Abstract
Fatigue is highly prevalent and causes serious disruption in quality of life. Although cross-sectional studies suggest childhood adversity is associated with adulthood fatigue, longitudinal evidence of this relationship and its specific biological mechanisms have not been established. This longitudinal study examined the association between early life stress and adulthood fatigue and tested whether this association was mediated by low-grade systemic inflammation as indexed by circulating C-reactive protein (CRP) and interleukin-6 (IL-6). In the Coronary Artery Risk Development in Young Adults (CARDIA) study, a population-based longitudinal study conducted in 4 US cities, early life stress was retrospectively assessed in 2716 African-American and white adults using the Risky Families Questionnaire at Year 15 examination (2000–2001, ages 33–45 years). Fatigue as indexed by a loss of subjective vitality using the Vitality Subscale of the 12-item Short Form Health Survey was assessed at both Years 15 and 20. While CRP was measured at both Years 15 and 20, IL-6 was measured only at Year 20. Early life stress assessed at Year 15 was associated with adulthood fatigue at Year 20 after adjustment for sociodemographic characteristics, body-mass index, medication use, medical comorbidity, smoking, alcohol consumption, physical activity, current stress, pain, sleep disturbance as well as Year 15 fatigue (adjusted beta 0.047, P=0.007). However, neither CRP nor IL-6 was a significant mediator of this association. In summary, early life stress assessed in adulthood was associated with fatigue 5 years later, but this association was not mediated by low-grade systemic inflammation.
Keywords: early life stress, fatigue, sickness behavior, systemic inflammation, C-reactive protein, interleukin-6, population-based longitudinal study, mediation
1. INTRODUCTION
Fatigue is a highly prevalent symptom with up to 38% of community-dwelling individuals suffering from this subjective sense of weariness, tiredness, lack of energy, and low vitality (Pawlikowska et al., 1994; Valdini, 1985). Fatigue is a symptom found with many major medical and psychiatric disorders – e.g. HIV/AIDS, cancer, multiple sclerosis, chronic fatigue syndrome, major depression, and schizophrenia – and causes serious disruption in quality of life (Amato et al., 2001; Anderson and Ferrans, 1997; Curt, 2000). However, it also occurs independently, in otherwise healthy individuals, and can lead to disability and cost for society. US workers with fatigue cost employers $136.4 billion annually in lost productivity (Ricci et al., 2007) – far higher compared to $61.2 billion for pain (Stewart et al., 2003b) and $44.0 billion for depression (Stewart et al., 2003a).
Evidence from community-based cross-sectional studies suggests that adverse experience in childhood is associated with elevated levels of fatigue in adulthood (Heim et al., 2006; McCauley et al., 1997; Romans et al., 2002). In particular, these studies suggest childhood abuse is associated with both the diagnosis of chronic fatigue syndrome and the symptom of fatigue. Additionally, a cross-sectional association between child maltreatment and fatigue among breast cancer survivors has been recently reported (Fagundes et al., 2011). However, to our knowledge, no longitudinal studies have evaluated whether severity of early life stress as indexed in adulthood is associated with the later development of fatigue. In addition, the biological mechanisms that mediate this relationship are not well known.
Basic research on neuroimmune interactions has suggested that inflammatory processes may trigger ‘sickness behavior’, a constellation of behavioral changes that include fatigue, sleep disturbance, hyperalgesia, and depressive-like symptoms, through the effects of proinflammatory cytokines on the central nervous system (Dantzer et al., 2008; Eisenberger et al., 2010; Irwin and Cole, 2011; Reichenberg et al., 2001). Our prior work using the Coronary Artery Risk Development in Young Adults (CARDIA) Study data showed a prospective association between plasma C-reactive protein (CRP), a biomarker of systemic inflammation, and fatigue five years later, a finding consistent with the notion of an underlying subclinical inflammatory process as an independent risk factor for the development of fatigue (Cho et al., 2009). Furthermore, previous studies have shown early life stress to be associated with systemic inflammation in adulthood (Danese et al., 2007; Miller et al., 2009). Based on this evidence, heightened inflammatory responses are a plausible mechanism for the link between early life stress and fatigue. However, to our knowledge, no previous studies have examined this pathway.
The goal of the current study was to investigate the role of early life stress in the development of fatigue, and to determine whether this association was mediated by inflammation. Based on previous work conducted by our group and others (Cho et al., 2009; Danese et al., 2007; Miller et al., 2009), we hypothesized that adulthood reports of early life stress would be associated with the later development of fatigue, and the association between early life stress and fatigue would be mediated by plasma CRP and interleukin-6 (IL-6) levels. Additionally, as comparisons, we assessed the associations between early life stress and the other symptoms of sickness behavior, namely pain, sleep disturbance, and depression, and the mediation of these associations by plasma CRP and IL-6 levels.
