Abstract
Patients with rheumatoid arthritis (RA) suffer from an excess burden of cardiovascular disease (CVD). CV risk scores for the general population may not accurately predict CV risk for patients with RA. A population-based inception cohort of patients who fulfilled 1987 American College of Rheumatology criteria for RA in 1988–2007 was followed until death, migration, or 12/31/2008. CV risk factors and CVD (myocardial infarction, CV death, angina, stroke, intermittent claudication and heart failure) were ascertained by medical record review. The 10 year predicted CVD risk was calculated using the general Framingham and the Reynolds risk scores. Standardized incidence ratios were calculated to compare observed and predicted CVD risks. The study included 525 patients with RA aged ≥30 years without prior CVD. The mean follow-up was 8.4 years, during which 84 patients developed CVD. The observed CVD risk was 2-fold higher than the Framingham risk score predicted in women and 65% higher in men, and the Reynolds risk score revealed similar deficits. Patients aged ≥75 years had observed CVD risk >3 times the Framingham predicted risk. Patients with positive rheumatoid factor or persistently elevated erythrocyte sedimentation rate also experienced more CVD events than predicted. In conclusion, the Framingham and Reynolds risk scores substantially underestimated CVD risk in patients with RA of both sexes, especially in older ages and in patients with positive rheumatoid factor. These data underscore the need for more accurate tools to predict CVD risk in patients with RA.
Keywords: rheumatoid arthritis, cardiovascular disease, risk scores
INTRODUCTION
Cardiovascular (CV) risk assessment tools designed for the general population, such as the Framingham risk score, may not predict the risk of cardiovascular disease (CVD) among patients with substantial comorbidities, such as rheumatoid arthritis (RA) 1. The profile of CV risk factors differs among patients with RA, who have a higher frequency of smoking and low body mass index compared to subjects without RA 2. In addition, many traditional CV risk factors, such as male sex, smoking, personal cardiac history and lipids, have less impact or paradoxical effects on the development of CVD in patients with RA 2,3. Finally, although inflammation plays a pivotal role in the increased risk of CVD in RA patients, it is not incorporated into many CV risk assessment tools 3,4. The Reynolds risk score includes C-reactive protein, so theoretically it may perform better in patients with RA, but this has not been assessed 5. The objective of this study was to assess the accuracy of the Framingham and Reynolds risk scores for predicting CVD events in patients with RA.
METHODS
This retrospective, population-based study was conducted using the resources of the Rochester Epidemiology Project, a medical records linkage system that allows ready access to the complete (in-patient and out-patient) medical records from all community medical providers 6. An incidence cohort of all residents of Olmsted County, Minnesota aged ≥18 years who first fulfilled 1987 American College of Rheumatology classification criteria for RA between 1/1/1988 and 12/31/2007 was identified and followed until death, migration, or 12/31/2008 7,8. For each patient, the earliest date of fulfillment of ≥4 American College of Rheumatology criteria for RA was considered the RA incidence date. An Olmsted County resident of similar age and sex without RA was selected for each RA patient, and the RA incidence date was used as the index date for each of these non-RA subjects. The institutional review boards of the Mayo Clinic and the Olmsted Medical Center approved this study.
The presence of CV risk factors at RA incidence were ascertained by medical record review including: smoking status (never, current or former); systolic and diastolic blood pressure measurements, use of antihypertensive medications, body mass index and diabetes mellitus (defined as at least 2 measurements of fasting plasma glucose ≥126 mg/dl or a 2-hour plasma glucose ≥ 200 mg/dl, physician diagnosis or documented use of insulin and/or oral hypoglycemic agents). The results of all fasting serum lipid measures, including total cholesterol, high density lipoprotein, low density lipoprotein and triglycerides were also abstracted from the medical records. For blood pressure, lipids and body mass index, the recorded value closest to RA incidence date within ± 1 year was used to calculate the risk of CVD. Note that 80% of the values were obtained between 90 days prior to 30 days after RA incidence date. Data on RA characteristics (rheumatoid factor positivity, erythrocyte sedimentation rate and C-reactive protein) were also collected. Persistently elevated erythrocyte sedimentation rate was defined as ≥3 measures of >40 mm/hr within the first year after RA incidence.
