Abstract
Objectives
Prescription rates for diabetic drugs vary considerably across the United States for Medicare beneficiaries. The goal of this study was to determine if non-clinical factors (patient race, ethnicity, gender, income) are associated with regional variation in pharmacotherapy decisions for diabetic patients enrolled in Medicare.
Methods
We performed a spatially-weighted, linear regression analysis of the entire diabetic population enrolled in Medicare Parts A, B, and D for the years 2006 through 2009. Our outcomes of interest were the percentage of diabetic patients being treated with metformin, a sulfonylurea, a thiazolidinedione, or insulin within a hospital referral region (HRR).
Results
Prescription rates for metformin, sulfonylureas, thiazolidinediones, and insulin varied more than two-fold between hospital referral region. Metformin prescription rates were increased in western states while prescription rates for sulfonylureas and insulins were highest in the South and Midwest. In contrast with these other diabetic drug classes, members of the thiazolidinedione drug class were prescribed more frequently in the Central United States (Great Plains, Colorado Rockies, Northern Texas, Oklahoma). Prescription rates for each drug class were increased in hospital referral regions with a lower household income. Referral regions with larger African American populations were associated with higher prescription rates for insulin (p<0.001) and lower prescription rates for metformin (p<0.001). Gender and Hispanic ethnicity were not associated with regional variation in prescription rates for the four major diabetic drug classes.
Conclusions
Geographic differences exist in the management of type 2 diabetes for Medicare enrollees. Prescription patterns were associated with household income and African American race. Further studies are necessary to identify local, unidentified factors that might be influencing provider management styles.
Keywords: Medicare, Diabetes Treatment, Race, Ethnicity, Gender, Metformin, Geographic Variation in Diabetes Care
Introduction
Diabetes currently affects one out of every four (10.9 million) Americans over the age of sixty-five and accounts for 32% of total Medicare expenditures.1,2 Reimbursements for the treatment of macrovascular and microvascular complications are predominantly responsible for the high costs associated with diabetes care.3,4 However, the incidence of these complications can be significantly reduced with pharmacotherapy that appropriately normalizes blood glucose levels.5,6
Clinicians have a variety of medications at their disposal to treat hyperglycemia, but the choice of drug therapy is influenced by a variety of clinical factors and patient preferences. The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommends metformin as the first-line treatment for type 2 diabetes for “its effect on glycemia, absence of weight gain or hypoglycemia, generally low level of side effects, high level of acceptance, and relatively low cost.”7-10 The ADA/EASD guidelines further recommend that sulfonylureas, thiazolidinediones, and insulins be used as supplemental agents or as the primary pharmacotherapy in diabetic patients with a contraindication to using metformin (eg. renal insufficiency).10
While it is often assumed that the decision to treat with a particular drug class is a function of the patient’s clinical situation, several recent studies have reported that disease severity and treatment decisions are associated with patient race and ethnicity.11,12 It is unknown whether similar associations exist for the U.S. Medicare population. In this study, we highlight regional variation in prescription rates for diabetic medications received by patients enrolled in Medicare. We subsequently performed a linear regression analysis of the prescription data to determine if non-clinical factors such as race, ethnicity, gender, and income are associated with geographic differences in the pharmacological management of diabetes for U.S. Medicare beneficiaries.
