Abstract
Observational studies have linked lower levels of omega-3 (n-3) polyunsaturated fatty acids (PUFAs) with inflammation and depression. This study was designed to determine whether n-3 supplementation would decrease serum cytokine production and depressive symptoms in 138 healthy middle-aged and older adults (average age=51.04, SD=7.76) who were sedentary and overweight (average BMI=30.59, SD= 4.50). This three-arm randomized, placebo-controlled, double-blind 4-month trial compared responses to (1) 2.5 g/d n-3 PUFAs, or (2) 1.25 g/d n-3 PUFAs, or (3) placebo capsules that mirrored the proportions of fatty acids in the typical American diet. Serum interleukin-6 decreased by 10% and 12% in our low and high dose n-3 groups, respectively, compared to a 36% increase in the placebo group. Similarly, low and high dose n-3 groups showed modest 0.2% and −2.3% changes in serum tumor necrosis factor alpha, compared to a 12% increase in the control group. Depressive symptoms were quite low at baseline and did not change significantly in response to supplementation. Our data suggest that n-3 PUFAs can reduce inflammation in overweight, sedentary middle-aged and older adults, and thus could have broad health benefits. These data provide a window into the ways in which the n-3 PUFAs may impact disease initiation, progression, and resolution.
ClinicalTrials.gov identifier: NCT00385723
Keywords: fish oil, omega-3, omega-6, interleukin-6, tumor necrosis factor alpha, psychoneuroimmunology, integrative medicine, nutritional neuroscience
1. Introduction
1.1 Inflammation, mental and physical health, and omega-3
Inflammation is a robust and reliable predictor of all-cause mortality in older adults (Krabbe et al., 2004). Proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) play a role in coronary heart disease (CHD), depression, type II diabetes, arthritis, osteoporosis, Alzheimer’s disease, periodontal disease, and frailty and functional decline (Ferrucci et al., 2006; Krabbe et al., 2004). Fish, the prime source for the long-chain omega-3 (n-3) polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), has generated considerable interest as a potential anti-inflammatory food (Lee et al., 2009; Wall et al., 2010).
The positive effects of fish and fish oil consumption have been demonstrated across such diverse conditions as depression, CHD, rheumatoid arthritis, and macular degeneration (Goldberg and Katz, 2007; Hallahan and Garland, 2005; Hibbeln, 1998; Johnson and Schaefer, 2006; Wall et al., 2010). Although the health benefits of fish oil may arise through a number of different mechanisms, reduced inflammation appears to be one common pathway (Shelton and Miller, 2010).
A number of epidemiological and observational studies have demonstrated that lower n-3 PUFA levels are associated with higher serum IL-6, TNF-α, and CRP (Farzaneh-Far et al., 2009; Ferrucci et al., 2006; Kalogeropoulos et al., 2010; Kiecolt-Glaser et al., 2007). In contrast, comparisons of supplemented and placebo groups in n-3 PUFA randomized controlled trials (RCTs), the gold standard for demonstrating causality, have not produced reliable serum cytokine differences (Calder et al., 2009; Fritsche, 2006; Kiecolt-Glaser et al., 2011; Sijben and Calder, 2007). Problematic methodological issues that muddy interpretation have included severely underpowered small treatment groups (e.g., 8–10 per group), low n–3 PUFA supplementation doses, insensitive cytokine assays, use of young and healthy subjects and/or highly-trained athletes, and very low levels of baseline inflammation. For example, serum cytokines did not differ significantly among 58 monks who received 0, 1.06, 2.13 or 3.19 g/d of n-3 PUFAs for a year (Blok et al., 1997); however, basal cytokine data did not differ between vegetarians and non-vegetarians even before supplementation, suggesting that the monks’ extremely healthy lifestyle limited the ability to see meaningful downward change.
The strongest RCT support for the n-3 PUFA’s anti-inflammatory properties has come from studies with older, hypertriglyceridemic or diabetic individuals with elevated inflammatory markers (Fritsche, 2006; Sijben and Calder, 2007; Wu, 2004; Yusof et al., 2008). Consequently, it has been suggested that cytokine production in healthy people is relatively insensitive to long-chain n-3 PUFAs (Sijben and Calder, 2007; Wu, 2004).
