Abstract
Background
Persons with occupational or recreational exposure to migratory birds may be at risk for infection with highly pathogenic avian influenza and other avian influenza viruses since wild birds are the natural reservoir of influenza A. Additionally, bird handlers may host avian and swine-origin influenza (pH1N1) virus co-infections, which generate reassortant viruses with high pathogenicity in mammals.
Objectives
We assessed the prevalence of avian and swine influenza viruses in US-based bird handlers and estimated their exposure to different orders of wild birds including waterfowl (Anseriformes), songbirds (Passeriformes), and shorebirds (Charadriiformes).
Study Design
Cross-sectional serologic survey accompanied by a questionnaire to estimate behavioral risk factors. This is first survey of US-based bird handlers who also work at international sites.
Results
401 participants were recruited and tested over the course of three years. One participant with occupational exposure to migratory birds had evidence of past infections with a H5N2 virus antigenically related to A/Nopi/MN/07/462960-02, which is the first case of this influenza subtype in a human host associated with exposure to wild rather than domestic birds. We detected no avian and swine-origin influenza virus co-infections. The exposure of bird handlers to songbirds was four times greater than to shorebirds or waterfowl.
Conclusions
Though rare, the transmission of avian influenza viruses from migratory birds to US-based bird handlers has potentially significant public health and economic consequences.
1. Background
The Asian lineage of highly pathogenic avian influenza viruses of subtype H5N1 (hereafter HPAIV) was first detected in southern China in 1996 and subsequently has caused much concern among the medical and public health community. Prior to 2002, HPAIV was almost exclusively found in poultry, but since then the virus have been isolated from a number of different species of wild and migratory birds1, 2. Because HPAIV currently has low transmissibility but high case fatality, an outbreak in humans is an event with low probability but high potential for negative impact on human health. Since ornithologists and others who work occupationally or recreationally with migratory birds have substantial exposure to zoonotic reservoirs of HPAIV and other subtypes of avian influenza virus (AIV), this population is at risk for wildlife-to-human AIV transmission3.
2. Objectives
Our goal was to examine the potential for AIV transmission from avian migrants to humans by conducting a serosurvey of individuals with significant and measurable contact with wild bird populations. By pairing serological analysis of blood samples for evidence of past infection with demographic and bird handling data collection from a questionnaire, we were able to identify subclinical cases of AIV infection that may have occurred and behavioral risk factors for the transmission of AIV from wild birds to the study participants. The study is unique in that we also screened participants for pandemic 2009 H1N1 (hereafter pH1N1) because co-infection with AIV and pH1N1 could lead to in vivo recombination, generating a reassortant strain with high pathogenicity in mammals4.
Study Design
Participants were recruited for participation via convenience sampling at three annual meetings (2008–2010) of the American Ornithologist’s Union. Anyone who fulfilled the inclusion criteria (at least 18 years of age, able to give informed consent in English, and affiliated with the wild bird handling community) was offered enrollment in the study. Following informed consent, all participants completed a comprehensive questionnaire about their basic demographic information and vaccination status. Participants were asked specific details about their bird handling habits as well as the locations and the duration of their handling.
A 10 ml venous blood sample was then collected from all willing participants. Antibody responses to AIV strains were detected by use of a microneutralization (MN) assay5–8 (Table 1). We screened H5N2, H7N2, and H9N2 in 2008, H5N2 and H7N3 in 2009, and H5N2, H7N2, and pH1N1 in 2010 to test for avian and swine influenza co-infection. In each year, we selected antisera from different H5N2 and H7 viruses to match the H5N2 and H7 viruses detected in US poultry in the previous 12 months. For example, we used A/Chukar/Minnesota/14591-6/95 (H5N2) in 2008 because antibody positivity to this strain was detected in US poultry workers in the spring of 20089 thus we hypothesized that the strain might also circulate in persons exposed to wild birds due to spillover from poultry to wild birds. Sera were considered positive at a MN titer of ≥1: 40 since such titers are correlated with a reduction of 50% of the risk of contracting an influenza infection9–12.
Table 1.
