. Viral RNA in WNV H8912-infected cells was more than 1000-fold lower than titers of wild-type WNV NY99 strain (Figure 1A, P < 0.01) at days 1, 4, 6 and 8 post-infection. Plaque assay results further confirmed the difference of viral load between these two virus strains at days 4 and 6 post-infection (Figure 1B, P < 0.01 or P < 0.05). Compared to Q-PCR analysis, the magnitude of viral load difference shown by plaque assay was reduced to 100-fold for H8912 at day 6 post-infection (Figure 1B). The plaque size of WNV H8912 was about two thirds of that of wild-type WNV NY99 (Figure 1C, P < 0.05). Finally, immunofluorescence staining for WNV antigen demonstrated over 80% infection rate for WNV NY99 at day 4 post-infection; while only about 10–12% of macrophage cells inoculated with WNV H8912 were positive for WNV antigen (Figure 1D).
Fig.1.
Comparison of infection between WNV H8912 and WNV NY99 in mouse macrophage cells. Mouse macrophage cells were infected with both WNV strains at an MOI of 1. A, Q-PCR was used to measure WNV infection in cell lysates. B-C, Supernatant was collected to measure viral titer (B) and plaque size (C) by plaque assay. D, Immunofluorescence photomicrographs of macrophage cells at day 4 after infection. Cells were stained with antibody to WNV antigen (green signal). Hoechst dye was used as a nuclear counterstaining (blue signal), and merged images are shown. **P < 0.01 or *P < 0.05 for NY99 vs. H8912. §§ P < 0.01 or §P < 0.05 for WNV-infected vs. mock. P values were calculated with a non-paired Student’s t test.
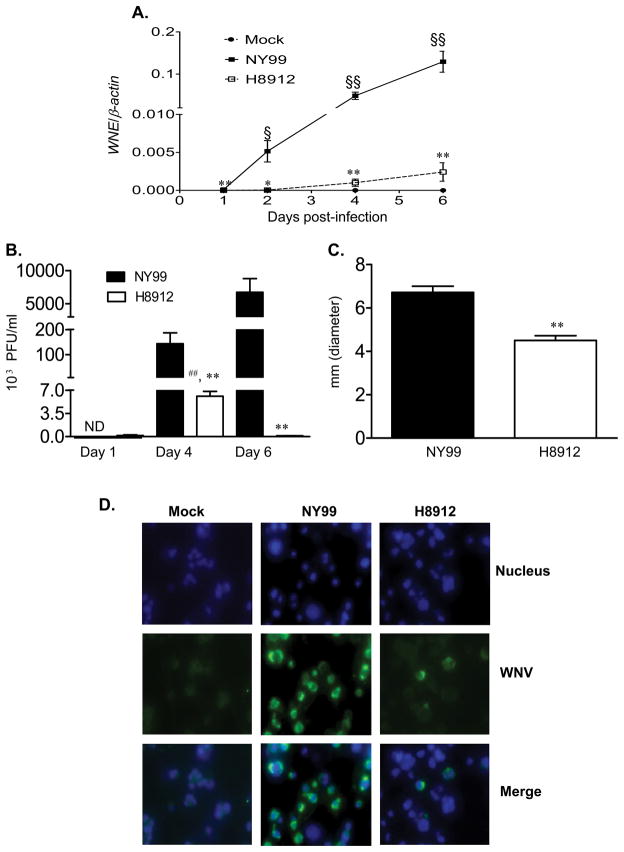
In WNV persistently infected hamsters, viral antigen was detected in renal tubular epithelial and vascular endothelial cells (Tesh et al., 2005). Here, to characterize the replication of a WNV isolate obtained from hamster urine in mouse kidney, a mouse kidney epithelial cell line was infected with WNV H8912 and its parent strain WNV NY99. Q-PCR analysis (Figure 2A) demonstrated that the viral load of WNV H8912-infected kidney epithelial cells was about 100-fold lower than those infected by wild-type WNV NY99 at days 1, 2, 4 and 6 post-infection (P < 0.05 or P < 0.01). Although WNV H8912 replication in kidney epithelial cells was shown to be non-productive by Q-PCR analysis (Figure 2A, P > 0.05), plaque assay results indicate that WNV H8912 titer was about 38-fold higher at day 4 post-infection than that of day 1 (Figure 2B, P < 0.05). The difference in viral loads between WNV NY99 and WNV H8912 was consistent in Q-PCR and plaque assays. Like infection in macrophage cells, WNV H8912 replication in kidney epithelial cells exhibited a smaller plaque size (about two thirds of those by WNV NY99, Figure 2C, P < 0.01). Moreover, immunofluoresence staining for WNV antigen in mouse kidney epithelial cells revealed that at day 4 post-infection, over 60% of the kidney epithelial cells were infected by WNV NY99; whereas, only about 8–9% of WNV H8912-infected kidney cells stained positive for WNV antigen (Figure 2D, P < 0.01). Overall, the WNV H8912 replication rate in both mouse macrophage cells and kidney epithelial cells was significantly reduced compared to wild-type WNV NY99.
