Abstract
Interleukin-12 (IL-12) is a major pro-inflammatory cytokine, which promotes cell-mediated immunity and TH1 differentiation. In vitro studies indicated suppression of IL-12 production by several stress-related factors, but no effects of behavioral stress were shown on plasma IL-12 levels. Therefore, in the current study we (i) examined the in vivo effects of various behavioral and pharmacological stress paradigms on baseline plasma IL-12 levels; (ii) compared these in vivo findings to those obtained following in vitro stimulation of leukocytes from the same rats; and (iii) assessed potential sexual dimorphism in these outcomes. The findings indicated that plasma IL-12 levels were significantly reduced by social confrontation, wet-cage exposure, surgery, and the administration of corticosterone, epinephrine, or prostaglandin-E2. Notably, most in vivo impacts on plasma levels were not evident when assessed in vitro. The IL-12-reducing effects of wet-cage exposure, and of corticosterone and epinephrine administration, were significantly greater in males than in females, although females exhibited greater total corticosterone levels following stress. The duration of acute stressors predicted the degree of IL-12 reduction, but more prolonged stressors did not. Furthermore, seven days of alternating behavioral stressors reduced plasma IL-12 levels more than fourteen days. These findings suggest animals’ behavioral habituation to stress conditions, or a specific immune mechanism restricting the duration of IL-12 reduction. Overall, our findings indicate a generic and robust stress-induced reduction in plasma IL-12 levels, and suggest epinephrine, corticosterone, and prostaglandin-E2, as potential mediators that should be scrutinized in vivo in the context of natural physiological stress responses.
Keywords: interleukin-12, stress, surgery, sex differences, in vivo, chronic, acute, social confrontation, induced production.
Introduction
Interleukin-12 (IL-12) is an important pro-inflammatory cytokine and a promoter of cell-mediated immunity. IL-12 is known to promote TH1 differentiation (i) by stimulating NK and CD +4 T cells to produce interferon-γ (IFN-γ); (ii) by stimulating the proliferation of undifferentiated T cells (Kubin et al., 1994; Trinchieri, 2003); and (iii) by supporting the expansion and activation of pre-activated TH1 cells (Perussia et al., 1992). IL-12 also coordinates between the innate and the adaptive immune systems by (i) directly stimulating leukocytes of both immune arms, including NK, T and B cells, and (ii) by stimulating NK cells of innate immunity (Trinchieri, 2003; Yoo et al., 2002) to enhance cytotoxicity and/or proliferation of CTLs and B cells (Curtsinger et al., 2003; Metzger et al., 1997). Finally, IL-12 has been shown to reduce tumor angiogenesis (Kanegane et al., 1998) by elevating CXC chemokine levels (e.g., IP-10 and Mig). Given the prevalent immunostimulatory and anti-angiogenic effects of IL-12, this cytokine is considered an important anti-tumor agent, in addition to being a prominent indicator of the TH1/TH2 cytokine balance.
Environmental and physiological stressors were shown to modulate numerous immune and inflammatory indices related to IL-12 levels. Surgical stress was shown to suppress CMI in humans (Bartal et al., 2010; Ogawa et al., 2000) and in animals (Glasner et al., 2010), as indicated by reduced number and activity of TH cells, CTLs, and NK cells, and by reduced expression of Fas ligand and CD11a on NK cells. Acute restraint stress was shown to reduce serum levels of IFN-γ and to suppress the expression of IFN-γ inducible genes, such as IP-10 (IFN-γ -inducible protein 10) and iNOS (inducible nitric oxide synthase). These effects were suggested to be mediated, at least partly, by reduced in vivo IL-12 levels (Curtin et al., 2009), but no direct evidence is available as of yet. In vitro studies suggested that several stress factors, including PGE2 (van der Pouw Kraan et al., 1995), catecholamines (Panina-Bordignon et al., 1997), and glucocorticoids (Elenkov et al., 1996), could reduce IL-12 production by leukocytes stimulated by biological response modifiers (e.g. LPS).
Chronic stress was repeatedly shown to suppress CMI and TH1 indices, and to promote cancer progression. For example, caregivers of Alzheimer patients exhibited lower percentages of total T and TH lymphocytes (Kiecolt-Glaser et al., 1987). In several animal models, chronic restraint stress had detrimental effects on immune functions, as was indicated by decreased gene expression of TH1 cytokines, including IFN-γ and IL-12p40, reduced numbers of CD4+ and CD8+ cells in the tumor's surrounding, and increased susceptibility to tumor development in squamous cell carcinoma (Saul et al., 2005). Others reported increased angiogenesis and tumor growth in an ovarian carcinoma model in the context of chronic stress (Thaker et al., 2006).
Several stress responses, including those affecting the immune system, were shown to be sexually dimorphic (Gaillard and Spinedi, 1998). Sex differences are prevalent in numerous immune and endocrine indices (Fish, 2008), and in disease susceptibility (Whitacre, 2001). For example, women are more vulnerable to several autoimmune diseases (e.g., multiple sclerosis and rheumatoid arthritis), and men are more susceptible to cardiovascular conditions. Both categories of diseases are associated with stress responses and immune perturbations. Thus, it is essential to address sexual dimorphism in the impact of stress on immunity, specifically on IL-12 levels which might mediate various immune modulations.
It is worthy to note that most studies addressing modulation of IL-12 production are based on in vitro or ex-vivo pharmacological approaches, which may not adequately reflect in vivo processes of stress-induced immunological perturbations. Additionally, many experiments are conducted on artificially-activated leukocytes (e.g., LPS-stimulated), rather than on naïve cells. This latter approach may bear major consequences, as different cytokine patterns were evident in induced vs. non-induced cultures (Greenfeld et al., 2007). To the best of our knowledge, no studies have assessed plasma IL-12 levels in the context of stress. Thus, the aims of the current study were to (i) examine the impact of various stress paradigms (including acute and chronic) on in vivo plasma levels of IL-12 in otherwise naïve animals; (ii) compare the in vivo findings to in vitro IL-12 induced production levels in the same rats; (iii) compare the effects of stress hormones administration (in presumably physiologically-relevant dosages) to those induced by behavioral stressors; and (iv) explore sex differences in these effects.
Materials and Methods
Animals and counterbalancing
Four month old male and female Fisher 344 (F344), and six month old (±1) male Long-Evans rats (Harlan Laboratories, Jerusalem, Israel), were housed 3-4 per cage in our vivarium with ad-lib access to food and water on a 12:12 light:dark cycle at 22 ± 1°C. Animals were handled daily during the last week prior to experimentation to reduce potential procedural stress. Body weight, sex, and the order of drug administration and blood withdrawal were counterbalanced across experimental groups in each study. In all studies blood was withdrawn during the second half of the light period, excluding Experiment 4 (chronic stress), in which blood was withdrawn at the beginning of the light period. Housing conditions were monitored by the Institutional Animal Care and Use Committee of Tel Aviv University, which also approved all studies described herein.