Antibody titers against avian and swine influenza viruses determined by microneutralization assays.
| Year | Virus and Titer | no. (%) |
|---|---|---|
| 2008 | A/Chukar/Minnesota/14191–7/98 (H5N2) <1:10 A/Turkey/Virginia/4569/02 (H7N2) <1:10 A/Turkey/Germany/49 (H9N2) <1:10 |
183 (100) 183 (100) 183 (100) |
| 2009 | A/Nopi/Minnesota/07/462960/02 (H5N2) <1:10 1:40 A/Blue-winged teal/Ohio/07 (H7N3) <1:10 1:10 |
122 (99.2) 1 (0.8) 122 (99.2) 1 (0.8) |
| 2010 | A/Mexico/4108/09 (H1N1) <1:10 1:10 1:20 1:40 1:80 1:160 1:320 1:640 A/Virginia/4529/02 (H7N2) <1:10 A/Turkey/Minnesota/38391-6/95 (H5N2) <1:10 |
47 (48.96) 11 (11.46) 11 (11.46) 5 (5.26) 10 (10.42) 8 (8.33) 3 (3.125) 1 (1.04) 96 (100%) 96 (100%) |
3. Results
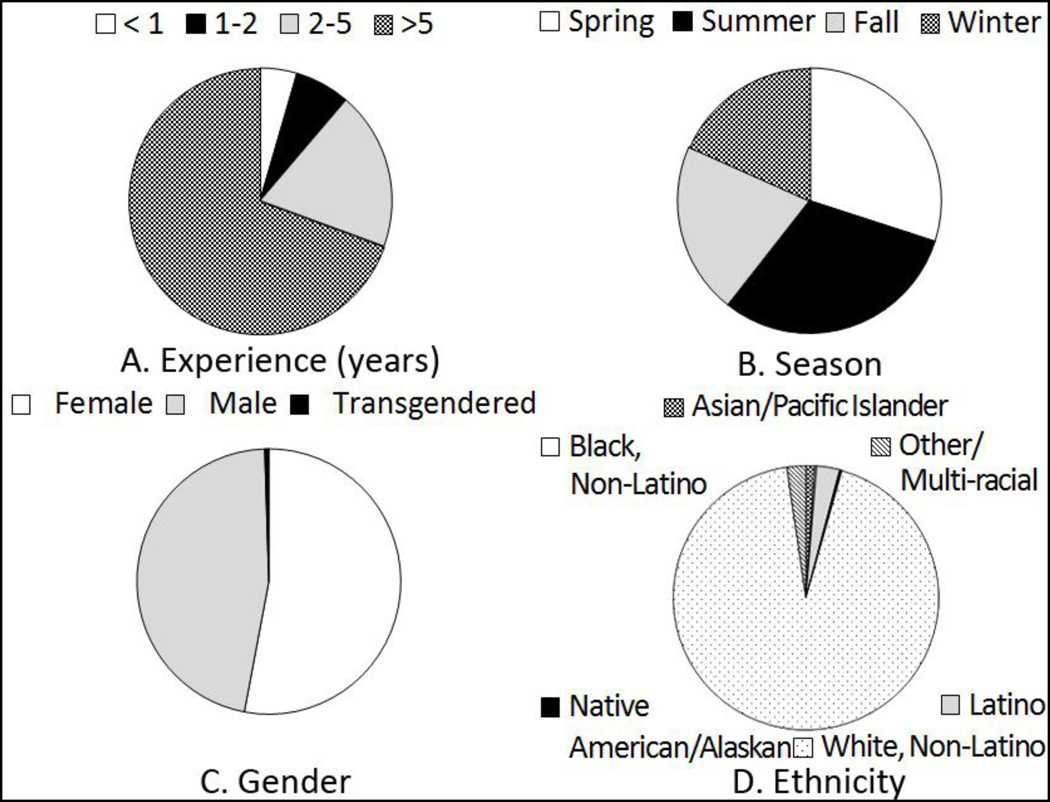
In total, 401 participants were enrolled in the study. The majority of study participants (69.08%, n=277) had handled migratory birds for at least five years and most exposure to wild migratory birds occurred in the summer (Fig. 1). The most common handling activities were banding and measuring (91.52%, n=367) followed by bleeding (60.60%, n=243) (Table 2). Exposure to avian migrants occurred primarily in the eastern or inland US, but 135 participants (33.67%) reported handling birds internationally, most often in the Americas and Caribbean (Table 3). Songbirds and perching birds were handled by 86% (n=344) of the participants whereas 22% (n=87) and 19% (n=77) were exposed to shorebirds and waterfowl, respectively. Additionally, 15 (3.7%) participants had occupational contact with poultry, while 11 (2.7%) had recreational contact with poultry (such as keeping backyard chickens). Even when using a very sensitive cut-off of 40 for the MN results13, only one individual tested positive (0.25%) for any of the selected AIVs, which was an H5N2 virus antigenically related to A/Nopi/Minnesota/07/462960/02 (Table 1). There was no evidence of AIV and pH1N1 co-infection in this cohort.