Fig. 2.
Comparison of infection between WNV H8912 and WNV NY99 in mouse kidney epithelial cells. Mouse kidney epithelial cells were infected with both WNV H8912 and its parent strain WNV NY99 at an MOI of 1. A, Q-PCR was used to measure WNV infection in cell lysates. B-C, Supernatant was collected to measure viral titer (B) and plaque size (C) by plaque assay. ND: not determined. D, Immunofluorescence photomicrographs of mouse kidney epithelial cells at day 4 post-infection. Infected cells and controls were stained with antibody to WNV antigen (green signal). Hoechst dye was used as a nuclear counterstaining (blue signal), and merged images are shown. **P < 0.01 or *P < 0.05 for WNV NY99 vs. WNV H8912. §§ P < 0.01 or §P < 0.05 for WNV-infected vs. mock. ## P < 0.01 compared to day 1 post-WNV H8912 infection. P values were calculated with a non-paired Student’s t test.
3.2. Innate cytokine expression in WNV H8912-infected macrophage cells and kidney epithelial cells
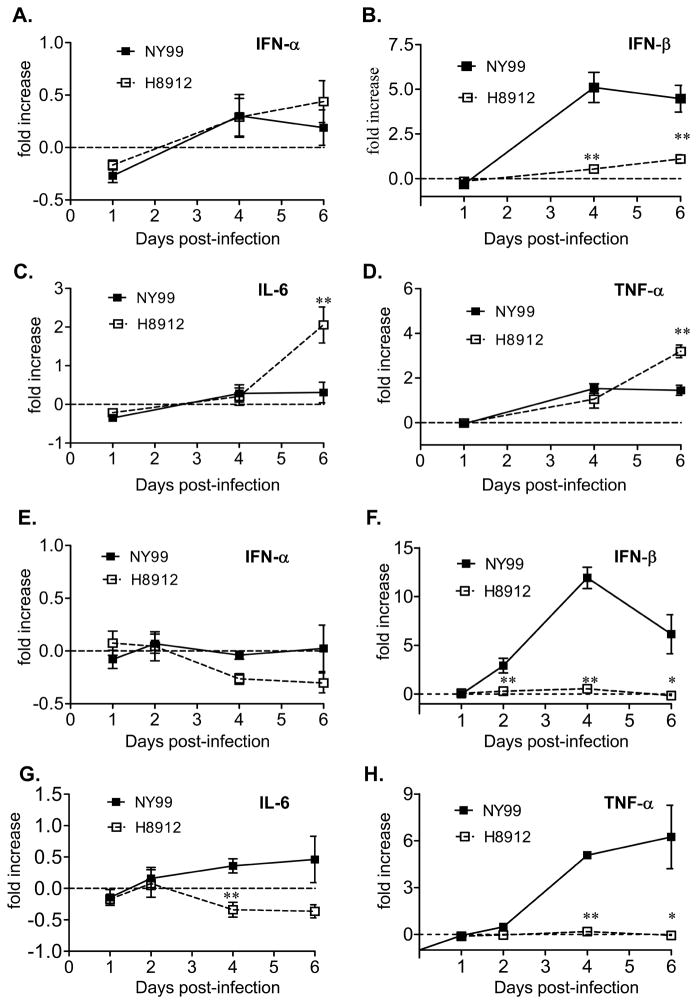
Innate cytokines play an important role in host defense and viral pathogenesis (Akira and Hemmi, 2003; Iwasaki and Medzhitov, 2004). We next measured cytokine production by Q-PCR. There was no significant difference in interferon (IFN)-α expression between WNV H8912 and NY99-infected macrophages at days 1 and 4 and 6 post-infection (Figure 3A, P > 0.05). IFN-β expression was about 3–4-fold lower in WNV H8912-infected cells compared to WNV NY99 at days 4 and 6 post-infection (Figure 3B, P < 0.01). Interestingly, proinflammatory cytokines, such as IL-6 and tumor necrosis factor (TNF)-α levels were higher in WNV H8912-infected macrophages cells than in cells infected by WNV NY99 at day 6 post-infection (Figures 3C & 3D, P < 0.01); no significant differences were detected at earlier time points. Similar to results in macrophage cells, there was no significant difference detected for IFN-β expression between WNV H8912 and NY99 infected kidney epithelial cells at days 1, 2, 4 and 6 post-infection (Figure 3E, P > 0.05). IFN-β levels in WNV H8912-infected cells were about 6–20-fold lower than that of WNV NY99-infected cells at days 2, 4 and 6 post-infection (Figure 3F, P < 0.01 or P < 0.05). WNV H8912 infection in the kidney epithelial cells did not induce IL-6 and TNF-α expression. In contrast, IL-6 and TNF-α expression was upregulated in WNV NY99-infected cells at day 4 and/or day 6 post-infection (Figures 3G & 3H, P < 0.01 or P < 0.05). Together, these results suggest that WNV H8912 infection in mouse macrophages and kidney epithelial cells induced a lower IFN-β, but a differential proinflammatory cytokine gene expression.