The presence of CVD (myocardial infarction, CV death, angina, stroke, intermittent claudication and heart failure) was ascertained throughout follow-up. Myocardial infarction was defined according to standardized criteria 9. CV death was defined using the underlying cause of death obtained from death certificates (International Classification of Diseases version 9, codes 350.0 – 459.9 and version 10, codes 100 – 199). Angina was ascertained by physician diagnosis. Stroke and intermittent claudication were defined using physician diagnosis verified by imaging, cerebrospinal fluid analysis, or autopsy 10. Heart failure was defined using the Framingham criteria 11.
Patients with CV outcomes prior to RA incidence/index date were excluded from the analysis. Descriptive statistics were used to summarize the demographics of the RA and non-RA cohorts. Chi-square and rank sum tests were used to examine differences in CV risk factors according to sex. The 10 year general Framingham risk score for CVD and the office-based 10 year Framingham risk score, which does not include laboratory values, were calculated according to published algorithms 1. In addition, the Reynolds risk score was calculated among patients with available C-reactive protein values 5. Observed follow-up was truncated at 10 years after RA incidence. For patients with <10 years of follow-up, the predicted risk of CVD was adjusted proportionately. Standardized incidence ratios, which are the ratios of the observed CVD in RA to the predicted CVD obtained from a risk score, were calculated assuming the predicted rates are fixed and the observed CVD events follow a Poisson distribution 12.
The accuracy of predictions was assessed using calibration (i.e., agreement between the observed and predicted event rates) and discrimination (i.e., whether patients are correctly ranked from low to high risk). Calibration was assessed graphically comparing observed event rate for patients in each decile of predicted event rates 13, 14. Discrimination was assessed using Harrell’s concordance (c) statistic 15. Analyses were conducted using R and SAS 9.2 (SAS institute, Cary, NC, USA).
RESULTS
The study included 525 patients with RA aged 30+ years without prior CVD (mean age of 57 years, 69% women) who were followed up for a mean of 8.4 years, and 536 patients without RA of similar age, sex and follow-up. Table 1 shows the characteristics at incidence/index date for patients with and without RA.
Table 1.
Characteristics and cardiovascular risk factors of patients with and without rheumatoid arthritis (RA) who had no prior cardiovascular disease.
| Criteria for CV risk score | RA (n = 525) | Non-RA (n = 524) |
|---|---|---|
| Age (years) mean ± SD | 54.6 ± 13.7 | 54.5 ± 13.5 |
| Rheumatoid factor positive | 357 (68%) | -- |
| Abnormal erythrocyte sedimentation rate at incidence (>30 mm/hr) | 203 (40%) | -- |
| Total cholesterol, (mg/dl) mean ± SD | 195.4 ± 35.7 | 205.8 ± 40.9 |
| HDL cholesterol, (mg/dl) mean ± SD | 54.2 ± 16.1 | 55.5 ± 16.5 |
| Systolic blood pressure (mmHg) mean ± SD | 131.5 ± 18.3 | 129.1 ± 19.8 |
| Blood pressure treatment | 125 (24%) | 109 (21%) |
| Current smoking | 98 (19%) | 91 (17%) |
| Diabetes mellitus | 45 (9%) | 38 (7%) |
| Body mass index (kg/m2) mean ± SD | 28.3 ± 6.2 | 28.0 ± 6.1 |
SD: Standard deviation
Both the general Framingham risk score and the office-based Framingham risk score were calculated The 2 assessments were similar, but the office-based Framingham risk score yielded estimates that were on average 2.4% higher (median 0.9% higher) than the general Framingham risk score. Given this reasonably close agreement, the office-based Framingham risk score was used for 90 RA patients without available lipid measures. If the observed risk exceeds the predicted risk, using the office-based Framingham risk score would slightly diminish the differences between observed and predicted risk. The median Framingham risk score was 5.9% for women (min: 0.6%, max: 67.8%) and 15.6% for men (min: 1.2%, max: 90.5%). The actual observed 10 year risk of CVD in women was 18.3% (95% confidence interval: 13.2%, 23.2%) and in men was 32.7% (95% confidence interval: 22.6%, 41.5%).