Fig. 1.
Participants’ demographic and bird handling characteristics.
Table 2.
Songbirds and perching birds were the species most commonly handled by the study participants, whose most frequent exposure to avian migrants consisted of banding and measuring birds.
| Handling Characteristics | no. (%) |
|---|---|
| Species Handled | |
| Passerines (songbirds and perching birds) | 344 (85.79%) |
| Waterfowl | 77 (19.20%) |
| Shorebirds | 87 (21.70%) |
| Raptors | 97 (24.19%) |
| Charadriiformes (auks, gulls, and waders) | 21 (5.37%) |
| Piciformes (woodpeckers) | 14 (3.58%) |
| Psittaciformes (parrots) | 9 (2.30%) |
| Apodiformes (hummingbirds and swifts) | 13 (3.32%) |
| Other | 50 (12.79%) |
| Handling Activities | |
| Banding and measuring | 367 (91.52%) |
| Cloacal swabbing | 98 (24.44%) |
| Bleeding | 243 (60.60%) |
| Specimen collection/preparation | 86 (21.45%) |
| General care | 17 (4.24%) |
| Surgery | 8 (2%) |
| Other | 14 (3.49%) |
Table 3.
Participants with contact with avian migrants outside the US most frequently worked with wild birds in the Americas and Caribbean.
| Region | No. (%) |
|---|---|
| Africa | 8 (2.0%) |
| Americas and the Caribbean | 111 (27.68%) |
| Europe and the Mediterranean | 12 (2.99%) |
| Western Pacific | 17 (4.24%) |
4. Discussion
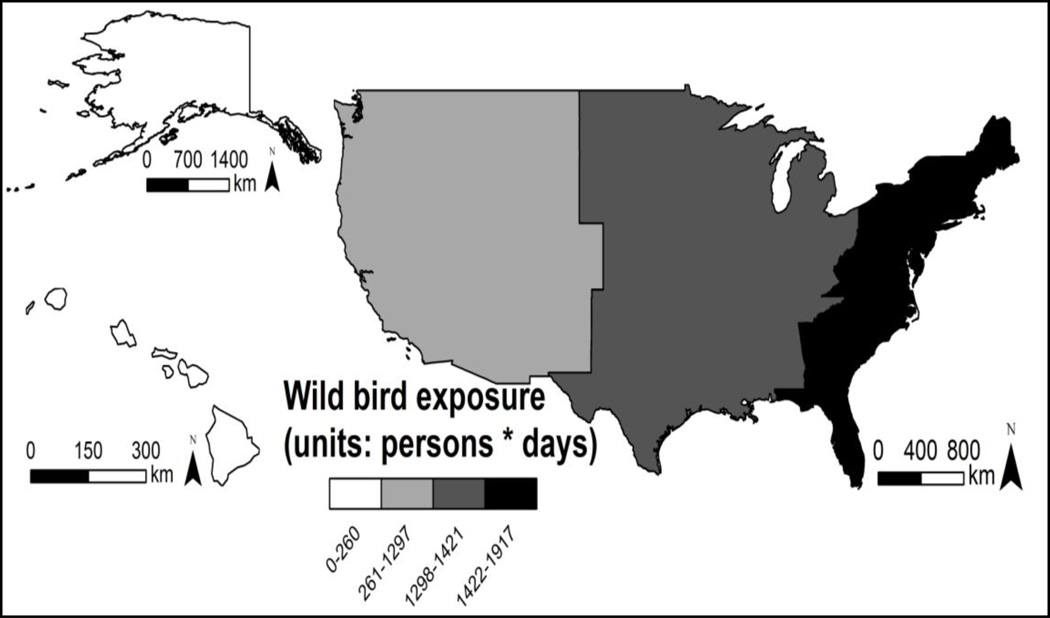
Although 1.4% of poultry workers in the eastern hemisphere are antibody positive for avian H5N114 and seropositivity to H5N2 has previously been detected in poultry workers in the US and Japan5, 9, 15–17, this is the first evidence of H5N2 infection in a person exposed only to wild birds. The seropositive participant indicated no occupational or recreational contact with poultry and had worked exclusively with wild migratory birds in the eastern US, which is a region where H5N2 circulates in migratory waterfowl18. Furthermore, our questionnaire results indicate that the exposure of bird handlers to wild birds is greatest in the eastern US (Fig. 2), so the potential exists for wild bird-to-human transmission of H5N2 in this part of the country.
Fig. 2.