Research Design and Methods
Study Population and Design
We studied the entire population of U.S. Medicare beneficiaries with diabetes who were enrolled in Medicare Parts A, B, and D (prescription drug coverage) for a continuous period of 12 months or longer between 2006 and 2009 (n = 8.8 million). The primary objective was to describe prescription rates across the United States for the major diabetic drug classes and to determine if non-clinical factors such as race, ethnicity, and income are associated with these treatment patterns. Beneficiaries were considered alive up to and including the month of their death. Enrollment was determined using the Medicare Enrollment Database. The data for this study and additional details of our study population are publicly available and summarized online (http://www.effectivehealthcare.ahrq.gov/) in the Data Points publication series produced as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program funded by the U.S. Department of Health & Human Services Agency for Healthcare Research and Quality.4,13,14
Identification of Diabetic Patients in the U.S. Medicare Population
Beneficiaries were determined to have diabetes if they had two or more ICD-9 (International Classification of Diseases, 9th Revision) codes or one ICD-9 inpatient claim consistent with such a diagnosis, a method similar to that used by the Centers for Disease Control and Prevention for the study of large administrative datasets. The ICD-9 codes used by CMS (Centers for Medicare and Medicaid Services) to identify diabetic enrollees for our cohort were: 250.00-03 (Diabetes mellitus without complication), 250.10-13 (Diabetes with ketoacidosis), 250.20-23 (Diabetes with hyperosmolarity), 250.30-33 (Diabetes with other coma), 250.40-43 (Diabetes with renal manifestations), 250.50-53 (Diabetes with ophthalmic manifestations), 250.60-63 (Diabetes with neurological manifestations), 250.70-73 (Diabetes with peripheral circulatory disorders), 250.80-83 (Diabetes with other specified manifestations), 250.90-93 (Diabetes with unspecified complication). Diabetic beneficiaries in our study qualified for Medicare coverage if they were 65 years of age or older, had chronic kidney disease requiring dialysis, and/or were disabled. For the years 2006-2009, approximately 84% of Medicare beneficiaries were sixty-five years of age or older. Patients with type 1 diabetes are estimated to account for less than 1% of our study population.15
Outcome of Interest
We measured the percentage of diabetic patients being treated with metformin, insulin, a thiazolidinedione, or a sulfonylurea within a hospital referral region (HRR). Prescriptions were identified by tracking Medicare reimbursements for diabetic medications filled by a pharmacy and received by the patient. Patients receiving combination therapy were counted in multiple prescription cohorts.
Geographic Unit of Analysis
The geographic unit of analysis was the Dartmouth Atlas of Health Care hospital referral region (n=306). Hospital referral regions contain at least one tertiary care hospital that performs major cardiovascular and neurosurgical procedures (www.dartmouthatlas.org). They reflect regional markets of care based upon referral patterns and can be used to understand how providers practicing within them utilize health services. Diabetic beneficiaries were assigned to a hospital referral region based upon their zip code of residence.
Spatially-Weighted Linear Analysis
We used spatially-weighted linear regression to identify associations between our covariates and prescription rates for metformin, insulin, thiazolidinediones, and sulfonylureas within a hospital referral region. We adjusted for spatial autocorrelation in our dataset using a spatial error function. Spatial autocorrelation is the geographic clustering of referral regions with similar prescription rates, and was indentified in our dataset after performing standard diagnostics for spatial dependence. The use of ordinary least squares (OLS) regression would have been statistically inappropriate for this linear regression analysis as it violates the regression assumption of independent observations when spatial autocorrelation exists.16-18 We performed regression analysis for the year 2007 for which we had the most comprehensive covariate and confounder data. Statistical analyses were conducted using Stata 11 (StataCorp LP: College Station, TX), GeoDa (Arizona State University: Tempe, AZ), and ArcGIS 9 (ERSI: Redlands, CA).
Covariates and Confounders
Each variable was measured at the level of the hospital referral region. We used data from the 2000 U.S. Census report, which was the most currently available and comprehensive census data at the time of our analysis, to measure the following independent x-variables: percentage African American, percentage Hispanic, percentage white, percentage female, age, and household income. We used the prevalence of peripheral arterial disease (PAD), prevalence of diabetic foot ulcers, and prevalence of lower extremity amputations as markers of systemic vascular disease.19,20 We did not have access to hemoglobin A1C and eGFR (estimated Glomerular Filtration Rate) data for our patient cohort. We also measured the per capita supply of physicians practicing within a hospital referral region as a marker of health care access. Physician estimates were provided by the Health Resources and Service Administration.
Maps: Percentage of Patients Receiving a Drug Class
We created maps showing the percentage of diabetic beneficiaries within a hospital referral region receiving one of the following drug classes: metformin, sulfonylureas, thiazolidinediones, and insulin. For 2007, hospital referral regions were divided into quartiles based upon their prescription rates. These percentage quartiles were subsequently used for our 2009 maps to illustrate changes in prescribing patterns over time.