Fig. 3.
Innate cytokine gene expression in mouse macrophages and kidney epithelial cells following infection with WNV H8912 and WNV NY99. Cytokine levels in macrophages (A-D) and kidney epithelial cells (E-H) were determined using Q-PCR at various time intervals post-infection. Fold of increase compared to mock-infected group was shown. **P < 0.01 or *P < 0.05 for WNV NY99 vs. WNV H8912. P values were calculated with a non-paired Student’s t test.
3.3. IL-6 production in WNV H8912-infected macrophages is dependent on activation and translocation of NF-κB
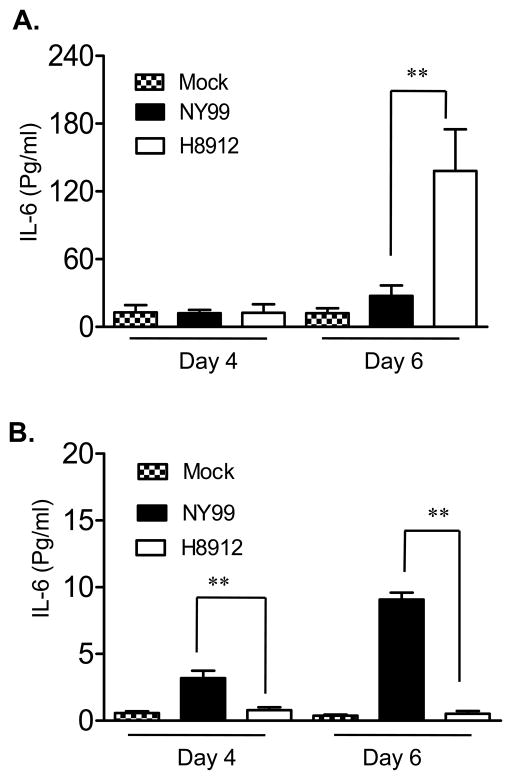
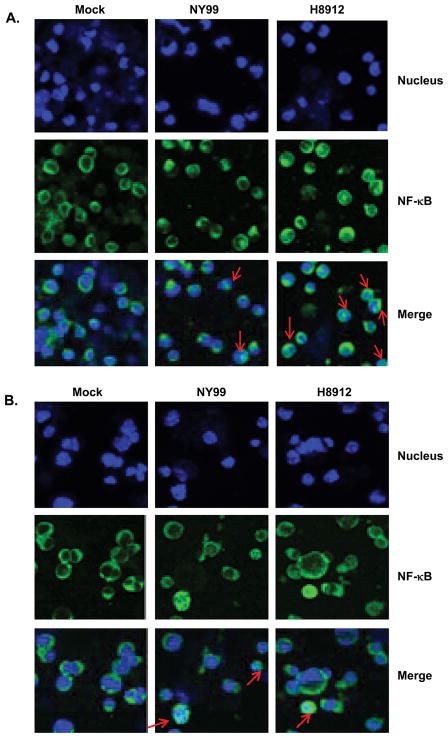
IL-6 has been shown to be involved in the regulation of T cell immunity (Conti et al., 2005; Dieli et al., 2004; Goodman et al., 2009; Harker et al., 2011). To confirm Q-PCR results on IL-6 gene expression, we measured IL-6 production by Bioplex. WNV H8912-infected macrophages produced four-fold more IL-6 compared to WNV NY99 (Figure 4A, P < 0.01) at 6 days post-infection. In kidney epithelial cells, WNV NY99 induced IL-6 production on days 4 and 6 post-infection; whereas WNV H8912 had no significant effect on IL-6 production as compared to non-infected cells (Figure 4B, P < 0.01). The induction of transcription factor NF-κB superfamily, such as p50 and p65, is responsible for expression of a variety of viral-induced chemokine and proinflammtory cytokine genes, such as IL-6 and TNF-α. Upon viral infections, the multicomponent IκB kinase (IKK) complexes, which are activated through RIG-I/TLR-3 pathway, phosphorylate NF-κB accessory inhibitory protein IκB. This leads to IκB proteolytic degradation, and then the relocalization of NF-κB into the nucleus and activation of target gene transcription (Li and Verma, 2002). To investigate the relationship between WNV-induced IL-6 and NF-κB activation, we analyzed the nuclear translocation of p65 in WNV-infected macrophages and kidney epithelial cells by immunofluorescence imaging. Mock, WNV NY99 and WNV H8912-infected macrophages (Figure 5A) or kidney epithelial cells (Figure 5B) were double labeled with NFκB-p65 antibody and hoechst dye for nuclear counterstaining. Although we detected about 7-fold lower infection rate in H8912-infected macrophages by immunofluorescence staining for WNV antigen at day 4 post-infection (Figure 1D), there was more nuclear translocation of p65 in the WNV-H8912-infected macrophages (12.3% ± 0.61%, P < 0.05, data not shown), compared to WNV NY99 (5.4% ± 1.6%) at day 3 post-infection. In WNV-H8912-infected kidney epithelial cells, the infection rate was 6–7-fold lower than that of WNV NY99-infected cells at day 4 post-infection (Figure 2D). There was also less nuclear translocation of p65 induced by WNV-H8912 (3.9% ± 0.3%, P < 0.05, data not shown) than by WNV NY99 (13.0% ± 2.85%) at day 3 post-infection. Overall, WNV H8912 infection induced a differential IL-6 production and NF-κB activation in mouse macrophage cells and kidney epithelial cells.