1.2 The present study
Although the health benefits of the n-3 PUFAs are well established, particularly their cardioprotective and antidepressant impacts, clear evidence that small dietary changes modulate inflammation in healthy people would provide a window into the ways in which dietary n-3 PUFAs alter disease initiation, progression, and resolution (Fritsche, 2006). Accordingly, this study assessed the impact of long-chain n-3 PUFA supplementation on inflammation in healthy sedentary overweight middle-aged and older adults. We hypothesized that n-3 PUFA supplementation would decrease our primary outcomes, proinflammatory cytokine production and depressive symptoms, in contrast to placebo.
2. Methods
2.1 Participants
The 138 participants, 45 men and 93 women, ranged in age from 40 to 85 (Table 1). Campus and community print and web-based announcements were used for recruitment. The institutional review board approved this study, and each participant provided informed consent.
Table 1.
Baseline characteristics
| Placebo (n=46) | 1.25 g/d (n=46) | 2.5 g/d (n=46) | |
|---|---|---|---|
| Age (years) | 51.1 (8.6) | 51.1 (8.0) | 51.0 (6.7) |
| Female | 36 (78%) | 28 (61%) | 29 (63%) |
| Race | |||
| White | 33 (72%) | 39 (85%) | 37 (80%) |
| Black | 9 (20%) | 5 (11%) | 8 (17%) |
| Asian | 2 (4%) | 1 (2%) | 1 (2%) |
| Other | 2 (4%) | 1 (2%) | 0 (0%) |
| Sagittal abdominal diameter (cm) | 22.8 (3.2) | 23.9 (3.4) | 22.9 (2.9) |
| CES-D | |||
| Median (IQR) | 5 (3–9) | 5 (2–11) | 6 (2–10) |
| Range | 0–50 | 0–43 | 0–32 |
Data are mean (SD) or n (%) except where noted. There were no significant differences on any of the participant characteristics (p > 0.1 for all tests).
The online screening form assessed health history, medications, and health behaviors. Exclusions included psychoactive drugs or mood altering medications, lipid-altering drugs, beta blockers, steroids, regular use of non-steroidal anti-inflammatory drugs other than an aspirin a day, ACE-inhibitors, prostaglandin inhibitors, heparin, warfarin, and alcohol/drug abuse (Buckley et al., 2004; Ferrucci et al., 2006). We also excluded pregnant or nursing women, vegetarians, diabetics, people who routinely took fish oil or flaxseed supplements or ate more than two portions of oily fish per week, smokers, and individuals with recurrent digestive problems, convulsive disorders, and autoimmune and/or inflammatory diseases. Individuals who typically engaged in 2 or more hours of vigorous physical activity per week, as well as individuals with a body mass index (BMI) less than 22.5 or greater than 40 were excluded (Fernandez-Real et al., 2003).
In addition, we used participants’ ability to follow the regimen as a criterion for study entry. Participants received a 7-day supply of placebo capsules (single blind) at the subsequent in-person screening session, and those who had taken less than 80% of the capsules a week later were dropped before randomization. We also verified height and weight at the screening visit.
2.2 Design and Study Components
Data collection for this double-blind placebo-controlled four month randomized clinical trial (RCT) began in September, 2006 and ended in February, 2011. Fasting blood samples were collected between 7:00 and 9:00 AM to control for diurnal variation.
2.21 Supplement and Placebo
This three-arm parallel group RCT compared responses to A) 2.496 g/d n-3, B) 1.25 g/d n-3, and placebo, or C) placebo. All participants took 6 pills (3 g oil) per day. For the two omega-3 groups, each 500 mg gel capsule contained 347.5 mg eicosapentaenoic acid (EPA) and 58 mg docosahexaenoic acid (DHA). Thus, for the high dose group the full daily supplement would equal 2085 mg/d of EPA and 348 mg/d of DHA. We chose the 7:1 EPA/DHA balance because of evidence that EPA has relatively stronger anti-inflammatory and antidepressant effects than DHA (Lin et al., 2010; Sijben and Calder, 2007). The placebo was a mixture of palm, olive, soy, canola, and coco butter oils that approximated the saturated:monounsaturated:polyunsaturated (SMP) ratio consumed by US adults, 37:42: 21 (USDA Continuing Survey of Food Intake by Individuals, 1994–1996) . OmegaBrite (Waltham, MA) supplied both the n-3 and the matching placebo; all pills were coated with a fuchsia coloring. OmegaBrite added a mild fish flavor to the placebo to help disguise any differences between the n-3 PUFA pills and the placebo, and we told participants about the fish flavoring to promote blindness (Stoll et al., 2001). Table 2 shows the results of our independent analysis of the fatty acid profile of the n-3 and placebo pills.