Wet-cage stress paradigm
Animals were placed in cages filled with 2 cm of room-temperature water, with ad-lib access to food and water, for duration of 1 to 20 hrs (varying between experiments). Our previous studies indicated that this stress paradigm significantly increased corticosterone (CORT) levels throughout the stress paradigm, an effect that completely dissipated within two hours of stress cessation (Levi et al., 2011).
Restraint stress
Each rat was placed for 10 hours in well-ventilated Plexiglas cylinders which confine movement without any potential harm to the animals, and with no access to food or water.
Cages mixing
F344 males from different cages were moved to a foreign cage for 10 hours in order to interfere with the social structure of the animals. The behavior of animals was monitored, and no prolonged fighting or injuries were evident.
Swim stress
A weight of 45 g/kg body weight was attached to the rat's tails. Each rat was then placed in a tank containing water, 60 cm deep at a temperature of 30°C for 3 minutes, which was followed by a 3 minutes rest period. This procedure was repeated five times consecutively (total of 30 min.). Animals underwent 2 sessions of swim stress, at the beginning and at the end of the dark period.
Social confrontation (SC) procedure
Social stress was induced using the resident–intruder confrontation procedure, as was previously described in our studies (Stefanski and Ben-Eliyahu, 1996). Shortly, this paradigm is based on the establishment of territoriality behavior by a Long-Evans male (resident) toward unfamiliar F344 male intruders (Stefanski et al., 2003). The Long-Evans male was housed with an ovariectomized F344 female rat, which was injected with estradiol (10 μg per animal) and progesterone (1 mg per animal) to induce estrus behavior and copulation with the “resident”. This procedure enhances the territoriality of the Long-Evans male. The insertion of two male F344 rats into the residents’ cage, at the beginning of the 12 h dark period, resulted in a confrontation between the resident and intruder males. The animals’ behavior during the confrontation period was monitored to enable termination of the study in case of extremely aggressive behavior (that was prevented by pre-screening of the residents). Blood was always withdrawn at the end of the dark period (either 12 or 36 hours later).
Experimental laparotomy
This procedure has been described elsewhere (Page et al., 1994). Briefly, rats were anesthetized with 2.5% isoflurane and a 4 cm midline abdominal incision was performed. The small intestine was externalized, rubbed with a PBS-soaked gauze pad, and left hydrated for 40 minutes. Finally, the intestine was internalized and the abdomen was sutured.
Blood withdrawal (from the tail or by cardiac puncture)
Except for exp. 4, blood was withdrawn by cardiac puncture. To this end, animals were anesthetized with an overdose of isoflurane, and one ml of blood was immediately withdrawn by cardiac puncture, within less than 3 minutes from disturbing the animals, using EDTA-containing syringes (1.8 mg/1ml blood). In exp. 4, 0.5 ml blood was collected from a small cut at the end of the tail into an EDTA-containing tube (1.8 mg/1ml blood). In both cases, blood was then centrifuged for 20 minutes at 930g 4°C for plasma separation, which was collected and stored at -20°C until assayed for IL-12 and/or CORT levels.
In vitro CpG-C-induced production of IL-12
Half a ml of whole blood was washed once with PBS (4-fold dilution, 10 min. at 456g, and supernatant removal to restore the original volume), and twice with complete media (see below). A 500 μl washed blood aliquot was then added to a well containing 500 μl of CM with CpG-C, reaching a final concentration of 5 μg CpG-C/ml. Samples were incubated at 100% humidity, 5% CO2, and 37°C for 20 h. The supernatants were then harvested and stored at -20°C until assayed for IL-12 levels.
In vitro spontaneous release of IL-12
To assess the spontaneous release of IL-12 without specific activation, a similar procedure to the above induced production approach was conducted, without adding CpG-C.
Assessment of IL-12 p70+p40 levels using ELISA
The anti rat IL-12 p70+p40 ELISA kit (BioSource International, Camarillo, CA) was used to assess IL-12 levels from plasma and supernatants, based on the manufacturer's instructions. The detection range of the kit is 15 to 1000 pg/ml. The intra assay coefficient of variability was less than 5%, as reported by the manufacturer.
Assessment of plasma corticosterone levels
Plasma CORT levels were measured by radioimmunoassay (RIA) (ImmuChem double antibody corticosterone 125I RIA kit, MP Biomedicals, Orangeburg, NY), per manufacturer's instructions. The intra assay coefficient of variability was less than 5%, as reported by the manufacturer.
Drugs and their administration
Drug sources
all drugs and substances (i.e., mannide monooleate, mineral oil, PGE2, epinephrine, and corticosterone), except for CpG-C and complete media, were obtained from Sigma-Aldrich, Rehovot, Israel. CpG-C was purchased from Coley Pharmaceuticals Canada, Ottawa, Canada and complete media was purchased from Biological Industries, Kibbutz Beit Haemek, Israel.
Slow-release emulsion
the emulsion is based on a mixture of PBS, mineral oil, and mannide monooleate (a non-ionic surface active emulsifier), in a 4:3:1 ratio, respectively. Drugs are dissolved in the PBS or the oil fraction of the emulsion before the emulsifying agent is added, and before a rigorous vortexing that is needed for creating the emulsion. Rats were always injected s.c. with 0.5 ml of the emulsion. Unpublished data from our laboratory have shown that drugs carried in this emulsion, were released slowly and were effective for at least 12 h.
Prostaglandin E2
the drug was dissolved in ethanol and diluted 1:10 in PBS. The drug was administered s.c. (0.8 mg/kg) in the slow-release emulsion (see above). This dose was shown in our previous studies to impact NK activity levels and MADB106 lung tumor retention levels, causing effect sizes similar to those of surgery (Yakar et al., 2003).
Epinephrine
the drug was dissolved in the PBS fraction of the slow-release emulsion (see above), and administered s.c. (0.5 or 1.5 mg/kg). This dose was shown in our previous studies to impact NK activity levels and MADB106 lung tumor retention levels, causing effect sizes similar to those of swim stress (Ben-Eliyahu et al., 2000).
Corticosterone
A fine powder of the drug was dissolved in corn oil, and administered s.c. (3 or 9 mg/kg, in a volume of 1 ml/kg). Our previous study in male rats indicated that one hour following administration of 3 mg/kg CORT, serum levels increased to approximately 700 ng/ml, halved after 3 h, and completely dissipated to baseline levels (50 ng/ml) by 6 hrs (Haim et al., 2003).
CpG-C oligodeoxynucleotides (ODNs)
CpG-C ODN (ODN 2395:5′-TCGTCGTTTTCGGCGCGCG CCG-3′) with a phosphorothioate backbone was used. CpG-C was dissolved in PBS and employed in a final concentration of 5 μg/ml in induced production experiments. CpG-C contained undetectable levels of endotoxin as measured by the limulus amebocyte lysate assay.
Complete Media (CM)
RPMI-1640 media supplemented with 10% heat-inactivated fetal calf serum (FCS), 50 μg/ml of gentamicin, 2 mM of L-glutamine, 0.1 mM of nonessential amino-acids, and 1 mM of sodium pyruvate.