The exposure of bird handlers to avian migrants is greatest in the eastern US. Colors indicate person days of wild bird exposure per US region (2008–2010).
Among the shortcomings of the study is that we did not collect sera from any control subjects, which makes it difficult to determine whether the MN titer of 1:40 to the H5N2 virus actually reflects infection by an avian virus as opposed to cross-reactive antibody responses arising from seasonal influenza infection or vaccination. Our participant with evidence of H5N2 infection did report receiving the seasonal vaccine within the previous 12 months. The relationship between vaccination against seasonal influenza and H5N2 seropositivity requires further study insofar as the two are correlated in some cohorts with poultry exposure15 but not others5, 12. Thus, additional serological surveys are needed to determine if putative H5N2 positivity represents reactivity to the neuraminidase antigen in the seasonal influenza vaccine. Furthermore, H5N2 antibody positivity increases with age in poultry workers16, with highest prevalence among people older than 40, possibly due to cross-reactivity with seasonal influenza antibodies19. At 27 years of age, our seropositive subject was younger than 79% of the study participants, so H5N2 seropositivity in this individual is not likely due to age-related cross-reactivity.
From the data collected, that transmission of AIV to humans who handle wild birds appears to be a rare event, but one that has potentially significant public health and economic consequences. For example, an outbreak of highly pathogenic H5N2 in a live-bird market in Texas in 2004 led to export restrictions that cost the US poultry industry hundreds of millions of dollars20. Such poultry outbreaks along with the transmission of H5N1 from wild birds and poultry to humans in the eastern hemisphere underscore the importance of preventing the spread of avian H5 viruses. The US Ornithological Council recognizes this risk does exist and has published practice guidelines for ornithologists and bird banders21 but compliance with these guidelines remains low6. For instance, at 30%, the seasonal influenza vaccination rate in our study participants was substantially lower than the US average of 43%22. In light of this, further efforts are needed to educate the ornithology community about the importance of influenza vaccination to protect the public from AIV or an avian and swine influenza reassortment event if it were to occur in a wild bird handler.
Acknowledgments
We thank Kirstin Chickering, Gregory C. Gray, and Gary Heil for technical assistance and advice and Alexander Klimov, Dennis Senne, and Richard Webby for AI viruses and antisera.
Funding
This study was supported by grants NIAID 1R01AI074059-01 and 3R01-TW005869 from the NIH Fogarty International Center and was conducted in the context of the Zoonotic Influenza Collaborative Network.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
None declared.
Ethical approval
Approval for this study was obtained from Medical IRB-2 at the University of California, Los Angeles.
References
- 1.Liu J, Xiao H, Lei F, Zhu Q, Qin K, Zhang XW, Zhang XL, Zhao D, Wang G, Feng Y, Ma J, Liu W, Wang J, Gao GF. Highly pathogenic H5N1 influenza virus infection in migratory birds. Science. 2005;309:1206. doi: 10.1126/science.1115273. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Smith GJD, Zhang SY, Qin K, Wang J, Li KS, Webster RG, Peiris JSM, Guan Y. H5N1 virus outbreak in migratory waterfowl. Nature. 2005;436:191–192. doi: 10.1038/nature03974. [DOI] [PubMed] [Google Scholar]
- 3.Gray GC, Ferguson DD, Lowther PE, Heil GL, Friary JA. A national study of US bird banders for evidence of avian influenza virus infections. Journal of Clinical Virology. 2011;51:132–135. doi: 10.1016/j.jcv.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun YP, Qin K, Wang JJ, Pu JA, Tang QD, Hu YX, Bi YH, Zhao XL, Yang HC, Shu YL, Liu JH. High genetic compatibility and increased pathogenicity of reassortants derived from avian H9N2 and pandemic H1N1/2009 influenza viruses. Proceedings of the National Academy of Sciences. 2011;108:4164–4169. doi: 10.1073/pnas.1019109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray GC, McCarthy T, Capuano AW, Setterquist SF, Alavanja MC, Lynch CF. Evidence for avian influenza A infections among Iowa's agricultural workers. Influenza and Other Respiratory Viruses. 2008;2:61–69. doi: 10.1111/j.1750-2659.