Results
There were approximately 8.8 million Medicare beneficiaries who satisfied the inclusion criteria for this study. The majority of patients (7.4 million, 84%) were eligible for Medicare benefits based upon their age, while the remaining diabetic patients in our cohort received Medicare coverage as a result of disabilities and/or end-stage renal disease. There was a slight female predominance in our dataset (4.8 million females, 55%) and the majority of diabetic beneficiaries were white (7.0 million, 79.5%). Further details of the study population are publicly available online (http://www.effectivehealthcare.ahrq.gov/).4,13,14
In Table 1, we report overall changes in prescription rates for our patient cohort. The percentage of beneficiaries receiving metformin increased from 33.1% to 37.2% between 2006 and 2009 (Table 1). However, this percentage varied more than two-fold between hospital referral region. During this same time period, the number of patients being treated with a sulfonylurea, thiazolidinedione, or insulin decreased (Table 1), and these trends were each statistically significant (p<0.001).
Table 1. Percentage of total Medicare beneficiaries with diabetes being prescribed a drug class, 2006-2009.
Table 1 lists the percentage of Medicare beneficiaries with diabetes receiving a particular medication for the years 2006 through 2009. The distribution of percentages for all 306 hospital referral regions is included in parentheses. Prescriptions increased for metformin and decreased for sulfonylureas, thiazolidinediones, and insulins during the study period.
| Drug Class | 2006* | 2007* | 2008* | 2009* |
|---|---|---|---|---|
| Biguanides | 33.1(24.7- 52.5) |
34.8(25.5- 52.7) |
36.0(25.8- 54.6) |
37.2(27.3- 55.4) a |
| Insulins | 24.4(14.0- 33.7) |
22.4(12.2- 30.0) |
22.8(12.5- 32.1) |
23.5(13.8- 33.6) a |
| Sulfonylureas | 33.0(18.2- 42.5) |
32.0(18.7- 40.2) |
30.7(19.8- 39.0) |
29.4(19.7- 37.3) a |
| Thiazolidinediones | 22.1(12.3- 36.7) |
19.5(11.2- 29.8) |
14.6(8.2- 24.8) |
13.0(7.0-23.2) a |
p<0.001 for percentage change between 2006 and 2009.
Percentages were calculated by dividing the total number of Medicare beneficiaries receiving drug “X” by the total number of Medicare beneficiaries with diabetes. The percentages for each drug class do not total 100% for a calendar year because patients receiving combination therapy were counted in the numerator of these calculations for multiple drug classes.
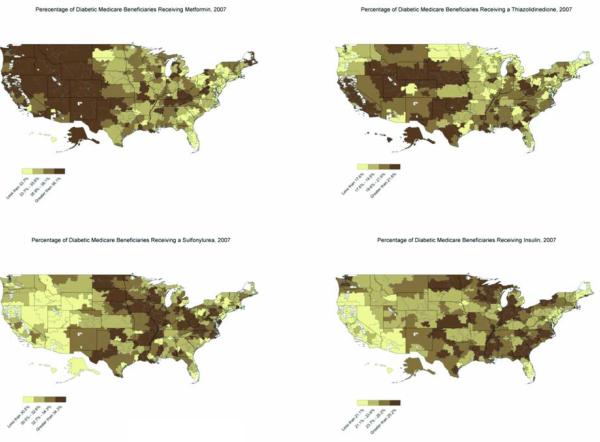
Prescription rates for each medication also varied considerably by location (Figures 1 and 2). Overall, the heaviest use of metformin was observed in western states with an increasing number of patients being treated with this medication in the eastern half of the country during the study period (Figures 1 and 2). In contrast with metformin, prescription rates for sulfonylureas and insulins were increased in the South and Midwest while provider utilization of thiazolidinediones was highest in the central United States in a region that included the Colorado Rockies, Great Plains, northern Texas, and Oklahoma (Figure 1).
Figure 1. Prescription Patterns for Diabetic Medications Received by U.S. Medicare Beneficiaries, 2007.