2. METHODS
2.1. Subjects
CARDIA is a longitudinal study of cardiovascular risk factors in white and African-American men and women aged 18 to 30 years at study inception (1985–1986). Full details of the study design and methods have been published previously (Friedman et al., 1988). Briefly, 5115 individuals were recruited from four US cities (Birmingham, Ala; Chicago, Ill; Minneapolis, Minn; and Oakland, Calif) to take part in the baseline clinical examination in 1985–1986. Of the 5115 individuals originally enrolled at Year 0, 3178 participated in both Years 15 and 20, the two CARDIA examinations considered for the current analysis as Year 15 included the assessment of early life stress, fatigue, and CRP, and Year 20 included fatigue, CRP, and IL-6. For the purpose of the current paper, Year 15 (2000–2001) was considered as the baseline and Year 20 (2005–2006) as the follow-up. We excluded 252 participants with missing values of either Year 15 measures of early life stress, CRP, or covariates or Year 20 measures of fatigue or IL-6. After this exclusion, 2926 participants remained. We additionally excluded 210 participants with CRP concentrations that could be suggestive of acute infection or inflammation (>10mg/L) at Year 15 (Myers et al., 2004), leaving 2716 participants for the analysis. This latter exclusion procedure would help ensure that the findings of the current study are about the effects of low-grade systemic inflammation.
2.2. Main Outcome Measure
Fatigue was measured at baseline and follow-up (Years 15 and 20, respectively) as low vitality using the Vitality Subscale of the 12-item Short Form Health Survey (SF-12) (Ware et al., 1996). The SF-12 is a valid and reliable shorter version of the SF-36 with their subscales being highly correlated in clinical and general populations (Han et al., 2002; Hurst et al., 1998; Jenkinson et al., 1997; Ware et al., 1996). The SF-12 Vitality Subscale consists of a single item (“Did you have a lot of energy?” referring to the past four weeks) on a six-point scale: All of the time, 0; Most of the time, 1; A good bit of the time, 2; Some of the time, 3; A little of the time, 4; and None of the time, 5. Higher scores on this scale reflect more severe fatigue. The SF-12 Vitality Subscale score was treated as a continuous variable given that ordinal variables with five or more categories are referred to as ‘quasi-interval’ and can be treated as if they were continuous (Powers and Xie, 2000).
To ensure the suitability of using the SF-12 Vitality Subscale as the main outcome measure, we assessed its convergent validity using two data sets we had access to: the Whitehall II study, a prospective cohort study of British civil servants (Marmot and Brunner, 2005), and the Moving Beyond Cancer (MBC) study, a multisite randomized controlled trial of behavioral interventions (Ganz et al., 2004). Both studies included the SF-36 and the latter also included the Fatigue Symptom Inventory (FSI) (Hann et al., 1998). Convergent validity was demonstrated by computing correlations between the individual energy item, “Did you have a lot of energy?”, and the full Vitality Subscale of the SF-36. High correlations were observed in 7888 healthy adults from the Whitehall II study (r=0.87; P<0.0001) and in 557 breast cancer patients from the MBC study (r=0.89; P<0.0001). The individual energy item, “Did you have a lot of energy?”, was also highly correlated with the FSI in the latter group (r=0.64; P<0.0001).
2.3. Secondary Outcome Measures
The secondary outcome measures included the other symptoms of sickness behavior, i.e., pain, sleep disturbance, and depression, and they were assessed at baseline and follow-up. Pain was measured using the Bodily Pain Subscale of SF-12 (score range 0–4), with higher scores reflecting more severe pain. A summary score of sleep quality was produced summing up five items of the sleep questionnaire (score range 0–8), a questionnaire of sleep quality specifically devised for the CARDIA study, with higher summary scores reflecting poorer sleep quality: daytime sleepiness (individual score 0 or 1), sleep onset problem (0 or 1), sleep maintenance problem (0 or 1), early awakening (0 or 1) and subjective sleep quality (0 to 4). Depressive symptoms were assessed using the Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, 1977), a valid and reliable measure of depressive symptoms in the general population, with a score range 0–60 and higher scores reflecting more severe depression. In order to evaluate depression as a separate construct from fatigue and sleep disturbance given that there is an overlap between these components of sickness behavior, a new variable was created for the summary score of the CES-D excluding two items describing fatigue and one describing sleep disturbance (“I felt that everything I did was an effort.”, “I could not ‘get going’.”, and “My sleep was restless.”). This variable was used in all analyses throughout this study unless stated otherwise.
2.4. Early Life Stress
Early life stress was assessed using the Risky Families Questionnaire (Taylor et al., 2006), which assessed various aspects of participants’ early family environment on 4-point scales from 1 (rarely or none of the time) to 4 (most or all of the time) at baseline (Year 15). The questionnaire items included whether the individual felt loved and cared for; was shown physical affection; was verbally abused; was physically abused; lived with a substance abuser; lived in a well-organized, well-managed household; and whether family members knew what the child was up to. The composite score from these 7 items ranges from 7 to 28 and higher values represent a more stressful family environment.