Statistical Analyses
One- or two- way factorial analysis of variance (ANOVA), and repeated measures ANOVA with a pre-determined significance level of 0.05 were conducted. Provided significant group differences were found, Fisher's protected least significant differences (Fisher's PLSD) contrasts were performed to compare pair-wise comparisons, based on priori hypotheses, and Tukey's Honestly Significant Differences (Tukey's HSD) were performed to compare unplanned pair-wise comparisons.
Procedures and Results
Various stress paradigms reduce baseline plasma IL-12 levels
Experiment 1
Wet-cage exposure reduced plasma IL-12 levels, but did not decrease IL-12 levels following in vitro induced production or spontaneous release
Design and procedure
Eleven female and twelve male rats were either subjected to 10 hrs of wet-cage exposure (see Methods), or served as home-cage controls. Blood was withdrawn by cardiac puncture 2 hrs following stress cessation for assessment of IL-12 levels in the (i) plasma, (ii) supernatant of in vitro cultures following CpG-C induced production (see Methods), and (iii) supernatant of in vitro cultures without CpG-C (spontaneous release) (see Methods).
Results
Plasma IL-12 levels
A 2×2 (stress × sex) ANOVA revealed a significant reduction in IL-12 levels following wet-cage exposure [F(1,19)= 54.09, p < 0.05]. No sex differences or stress by sex interaction were evident (Fig. 1a). Fisher's PLSD pair-wise comparisons indicated a significant reduction by wet-cage exposure, for each sex (p < 0.05), compared to the respective control group.
Fig. 1. The effects of wet-cage exposure on IL-12 levels. A comparison between plasma, in vitro CpG-C induced production, and in vitro spontaneous release levels.
(A) Ten hrs of wet-cage exposure significantly reduced plasma IL-12 levels in both sexes. (B) No significant effects were evident in IL-12 levels when assessed following in vitro induced production. (C) No significant effects were evident in IL-12 levels when in vitro spontaneous release was assessed. * indicates a significant reduction compared to the respective control group. Data are presented as mean + SEM.
IL-12 supernatant levels following CpG-C induced production
A 2×2 (stress × sex) ANOVA did not reveal any significant effect (Fig. 1b).
IL-12 supernatant levels following in vitro spontaneous release
A 2×2 (stress × sex) ANOVA did not reveal any significant effect (Fig. 1c).
Experiment 2
Time course of the impact of wet-cage exposure on plasma levels of IL-12 and corticosterone
Design and procedure
Sixty-four male rats were subjected to different durations of stress paradigm that included 9 hrs of wet cage paradigm, then a 2 hrs recess at the home cage, and again a 9 hrs of wet cage exposure. Blood was withdrawn by cardiac puncture at different time points, once from each rat, at 1, 3, 9, 11, 12, 14, 20, or 22 hrs following the initiation of the stress paradigm (as detailed in Fig. 2). To prevent potential effects of circadian rhythm in IL-12 and corticosterone levels, the study was designed such that blood samples from all groups were collected at approximately the same time of the day (counterbalanced between groups), and an additional 8 home-cage control rats were simultaneously sampled for baseline levels. Corticosterone levels were assessed in all animals, while IL-12 levels were assessed only in animals sampled at the 1, 3, 9 and 20 h time points, and in control animals.
Fig. 2. Time course of wet-cage exposure on plasma IL-12 and CORT levels.
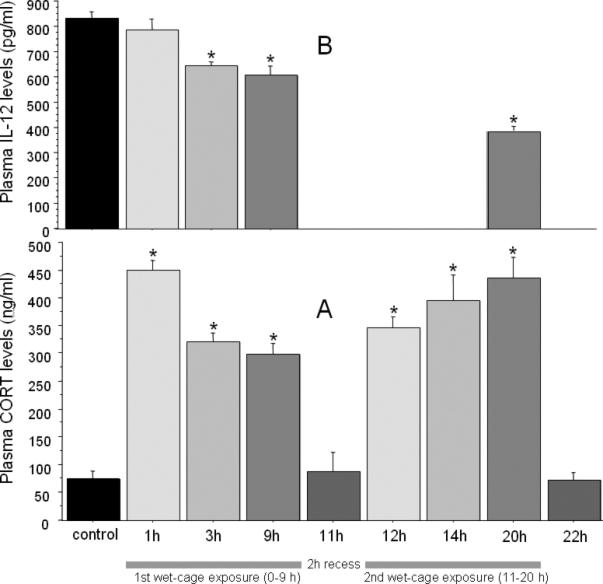
(A) CORT levels were elevated by wet-cage exposure during all time points within the stress sessions. Two hours following stress cessation (at 11 and 22 h) CORT levels retuned to baseline levels. (B) Plasma IL-12 levels did not decrease following 1 hr of wet-cage exposure, but significantly decreased in a time dependent manner at 3, 9, and 20 hrs following wet-cage exposure. * indicates a significant difference from control levels. Data are presented as mean + SEM.
Results
CORT levels
One-way ANOVA revealed significant group differences in CORT levels [F(8,63)= 33.29, p < 0.05]. Specifically, Tukey's HSD post hoc pair-wise comparisons indicated a significant and robust elevation in CORT levels following 1, 3 and 9 hrs of wet-cage exposure, which was significantly greater at 1 hr than at 3 and 9 hrs. After two hours of recess, CORT levels returned to baseline levels. In the second stress session, CORT levels were elevated once again at all time points measured (12, 14 and 20 h) and decreased back to baseline levels 2 hrs following stress cessation, (p < 0.05; Fig. 2 A).
IL-12 levels
One-way ANOVA revealed significant group differences in IL-12 levels [F(4,35)= 33.37, p < 0.05]. Specifically, Tukey's HSD post hoc pair-wise comparisons indicated a significant reduction in IL-12 levels following 3, 9 or 20 hrs of wet-cage exposure compared to control levels, but not following 1 hr of stress. Although no differences were evident between the 3 and 9 h time points, a further reduction in IL-12 levels was evident at the 20 h time point compared to the 3 and 9 h groups (p < 0.05; Fig. 2 B).
Experiment 3
A prolonged reduction in plasma IL-12 levels following social confrontation (SC) and surgery
Design and procedure
Twenty-nine female and 41 male rats served as home-cage controls, or were subjected to surgery (laparotomy) or to social confrontation (only males, given the nature of the procedure). Stress was initiated at 6, 12, 24, 36 or 48 hrs before blood withdrawal, so that blood collection was conducted at the beginning of the light period in all animals, to avoid potential effects of circadian rhythm on plasma IL-12 levels (n = 5-6 per group in each sex).