A total of 84 patients developed CVD during follow-up, but the Framingham risk score predicted only 45.7 CVD events among these patients (Table 2). The standardized incidence ratio was elevated overall and for both sexes, indicating the Framingham risk score significantly underestimated the CV risk in RA in both women (by 102%) and men (by 65%). Analysis of age groups revealed the standardized incidence ratio were >1 for all age groups with patients aged ≥75 years demonstrating substantially higher CV risk than predicted. Among the non-RA patients age 30–74 years, 47 developed CVD during follow-up; 40.0 events were predicted by the Framingham risk score. Thus, the risk score performed well in patients without RA from our community (standardized incidence ratio: 1.18; 95% confidence interval: 0.88, 1.56).
Table 2.
Comparison of observed and predicted cardiovascular (CV) risk in 525 patients with rheumatoid arthritis (RA)
| Group | N | Cardiovascular Events* | standardized incidence ratio (95% confidence interval) | p-value | |
|---|---|---|---|---|---|
| Observed | Predicted | ||||
| Total | 525 | 84 | 45.7 | 1.84 (1.49, 2.28) | <0.001 |
| Female | 371 | 47 | 23.3 | 2.02 (1.52, 2.69) | <0.001 |
| Male | 154 | 37 | 22.4 | 1.65 (1.20, 2.28) | 0.002 |
| 45 years | 148 | 4 | 3.6 | 1.11 (0.42, 2.96) | 0.83 |
| 45–54 | 141 | 13 | 9.1 | 1.42 (0.83, 2.45) | 0.20 |
| 55–64 | 109 | 20 | 10.6 | 1.97 (1.29, 3.03) | 0.002 |
| 65–74 | 79 | 22 | 15.4 | 1.43 (0.94, 2.17) | 0.092 |
| Age 75+ | 48 | 23 | 6.9 | 3.48 (2.33, 5.19) | <0.001 |
| Rheumatoid factor negative | 168 | 21 | 19.9 | 1.06 (0.69, 1.62) | 0.80 |
| Rheumatoid factor positive | 357 | 63 | 25.8 | 2.45 (1.91, 3.13) | <0.001 |
| Without persistently elevated erythrocyte sedimentation rate | 468 | 57 | 39.8 | 1.46 (1.13, 1.89) | 0.004 |
| Persistently elevated erythrocyte sedimentation rate** | 57 | 26 | 5.9 | 4.43 (3.02, 6.51) | <0.001 |
Number of patients
Persistently elevated erythrocyte sedimentation rate defined as ≥3 measures of >40 within the first year after RA incidence date.
The discrepancies between observed and predicted CVD risk differed according to RA disease characteristics. No difference between observed and predicted CV risk was noted for rheumatoid factor negative patients with RA. However, the observed CV risk was more than two-fold greater than the predicted CV risk among patients with rheumatoid factor positive RA. Also, the observed risk of CVD among patients with persistently elevated erythrocyte sedimentation rate was four-fold greater than expected, whereas the difference between the observed and predicted CVD risk for patients without persistently elevated erythrocyte sedimentation rate was less pronounced. C-reactive protein measures were only available in a subset of 167 patients, but the results were similar to those for erythrocyte sedimentation rate. Patients with C-reactive protein ≥ 10 mg/L had observed CVD risk greater than predicted (standardized incidence ratio: 1.62; 95% confidence interval: 0.81, 3.24), but this difference did not reach statistical significance (p=0.17). There was no evidence of greater than expected CVD risk among patients with C-reactive protein < 10 mg/L at RA incidence (standardized incidence ratio: 0.81; 95% confidence interval: 0.26, 2.50).
Figure 1 shows the observed and predicted 10 year risk of CVD according to deciles of the predicted risk obtained from the Framingham risk score. The observed CVD risk is higher than the predicted CVD risk in 6 of the 10 deciles. This indicates the Framingham risk score is poorly calibrated for patients with RA. In addition, the widely varying levels of observed CVD risk in deciles 1 – 4 indicate the Framingham risk score does not properly rank the patients with RA from lowest to highest CVD risk. While overall the discrimination is good (c-statistic: 0.786; 95% confidence interval: 0.721, 0.851), discrimination in the lowest 4 deciles is much poorer (c-statistic: 0.562; 95% confidence interval: 0.363, 0.761). Similarly, discrimination among RA patients with an intermediate Framingham risk (i.e., 10–20%) is also low (c-statistic: 0.505; 95% confidence interval: 0.389, 0.621). Since a c statistic of 0.5 indicates the discrimination is no better than chance, these data indicate that the discrimination is poor among RA patients with low or intermediate risk scores.