Fig. 4.
IL-6 production in mouse macrophage cells and kidney epithelial cells following WNV infection. Culture supernatant was harvested from mock, WNV NY99 or WNV H8912 infected macrophage cells (A) and kidney epithelial cells (B) at days 4 and 6 post-infection. IL-6 production was measured by Bioplex. Data are presented as means ± SEM, n = 6. **P < 0.01 compared to WNV NY99 strain-infected cells.
Fig. 5.
Nuclear translocation of NF-κB in WNV-infected macrophages and kidney epithelial cells. Immunofluorescence photomicrographs of mouse macrophage cells (A) and kidney epithelial cells (B) infected with mock, WNV NY99 and WNV H8912 (MOI =1). Mock-infected and WNV NY99 or H8912-post-infected cells were double labeled on day 3 post-infection with NFκB-p65 antibody (Green signal) and Hoechst dye for nuclear counterstaining (blue signal). Arrow points to cells in which NF-κB translocation to the nucleus was detected.
4. Discussion
Although increasing evidence indicates that persistent WNV infection occurs in humans and animals (Murray et al., 2010; Penn et al., 2006; Pogodina et al., 1983; Siddharthan et al., 2009; Tesh et al., 2005), the mechanisms by which the virus survives in the host and develops chronic infection are poorly understood. WNV-infected hamsters developed chronic renal infection and persistent shedding of virus in urine for up to 8 months. The recovered WNV isolates and their subsequent three passages through hamsters produced a chronic, asymptomatic renal infection when inoculated again into adult hamsters. Full genomic sequencing of WNV isolates from hamster urine revealed that they exhibit 0.08 to 0.26% nucleotide divergence when compared to the wild-type parental strain. This suggests that some of the genetic changes may be associated with a persistent renal infection (Tesh et al., 2005; Tesh and Xiao, 2009). In this study, we found that the WNV isolate cultured from the urine of a persistently-infected hamster-WNV H8912 had a significantly reduced replication rate and exhibited a smaller plaque size than WNV NY99 in both mouse macrophage and kidney epithelial cells. The susceptibility of macrophages to a productive WNV infection in vitro (Cardosa et al., 1983) usually indicates a role for these cells in initial WNV replication rate and propagation in the host (Rios et al., 2006). Thus, our findings indicate that WNV H8912, when inoculated into adult mice, may potentially develop a lower infection rate in the peripheral tissues.