Results
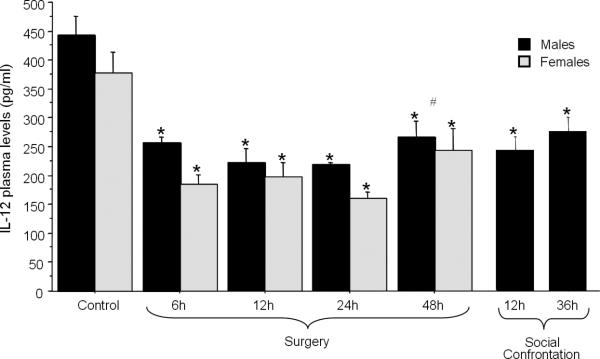
As only males were subjected to social confrontation, while surgery was conducted in both sexes, two different ANOVAs were used (although the data is presented together in Fig. 3). A 2×5 (sex × group {time following surgery or control}) ANOVA indicated significant group differences in plasma IL-12 levels [F(4,44)= 22.245, p < 0.05], a main effect for sex [F(1,44)= 6.64, p < 0.05], males having higher IL-12 levels, and no interaction. Specifically, Tukey's HSD post hoc pair-wise comparisons indicated a significant suppression of IL-12 levels at all time points tested following surgery, in both sexes, relative to the respective control group (p < 0.05). Additionally, 48 hrs post-operatively, IL-12 levels were higher than 24 hrs post-operatively, indicating initiation of a recovery phase (Fig. 3). Regarding to social confrontation paradigm, a one-way ANOVA (12 hrs SC, 36 hrs SC, and male control) showed significant group differences [F(2,28)= 12.442, p < 0.05], and a reduction in IL-12 levels by SC at both time points (p < 0.05; Fig. 3).
Fig. 3. The effects of surgery and SC on plasma IL-12 levels.
IL-12 levels reduced in all time points measured following either surgery or SC (marked by *). Forty eight hrs post-operatively, IL-12 levels were higher than 24 hrs postoperatively (marked by #), implying on a commencing recovery. A significant effect for sex was evident, as males had higher levels of IL-12 compared to females. Data are presented as mean + SEM.
Experiment 4
The impacts of chronic vs. acute stress on plasma IL-12 levels
Design and procedure
Twenty-four male rats were divided to three groups (n = 8 per group) which were subjected to acute stress, chronic stress, or served as controls (see Fig. 4b for experimental schedule and design). The acute stress group was subjected to one trial of 10 hrs wet-cage exposure on the second half of the seventh day. The chronic stress group was subjected to 14 days of alternating stress paradigms, including wet-cage exposure, restraint stress, cages mixing, and forced swim stress (see Methods). As in the acute group, the chronic group was subjected to a 10 hrs wet-cage exposure on the second half of the seventh day, and on the second half of the fourteenth day. Six blood samples were repeatedly withdrawn from the tail of each rat in a weekly interval (on days 0, 7, 14, 21, 28, 35), at the same time of the day (beginning of the light period). The second blood withdrawal (“week 1”) was conducted immediately after the stress session in both the acute and the chronic groups.
Fig. 4.
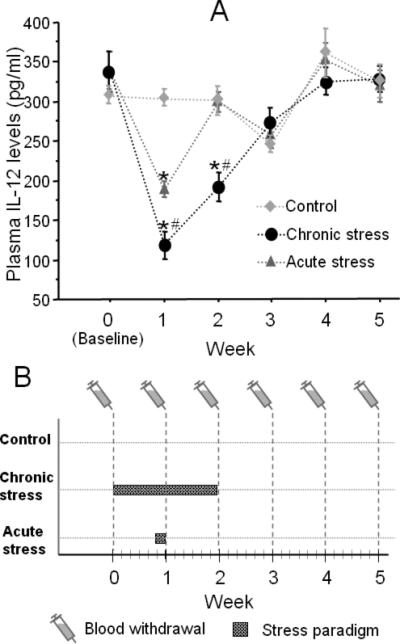
(A) The effects of chronic vs. acute stress on plasma IL-12 levels. Both acute stress (10 hrs of wet-cage exposure) and chronic stress (two weeks of daily alternating stress paradigms) reduced plasma IL-12 levels. Seven days of chronic stress had a greater impact than one day of acute stress (see week 1). However, 7 days of chronic stress reduced IL-12 levels greater than 14 days of stress. * indicates a significant difference from control levels within each week. # indicates a significant difference between chronic and acute groups within each week. Data are presented as mean + SEM. (B) Schematic representation of the design and procedure of exp. 4. The control group was not subjected to stress, the acute group was subjected to 10 hrs of stress on day 6, and the chronic stress group was subjected to two weeks of alternating stress paradigms. Blood was withdrawn via the tail every week from all animals.
Results
A 3×6 (group, a between subjects variable, × time point, a within subjects variable) repeated measures ANOVA revealed a significant interaction between groups and time points of blood sampling [F(10,105)= 9.29, p < 0.05]. Specifically, Tukey's HSD post hoc pair-wise comparisons indicated a significant decrease in IL-12 levels following both acute and chronic stress, at week 1, compared to control levels, showing a greater reduction in the chronic stress group compared to the acute stress group. At the third measurement (week 2), IL-12 levels remained significantly lower than controls only in the chronic stress group. Interestingly, IL-12 levels were more profoundly reduced following one week of chronic stress, compared to two weeks of the chronic stress paradigm. In both chronic and acute stress groups, IL-12 levels returned to baseline within one week following stress cessation, and maintained these levels thereafter (p < 0.05; Fig. 4a).
Pharmacological paradigms and their comparison to behavioral stress
Experiment 5
A comparison between the effects of epinephrine administration and wet-cage exposure on plasma IL-12 levels
Design and procedure
Forty-seven male rats were divided to five groups that were either subjected to 3 or 10 hrs of wet-cage exposure, injected s.c. with 0.5 or 1.5 mg/kg epinephrine (in SRE), or served as controls (injected with vehicle). Blood was withdrawn by cardiac puncture from all rats at the end of the light period, 12 hrs following stress initiation or epinephrine/vehicle administration.
Results
One-way ANOVA revealed significant group differences [F(4,42)= 20.8, p < 0.05]. Specifically, Tukey's HSD post hoc pair-wise comparisons indicated a significant reduction in IL-12 levels following 3 hrs of wet-cage exposure compared to control levels, and a significantly greater reduction following 10 hrs of this paradigm. Similarly, IL-12 levels were significantly reduced by administration of 0.5 mg/kg epinephrine compared to control levels, and were reduced more profoundly following administration of 1.5 mg/kg. It is worthy to note that the magnitude of the IL-12 reducing effects of 3 hrs of wet-cage exposure was very similar to those of 0.5 mg/kg epinephrine administration, as was the magnitude of the IL-12 reducing effects of 10 hrs of wet-cage exposure to those of 1.5 mg/kg epinephrine administration (p < 0.05; Fig. 5).
Fig. 5. The effects of behavioral stress and epinephrine administration on plasma IL-12 levels.