2.5. C-Reactive Protein and Interleukin-6
CRP was measured at follow-up (Year 20) using a BNII nephelometer (Dade Behring, Deerfield, Ill) utilizing a particle enhanced immunoepholometric assay. The assay range is 0.175–1100 mg/L, intrassay coefficients of variation range from 2.3–4.4% and inter-assay coefficients of variation range from 2.1–5.7%. IL-6 was measured by ultra-sensitive ELISA (R&D Systems, Minneapolis, MN). The lower detection limit is <0.10 pg/mL, with a detection range of 0.156–10.0 pg/mL and a routine inter-assay coefficient of variation in the lab of 10.0%. Given the skewed distribution of CRP and IL-6, the values were log transformed and used in all analyses as continuous variables.
2.6. Potential Biobehavioral Confounders
Covariates were chosen based on the published recommendations on the assessment of control variables for the studies investigating linkages between behavioral factors and inflammation (O’Connor et al., 2009), and all of them were assessed at baseline (Year 15). Variables known to exert confounding effects for the association between biobehavioral factors and inflammation include age, gender, ethnicity, socioeconomic status (often represented by education), smoking, alcohol consumption, body-mass index (BMI), exercise, and medications such as aspirin and statins. For the current analysis, smoking status, originally classified as never, former or current smoker, was re-categorized as a binary variable (current smoker or not). Medication use was defined as the use of prescription medications that may affect systemic inflammatory state such antihypertensives, antihyperlipidemics, antiasthmatics, and aspirin. Presence of medical comorbidity was defined by one of the following criteria: 1) lifetime history of chronic medical conditions often comorbid with fatigue (e.g., coronary heart disease, diabetes, renal failure, hepatitis, cancer, hypothyroidism, inflammatory bowel diseases, and autoimmune diseases); or 2) past year history of acute or subacute medical conditions often comorbid with fatigue (e.g., asthma, pneumonia, and deep vein thrombosis). All the other variables were used in the original format without further reduction or modification. They were binary (gender and ethnicity [White and African-American]) or continuous (age, years of education, BMI, self-reported average daily alcohol consumption, physical activity level, and current stress). Physical activity level was assessed with the CARDIA Physical Activity History Questionnaire (Sidney et al., 1991), eliciting frequency of participation in a range of specific heavy and moderate intensity activities during the previous 12 months. Physical activity score was computed and expressed in ‘exercise units’ (EU). For reference, 300 EU roughly approximates the American College of Sports Medicine recommendations for the amount of exercise needed to support weight loss (5 sessions of 300 kcal of energy expenditure weekly) (Parker et al., 2007). Current stress was assessed with the Chronic Burden Questionnaire (Janicki-Deverts et al., 2007). Participants indicated whether (yes, no) they had been experiencing ongoing problems (lasting >=6 months) in the domains of work, finances, relationships, personal health and health of close others, and to what extent the ongoing problems were stressful (1 = no problem; 2 = yes, but not very stressful; 3 = yes, moderately stressful; 4 = yes, very stressful). For the purposes of the present study, the average chronic burden score was calculated by taking the average of scores from the domains of work, finances, relationships, and health of close others.
2.6. Analysis
Baseline characteristics of 2716 participants were described and their associations with fatigue at follow-up were described using correlation or t-test. Then the study hypotheses were tested by performing linear regression analyses with standardized regression coefficients (beta), which facilitate comparison across models. The independent variable was early life stress retrospectively assessed at baseline (Year 15). The primary dependent variable was fatigue at follow-up (Year 20) and the secondary dependent variables were pain, sleep disturbance, and depression (CES-D without fatigue and sleep disturbance items) at follow-up. Selection of covariates for multivariable analysis relied on empirical evidence rather than predetermined P-value criteria; the latter approach, which selects factors for inclusion in a multivariable model only if the factors are ‘statistically significant’ in bivariate screening, is considered less optimal (Steyerberg et al., 2000). All covariates were assessed at baseline. Model 1 included sociodemographic variables (age, gender, ethnicity, and education). Model 2 further included biomedical variables (BMI, medical comorbidity, and medication use). Model 3 further included health-related behaviors (smoking, alcohol consumption, and physical activity). Model 4 further included current stress. Model 5 further included the other sickness behavior symptoms (pain, sleep disturbance, and depression). Finally, Model 6 further included baseline measure of fatigue in addition to all the above covariates. This procedure would examine the effect of early life stress assessed at baseline on fatigue measured at follow-up regardless of baseline fatigue, thus testing whether the association between early life stress and subsequent fatigue was independent of fatigue at the time of assessment of early life stress. Age, gender, ethnicity, and education were tested for potential effect modification. To avoid any artifact due to different sample sizes between the nested models, participants missing the covariates were excluded. The same multivariable approach was used for the analyses of each secondary outcome measure (pain, sleep disturbance, and depression assessed at follow-up). We also hypothesized that adulthood plasma CRP and IL-6 would be on the causal pathway from early life stress to adulthood fatigue (or the secondary outcome variables). To test this hypothesis, the Sobel–Goodman mediation test was performed (MacKinnon et al., 2002), using early life stress assessed at baseline as the independent variable, fatigue measured at follow-up as the dependent variable, and CRP measured at baseline as the mediating variable. Another Sobel-Goodman test was performed with IL-6 measured at follow-up as the mediating variable as IL-6 was not available at baseline. According to Baron and Kenny, perfect mediation holds if the independent variable has no effect on the dependent variable when the mediating variable is controlled for (Baron and Kenny, 1986). Because many factors affect outcome variables such as fatigue and the other symptoms of sickness behavior, a more realistic goal in evaluating a mediator is to consider whether the mediator significantly attenuates the relationship (partial mediation).