Both cell-mediated and humoral responses contribute to protective immunity against WNV infection. B cells and specific antibodies are critical in the control of disseminated WNV infection, but they are insufficient to eliminate the virus from the host (Diamond et al., 2003a; Diamond et al., 2003b; Diamond et al., 2003c; Roehrig et al., 2001). T cells may either provide help for antibody responses or are directly involved in clearing infection from peripheral tissues and CNS, and in preventing viral persistence (Brien et al., 2007; Shrestha and Diamond, 2004; Sitati and Diamond, 2006). Proinflammatory cytokines are involved in the regulation of host adaptive immunity to microbial infection. For example, TNF-α or IL-6 act synergistically with WNV to modulate the expression of immune recognition molecules on antigen presenting cells, leading to an increased recognition by the virus-specific cytotoxic T cells (King et al., 2003). IL-6 is known to be involved in the development of T cell-mediated immune responses (Conti et al., 2005; Dieli et al., 2004). In a recent study, IL-6 was shown to cause up-regulation of the transcription factor Bcl6 and enhanced T follicular helper cell responses at the late stages of chronic viral infection, which resulted in an escalation of germinal center reactions and improved antibody responses (Harker et al., 2011). Here, we noted that WNV H8912 induced much higher IL-6 and TNF-α expression in macrophages than in kidney epithelial cells. These results may suggest that WNV H8912 infection can evoke a stronger cell-mediated immune response in macrophage cell enriched lymphoid organs than in non-lymphoid organs, such as the kidneys. Furthermore, WNV H8912 infection in kidney epithelial cells barely induced IFN-β gene expression which could be partially due to a lower replication rate in these cells as reported in an early study (Fredericksen and Gale, 2006). Interestingly, we also noted that WNV H8912 replicated more efficiently in the type 1 IFN incompetent baby hamster kidney (BHK) cells than in mouse macrophages and kidney epithelial cells. Viral RNA in WNV H8912-infected BHK cells was only 2–3-fold lower than titers of wild-type WNV NY99 strain at day 4 post-infection (data not shown). These results further suggest that type 1 IFN signaling is important in control of WNV H8912 replication. Taken together, WNV H8912 induced immune responses in the kidneys may not be sufficient to completely clear virus and this may ultimately lead to a tissue specific persistent WNV infection.
The mechanisms by which WNV H8912 induces a differential proinflammatory cytokine production in macrophages and kidney epithelial cells are still under investigation. The lack of cytokine induction in the mouse kidney epithelial cells after WNV H8912 infection could be attributed to the very low replication rate in these cells. Alternatively, innate immune cells, like macrophage cells express more pathogen recognition receptors than kidney epithelial cells (data not shown here). TLR3, RIG-I and MDA-mediated signaling pathways have been reported to contribute to WNV recognition (Fredericksen and Gale, 2006; Fredericksen et al., 2008; Wang et al., 2004). Here, we found that siRNA knockdown of TLR3 and RIG-I signaling reduced IL-6 production in WNV H8912-infected macrophages (Supplementary Figures 1A & 1B, P < 0.05), suggesting a role of these pathogen recognition receptors in WNV H8912 recognition. It was also noted that WNV H8912 infection in macrophage cells had a greater upregulation of TNF-α and IL-6 expression. The nonstructural (NS) proteins of flaviviruses are associated with participation in evading or antagonizing host innate immune defenses (Moriyama et al., 2007; Scholle and Mason, 2005; Wilson et al., 2008). Compared to its parent strain, WNV H8912 has amino acid substitutions in several coding and non-coding regions, including the NS proteins. These changes may contribute to the higher proinflammatory cytokine gene expression in WNV H8912-infected macrophages than those infected by WNV NY99.
WNV is now the leading cause of arboviral encephalitis in the U.S., but there is no specific therapeutic agent for treatment of the infection or an approved vaccine for its prevention. Many WNV convalescent patients have significant long-term morbidity following their acute illness (Carson et al., 2006; Cook et al., 2010; Ou and Ratard, 2005; Ravindra et al., 2004; Sadek et al., 2010). The underlying mechanism of long-term sequelae is not clearly understood. A recent study (Murray et al., 2010) reported detection of WNV RNA in urine samples of some convalescent WN encephalitis patients several years after their initial illness. Among this group, 80% reported chronic neurological symptoms and 20% had renal failure, suggesting an association of persistent renal infection with WNV-induced chronic neurological diseases. In hamsters, persistent WNV infection developed preferentially in the kidneys and brain with neurological sequelae and mild renal histopathological changes. These findings partially mimic observations in some WNV convalescent patients with long-term morbidity (Siddharthan et al., 2009; Tesh and Xiao, 2009; Tonry et al., 2005). However, there is an obstacle to study host immunity to chronic WNV infection in the hamster model due to a limited availability of reagents. Mice are easier to work with, amenable to immunological manipulation and are relatively inexpensive. It was shown in a recent study that following systemic infection with wild-type WNV infection in mice, the virus persisted in the CNS and peripheral tissues for up to 6 months post-infection with subclinical infections. Moreover, the frequency of persistence was tissue dependent in the following order: skin, spinal cord, brain, lymphoid tissues, kidney and heart (Appler et al., 2010). Although viruses were recovered from the persistently-infected tissues, full genomic sequencing of these WNV isolates is not revealed. Defining a murine model of WNV persistence, using a well characterized WNV isolate would be timely and important. In this study, we showed that a strain of WNV, isolated from the urine of a persistently-infected hamster was highly attenuated in both mouse macrophage and kidney epithelial cell lines. We also found that the WNV isolate obtained from hamster urine induced a differential proinflammatory cytokine response in the two cell types. Our results from the in vitro mouse model provide insight into the reduced neurovirulence of a WNV strain isolated from the urine of persistently-infected hamsters (Tesh et al., 2005). A better understanding of the immune mechanism of WNV persistence should lead to more effective and safer therapeutic approaches to alleviate persistent infection and to prevent the neurologic sequellae associated with it.