Figure 1.
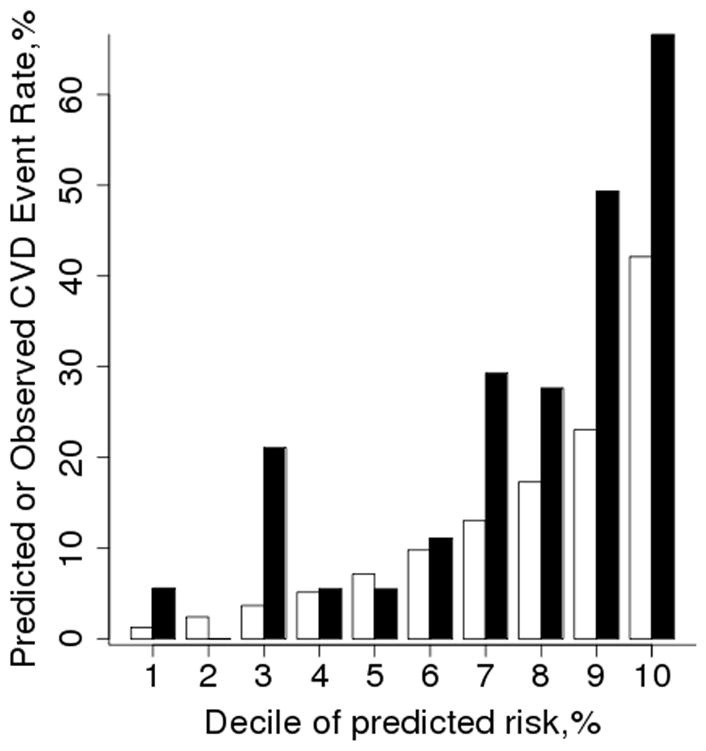
Comparison of observed and predicted 10 year risk of cardiovascular disease (CVD) according to deciles of predicted risk (clear bars) obtained from the Framingham risk score. The observed risk (black bars) was obtained using Kaplan-Meier methods.
Figure 2 displays the excess observed risk, which is the difference between the observed and predicted risk. In addition, adjusted predicted risks were obtained by multiplying the Framingham risk score predictions by 1.8 in an attempt to improve calibration. This adjustment improved calibration for deciles 5 – 8, overcorrected the last decile and had little impact on deciles 1 – 4. Furthermore, the use of a multiplier has no effect on discrimination.
Figure 2.
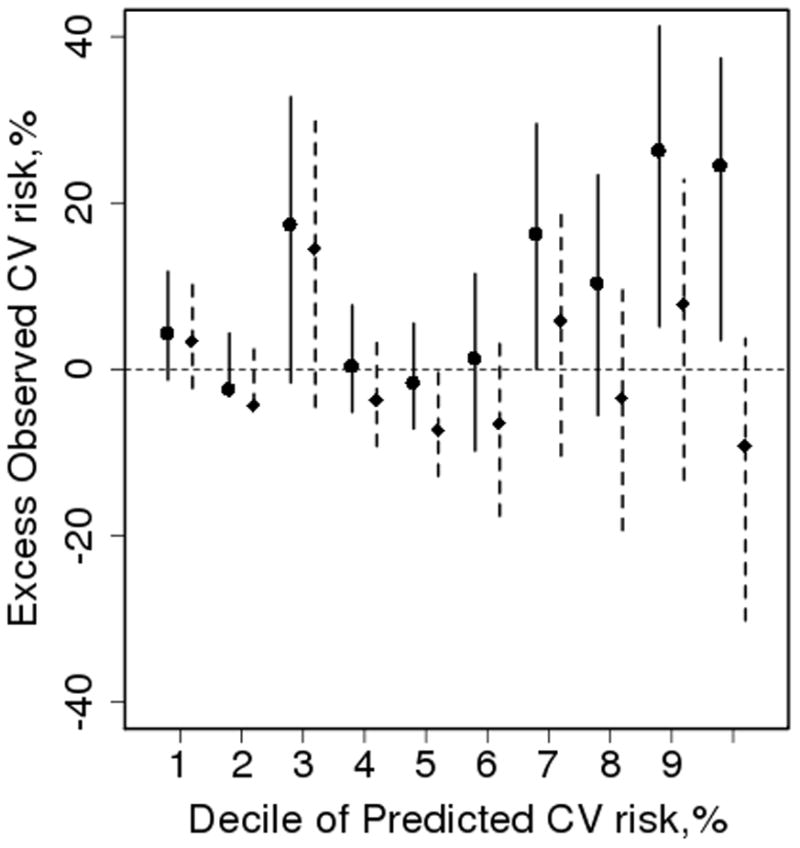
Excess observed vs. predicted 10 year risk of cardiovascular disease (CVD) according to deciles of predicted risk obtained from the Framingham risk score. The mean predicted risk for each decile was subtracted from the Kaplan-Meier estimate of 10 year observed risk with a 95% confidence interval. The solid interval is the actual and the dashed interval was obtained by multiplying the Framingham predicted risk by the overall standardized incidence ratio of 1.8 in an attempt to improve calibration of the Framingham risk score estimates.
Another suggestion for improving calibration is to assume each patient with RA has a risk of CVD equivalent to someone who is 10 years older in the general population 16. This method did improve overall calibration (standardized incidence ratio: 1.20; 95% confidence interval: 0.97, 1.49). Calibration differed by sex, with poor calibration for women (standardized incidence ratio: 1.39; 1.04, 1.85; p=0.024), but not for men (standardized incidence ratio: 1.03; 95% confidence interval: 0.74, 1.42; p=0.87). Furthermore, calibration remained poor for patients with actual age 75+ years (standardized incidence ratio: 2.54; 95% confidence interval: 1.70, 3.79; p<0.001). While the calibration on average improved with this method, it had no effect on discrimination.