3. RESULTS
3.1. Baseline Characteristics
Table 1 describes baseline characteristics of the participants and their associations with fatigue at follow-up (Year 20) using correlation or t-test. Fatigue score at follow-up was significantly positively correlated with baseline characteristics such as early life stress, BMI, current stress, fatigue, pain, sleep disturbance, and depression; and significantly negatively correlated with education years and physical activity level. Mean fatigue score at follow-up was significantly higher for female participants, those using medications, those with medical comorbidity, and current smokers at baseline.
Table 1.
Characteristics of 2716 participants at baseline (CARDIA Year 15) and their association with fatigue at follow-up (CARDIA Year 20)
| Variable (range) | Mean (SD) or N (%) | Correlation coefficient or Mean fatigue score* | P from correlation or t-test* |
|---|---|---|---|
| Early life stress (7–28), mean (SD) | 11.6 (4.1) | 0.138 | <0.0001 |
|
| |||
| Age (33–45 years), mean (SD) | 40.3 (3.6) | −0.001 | 0.970 |
|
| |||
| Gender, N (%) | |||
| Male | 1232 (45.4) | 1.75 | <0.0001 |
| Female | 1484 (54.6) | 2.06 | |
|
| |||
| Ethnicity, N (%) | |||
| White | 1556 (57.3) | 1.94 | 0.350 |
| African-American | 1160 (42.7) | 1.90 | |
|
| |||
| Education (4–20 years), mean (SD) | 15.1 (2.5) | −0.044 | 0.023 |
|
| |||
| BMI (15.7–58.4 kg/m2), mean (SD) | 28.0 (6.1) | 0.116 | <0.0001 |
|
| |||
| Medication use, N (%) | |||
| No | 2283 (84.1) | 1.88 | 0.0001 |
| Yes | 433 (15.9) | 2.12 | |
|
| |||
| Medical comorbidity, N (%) | |||
| No | 2070 (76.2) | 1.84 | <0.0001 |
| Yes | 646 (23.8) | 2.19 | |
|
| |||
| Current smoker, N (%) | |||
| No | 2180 (80.3) | 1.89 | 0.005 |
| Yes | 536(19.7) | 2.05 | |
|
| |||
| Daily alcohol consumption (0–564.9 mL), mean (SD) | 11.1 (26.1) | −0.024 | 0.215 |
|
| |||
| Physical activity score (0–1818), mean (SD) | 360 (284) | −0.195 | <0.0001 |
|
| |||
| Current stress (1–4), mean (SD) | 1.8 (0.6) | 0.245 | <0.0001 |
|
| |||
| Fatigue score (0–5), mean (SD) | 1.9 (1.1) | 0.471 | <0.0001 |
|
| |||
| Pain score (0–4), mean (SD) | 0.5 (0.9) | 0.183 | <0.0001 |
|
| |||
| Sleep quality score (0–8), mean (SD) | 2.2 (1.7) | 0.244 | <0.0001 |
|
| |||
| Depression (total CES-D score) (0–60), mean (SD) | 8.8 (7.7) | 0.275 | <0.0001 |
|
| |||
| CES-D without fatigue and sleep disturbance items (0–54), mean (SD) | 7.1 (6.6) | 0.258 | <0.0001 |
BMI = body mass index; CES-D = Center for Epidemiological Studies Depression Scale; SD = standard deviation
Correlation coefficient between the baseline variable and fatigue at follow-up when the baseline variable is continuous (P-value from correlation); and mean fatigue score at follow-up by each category of the baseline variable when the baseline variable is categorical (P-value from t-test).
3.2. Cross-sectional Association between Early Life Stress and Fatigue
Early life stress was cross-sectionally associated with fatigue at baseline (Year 15) (unadjusted beta 0.142, P<0.001). Although this association remained significant after adjusting for age, gender, ethnicity, education, BMI, medication use, medical comorbidity, smoking, alcohol consumption, physical activity, current stress, pain, and sleep disturbance (adjusted beta 0.042, P=0.017), the additional adjustment for depression (i.e., CES-D score without fatigue and sleep items) rendered the association non-significant (adjusted beta −0.005, P=0.761).