Supplementary Material
TLR3 and RIG-I are responsible for higher IL-6 production in WNV H8912–infected macrophage cells. The macrophages were transfected with siRNAs to TLR3 (A, siTLR3), or siRNA to RIG-I (B, siRIG-I) as described in Materials & Methods. Cells treated with the same concentration of scrambled siRNA were used as controls. Culture supernatant was harvested from mock and WNV H8912-infected cells at day 6 post-infection. IL-6 production was measured by Bioplex. Fold of increase compared to mock-infected group was shown. Data are presented as means ± SEM, n = 6. *P < 0.05 compared to control vector-treated cells.
Highlights.
WNV H8912 was attenuated in mouse macrophages and kidney epithelial cells.
WNV H8912 induced a differential IL-6 and TNF-α gene expression in both cell types.
IL-6 production in WNV H8912-infected macrophages was dependent on NF-κB activation.
Acknowledgments
This work was supported by NIH grant R01AI072060 and start-up funds from the University of Texas Medical Branch to T. Wang. R.B. Tesh was supported by NIH contract HHSN 2722010000401/HHSN 27200004/D04. G. Xie was supported by a James W. McLaughlin Predoctoral Fellowship. We thank Ms. Mardelle Susman for assisting in manuscript preparation.
Abbreviations used in this paper
- BHK
baby hamster kidney
- CNS
central nervous system
- DMEM
dulbecco’s modified eagle’s medium
- E
envelope
- FBS
fetal bovine serum
- IFN
interferon
- IL
interleukin
- MOI
multiplicity of infection
- NF-κB
nuclear factor kappa B
- PBS
phosphate buffered saline
- PFU
plaque forming unit
- Q-PCR
quantitative PCR
- RIG-I
retinoic acid inducible gene I
- TLR
toll like receptor
- TNF-α
tumor necrosis factor α
- WNV
West Nile virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85(2):85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Appler KK, Brown AN, Stewart BS, Behr MJ, Demarest VL, Wong SJ, Bernard KA. Persistence of West Nile virus in the central nervous system and periphery of mice. PLoS One. 2010;5(5):e10649. doi: 10.1371/journal.pone.0010649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley DW, Li L, Suderman MT, Barrett AD. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology. 2002;296(1):17–23. doi: 10.1006/viro.2002.1372. [DOI] [PubMed] [Google Scholar]
- Brien JD, Uhrlaub JL, Nikolich-Zugich J. Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur J Immunol. 2007;37(7):1855–63. doi: 10.1002/eji.200737196. [DOI] [PubMed] [Google Scholar]
- Busch MP, Kleinman SH, Tobler LH, Kamel HT, Norris PJ, Walsh I, Matud JL, Prince HE, Lanciotti RS, Wright DJ, Linnen JM, Caglioti S. Virus and antibody dynamics in acute west nile virus infection. J Infect Dis. 2008;198(7):984–93. doi: 10.1086/591467. [DOI] [PubMed] [Google Scholar]
- Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2(9):519–29. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- Cardosa MJ, Porterfield JS, Gordon S. Complement receptor mediates enhanced flavivirus replication in macrophages. J Exp Med. 1983;158(1):258–63. doi: 10.1084/jem.158.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson PJ, Konewko P, Wold KS, Mariani P, Goli S, Bergloff P, Crosby RD. Long-term clinical and neuropsychological outcomes of West Nile virus infection. Clin Infect Dis. 2006;43(6):723–30. doi: 10.1086/506939. [DOI] [PubMed] [Google Scholar]
- Conti L, Casetti R, Cardone M, Varano B, Martino A, Belardelli F, Poccia F, Gessani S. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J Immunol. 2005;174(1):252–60. doi: 10.4049/jimmunol.174.1.252. [DOI] [PubMed] [Google Scholar]
- Cook RL, Xu X, Yablonsky EJ, Sakata N, Tripp JH, Hess R, Piazza P, Rinaldo CR. Demographic and clinical factors associated with persistent symptoms after West Nile virus infection. Am J Trop Med Hyg. 2010;83(5):1133–6. doi: 10.4269/ajtmh.2010.09-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, Bowen R, Bunning ML. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75(9):4040–7. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Shrestha B, Marri A, Mahan D, Engle M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol. 2003a;77(4):2578–86. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 2003b;16(3):259–78. doi: 10.1089/088282403322396082. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med. 2003c;198(12):1853–62. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieli F, Caccamo N, Meraviglia S, Ivanyi J, Sireci G, Bonanno CT, Ferlazzo V, La Mendola C, Salerno A. Reciprocal stimulation of gammadelta T cells and dendritic cells during the anti-mycobacterial immune response. Eur J Immunol. 2004;34(11):3227–35. doi: 10.1002/eji.200425368. [DOI] [PubMed] [Google Scholar]