A total of 290 women with RA did not have prior CVD using the Reynolds definition (i.e., myocardial infarction, revascularization procedures, cardiovascular death, or stroke) and were age >45 years, which is a requirement for assessment with the Reynolds risk score. Of these, only 92 had available C-reactive protein values (median 9.5 mg/L; 25th percentile 3.0; 75th percentile 28.0 mg/L). The standardized incidence ratio was 1.62 (95% confidence interval: 0.67, 3.88; p=0.28). However, women with C-reactive protein ≥ 15 mg/L had observed CVD risk greater than predicted (standardized incidence ratio: 2.67; 95% confidence interval: 1.01, 7.11; p=0.049), indicating the Reynolds risk score underestimated risk of CVD in RA women with elevated C-reactive protein. There was no evidence of greater than expected CVD risk among women with C-reactive protein < 15 mg/L at RA incidence (standardized incidence ratio: 0.63; 95% confidence interval: 0.09, 4.45).
An additional analysis was performed using erythrocyte sedimentation rate values to impute C-reactive protein values allowing examination of the utility of the Reynolds risk score in a larger sample of patients with RA. A linear regression model was used to model the association between erythrocyte sedimentation rate and log transformed C-reactive protein (R-square=0.42; p<0.001). The standardized incidence ratio was 2.00 (95% confidence interval: 1.44, 2.78; p<0.001), indicating the Reynolds risk score underestimated the risk of CVD in women with RA.
In a sensitivity analysis, all women with RA were assumed to have C-reactive protein of 50 mg/L. This high value, which is ~90th percentile of the available C-reactive protein values, was chosen to examine the potential of the Reynolds score to accurately assess risk of CVD in women with RA. The standardized incidence ratio was 1.48 (95% confidence interval; 1.07, 2.06; p=0.02), indicating the Reynolds risk score cannot adequately predict the risk of CVD in women with RA. The Reynolds risk score for men was not assessed due to the small number of men with available C-reactive protein values in this study.