Table 2.
Fatty acid composition of the dietary supplements as determined by independent analysis
| Placebo | Supplement | ||
|---|---|---|---|
|
| |||
| % fatty acid | % fatty acid | ||
| C14:0 | myristic acid | 3.1 | 0.0 |
| C16:0 | palmitic acid | 16.4 | 0.1 |
| C18:0 | stearic acid | 3.2 | 0.5 |
| C18:1n9 | oleic acid | 48.7 | 0.7 |
| C18:1n7 | vaccenic acid | 1.6 | 0.3 |
| C18:2n6 | linoleic acid | 21.5 | 0.2 |
| C18:3n3 | alpha linolenic acid | 3.3 | 0.2 |
| C18:4n3 | stearidonic acid | 0.1 | 6.4 |
| C20:4n6 | arachidonic acid | 0.1 | 3.2 |
| C20:4n3 | eicosatetraenoic acid | 0.0 | 1.0 |
| C20:5n3 | eicosapentaenoic acid | 1.0 | 76.8 |
| C22:6n3 | docosahexaenoic acid | 0.1 | 8.5 |
2.22 Randomization and Masking
Before a participant left the baseline session, s/he was randomly assigned to one of three treatment groups using a permuted block randomization sequence prepared and maintained by the data manager who had no involvement in data collection or biological laboratory analyses; she was the only person who had access to the randomization list. The active and placebo study medications were packaged according to the randomization sequence, so that a participant was randomized to the next available individual supply of study medication. At each subsequent study visit, participants returned unused pills and received the next month’s supply. Blinding was assessed at the final visit.
2.3 Health-Related Behaviors
Participants’ height and weight were assessed at the screening visit, and participants were weighed during each subsequent visit. At each study visit, participants were evaluated for changes in fatty acid composition of plasma and PBMCs, mood, and proinflammatory cytokines.
Adipose tissue in the abdomen may secrete up to three times as much IL-6 as other subcutaneous fat tissues (Browning, 2003). Sagittal abdominal diameter measurements provided data on abdominal fat. Sagittal abdominal diameter was assessed by measuring the distance from the small of the back to the upper abdomen midway between the top of the pelvis and the base of the ribs when the participant was lying on their back. Validational studies using computerized axial tomography and dual-energy X-ray absorptiometry have demonstrated sagittal abdominal diameter’s utility as a noninvasive central adiposity measure (Clasey et al., 1999).
The Women’s Health Initiative Food Frequency Questionnaire (FFQ), completed during the screening session and repeated at the end of the trial, provided data on the type, frequency, and quantity of foods and beverages consumed in the past 90 days (Patterson et al., 1999). The precision is similar to other FFQs, and means are within 10% of dietary records or recalls. Software calculations estimated dietary intake of energy, macro- and micronutrients, as well as intake of key food groups using the Nutrition Data Systems for Research. Participants were asked not to change their diet during the trial were told not to take any fish oil or other n-3 supplements other than those provided by the study.
The Pittsburgh Sleep Quality Index (PSQI) assessed sleep quality and disturbances over a one-month interval; it has good diagnostic sensitivity and specificity (Buysse et al., 1989). We used the full scale at baseline and at 4 months. In addition, we also assessed sleep efficiency for the prior night (Vgontzas et al., 1999).
The Community Healthy Activities Model Program for Seniors Questionnaire (CHAMPS) assessed the weekly frequency and duration of various physical activities at baseline and 4 months. An excellent instrument for middle-aged and older populations, it has an strong history of validation and testing, and it is sensitive to relatively small changes in physical activity (Harada et al., 2001; Resnicow et al., 2003; Stewart et al., 2001a; Stewart et al., 2001b).