- Ding X, Wu X, Duan T, Siirin M, Guzman H, Yang Z, Tesh RB, Xiao SY. Nucleotide and amino acid changes in West Nile virus strains exhibiting renal tropism in hamsters. Am J Trop Med Hyg. 2005;73(4):803–7. [PubMed] [Google Scholar]
- Fredericksen BL, Gale M., Jr West Nile virus evades activation of interferon regulatory factor 3 through RIG-I-dependent and -independent pathways without antagonizing host defense signaling. J Virol. 2006;80(6):2913–23. doi: 10.1128/JVI.80.6.2913-2923.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericksen BL, Keller BC, Fornek J, Katze MG, Gale M., Jr Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J Virol. 2008;82(2):609–16. doi: 10.1128/JVI.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183(5):3170–6. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45(8):1039–46. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334(6057):825–9. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henchal EA, Gentry MK, McCown JM, Brandt WE. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg. 1982;31(4):830–6. doi: 10.4269/ajtmh.1982.31.830. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- King NJ, Shrestha B, Kesson AM. Immune modulation by flaviviruses. Adv Virus Res. 2003;60:121–55. doi: 10.1016/s0065-3527(03)60004-7. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Bernard KA. West Nile virus infection in birds and mammals. Ann N Y Acad Sci. 2001;951:84–93. doi: 10.1111/j.1749-6632.2001.tb02687.x. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38(11):4066–71. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Moriyama M, Matsumura H, Nirei K, Arakawa Y, Yamagami H, Ogawa M, Kaneko M, Matsuoka S, Amaki S, Tanaka N. Factors influencing treatment efficacy of 24-week combination therapy with interferon alpha-2b plus ribavirin for chronic hepatitis C. Dig Dis Sci. 2007;52(9):2418–26. doi: 10.1007/s10620-006-9693-0. [DOI] [PubMed] [Google Scholar]
- Murray K, Walker C, Herrington E, Lewis JA, McCormick J, Beasley DW, Tesh RB, Fisher-Hoch S. Persistent infection with West Nile virus years after initial infection. J Infect Dis. 2010;201(1):2–4. doi: 10.1086/648731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou AC, Ratard RC. One-year sequelae in patients with West Nile Virus encephalitis and meningitis in Louisiana. J La State Med Soc. 2005;157(1):42–6. [PubMed] [Google Scholar]
- Papa A, Danis K, Athanasiadou A, Delianidou M, Panagiotopoulos T. Persistence of West Nile virus immunoglobulin M antibodies, Greece. J Med Virol. 2011;83(10):1857–60. doi: 10.1002/jmv.22190. [DOI] [PubMed] [Google Scholar]
- Penn RG, Guarner J, Sejvar JJ, Hartman H, McComb RD, Nevins DL, Bhatnagar J, Zaki SR. Persistent neuroinvasive West Nile virus infection in an immunocompromised patient. Clin Infect Dis. 2006;42(5):680–3. doi: 10.1086/500216. [DOI] [PubMed] [Google Scholar]
- Pletnev AG, Swayne DE, Speicher J, Rumyantsev AA, Murphy BR. Chimeric West Nile/dengue virus vaccine candidate: Preclinical evaluation in mice, geese and monkeys for safety and immunogenicity. Vaccine. 2006;24(40–41):6392–404. doi: 10.1016/j.vaccine.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Pogodina VV, Bochkova NG, Levina LS. Persistence of tick-borne encephalitis virus in monkeys. VII. Some features of the immune response. Acta Virol. 1984;28(5):407–15. [PubMed] [Google Scholar]
- Pogodina VV, Frolova MP, Malenko GV, Fokina GI, Koreshkova GV, Kiseleva LL, Bochkova NG, Ralph NM. Study on West Nile virus persistence in monkeys. Arch Virol. 1983;75(1–2):71–86. doi: 10.1007/BF01314128. [DOI] [PubMed] [Google Scholar]
- Ratterree MS, Gutierrez RA, Travassos da Rosa AP, Dille BJ, Beasley DW, Bohm RP, Desai SM, Didier PJ, Bikenmeyer LG, Dawson GJ, Leary TP, Schochetman G, Phillippi-Falkenstein K, Arroyo J, Barrett AD, Tesh RB. Experimental infection of rhesus macaques with West Nile virus: level and duration of viremia and kinetics of the antibody response after infection. J Infect Dis. 2004;189(4):669–76. doi: 10.1086/381461. [DOI] [PubMed] [Google Scholar]