3.3. Longitudinal Association between Early Life Stress and Fatigue
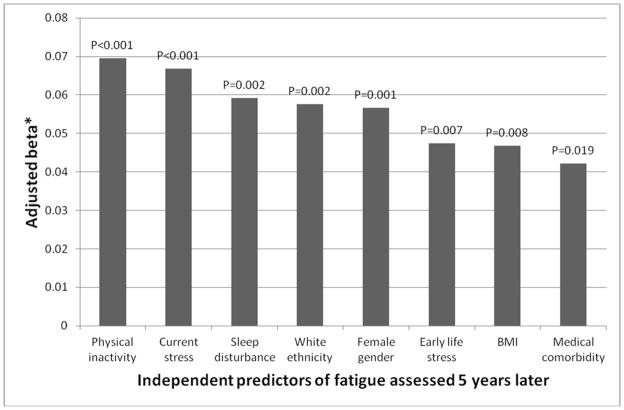
As shown in Table 2, early life stress retrospectively assessed at baseline (Year 15) was associated with fatigue at follow-up (Year 20) (unadjusted beta 0.138, P<0.001). Table 2 also describes the contribution of the six sets of covariates to the association between early life stress and fatigue. This association remained highly significant after adjusting for all assessed covariates including sociodemographic characteristics, BMI, medication use, medical comorbidity, smoking, alcohol consumption, physical activity, current stress, pain, sleep disturbance, depression (CES-D score without fatigue and sleep items), and baseline fatigue (adjusted beta 0.047, P=0.007). No effect modification was observed for age (P = 0.233), gender (P = 0.849), ethnicity (P = 0.070), or education (P = 0.935). Of note, as shown in Figure 1, the fully adjusted multivariable model indicated that, in addition to early life stress, the following variables were independent predictors of fatigue at follow up: baseline fatigue (adjusted beta 0.373, P<0.001), physical inactivity (adjusted beta 0.070, P<0.001), current stress (adjusted beta 0.067, P<0.001), sleep disturbance (adjusted beta 0.059, P=0.002), White ethnicity (adjusted beta 0.058, P=0.002), female gender (adjusted beta 0.057, P=0.001), BMI (adjusted beta 0.047, P=0.008), and medical comorbidity (adjusted beta 0.042, P=0.019).
Table 2.
Association of early life stress retrospectively assessed at baseline (CARDIA Year 15) with fatigue assessed at follow-up as an adult (CARDIA Year 20) (N=2716)
| Adjustment* | Beta | P |
|---|---|---|
| Unadjusted model | 0.138 | <0.001 |
| Model 1: Sociodemographic variables | 0.126 | <0.001 |
| Model 2: Model 1 + Biomedical factors | 0.120 | <0.001 |
| Model 3: Model 2 + Health-related behaviors | 0.116 | <0.001 |
| Model 4: Model 3 + Current stress | 0.080 | <0.001 |
| Model 5: Model 4 + Sickness behavior symptoms | 0.045 | 0.016 |
| Model 6†: Model 5 + Baseline fatigue | 0.047 | 0.007 |
Beta = standardized regression coefficient expressing the change in standardized fatigue score per one standard deviation in early life stress measured as Early Family Environment
Sociodemographic variables include age, gender, ethnicity, and education. Biomedical factors include body-mass index, medication use, and medical comorbidity. Health-related behaviors include smoking, alcohol consumption, and physical activity. Current stress means ongoing adversity present for 6 months or more assessed by the Chronic Burden Questionnaire. Sickness behavior symptoms include pain, sleep disturbance, and depression (CES-D score without fatigue and sleep items).
No effect modification was observed for age (P = 0.233), gender (P = 0.849), ethnicity (P = 0.070), or education (P = 0.935).
Figure 1. Independent baseline predictors of fatigue at follow-up 5 years later.
Beta = standardized regression coefficient expressing the change in standardized outcome score per one standard deviation in early life stress measured as Early Family Environment; BMI = body-mass index
* The figure shows significant baseline predictors of fatigue assessed 5 years later according to the multivariable model including the following variables: early life stress, age, gender, ethnicity, education, body-mass index, medication use, medical comorbidity, smoking, alcohol consumption, physical activity, current stress, pain, sleep disturbance, depression (CES-D score without fatigue and sleep items), and fatigue (all assessed at baseline, i.e., CARDIA Year 15). Hence, the beta was adjusted for all the above variables in testing the independent effect for each individual predictor. Baseline fatigue was obviously the strongest predictor of fatigue at follow-up (adjusted beta 0.373, P<0.001) but not shown in this figure.
When each of the secondary outcome measures was used as the dependent variable, the fully adjusted multivariable models revealed a significant association only for depression (i.e., CES-D score without fatigue and sleep items; adjusted beta 0.094, P<0.001). Adjusted betas for pain and sleep disturbance were respectively 0.003 (P=0.862) and 0.022 (P=0.214).