Figure 1 illustrates regional variation in the prescription rates of metformin, sulfonylureas, thiazolidinediones, and sulfonylureas for the year 2007. These maps show the percentage of diabetic patients in each of the 306 hospital referral regions receiving a particular drug class. Patients receiving combination therapy were counted as receiving multiple diabetic medications. The heaviest use of metformin was observed in western states while prescription rates for sulfonylureas and insulins were increased in the South and Midwest. In contrast with these medications, provider utilization of thiazolidinediones was highest in the central United States in a region that included the Colorado Rockies, Great Plains, northern Texas, and Oklahoma.
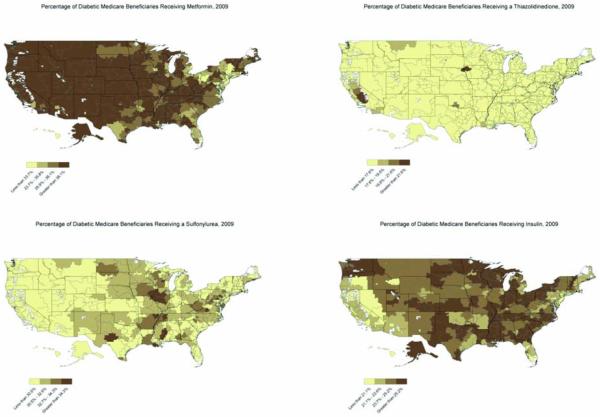
Figure 2. Prescription Patterns for Diabetic Medications Received by U.S. Medicare Beneficiaries, 2009.
Figure 2 illustrates regional variation and temporal changes in the prescription rates of metformin, sulfonylureas, thiazolidinediones, and sulfonylureas between 2007 and 2009. These maps show the percentage of diabetic patients in each of the 306 hospital referral regions receiving a particular drug class. Patients receiving combination therapy were counted as receiving multiple diabetic medications. A comparison of the 2007 and 2009 maps shows that prescriptions increased for metformin and decreased for sulfonylureas and thiazolidinediones during the study period. The prescription rate for insulin was slightly higher in 2009 compared to 2007, but was decreased from 2006.
Table 2 reports the results of our regression analysis. Treatment decisions were associated with several non-clinical factors after adjusting for potential confounders including physician supply, patient age, prevalence of peripheral arterial disease, prevalence of diabetic foot ulcers, and prevalence of lower extremity amputations within a hospital referral region. Diabetic patients living in a hospital referral region with a lower household income were more likely to be prescribed an oral hypoglycemic or insulin (p<0.001 for metformin, insulin, thiazolidinediones; p=0.14 for sulfonylureas). Insulin was also prescribed more frequently in hospital referral regions with larger African American populations (p<0.001). However, prescription rates for metformin (p<0.001), sulfonylureas (p=0.04), and thiazolidinediones (p<0.001) were decreased in these same referral areas (Table 3). Gender and Hispanic ethnicity were not associated with geographic variation in prescription rates for the four major diabetic drug classes.
Table 2. Select coefficients (z-scores) and p-values for spatially-weighted linear regression analysis of Diabetic prescriptions for U.S. Medicare beneficiaries, 2007.
Table 2 reports the results of our spatially-weighted linear regression analysis. We created regression equations for the year 2007 because we had the most comprehensive covariate and confounder data for that year. The sign of the coefficient indicates the direction of the association with positive coefficients reflecting a positive correlation and negative coefficients reflecting a negative correlation. Coefficients do not provide any information about the magnitude of an association or its statistical significance. P-values for each covariate are listed in parentheses. Each variable was included in our multivariate regression equation. Prescription rates for each drug class were increased in hospital referral regions with a lower household income. Referral regions with larger African American populations were associated with higher prescription rates for insulin and lower prescription rates for metformin. Gender and Hispanic ethnicity were not associated with regional variation in prescription rates for the four major diabetic drug classes.