- Ravindra KV, Freifeld AG, Kalil AC, Mercer DF, Grant WJ, Botha JF, Wrenshall LE, Stevens RB. West Nile virus-associated encephalitis in recipients of renal and pancreas transplants: case series and literature review. Clin Infect Dis. 2004;38(9):1257–60. doi: 10.1086/383325. [DOI] [PubMed] [Google Scholar]
- Rios M, Zhang MJ, Grinev A, Srinivasan K, Daniel S, Wood O, Hewlett IK, Dayton AI. Monocytes-macrophages are a potential target in human infection with West Nile virus through blood transfusion. Transfusion. 2006;46(4):659–67. doi: 10.1111/j.1537-2995.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- Roehrig JT, Staudinger LA, Hunt AR, Mathews JH, Blair CD. Antibody prophylaxis and therapy for flavivirus encephalitis infections. Ann N Y Acad Sci. 2001;951:286–97. doi: 10.1111/j.1749-6632.2001.tb02704.x. [DOI] [PubMed] [Google Scholar]
- Sadek JR, Pergam SA, Harrington JA, Echevarria LA, Davis LE, Goade D, Harnar J, Nofchissey RA, Sewell CM, Ettestad P, Haaland KY. Persistent neuropsychological impairment associated with West Nile virus infection. J Clin Exp Neuropsychol. 2010;32(1):81–7. doi: 10.1080/13803390902881918. [DOI] [PubMed] [Google Scholar]
- Scholle F, Mason PW. West Nile virus replication interferes with both poly(I:C)-induced interferon gene transcription and response to interferon treatment. Virology. 2005;342(1):77–87. doi: 10.1016/j.virol.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Diamond MS. Role of CD8+ T cells in control of West Nile virus infection. J Virol. 2004;78(15):8312–21. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddharthan V, Wang H, Motter NE, Hall JO, Skinner RD, Skirpstunas RT, Morrey JD. Persistent West Nile virus associated with a neurological sequela in hamsters identified by motor unit number estimation. J Virol. 2009;83(9):4251–61. doi: 10.1128/JVI.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitati EM, Diamond MS. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J Virol. 2006;80(24):12060–9. doi: 10.1128/JVI.01650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh RB, Siirin M, Guzman H, Travassos da Rosa AP, Wu X, Duan T, Lei H, Nunes MR, Xiao SY. Persistent West Nile virus infection in the golden hamster: studies on its mechanism and possible implications for other flavivirus infections. J Infect Dis. 2005;192(2):287–95. doi: 10.1086/431153. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Xiao SY. Persistence of West Nile virus infection in vertebrates. In: Diamond MS, editor. West Nile Encephalitis Virus Infection Viral Pathogenesis and Host Response. Springer; New York: 2009. pp. 361–377. [Google Scholar]
- Tonry JH, Xiao SY, Siirin M, Chen H, da Rosa AP, Tesh RB. Persistent shedding of West Nile virus in urine of experimentally infected hamsters. Am J Trop Med Hyg. 2005;72(3):320–4. [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10(12):1366–73. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Wilson JR, de Sessions PF, Leon MA, Scholle F. West Nile virus nonstructural protein 1 inhibits TLR3 signal transduction. J Virol. 2008;82(17):8262–71. doi: 10.1128/JVI.00226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Lu L, Guzman H, Tesh RB, Xiao SY. Persistent infection and associated nucleotide changes of West Nile virus serially passaged in hamsters. J Gen Virol. 2008;89(Pt 12):3073–9. doi: 10.1099/vir.0.2008/003210-0. [DOI] [PubMed] [Google Scholar]
- Xiao SY, Guzman H, Zhang H, Travassos da Rosa AP, Tesh RB. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg Infect Dis. 2001;7(4):714–21. doi: 10.3201/eid0704.010420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TLR3 and RIG-I are responsible for higher IL-6 production in WNV H8912–infected macrophage cells. The macrophages were transfected with siRNAs to TLR3 (A, siTLR3), or siRNA to RIG-I (B, siRIG-I) as described in Materials & Methods. Cells treated with the same concentration of scrambled siRNA were used as controls. Culture supernatant was harvested from mock and WNV H8912-infected cells at day 6 post-infection. IL-6 production was measured by Bioplex. Fold of increase compared to mock-infected group was shown. Data are presented as means ± SEM, n = 6. *P < 0.05 compared to control vector-treated cells.