3.4. Mediation of the Association between Early Life Stress and Fatigue by CRP and IL-6
As shown in Table 3, plasma CRP levels did not mediate the association between early life stress and adulthood fatigue with or without adjustment for sociodemographic variables. Although early life stress assessed at baseline (independent variable) accounted for variation in adulthood fatigue assessed at follow-up (dependent variable) as noted by beta 0.138 (P<0.001), early life stress was not associated with CRP measured at baseline (mediator) as noted by beta 0.021 (P=0.270). Consequently, when CRP was added to the model, its presence did not reduce the strength of the association between early life stress and fatigue at follow-up as noted by the change of beta from 0.138 (P<0.001) to 0.136 (P<0.001). The Sobel-Goodman mediation test suggested that just 1.4% of the total effect of early life stress on fatigue was mediated by CRP (Z=1.075, P=0.282). Similarly, when CRP measured at follow-up was tested as a mediator, no significant mediation was observed (2.7%, Z=1.631, P=0.103).
Table 3.
Sobel–Goodman mediation tests with Year 15 early life stress as independent variable, Year 20 sickness behavior symptoms as dependent variables, and Year 15 CRP as mediating variable
| Sickness behavior symptom | Model with IV and DV | Model with IV, DV and MV | % mediated by CRP | P | % mediated by CRP after adjustment* | P | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Beta | P | Beta | P | |||||
| Fatigue | 0.138 | <0.001 | 0.136 | <0.001 | 1.4 | 0.282 | 0.2 | 0.869 |
| Pain | 0.115 | <0.001 | 0.112 | <0.001 | 2.0 | 0.303 | 0.2 | 0.939 |
| Sleep disturbance | 0.126 | <0.001 | 0.124 | <0.001 | 1.4 | 0.271 | 0.2 | 0.823 |
| Depression† | 0.254 | <0.001 | 0.253 | <0.001 | 0.5 | 0.274 | 0.05 | 0.818 |
CRP = C-reactive protein; IV = independent variable; DV = dependent variable; MV = mediating variable; Beta = standardized regression coefficient expressing the change in standardized outcome score per one standard deviation in early life stress measured as Early Family Environment
Adjusted for sociodemographic variables (age, gender, ethnicity, and education)
Center for Epidemiological Studies Depression Scale (CES-D) score excluding 2 fatigue items and 1 sleep item
As shown in Table 4, plasma IL-6 levels significantly mediated the association between early life stress and adulthood fatigue in a small magnitude, but when the mediation model was adjusted for sociodemographic variables, the mediation by IL-6 was no longer significant. First, early life stress assessed at baseline (independent variable) accounted for variation in adulthood fatigue assessed at follow-up (dependent variable) as noted by beta 0.138 (P<0.001). Second, early life stress accounted for variation in IL-6 measured at follow-up (mediator) as noted by beta 0.062 (P=0.001). Third, when IL-6 was added to the model, its presence reduced the strength of the association between early life stress and fatigue at follow-up as noted by the change of beta from 0.138 (P<0.001) to 0.133 (P<0.001). The Sobel-Goodman mediation test suggested that 3.2% of the total effect of early life stress on fatigue was mediated by IL-6 (Z=2.460, P=0.014). However, when sociodemographic variables – age, gender, ethnicity, and education – were added to the mediation model as covariates, the Sobel-Goodman mediation test suggested that just 2.3% of the total effect of early life stress on fatigue was mediated by IL-6 (Z=1.796, P=0.072). As further shown in Table 4, while IL-6 significantly mediated the effect of early life stress on pain, sleep disturbance, and depression, the adjustment for sociodemographic variables attenuated these mediations to non-significance. Of note, IL-6 was available only at follow-up.
Table 4.
Sobel–Goodman mediation tests with Year 15 early life stress as independent variable, Year 20 sickness behavior symptoms as dependent variables, and Year 20 IL-6 as mediating variable
| Sickness behavior symptom | Model with IV and DV | Model with IV, DV and MV | % mediated by IL-6 | P | % mediated by IL-6 after adjustment* | P | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Beta | P | Beta | P | |||||
| Fatigue | 0.138 | <0.001 | 0.133 | <0.001 | 3.2 | 0.014 | 2.3 | 0.072 |
| Pain | 0.115 | <0.001 | 0.107 | <0.001 | 6.3 | 0.004 | 4.2 | 0.059 |
| Sleep disturbance | 0.126 | <0.001 | 0.122 | <0.001 | 3.2 | 0.019 | 1.2 | 0.191 |
| Depression† | 0.254 | <0.001 | 0.249 | <0.001 | 2.0 | 0.009 | 0.8 | 0.120 |
IL-6 = interleukin-6; IV = independent variable; DV = dependent variable; MV = mediating variable; Beta = standardized regression coefficient expressing the change in standardized outcome score per one standard deviation in early life stress measured as Early Family Environment
Adjusted for sociodemographic variables (age, gender, ethnicity, and education)
Center for Epidemiological Studies Depression Scale (CES-D) score excluding 2 fatigue items and 1 sleep item
4. DISCUSSION
In a community sample, early life stress as indexed by self-report of a risky early family environment was longitudinally associated with fatigue and this association was independent of several covariates including sociodemographic characteristics, BMI, medication use, presence of medical comorbidity, smoking, alcohol consumption, physical activity, current stress, pain, sleep disturbance, depression, and fatigue assessed at the time of the assessment of early life stress. Of note, those participants who reported higher early life stress at baseline were feeling more fatigued at baseline, indicating a cross-sectional association; but even when this was taken into account by including baseline fatigue as an additional covariate in the multivariable model, early life stress was shown to be a highly statistically significant risk factor for fatigue at follow-up. The same was true for depression, another symptom of sickness behavior, but not for pain and sleep disturbance. However, plasma levels of CRP and IL-6 did not mediate the longitudinal association between early life stress and adulthood fatigue. In summary, early life stress retrospectively assessed in adulthood was associated with the development of fatigue five years later, but this association was not mediated by low-grade systemic inflammation.