| Biguanides | Insulin | Sulfonylureas | Thiazolidinediones | |
|---|---|---|---|---|
|
Non-Clinical Variables |
||||
| Percentage African American |
−3.2 (p<0.001) |
3.9 (p<0.001) | −2.0 (p=0.04) | −3.5 (p<0.001) |
| Percentage Hispanic | −0.14 (p=0.89) |
−0.35 (p=0.73) |
0.67 (p=0.50) | −0.65 (p=0.52) |
| Percentage Caucasian | 0.20 (p=0.84) |
5.3 (p<0.001) | 0.55 (p=0.58) | −5.0 (p<0.001) |
| Percentage Female | −1.4 (p=0.15) |
0.23 (p=0.82) | 0.78 (p=0.43) | 0.61 (p=0.54) |
| Age | −4.8 (p<0.001) |
−4.7 (p<0.001) |
0.41 (p=0.68) | −3.6 (p<0.001) |
| Household Income | −4.6 (p<0.001) |
−4.0 (p<0.001) |
−1.5 (p=0.14) | −4.0 (p<0.001) |
| Clinical Variables | ||||
| Total Physicians | 1.1 (p=0.28) | 0.51 (p=0.61) | −2.0 (p=0.04) | −1.1 (p=0.27) |
| Prevalence of Diabetic Foot Ulcers |
−2.6 (p=0.008) |
−1.9 (p=0.06) | −0.65 (p=0.52) | −2.5 (p=0.01) |
| Prevalence of Lower Extremity Amputations |
1.2 (p=0.23) | 6.4 (p<0.001) | 3.7 (p<0.001) | −1.0 (p=0.31) |
| Prevalence of Peripheral Arterial Disease |
−2.7 (p=0.007) |
0.34 (p=0.73) | −0.68 (p=0.49) | −1.9 (p=0.06) |
| Hospital Discharges | −2.1 (p=0.03) |
0.03 (p=0.98) | 2.5 (p=0.01) | −0.001 (p=0.99) |
Discussion
For Medicare patients, prescription rates for diabetic medications varied considerably by geographic location and demonstrated statistically significant trends during our four-year study period. The percentage of Medicare beneficiaries with diabetes receiving metformin increased by 4.1% (33.1% to 37.2%, p<0.001) between 2006 and 2009, which represents a 12.3% rise in the prescription rate for this drug. This trend coincided with updated ADA/EASD guidelines in 2006, which officially endorsed metformin as the first-line treatment for type 2 diabetes.10 The increased utilization of metformin amongst U.S. Medicare beneficiaries is also consistent with the prescription patterns observed for individuals with private insurance and U.S. veterans during the past decade.21-23 In contrast with metformin, prescription rates for sulfonylureas, thiazolidinediones, and insulins decreased during the study period.
The declining utilization of sulfonylureas is likely the result of clinicians increasingly replacing this drug class with metformin as the first-line treatment for type 2 diabetes.12 In support of this hypothesis is the observation that prescription rates for sulfonylureas were generally lower in western states where metfomin utilization was high. Prescription rates for thiazolidinediones exhibited a more marked decline between 2006 and 2009 compared to the other diabetic drug classes, and this trend was presumably driven by new FDA warnings regarding the potential deleterious cardiac effects of rosiglitazone, a member of the thiazolidinedione drug class.24-26 We also observed a minor, but statistically significant decrease in insulin prescriptions, which coincided with multiple studies highlighting the deleterious effects of hypoglycemia in the elderly. A low blood glucose level in elderly patients may induce a variety of fatal and non-fatal events including stroke, myocardial infarction, and ventricular arrhythmias. Patients can also develop unsteadiness and weakness resulting in falls, which is a significant cause of morbidity and mortality within the Medicare population.27-32 However, the observation that insulin prescriptions failed to sharply decline during the study period despite these published concerns over hypoglycemia suggests that a constant percentage (approximately 20%) of type 2 diabetic beneficiaries are insulin-dependent.
Even more striking than these trends in prescribing patterns over time was the significant geographic variation observed in the prescription rates for each of the diabetic drug classes. Moreover, hospital referral regions with similar prescription rates appeared to cluster together over large areas of land involving multiple states. Prescription rates for metformin were particularly high in hospital referral regions located in western states such as California, Washington, Oregon, New Mexico, Arizona, Colorado, Wyoming, and Idaho compared to the eastern half of the country for 2007. In contrast, sulfonylureas and insulins appeared to exhibit the opposite pattern with higher prescription rates in the South and Midwest.