The modified version of the Health Review, administered monthly, provided data on infectious illness symptoms as well as possible supplementation side effects (Jenkins et al., 1980; Orts et al., 1995). Developed to provide a simple reliable and valid method for periodic assessment of infectious illness, the symptoms assessed also include the primary gastrointestinal side effects described for n-3 PUFA supplementation.
2.4 Depressive Symptoms
The Center for Epidemiological Studies Depression Scale (CES-D), administered at all visits, has been used extensively to measure depressive symptomatology (Basco et al., 1997; Radloff, 1977). Studies have shown acceptable test-retest reliability and excellent construct validity (Basco et al., 1997).
2.5 Fatty Acid Analyses
Lipids were extracted from plasma and PBMCs using chloroform: methanol (2:1, v/v) with 0.2 vol. 0.88% KCl (Bligh and Dyer, 1959). Fatty acid methyl esters of the fractions were prepared by incubating the fractions with tetramethylguanidine at 100°C (Shantha et al., 1993) and analyzed by gas chromatography (Shimadzu, Columbia, MD) using a 30-m Omegawax 320 (Supelco-Sigma) capillary column. The helium flow rate was 30 ml/min and oven temperature ramped beginning at 175°C and held for 4 min then increased to 220°C at a rate of 3°C/min as previously described (Belury and Kempa-Steczko, 1997). Retention times were compared to authentic standards for fatty acid methyl esters (Supelco-Sigma, St. Louis, MO and Matreya, Inc, Pleasant Gap, PA).
2.6 Immunological Assays
Serum levels of IL-6 and TNF-α were multiplexed and measured using an electrochemilluminescence method with Meso Scale Discovery kits, and read using the Meso Scale Discovery Sector Imager 2400. Each subject’s stored samples were assayed for all the cytokine markers in one run, thus using the same controls for all time points for each person. Sensitivity for these serum cytokines was 0.3 pg/ml. The intra-assay coefficient of variation for IL-6 was 2.8%, and the inter-assay coefficient of variation was 12.5%; corresponding values for TNF-α were 4.3% and 12.1%.
2.7 Sample Size
Sample size was based on detection of conservative effect sizes for the lower dose versus placebo comparisons for the primary outcome of cytokine levels. The literature suggested that the higher dose would have a greater effect, thus we expected the higher dose versus placebo contrast to have more power than low versus placebo. All power analyses were based on contrasts within mixed effect linear models with two-sided alpha=0.05 and assumed a 10% attrition rate. Our conservative estimated effect size was a decrease in IL-6 of 0.45 pg/mL in the low dose group, extrapolated from results from Ferrucci et al. (Ferrucci et al., 2006). Pilot data from our lab provided an estimate of standard deviation of 0.88 pg/mL, thus to achieve 85% power a sample size of 46 in each group was required.
2.8 Statistical Methods
Mixed models were used to test the effects of supplementation on proinflammatory cytokine levels and depressive symptoms (Diggle et al., 2002). This type of model treats the responses from each subject as repeated measures, accounting for the within-subject correlation. Compound symmetry variance-covariance structure was used to account for the correlation within subjects. In models for serum cytokines random effects for the assay plate and assay lot were also included. To test for treatment group differences, models included the effects of visit, treatment group, and their interaction. Outcome variables were natural log transformed when residual analyses suggested violation of the normality assumption. P-values from between-group comparisons were Bonferroni-adjusted within outcome model since three pairwise group comparisons were performed for each model.
Differences in the proportions experiencing nonserious adverse events between treatment groups were evaluated using Fisher’s exact test. James’ blinding indices were calculated using participant and experimenter guesses about treatment assignment at the final study visit (James et al., 1996). Alpha was set to 0.05, and two- sided tests were conducted. All analyses were carried out in SAS version 9.1 (SAS Institute, Cary, NC).
3. Results
3.1 Study Population, Diet, and Health Behaviors
Randomized groups were equivalent on key dimensions (Table 1). Randomization produced groups that did not differ on age, baseline FFQ dietary variables, depression, and sleep quality, p > 0.2 for all tests. Using BMI cut points of 25 and 30 kg/m2, 125 (91%) were overweight, and 65 (47%) were obese, respectively. There were small differences between groups on weight at baseline (p = 0.002), with the placebo group having the lowest average weight, however there were not significant differences in either sagittal abdominal diameter or BMI (p > 0.05 for both).