DISCUSSION
The Framingham risk score substantially underestimated CVD risk in patients with RA of both sexes, especially in older ages and among patients with positive rheumatoid factor and those with persistently elevated erythrocyte sedimentation rate. This indicates that RA disease severity and inflammation play a role in CVD risk that is not accounted for in the Framingham risk score. In addition, the Reynolds risk score underestimated CVD risk in women with RA, despite its inclusion of C-reactive protein.
Despite numerous assessments among patients with high risk of CVD, such as those with diabetes, chronic kidney disease and systemic lupus erythematosus, the accuracy of Framingham risk score predictions among patients with RA has not been previously examined 17–19. The poor discrimination and calibration of the Framingham risk score among patients with RA could result in missed opportunities for preventive interventions and may provide a false sense of security regarding CVD risk for both the patients and their physicians.
The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) and other commonly used guidelines recommend that preventive strategies should be initiated for patients with risks >20% and should not be considered for patients with risks <10% 20. However, RA patients with predicted risks as low as 11.1% actually have risks 1.8 times higher than predicted (i.e., 11.1% × 1.8 = 20%), which would classify them as high risk (≥ 20%) patients warranting initiation of more aggressive intervention. To address this, it has been suggested that a simple multiplier could be used as a means to improve CVD risk prediction in patients with RA 21. This would improve calibration on average, but discrimination would not improve.
The differences between observed and predicted CVD risk among patients with RA may be due to differential effects of traditional CV risk factors among patients with RA. Despite the increased prevalence of smoking among patients with RA, smoking seems to have less effect on CVD among patients with RA 2. In addition, total cholesterol and LDL tend to decrease around the onset of RA and have been shown to have a paradoxical association with the risk of CVD in patients with RA 3,22,23.
RA disease severity and inflammation may also influence CVD risk in RA, as evidenced by the large discrepancies between observed and predicted CVD risk among patients with rheumatoid factor positive RA and among those with persistently elevated erythrocyte sedimentation rate. Markers of systemic inflammation, such as C-reactive protein, interleukin-6, tumor necrosis factor alpha, and others have been shown to be associated with CVD in patients with RA and in the general population 24–26. While adding C-reactive protein to the Framingham risk score has been shown to provide only modest incremental clinical utility in risk prediction for the general population, it could play a larger role in assessing the risk of CVD among patients with RA 27. However, C-reactive protein is included in the Reynolds risk score and it did not perform any better among patients with RA than the Framingham risk score did.
Other factors that may influence CVD risk in RA include the loss of function and muscle mass that commonly occurs after diagnosis of RA. Furthermore, therapies used to treat RA, such as corticosteroids, disease modifying anti-rheumatic medications and biologic response modifiers may have disparate impact on CVD risk 28–30. Since there is likely considerable confounding of these factors, randomized clinical trials are needed to help disentangle the drivers of CVD risk and the most effective interventions to improve CVD outcomes in patients with RA.
Strengths of this study include its population-based cohort of patients who meet objective criteria for RA, and the complete ascertainment of clinically identified CVD events obtained from comprehensive (inpatient and outpatient) medical records. Potential study limitations include the predominately white population of Olmsted County, Minnesota, which may limit the generalizability of these results to more diverse populations. In addition, the retrospective study design is a limitation, and lipid measures were not available for some patients. For these patients, the office-based Framingham risk score was used, which may slightly diminish the differences between observed and predicted risks, since the office-based Framingham risk score was slightly higher than the general Framingham risk score in patients for whom both could be assessed.