It is not entirely clear why prescription rates for various medications should vary by geographic location. Our regression analysis identified that individuals living in lower income hospital referral regions were more likely to receive metformin, sulfonylureas, thiazolidinediones, and insulins as part of their treatment regimen compared to higher income locations. These associations may suggest that patients with decreased income are at greater risk for having poorly controlled diabetes, which necessitates the use of a greater number of medications to adequately manage their hyperglycemia. We also found that referral areas with larger African American populations had higher prescription rates for insulin and lower prescription rates for the other drug classes. Insulin is often prescribed for patients with poorly controlled diabetes who have failed to achieve adequate glycemic control with oral hypoglycemics alone. Poor medication adherence amongst African Americans and lower income Medicare patients is unlikely to explain these associations since prescription rates were calculated based upon reimbursements for medications received by the patient. However, it is possible that once patients received their medication from the pharmacy that they failed to properly use the medication as directed by their physician.
An alternative and more likely explanation for these findings is that providers have different management styles and criteria for prescribing diabetic medications. Variability in provider approaches to diabetes care may be a function of the physician’s training, their interpretation of clinical data, and which drug classes they have experience with and feel most comfortable prescribing to their patients. Medication side-effects may also guide therapy in some practices. Florez and colleagues presented data in 2010 which showed that metformin-associated gastrointestinal side-effects such as nausea, bloating, and abdominal pain were associated with decreased metformin adherence. Certain patient comorbidities such as renal insufficiency and heart disease may also affect provider management decisions. Furthermore, treatment guidelines appear to influence drug choice as well with prescriptions for metformin increasing nationally following the 2006 revised ADA/EASD treatment algorithm for type 2 diabetes.10
Limitations
There are several limitations with our data analysis. First, we assigned patients to a hospital referral region based upon their zip code of residence, which presumes that patients seek care in local health care facilities. Therefore, the associations presented in Table 2 could be distorted by patients seeking care in a hospital referral region different from the one that was geographically inferred. The advantage of dividing the United States into 306 distinct units of care was that we were able to evaluate provider management styles within those locations.
Second, we were unable to evaluate potential confounders such as renal disease in our regression analysis, which is a contraindication to using metformin and could affect drug utilization patterns. However, in order for renal disease to be a confounder, the incidence of this complication would have had to decrease in portions of the Midwest, Southeast, and Mid-Atlantic states in order to explain the increase in metformin prescriptions observed in these regions during the study period. We also accounted for the prevalence of peripheral arterial disease, diabetic foot ulcers, and lower extremity amputations, which are markers of macrovascular and microvascular disease including impaired renal function.19,20
Conclusions
The anticipated rise in Medicare beneficiaries with type 2 diabetes over the next few decades makes it critically important that physicians manage disease according to evidence-based guidelines. The treatment recommendations published by the American Diabetes Association and the European Association for the Study of Diabetes, when not contraindicated by other patient factors, should reduce the incidence of macrovascular and microvascular complications in patients, which drive up overall Medicare costs.3,4,6 While the number of beneficiaries receiving metformin has been increasing, its use varies greatly across the United States. This variation and that observed for the other major diabetic drug classes is not random and is associated with the non-clinical factors of household income and African American race. It will be important to further evaluate the role that these non-clinical factors play in provider management styles so that interventions can occur to ensure that all diabetic patients are receiving the appropriate medical care for their disease. This paper also provides a starting point for researchers to conduct regional studies of Medicare prescription data to search for local, unidentified factors that may be influencing diabetes pharmacotherapy decisions.
Acknowledgements
Dr. Margolis and Michael Sargen had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Financial Disclosures: This study was funded in part by NIH grant K24AR02212 (Dr. Margolis). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no relevant conflicts of interest concerning the content or message of this manuscript.