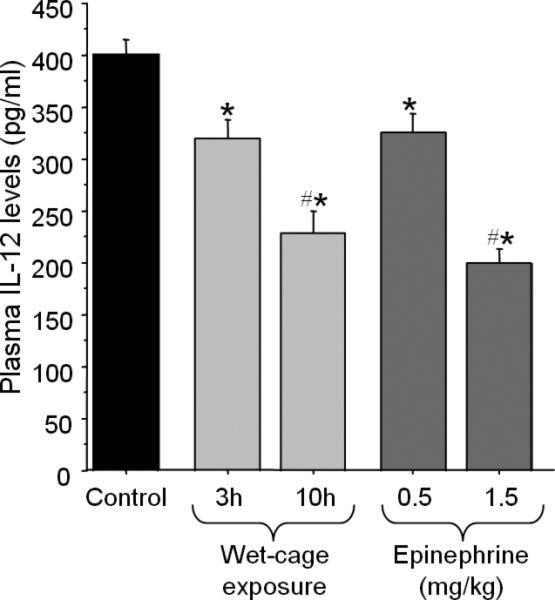
Both wet-cage exposure (3 or 10 hrs duration) and epinephrine administration (0.5 mg/kg or 1.5 mg/kg) significantly reduced plasma IL-12 levels in a time/dose dependent manner, having similar magnitude of effects. * indicates a significant difference from control levels. # indicates a significant difference between the two durations/dosages. Data are presented as mean + SEM.
Experiment 6
The impacts of wet-cage exposure and the administration of PGE2, CORT, or epinephrine on plasma IL-12 levels and on in vitro induced production of IL-12
Design and procedure
Thirty-six female and 35 male rats were subjected to 10 hrs of wet-cage exposure, or were injected s.c. with PGE2 (0.8 mg/kg in SRE), epinephrine (0.5 mg/kg in SRE), CORT (9 mg/kg) or vehicle (SRE). PGE2, epinephrine, and vehicle were administered in SRE either at 10 or 5 hrs prior to blood withdrawal. Given the short half-life of CORT, the 10 h groups were injected with CORT twice, at 10 and 5 hrs before blood withdrawal, and the 5 h CORT groups received CORT only once (5 hrs before blood withdrawal). Immediately upon completing the 10 h wet-cage exposure, all animals underwent blood withdrawal for assessment of IL-12 levels in plasma and preparation of in vitro cultures of CpG-C induced IL-12 production (see Methods).
Results
Plasma IL-12 levels
The two control groups (vehicle administration at the 5 or at the 10 h time points) showed very similar levels of IL-12, and were thus combined for further analysis and presentation. A 2×8 (sex × group) ANOVA revealed significant group differences [F(7,55)= 27.221, p < 0.05]. This effect was greater in males than in females [F(1,55)= 40.442, p < 0.05] in the wet-cage exposure and when animals were injected with either CORT or epinephrine (p < 0.05), but not with PGE2 or among the controls, as indicated by Fisher's PLSD post hoc comparisons (Fig. 6a). In addition, a significant reduction in IL-12 levels was evident in all conditions in comparison to the control group (in both sexes), (p < 0.05; Fig. 6a).
Fig. 6.
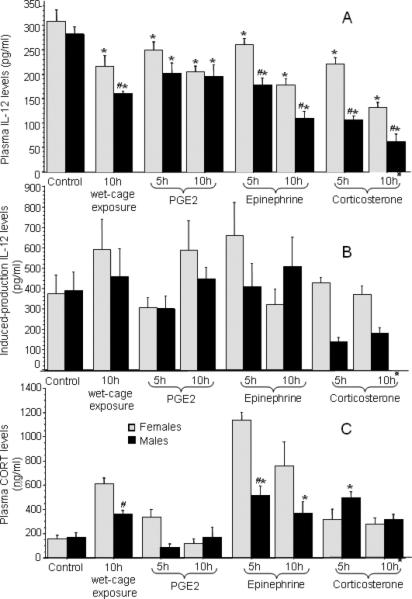
(A) The effects of behavioral stress vs. agonist administration on plasma IL-12 levels. IL-12 levels were reduced by all agonists and by exposure to wet-cage paradigm. This reduction was greater in males than in females in the groups of wet-cage exposure and the administration of epinephrine and CORT. * indicates a significant reduction compared to control, and # indicates a significant difference between females and males. these groups were injected twice given the short half life of CORT. (B) The effects of behavioral stress vs. agonist administration on induced-production IL-12 levels. No significant effects were evident in this method of IL-12 levels assessment, and a different pattern of results was evident compared to plasma IL-12 levels. (C) The effects of behavioral stress vs. agonists administration on plasma CORT levels CORT levels were elevated by exposure to wet-cage paradigm and by the administration of epinephrine and CORT. In females, the administration of epinephrine elevated CORT levels more than the administration of CORT and the wet-cage paradigm. * indicates a significant elevation compared to the respective control. # indicates a significant difference between females and males. Data are presented as mean + SEM.
IL-12 supernatant levels following CpG-C induced production
Similar to our results in the plasma, the two control groups showed very similar levels of IL-12, and were thus combined for further analysis and presentation. A 2×8 (sex × group) ANOVA on induced-production IL-12 levels did not reveal any significant effects (Fig. 6b). This is in contrast to the plasma levels of IL-12, which showed significant differences (see above). Moreover, the pattern of the effects was different from those evident in plasma levels. For example, wet-cage exposure significantly reduced plasma IL-12 levels by approximately 50%, but no such effects were evident in induced-production IL-12 levels.
CORT levels
As in the IL-12 analysis, the two control groups showed very similar levels of CORT, and were thus combined for further analysis and presentation. A 2×8 (sex × group) ANOVA showed a significant elevation in CORT levels following wet-cage exposure, and CORT and epinephrine injection [F(7,51)= 19.56, p < 0.05]. In addition, a significant interaction between sex and group was evident [F(7,51)= 6.16, p < 0.05], such that wet-cage exposure and administration of epinephrine induced higher CORT levels in females. Interestingly, in females, epinephrine administration elevated CORT levels more profoundly than the wet-cage exposure and the administration of CORT itself (p < 0.05; Fig. 6c).
Experiment 7
Time course and dose dependent impacts of CORT administration on plasma IL-12 levels
Design and procedure
Thirty-five female and 39 male rats were injected s.c. with either 3 or 9 mg/kg of CORT, or with vehicle (corn oil). Blood withdrawal for assessment of plasma IL-12 levels was conducted either at 5, 10 or 29 hrs following 3 mg/kg CORT/vehicle administration, or at 5 hrs after 9 mg/kg of CORT administration (see design in Fig 7).
Fig. 7. Time course and dose dependent effects of CORT administration on plasma IL-12 levels.