Acknowledgments
Funding: This work was funded by grants from Pfizer, Inc. and the National Institutes of Health, NIAMS (R01 AR46849), and made possible by the Rochester Epidemiology Project (R01 AG034676 from the National Institute on Aging).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez A, Kremers HM, Crowson CS, Ballman KV, Roger VL, Jacobsen SJ, O’Fallon WM, Gabriel SE. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 2008;67:64–69. doi: 10.1136/ard.2006.059980. [DOI] [PubMed] [Google Scholar]
- 3.Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-Gibbon PD, Therneau TM, Gabriel SE. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70(3):482–487. doi: 10.1136/ard.2010.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pieringer H, Pichler M. Cardiovascular morbidity and mortality in patients with rheumatoid arthritis: vascular alterations and possible clinical implications. QJM. 2011;104:13–26. doi: 10.1093/qjmed/hcq203. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 6.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30:819–834. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Jr, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 8.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62:1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mascioli SR, Jacobs DR, Jr, Kottke TE. Diagnostic criteria for hospitalized acute myocardial infarction: the Minnesota experience. Int J Epidemiol. 1989;18:76–83. doi: 10.1093/ije/18.1.76. [DOI] [PubMed] [Google Scholar]
- 10.Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: Increase in venous thromboembolic events? Arthritis Rheum. 2012;64:53–61. doi: 10.1002/art.33322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 12.Cox DR. Some simple approximate tests for Poisson variates. Biometrika. 1953;40:354–360. [Google Scholar]
- 13.Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121:1768–1777. doi: 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 14.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 16.Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet. 2006;368:29–36. doi: 10.1016/S0140-6736(06)68967-8. [DOI] [PubMed] [Google Scholar]
- 17.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007;50:217–224. doi: 10.1016/j.jacc.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 18.Coleman RL, Stevens RJ, Retnakaran R, Holman RR. Framingham, SCORE, and DECODE risk equations do not provide reliable cardiovascular risk estimates in type 2 diabetes. Diabetes Care. 2007;30:1292–1293. doi: 10.2337/dc06-1358. [DOI] [PubMed] [Google Scholar]
- 19.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, Cote R, Grover SA, Fortin PR, Clarke AE, Senecal JL. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 21.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, McInnes IB, Haentzschel H, Gonzalez-Gay MA, Provan S, Semb A, Sidiropoulos P, Kitas G, Smulders YM, Soubrier M, Szekanecz Z, Sattar N, Nurmohamed MT. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–331. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 22.Myasoedova E, Crowson CS, Maradit-Kremers H, Fitz-Gibbon PD, Therneau TM, Gabriel SE. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010;69:1310–1314. doi: 10.1136/ard.2009.122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters MJ, Voskuyl AE, Sattar N, Dijkmans BA, Smulders YM, Nurmohamed MT. The interplay between inflammation, lipids and cardiovascular risk in rheumatoid arthritis: why ratios may be better. Int J Clin Pract. 2010;64:1440–1443. doi: 10.1111/j.1742-1241.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- 24.Rho YH, Chung CP, Oeser A, Solus J, Asanuma Y, Sokka T, Pincus T, Raggi P, Gebretsadik T, Shintani A, Stein CM. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61:1580–1585. doi: 10.1002/art.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Leuven SI, Franssen R, Kastelein JJ, Levi M, Stroes ES, Tak PP. Systemic inflammation as a risk factor for atherothrombosis. Rheumatology (Oxford) 2008;47:3–7. doi: 10.1093/rheumatology/kem202. [DOI] [PubMed] [Google Scholar]
- 26.Sattar N, McInnes IB. Vascular comorbidity in rheumatoid arthritis: potential mechanisms and solutions. Curr Opin Rheumatol. 2005;17:286–292. doi: 10.1097/01.bor.0000158150.57154.f9. [DOI] [PubMed] [Google Scholar]
- 27.Shah T, Casas JP, Cooper JA, Tzoulaki I, Sofat R, McCormack V, Smeeth L, Deanfield JE, Lowe GD, Rumley A, Fowkes FG, Humphries SE, Hingorani AD. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol. 2009;38:217–231. doi: 10.1093/ije/dyn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Listing J, Strangfeld A, Kekow J, Schneider M, Kapelle A, Wassenberg S, Zink A. Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum. 2008;58:667–677. doi: 10.1002/art.23281. [DOI] [PubMed] [Google Scholar]
- 29.Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E, Ostor AJ, Edwards CJ. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2010;49:295–307. doi: 10.1093/rheumatology/kep366. [DOI] [PubMed] [Google Scholar]
- 30.Davis JM, 3rd, Maradit Kremers H, Crowson CS, Nicola PJ, Ballman KV, Therneau TM, Roger VL, Gabriel SE. Glucocorticoids and cardiovascular events in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2007;56:820–830. doi: 10.1002/art.22418. [DOI] [PubMed] [Google Scholar]