References
- 1.Ashkenazy R, Abrahamson MJ. Medicare coverage for patients with diabetes. A national plan with individual consequences. J Gen Intern Med. 2006;21:386–92. doi: 10.1111/j.1525-1497.2006.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Diabetes Statistics. 2011 NIH Publication No. 11-3892. [Google Scholar]
- 3.Economic costs of diabetes in the U.S. In 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 4.Margolis DJ, Malay DS, Hoffstad OJ, et al. Economic Burden of Diabetic Foot Ulcers and Amputations: Data Points #3. 2011 [PubMed] [Google Scholar]
- 5.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 6.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342–9. doi: 10.1056/NEJMcp021106. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Goodman AM, The Multicenter Metformin Study Group Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:541–9. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 10.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963–72. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 11.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999;22:403–8. doi: 10.2337/diacare.22.3.403. [DOI] [PubMed] [Google Scholar]
- 12.Desai NR, Shrank WH, Fischer MA, et al. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med. 2012;125:302, e1–7. doi: 10.1016/j.amjmed.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margolis DJ, Malay DS, Hoffstad OJ, et al. Incidence of Diabetic Foot Ulcer and Lower Extremity Amputation Among Medicare Beneficiaries, 2006 to 2008: Data Points #2. 2011 [PubMed] [Google Scholar]
- 14.Margolis DJ, Malay DS, Hoffstad OJ, et al. Prevalence of Diabetes, Diabetic Foot Ulcer, and Lower Extremity Amputation Among Medicare Beneficiaries, 2006 to 2008: Data Points #1. 2011 [PubMed] [Google Scholar]
- 15.Niefeld MR, Braunstein JB, Wu AW, Saudek CD, Weller WE, Anderson GF. Preventable hospitalization among elderly Medicare beneficiaries with type 2 diabetes. Diabetes Care. 2003;26:1344–9. doi: 10.2337/diacare.26.5.1344. [DOI] [PubMed] [Google Scholar]
- 16.Spatial Autocorrelation . Elsevier; [(Accessed December 16, 2011, 2009]. 2009. at http://www.elsevierdirect.com/brochures/hugy/SampleContent/Spatial-Autocorrelation.pdf. [Google Scholar]
- 17.Havard S, Deguen S, Zmirou-Navier D, Schillinger C, Bard D. Traffic-related air pollution and socioeconomic status: a spatial autocorrelation study to assess environmental equity on a small-area scale. Epidemiology. 2009;20:223–30. doi: 10.1097/EDE.0b013e31819464e1. [DOI] [PubMed] [Google Scholar]
- 18.Tsai PJ, Lin ML, Chu CM, Perng CH. Spatial autocorrelation analysis of health care hotspots in Taiwan in 2006. BMC Public Health. 2009;9:464. doi: 10.1186/1471-2458-9-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Escobedo J, Rana JS, Lombardero MS, et al. Association between albuminuria and duration of diabetes and myocardial dysfunction and peripheral arterial disease among patients with stable coronary artery disease in the BARI 2D study. Mayo Clin Proc. 2010;85:41–6. doi: 10.4065/mcp.2009.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–43. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 21.Cohen FJ, Neslusan CA, Conklin JE, Song X. Recent antihyperglycemic prescribing trends for US privately insured patients with type 2 diabetes. Diabetes Care. 2003;26:1847–51. doi: 10.2337/diacare.26.6.1847. [DOI] [PubMed] [Google Scholar]
- 22.Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994-2007. Arch Intern Med. 2008;168:2088–94. doi: 10.1001/archinte.168.19.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huizinga MM, Roumie CL, Elasy TA, et al. Changing incident diabetes regimens: a Veterans Administration cohort study from 2000 to 2005. Diabetes Care. 2007;30:e85. doi: 10.2337/dc07-0650. [DOI] [PubMed] [Google Scholar]
- 24.Woodcock J, Sharfstein JM, Hamburg M. Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. N Engl J Med. 2010;363:1489–91. doi: 10.1056/NEJMp1010788. [DOI] [PubMed] [Google Scholar]
- 25.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes--an interim analysis. N Engl J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 26.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 27.Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care. 2005;28:2948–61. doi: 10.2337/diacare.28.12.2948. [DOI] [PubMed] [Google Scholar]
- 28.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr., Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–72. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26:1485–9. doi: 10.2337/diacare.26.5.1485. [DOI] [PubMed] [Google Scholar]
- 30.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–8. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 32.Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011;154:554–9. doi: 10.7326/0003-4819-154-8-201104190-00007. [DOI] [PubMed] [Google Scholar]