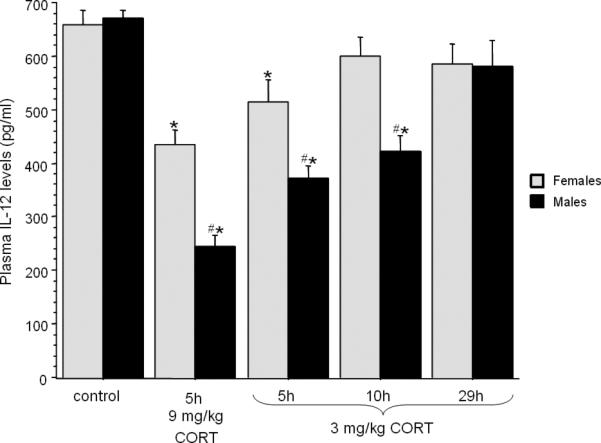
CORT reduced plasma IL-12 levels in a dose-dependent manner, with a greater effect in males than in females. Five hrs following CORT administration IL-12 levels were significantly reduced both in females and males, as opposed to 10 hrs following its administration, in which IL-12 levels remained low only in males. Twenty nine hours following CORT administration the reduction in IL-12 levels completely dissipated in both sexes. * indicates a significant reduction compared to control. # indicates a significant difference between sexes. Data are presented as mean + SEM.
Results
The three control groups (vehicle at 5, 10 or 29 h time points) showed very similar levels of IL-12, and were thus combined for further analysis and presentation. A 2×5 (sex × CORT regimen) ANOVA indicated a significant main effect for CORT regimen (dose and time effects) [F(4,64)=34.977, p < 0.05], and a significant interaction of CORT regimen with sex [F(4,64)= 4.938, p < 0.05], with males showing a greater reduction in plasma IL-12 levels than females at 5 and 10 hrs following CORT administration. As indicated by Fisher's PLSD, IL-12 levels in females were significantly reduced compared to females baseline levels at the 5 hrs time point (at both dosages), but not at 10 h. In contrast, males showed a significant reduction at both the 5 and 10 h time points. The reduction in IL-12 levels completely dissipated in both sexes, at 29 hrs post injection (p < 0.05; Fig. 7).
Discussion
To the best of our knowledge, this study is the first to assess the impact of stress on baseline plasma IL-12 levels in otherwise naïve animals. Previous studies addressing the impact of stress or stress hormones on IL-12 were based on (i) in vitro experiments, exposing cells to biological-response-modifiers (BRMs, e.g., LPS) (Panina-Bordignon et al., 1997) and assessing IL-12 production in these artificial conditions; or (ii) employing animals subjected to BRMs in vivo (Curtin et al., 2009). Such studies may not accurately and comprehensively reflect stress-related changes in plasma baseline cytokine levels. Specifically, numerous physiological indices, which are continuously fluctuating and interacting in vivo in response to stressors, are not mirrored in the in vitro milieu. These include alterations in cell distribution and changes in numerous soluble factors. Additionally, some cytokine-secreting cells are absent in vitro, including epithelial cells or immunocytes that are restricted to specific immune compartments (e.g., liver PIT cells). Finally, leukocytes activated by BRMs in vitro or in vivo may respond differently to stress hormones (or other modulating factors) compared to non-activated leukocytes.
Indeed, our study clearly indicates that in vitro production of IL-12 (spontaneously or in response to CpG-C) yielded different patterns of effects compared to those evident in the plasma in the same animals. In both exp. 1 and 6, the effects of wet-cage exposure or of pharmacological manipulations (injection of epinephrine, PGE2, or CORT) on IL-12 in vitro production vs. its plasma levels showed inconsistent patterns (IL-12 levels being suppressed only in the plasma). Thus, as each approach clearly has its own advantages, in vivo levels and induced production measures should be considered complementary rather than interchangeable when studying cytokines modulation.
In the current study we chose to use CpG-C ODN as a BRM for the in vitro induced production study, as it is a widespread natural BRM that potently induce the production of IL-12 and type-1 interferons. Specifically, CpG-C is a characteristic motif of bacterial DNA that stimulates the intracellular Toll-like receptor 9 (TLR9), eventually leading to the activation of B, NK, and antigen-presenting cells. CpG-C induces the release of various TH1 cytokines dendritic cells and macrophages, and is becoming a widespread immunoadjuvant given its relatively mild adverse effects (Weiner, 2000). We compared in vitro IL-12 production by leukocytes exposed to CpG-C, Poly I:C and LPS at various concentrations (unpublished data from our laboratory) and found CpG-C to be the most consistent and potent inducer of IL-12 production.
All stress paradigms and stress hormones employed in this study resulted in reduced plasma IL-12 levels, including the wet-cage paradigm, social confrontation, surgery, and the administration of PGE2, epinephrine, and CORT. These findings suggest the generalizability and robustness of the impact of stress on this important TH1 cytokine. The reduction in plasma IL-12 levels was evident as early as 3 hrs following stress initiation, but not earlier (i.e., 1 hr following stress; exp. 2). As epinephrine and CORT levels rise within seconds to minutes following stress initiation, the delay in reduced IL-12 levels in the current study is probably a result of the prolonged half-life time of IL-12 (approximately 2-5 hrs in rats) (Stern et al., 1996; Trinchieri and Scott, 1994), while its production may have ceased shortly upon stress initiation. Additionally, when employing acute stressors in the current study (hours to a few days), a longer duration of stress exposure, or a higher dose of stress hormones, resulted in a greater reduction in IL-12 levels (Fig. 2, 4, 5, 7). On the other hand, when stress exposure lasted for days (specifically, animals subjected to 12 vs 36 hrs of SC, or studied 12-48 hrs following surgery), similar magnitude of IL-12 reduction along the different time points was observed. Moreover, seven days of the alternating stress paradigm caused a significantly greater reduction in IL-12 levels compared to 14 days of the same paradigm (exp. 4). Thus, a general mechanism of animals’ adjustment to stress conditions, or a specific mechanism that counteracts long-term reductions in IL-12 levels, is implied.
A complete recovery of IL-12 levels following stress cessation occurred within approximately one day following CORT administration, and within less than one week following 14 days of chronic stress (shorter post-stress intervals were not assessed in this study). Although the neuroendocrine perturbations caused by surgery are continuous, and do not vanish immediately, a partial recovery of IL-12 levels was evident at 48 hrs post-operatively, and a complete recovery was seen at 96 hrs. Taken together, a reduction in IL-12 levels seems to last for approximately a day. Nevertheless, such reduction may still bear pivotal implications in specific circumstances, such as the critical perioperative period in cancer patients, when the fate of metastases may be determined within a short perioperative period (Ben-Eliyahu, 2003; Colacchio et al., 1994). Certain immune responses that bear long-term immunological consequences, such as antigen presentation, may also yield long-term effects of diminished IL-12 levels. Finally, IL-12 reduction may have prolonged consequences through its downstream impact on TH1/TH2 cell ratio. These potential long-term effects are beyond the scope of this study.
Sex differences were prevalent in some experiments conducted in this study. Specifically, IL-12 levels were reduced more profoundly in males than in females following various paradigms, including the wet-cage exposure and the administration of epinephrine and CORT, but not following PGE2 administration or surgery. On the other hand, CORT levels were significantly higher in females following the wet-cage paradigm and epinephrine administration. As CORT was shown to reduce IL-12 levels both in this study and in in vitro studies (DeKruyff et al., 1998), these results seem paradoxical. However, they can be explained by the reported higher levels of corticosteroid-binding globulin (CBG) (Kurabekova et al., 1988) and CORT metabolism (Kitay, 1961) in females, which potentially expose them to lower physiologic levels of free (active) CORT. The sexual dimorphism evident in this study, which occurred in some but not other stress paradigms, further suggests that multiple mechanisms underlie the impact of stress and stress hormones on plasma IL-12 levels.