Derived from a community-based prospective study, the current data extend the findings of the prior cross-sectional studies (Heim et al., 2006; McCauley et al., 1997; Romans et al., 2002) and provide some longitudinal evidence on the association between early life stress and adulthood fatigue. The following features further strengthen the current findings. First, selection bias was less likely than in previous studies, given that the study sample was randomly chosen from the community. Second, as noted above, the association between early life stress and fatigue was independent of a series of confounding variables such as socioeconomic status, obesity, medical comorbidity, smoking, physical activity, current stress, and the other sickness behavior symptoms.
The following limitations should be considered. First, early life stress was assessed retrospectively relying upon the memory of the participants. However, the assessment of early life stress predated the measurement of the fatigue outcome by five years, thus minimizing the possibility of information bias; and current stress and current depression were taken into account in order to minimize the differential recall of past experiences by the current factors. Second, the assessment of fatigue relied on a single item rather than a composite measure that evaluates the multidimensional nature of this construct. Thus, the current findings should be interpreted taking this limitation into account and future research should employ a more nuanced measure of fatigue such as the Multidimensional Fatigue Symptom Inventory (Smets et al., 1995). Third, because the measurement of CRP and IL-6 temporally coincided with the assessment of independent and dependent variable respectively, there was some limitation in interpreting the directions of effects. Ideally, the assessment of mediators should be conducted temporally after that of independent variables and before that of dependent variables.
The current study demonstrates that the presence of childhood stress is associated with the onset of fatigue in adults. Future research should focus on elucidating the psychological and biological mechanisms of this relationship, which have the potential to inform the development of targeted psychological and/or biological interventions for the prevention of fatigue in community dwelling adults.
Research Highlight.
Early life stress was longitudinally associated with the development of fatigue but this association was not mediated by low-grade systemic inflammation.
Acknowledgments
The Coronary Artery Risk Development in Young Adults study was supported (or partially supported) by the following contracts from the National Heart, Lung and Blood Institute: University of Alabama at Birmingham, Coordinating Center, N01-HC-95095; University of Alabama at Birmingham, Field Center, N01-HC- 48047; University of Minnesota, Field Center and Diet Reading Center (Year 20 Exam), N01-HC-48048; Northwestern Univer- sity, Field Center, N01-HC-48049; Kaiser Foundation Research Institute, N01-HC-48050; University of California, Irvine, Echocardiography Reading Center (Years 5 and 10), N01-HC-45134; Harbor-University of California, Los Angeles (UCLA) Research Education Institute, Computed Tomography Reading Center (Year 15 Exam), N01-HC-05187; Wake Forest University (Year 20 Exam), N01-HC-45205; and New England Medical Center (Year 20 Exam), N01-HC-45204. The work on this manuscript was supported by the UCLA Friends of the Semel Institute Fellowship and in part by R01-AG034588; R01-AG026364; R01-119159; R01-HL079955; P30-AG028748; R01-MH091352 to MI, and the Cousins Center for Psychoneuroimmunology.