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill JS, Webby R, Gilchrist MJR, Gray GC. Avian influenza among waterfowl hunters and wildlife professionals. Emerging Infectious Diseases. 2006;12:1284–1286. doi: 10.3201/eid1208.060492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu XH, Lim W, Fukuda K, Cox NJ, Katz JM. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. Journal of Clinical Microbiology. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortiz EJ, Kochel TJ, Capuano A, Setterquist S, Gray GC. Avian influenza and poultry workers, Perum 2006. Influenza and Other Respiratory Viruses. 2007;1:65–69. doi: 10.1111/j.1750-2659.2007.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayali G, Ortiz EJ, Chorazy ML, Gray GC. Evidence of previous avian influenza infection among US turkey workers. Zoonoses and Public Health. 2010;57:265–272. doi: 10.1111/j.1863-2378.2009.01231.x. [DOI] [PubMed] [Google Scholar]
- 10.Uddin M, Cherkowski GC, Liu G, Zhang J, Monto AS, Aiello AE. Demographic and socioeconomic determinants of influenza vaccination disparities among university students. Journal of Epidemiology and Community Health. 2010;64:808–813. doi: 10.1136/jech.2009.090852. [DOI] [PubMed] [Google Scholar]
- 11.Bandaranayake D, Huang QS, Bissielo A, Wood T, Mackereth G, Baker MG, Beasley R, Reid S, Roberts S, Hope V, Team HNSI. Risk factors and immunity in a nationally representative population following the 2009 influenza A(H1N1) pandemic. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers KP, Olsen CW, Setterquist SF, Capuano AW, Donham KJ, Thacker EL, Merchant JA, Gray GC. Are swine workers in the United States at increased risk of infection with zoonotic influenza virus? Clinical Infectious Diseases. 2006;42:14–20. doi: 10.1086/498977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaudoin A, Gramer M, Gray GC, Capuano A, Setterquist S, Bender J. Serologic survey of swine workers for exposure to H2N3 swine influenza A. Influenza and Other Respiratory Viruses. 2010;4:163–170. doi: 10.1111/j.1750-2659.2009.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, Parides MK, Palese P. Seroevidence for H5N1 influenza infections in humans: meta-analysis. Science. 2012;335:1463. doi: 10.1126/science.1218888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogata T, Yamazaki Y, Okabe N, Nakamura Y, Tashiro M, Nagata N, Itamura S, Yasui Y, Nakashima K, Doi M, Izumi Y, Fujieda T, Yamato S, Kawada Y. Human H5N2 avian influenza infection in Japan and the factors associated with high H5N2-neutralizing antibody titer. Journal of Epidemiology. 2008;18:160–166. doi: 10.2188/jea.JE2007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki Y, Doy M, Okabe N, Yasui Y, Nakashima K, Fujieda T, Yamato S, Kawata Y, Ogata T. Serological survey of avian H5N2-subtype influenza virus infections in human populations. Archives of Virology. 2009;154:421–427. doi: 10.1007/s00705-009-0319-7. [DOI] [PubMed] [Google Scholar]
- 17.Myers KP, Setterquist SF, Capuano AW, Gray GC. Infection due to three avian influenza subtypes in United States veterinarians. Clinical Infectious Diseases. 2007;45:4–9. doi: 10.1086/518579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deibel R, Emord DE, Dukelow W, Hinshaw VS, Wood JM. Influenza viruses and paramyxoviruses in ducks in the Atlantic flyway, 1977–1983, including an H5N2 isolate related to the virulent chicken virus. Avian Diseases. 1985;29:970–985. [PubMed] [Google Scholar]
- 19.Skountzou I, Koutsonanos DG, Kim JH, Powers R, Satyabhama L, Masseoud F, Weldon WC, Martin MD, Mittler RS, Compans R, Jacob J. Immunity to pre-1950 H1N1 influenza viruses confers cross-protection against the pandemic swine-origin 2009 A (H1N1) influenza virus. J. Immunol. 2010;185:1642–1649. doi: 10.4049/jimmunol.1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelzel AM, McCluskey BJ, Scott AE. Review of the highly pathogenic avian influenza outbreak in Texas, 2004. Javma-Journal of the American Veterinary Medical Association. 2006;228:1869–1875. doi: 10.2460/javma.228.12.1869. [DOI] [PubMed] [Google Scholar]
- 21.US Ornithological Council. West Nile Virus, Highly Pathogenic Avian Influenza H5N1, and other zoonotic diseases: what ornithologists and bird banders should know. Washington, DC: US Ornithological Council; 2010. [Google Scholar]
- 22.Centers for Disease Control and Prevention. Final state-level influenza vaccination coverage estimates for the 2010–11 season–United States, National Immunization Survey and Behavioral Risk Factor Surveillance System, August 2010–May 2011. Atlanta: CDC; 2011. [Google Scholar]