Most autoimmune diseases are more prevalent in women. It was suggested that sex hormones play a role in these dimorphisms, as in vitro studies indicated modulations of cytokine levels by estrogens and androgens (Araneo et al., 1991; Gilmore et al., 1997; Wynn et al., 1990), and as the abundance of estrogens during pregnancy often reduces symptoms of autoimmune diseases. Interestingly, several autoimmune diseases were shown to be exacerbated by TH1 activation (Abbas et al., 1996; Saoudi et al., 1993; Scott et al., 1994), which is known to be regulated by both stress and sex hormones. In the context of frequent stress responses characterizing modern lifestyle, a lower sensitivity of females to stress, which is indicated herein by higher IL-12 levels, is congruent with the hypothesis that higher IL-12 levels in women (and potentially other TH1 and pro-inflammatory cytokines (Nguyen et al., 2003)) may contribute to their increased prevalence of autoimmune diseases, most likely in conjunction with other necessary mediating responses.
This study suggests specific doses of epinephrine, CORT, and PGE2 as presumably physiologically relevant doses, as each of them reduced IL-12 levels to a similar extent as did the behavioral stress paradigms (within the same study). For example, 1.5 mg/kg of epinephrine (in a slow release emulsion) was as effective as 10 hrs exposure to the wet-cage paradigm. These doses were similarly shown to be physiologically relevant regarding other immune indices that we have studied, including NK activity and lung tumor retention (Ben-Eliyahu et al., 2000; Yakar et al., 2003). However, it should be noted that a similar impact on IL-12 levels does not indicate that the same mechanism underlies the behavioral and the pharmacological stress paradigms. Specifically, CORT is secreted and acts systemically, and its levels are easily measurable. Therefore, plasma CORT levels following CORT administration and following wet-cage exposure could justifiably be compared, and were found to be similar following 3 mg/kg of CORT administration (Haim et al., 2003). On the other hand, epinephrine and PGE are secreted locally in relatively high levels, and have short half-lives; thus, their effective levels are difficult to assess, and a systemic administration of these hormones does not accurately simulate their natural secretion pattern or their potential effects.
It was previously shown that injection of epinephrine to the Paraventricular nucleus (PVN) induces the release of CORT from the adrenal gland (Leibowitz et al., 1988), and that the secretion of CORT induced by IL-1 is a catecholamine-dependent process at the adrenal gland level (Gwosdow et al., 1992; O'Connell et al., 1994). In the present study, plasma CORT levels were significantly elevated by systemic epinephrine administration, reaching higher CORT levels than those reached following CORT administration or wet-cage exposure (Fig. 6c). However, epinephrine administration reduced IL-12 levels to a lesser extent than did CORT administration. These results further suggest that different mechanisms impact IL-12 levels in pharmacological and physiological stress paradigms. Therefore, elucidating specific neuroendocrine mechanisms of stress-induced suppression of IL-12 levels should clearly include approaches that selectively counteract the endogenous rise of specific stress hormones in the context of behavioral stress responses. Given that CAs, PGE, and CORT mutually affect each other's levels, studying the unique impact of each of them in vivo is not straightforward.
In conclusion, this study demonstrates, for the first time, an in vivo reduction in baseline plasma IL-12 levels by various stress paradigms and stress hormones, in both females and males. The incongruent outcomes obtained when assessing IL-12 levels in the plasma versus following in vitro induced production, indicate that these approaches may reflect different aspects of immunity and thus, should be considered complementary in studying cytokine regulation. Lastly, the findings of this paper suggest several potential neuroendocrine mediating mechanisms of the impacts of stress, including epinephrine, CORT, and PGE2, but are insufficient to exclusively implicate any of them in the context of natural stress responses.
Research Highlight.
Behavioral stressors, surgery, and stress hormones reduce baseline plasma IL-12 levels, novel effects that were not all evident in the induced production approach
Acknowledgment
This work was supported by NIH/NCI grant # CA125456 (SBE), and by the Israel-USA bi-national Science Foundation # 2005331 (SBE & GGP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
references
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383(6603):787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Araneo BA, Dowell T, Diegel M, Daynes RA. Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and gamma-interferon, but not IL-2 by activated murine T cells. Blood. 1991;78(3):688–699. [PubMed] [Google Scholar]
- Bartal I, Melamed R, Greenfeld K, Atzil S, Glasner A, Domankevich V, Naor R, Beilin B, Yardeni IZ, Ben-Eliyahu S. Immune perturbations in patients along the perioperative period: alterations in cell surface markers and leukocyte subtypes before and after surgery. Brain Behav Immun. 2010;24(3):376–386. doi: 10.1016/j.bbi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S. The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav Immun. 2003;17(Suppl 1):S27–36. doi: 10.1016/s0889-1591(02)00063-6. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8(3):154–164. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- Colacchio TA, Yeager MP, Hildebrandt LW. Perioperative immunomodulation in cancer surgery. Am J Surg. 1994;167:1. doi: 10.1016/0002-9610(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Curtin NM, Boyle NT, Mills KH, Connor TJ. Psychological stress suppresses innate IFN-gamma production via glucocorticoid receptor activation: reversal by the anxiolytic chlordiazepoxide. Brain Behav Immun. 2009;23(4):535. doi: 10.1016/j.bbi.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197(9):1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKruyff RH, Fang Y, Umetsu DT. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol. 1998;160(5):2231–2237. [PubMed] [Google Scholar]
- Elenkov IJ, Papanicolaou DA, Wilder RL, Chrousos GP. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians. 1996;108(5):374–381. [PubMed] [Google Scholar]
- Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard RC, Spinedi E. Sex- and stress-steroids interactions and the immune system: evidence for a neuroendocrine-immunological sexual dimorphism. Domest Anim Endocrinol. 1998;15(5):345–352. doi: 10.1016/s0739-7240(98)00028-9. [DOI] [PubMed] [Google Scholar]
- Gilmore W, Weiner LP, Correale J. Effect of estradiol on cytokine secretion by proteolipid protein-specific T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol. 1997;158:1. [PubMed] [Google Scholar]
- Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, Meiboom H, Ben-Eliyahu S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184(5):2449–2457. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- Greenfeld K, Avraham R, Benish M, Goldfarb Y, Rosenne E, Shapira Y, Rudich T, Ben-Eliyahu S. Immune suppression while awaiting surgery and following it: dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav Immun. 2007;21(4):503–513. doi: 10.1016/j.bbi.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Gwosdow AR, O'Connell NA, Spencer JA, Kumar MS, Agarwal RK, Bode HH, Abou-Samra AB. Interleukin-1-induced corticosterone release occurs by an adrenergic mechanism from rat adrenal gland. Am J Physiol. 263(3 Pt 1):E461–466. doi: 10.1152/ajpendo.1992.263.3.E461. [DOI] [PubMed] [Google Scholar]
- Haim S, Shakhar G, Rossene E, Taylor AN, Ben-Eliyahu S. Serum levels of sex hormones and corticosterone throughout 4- and 5-day estrous cycles in Fischer 344 rats and their simulation in ovariectomized females. J Endocrinol Invest. 2003;26(10):1013–1022. doi: 10.1007/BF03348201. [DOI] [PubMed] [Google Scholar]
- Kanegane C, Sgadari C, Kanegane H, Teruya-Feldstein J, Yao L, Gupta G, Farber JM, Liao F, Liu L, Tosato G. Contribution of the CXC chemokines IP-10 and Mig to the antitumor effects of IL-12. J Leukoc Biol. 1998;64(3):384–392. doi: 10.1002/jlb.64.3.384. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Shuttleworth EC, Dyer CS, Ogrocki P, Speicher CE. Chronic stress and immunity in family caregivers of Alzheimer's disease victims. Psychosom Med. 1987;49(5):523–535. doi: 10.1097/00006842-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- Kubin M, Kamoun M, Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994;180(1):211–222. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurabekova RM, Mataradze GD, Rozen VB. Mechanisms of sex differentiation of the level of corticosteroid-binding globulin in rats]. Probl Endokrinol (Mosk) 1988;34(6):66–70. [PubMed] [Google Scholar]
- Leibowitz SF, Sladek C, Spencer L, Tempel D. Neuropeptide Y, epinephrine and norepinephrine in the paraventricular nucleus: stimulation of feeding and the release of corticosterone, vasopressin and glucose. Brain Res Bull. 1988;21(6):905–912. doi: 10.1016/0361-9230(88)90025-1. [DOI] [PubMed] [Google Scholar]
- Levi B, Benish M, Goldfarb Y, Sorski L, Melamed R, Rosenne E, Ben-Eliyahu S. Continuous stress disrupts immunostimulatory effects of IL-12. Brain Behav Immun. 2011;25(4):727–735. doi: 10.1016/j.bbi.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger DW, McNutt RM, Collins JT, Buchanan JM, Van Cleave VH, Dunnick WA. Interleukin-12 acts as an adjuvant for humoral immunity through interferon-gamma-dependent and -independent mechanisms. Eur J Immunol. 1997;27(8):1958–1965. doi: 10.1002/eji.1830270820. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Ramanathan M, Weinstock-Guttman B, Baier M, Brownscheidle C, Jacobs LD. Sex differences in in vitro pro-inflammatory cytokine production from peripheral blood of multiple sclerosis patients. J Neurol Sci. 2003;209(1-2):93–99. doi: 10.1016/s0022-510x(03)00004-2. [DOI] [PubMed] [Google Scholar]
- O'Connell NA, Kumar A, Chatzipanteli K, Mohan A, Agarwal RK, Head C, Bornstein SR, Abou-Samra AB, Gwosdow AR. Interleukin-1 regulates corticosterone secretion from the rat adrenal gland through a catecholamine-dependent and prostaglandin E2-independent mechanism. Endocrinology. 1994;135(1):460–467. doi: 10.1210/endo.135.1.8013385. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Hirai M, Katsube T, Murayama M, Hamaguchi K, Shimakawa T, Naritake Y, Hosokawa T, Kajiwara T. Suppression of cellular immunity by surgical stress. Surgery. 2000;127(3):329–336. doi: 10.1067/msy.2000.103498. [DOI] [PubMed] [Google Scholar]
- Page GG, Ben-Eliyahu S, Liebeskind JC. The role of LGL/NK cells in surgery-induced promotion of metastasis and its attenuation by morphine. Brain Behav Immun. 1994;8(3):241–250. doi: 10.1006/brbi.1994.1022. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P, Mazzeo D, Lucia PD, D'Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest. 1997;100(6):1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B, Chan SH, D'Andrea A, Tsuji K, Santoli D, Pospisil M, Young D, Wolf SF, Trinchieri G. Natural killer (NK) cell stimulatory factor or IL-12 has differential effects on the proliferation of TCR-alpha beta+, TCR-gamma delta+ T lymphocytes, and NK cells. J Immunol. 1992;149(11):3495–3502. [PubMed] [Google Scholar]
- Saoudi A, Kuhn J, Huygen K, de Kozak Y, Velu T, Goldman M, Druet P, Bellon B. TH2 activated cells prevent experimental autoimmune uveoretinitis, a TH1-dependent autoimmune disease. Eur J Immunol. 1993;23(12):3096–3103. doi: 10.1002/eji.1830231208. [DOI] [PubMed] [Google Scholar]
- Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, Malarkey WB, Lehman A, Lemeshow S, Dhabhar FS. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97(23):1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B, Liblau R, Degermann S, Marconi LA, Ogata L, Caton AJ, McDevitt HO, Lo D. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994;1(1):73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Stefanski V, Ben-Eliyahu S. Social confrontation and tumor metastasis in rats: defeat and beta-adrenergic mechanisms. Physiol Behav. 1996;60(1):277–282. doi: 10.1016/0031-9384(96)00014-5. [DOI] [PubMed] [Google Scholar]
- Stefanski V, Peschel A, Reber S. Social stress affects migration of blood T cells into lymphoid organs. J Neuroimmunol. 2003;138(1-2):17–24. doi: 10.1016/s0165-5728(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Stern AS, Magram J, Presky DH. Interleukin-12 an integral cytokine in the immune response. Life Sci. 1996 doi: 10.1016/s0024-3205(96)80003-8. [DOI] [PubMed] [Google Scholar]
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Scott P. The role of interleukin 12 in the immune response, disease and therapy. Immunol Today. 1994;15(10):460–463. doi: 10.1016/0167-5699(94)90189-9. [DOI] [PubMed] [Google Scholar]
- van der Pouw Kraan TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181(2):775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner GJ. The immunobiology and clinical potential of immunostimulatory CpG oligodeoxynucleotides. J Leukoc Biol. 2000;68(4):455–463. [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2(9):777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Wynn DR, Rodriguez M, O'Fallon WM, Kurland LT. A reappraisal of the epidemiology of multiple sclerosis in Olmsted County, Minnesota. Neurology. 1990;40(5):780–786. doi: 10.1212/wnl.40.5.780. [DOI] [PubMed] [Google Scholar]
- Yakar I, Melamed R, Shakhar G, Shakhar K, Rosenne E, Abudarham N, Page GG, Ben-Eliyahu S. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10(4):469–479. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Yoo JK, Cho JH, Lee SW, Sung YC. IL-12 provides proliferation and survival signals to murine CD4+ T cells through phosphatidylinositol 3-kinase/Akt signaling pathway. J Immunol. 2002;169(7):3637–3643. doi: 10.4049/jimmunol.169.7.3637. [DOI] [PubMed] [Google Scholar]