Footnotes
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amato MP, Ponziani G, Rossi F, Liedl CL, Stefanile C, Rossi L. Quality of life in multiple sclerosis: the impact of depression, fatigue and disability. Mult Scler. 2001;7:340–344. doi: 10.1177/135245850100700511. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Ferrans CE. The quality of life of persons with chronic fatigue syndrome. J Nerv Ment Dis. 1997;185:359–367. doi: 10.1097/00005053-199706000-00001. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Seeman TE, Bower JE, Kiefe CI, Irwin MR. Prospective association between C-reactive protein and fatigue in the coronary artery risk development in young adults study. Biol Psychiatry. 2009;66:871–878. doi: 10.1016/j.biopsych.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curt G. Impact of fatigue on quality of life in oncology patients. Semin Hematol. 2000;37:14–17. doi: 10.1016/s0037-1963(00)90063-5. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Lindgren ME, Shapiro CL, Kiecolt-Glaser JK. Child maltreatment and breast cancer survivors: Social support makes a difference for quality of life, fatigue and cancer stress. Eur J Cancer. 2011 doi: 10.1016/j.ejca.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, Bower JE, Belin TR. Quality of Life at the End of Primary Treatment of Breast Cancer: First Results From the Moving Beyond Cancer Randomized Trial. J Natl Cancer Inst. 2004;96:376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- Han C, Pulling CC, Telke SE, Hullsiek KH. Assessing the utility of five domains in SF-12 Health Status Questionnaire in an AIDS clinical trial. AIDS. 2002;16:431. doi: 10.1097/00002030-200202150-00015. [DOI] [PubMed] [Google Scholar]
- Hann DM, Jacobsen PB, Azzarello LM, Martin SC, Curran SL, Fields KK, Greenberg H, Lyman G. Measurement of Fatigue in Cancer Patients: Development and Validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- Heim C, Wagner D, Maloney E, Papanicolaou DA, Solomon L, Jones JF, Unger ER, Reeves WC. Early adverse experience and risk for chronic fatigue syndrome: Results from a population-based study. Arch Gen Psychiatry. 2006;63:1258–1266. doi: 10.1001/archpsyc.63.11.1258. [DOI] [PubMed] [Google Scholar]
- Hurst NP, Ruta DA, Kind P. Comparison of the MOS short form-12 (SF12) health status questionnaire with the SF36 in patients with rheumatoid arthritis. Rheumatology (Oxford) 1998;37:862–869. doi: 10.1093/rheumatology/37.8.862. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature reviews Immunology. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki-Deverts D, Cohen S, Adler NE, Schwartz JE, Matthews KA, Seeman TE. Socioeconomic status is related to urinary catecholamines in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosom Med. 2007;69:514–520. doi: 10.1097/PSY.0b013e3180f60645. [DOI] [PubMed] [Google Scholar]
- Jenkinson C, Layte R, Jenkinson D, Lawrence K, Petersen S, Paice C, Stradling J. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health. 1997;19:179–186. doi: 10.1093/oxfordjournals.pubmed.a024606. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A Comparison of Methods to Test Mediation and Other Intervening Variable Effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M, Brunner E. Cohort Profile: The Whitehall II study. Int J Epidemiol. 2005;34:251–256. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EB. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA. 1997;277:1362–1368. [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers GL, Rifai N, Tracy RP, Roberts WL, Alexander RW, Biasucci LM, Catravas JD, Cole TG, Cooper GR, Khan BV, Kimberly MM, Stein EA, Taubert KA, Warnick GR, Waymack PP. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: Report From the Laboratory Science Discussion Group. Circulation. 2004;110:e545–549. doi: 10.1161/01.CIR.0000148980.87579.5E. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker ED, Schmitz KH, Jacobs DR, Jr, Dengel DR, Schreiner PJ. Physical Activity in Young Adults and Incident Hypertension Over 15 Years of Follow-Up: The CARDIA Study. Am J Public Health. 2007;97:703–709. doi: 10.2105/AJPH.2004.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlikowska T, Chalder T, Hirsch SR, Wallace P, Wright DJ, Wessely SC. Population based study of fatigue and psychological distress. BMJ. 1994;308:763–766. doi: 10.1136/bmj.308.6931.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers DA, Xie Y. Statistical methods for categorical data analysis. Academic Press; San Diego: 2000. [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385. [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Ricci JA, Chee E, Lorandeau AL, Berger J. Fatigue in the US Workforce: Prevalence and Implications for Lost Productive Work Time. J Occup Environ Med. 2007;49:1–10. doi: 10.1097/01.jom.0000249782.60321.2a. [DOI] [PubMed] [Google Scholar]
- Romans S, Belaise C, Martin J, Morris E, Raffi A. Childhood abuse and later medical disorders in women. An epidemiological study. Psychother Psychosom. 2002;71:141–150. doi: 10.1159/000056281. [DOI] [PubMed] [Google Scholar]
- Sidney S, Jacobs DR, Haskell WL, Armstrong MA, Dimicco A, Oberman A, Savage PJ, Slattery ML, Sternfeld B, Van Hom L. Comparison of Two Methods of Assessing Physical Activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133(12):1231–1245. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- Smets E, Garssen B, Bonke B, De Haes J. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of Lost Productive Work Time Among US Workers With Depression. JAMA. 2003a;289:3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost Productive Time and Cost Due to Common Pain Conditions in the US Workforce. JAMA. 2003b;290:2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19:1059–1079. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of Early Life Stress and Psychological Functioning to Adult C-Reactive Protein in the Coronary Artery Risk Development in Young Adults Study. Biol Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Valdini AF. Fatigue of Unknown Aetiology-- a Review. Fam Pract. 1985;2:48–53. doi: 10.1093/fampra/2.1.48. [DOI] [PubMed] [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]