Analyses of FFQ data at the last visit revealed no differences among the groups in reported changes in intake of calories, fiber, total fat, protein, saturated fat, monounsaturated fat, polyunsaturated fat, omega-3 fatty acids, or linoleic acid during the study period, p > .11 for all tests. Similarly, sleep and exercise did not show differential group changes, p > .26 for both. Both the lower and higher n-3 groups had modest but statistically significant increases in weight across the trial (average pounds gained: 2.1 lbs and 2.5 lbs in the two groups, respectively), compared to no change in the placebo group (average pounds gained: 0.39 lbs). However, the increased weight would have theoretically fueled inflammation in this overweight sample which was not observed.
Few participants reported taking any medication during the study, and the numbers did not differ among groups. The most common medications were multivitamins (n=42), NSAIDs (n=29; 15 aspirin, 8 ibuprofen, 5 naproxen, 1 meloxicam), antihistamines (n=13), estrogen with or without progesterone (n=10), and levothyroxine (n=10).
3.2 Protocol Adherence and Blinding
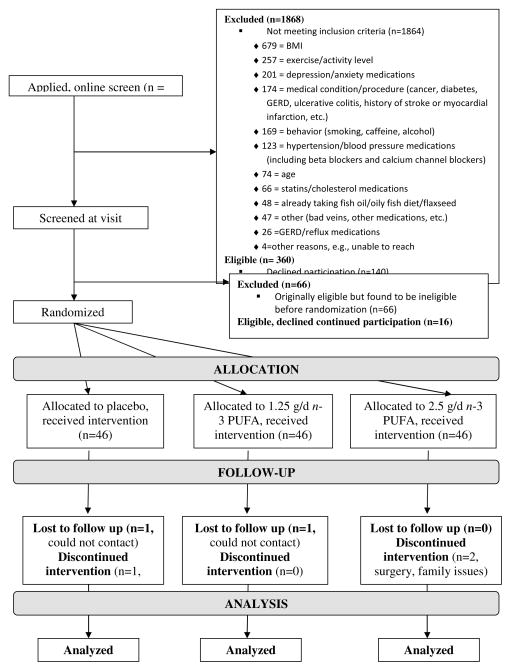
Of the 138 randomized subjects, 133 (96%) completed all five visits (Figure 1). Protocol adherence was measured by the number of pills returned at each study visit. There was no difference in adherence between the active and placebo groups, with 3.3%, 2.0%, and 2.6% percent of pills returned in the placebo,1.25 g/d, and 2.5 g/d groups, respectively (p=0.31). The mean number of pills taken per day was 5.8 in the placebo and 2.5 g/d groups, and 5.9 in the 1.25 g/d group.
Figure 1.
Screening, randomization, and participant flow by group
The James’ blinding index for participants at the end of the study was 0.60 (95% CI: 0.53–0.68, n=131) (James et al., 1996). For the two experimenters the James’ blinding indices were 0.83 (95% CI: 0.77–0.88, n=130) and 0.81 (95% CI: 0.75–0.87, n=116), respectively. Blinding is considered adequate if the index is greater than 0.5.
3.3 Safety and Tolerability
Nonserious adverse events reported by at least one subject are summarized in Table 4. There were no significant group differences on any dimension.
Table 4.
Nonserious adverse events reported by at least one study subject during trial.
| Placebo (n=46) | 1.25 g/d n-3 (n=46) | 2.5 g/d n-3 (n=46) | p-value | |
|---|---|---|---|---|
| Ear ache | 6 (13%) | 4 (9%) | 2 (4%) | 0.39 |
| Sore throat | 3 (7%) | 10 (22%) | 8 (17%) | 0.10 |
| Swollen glands | 1 (2%) | 0 (0%) | 1 (2%) | 1.00 |
| Stuffy nose | 18 (39%) | 20 (43%) | 23 (50%) | 0.60 |
| Wheeze | 3 (7%) | 1 (2%) | 2 (4%) | 0.87 |
| Dry cough | 10 (22%) | 10 (22%) | 6 (13%) | 0.50 |
| Wet cough | 8 (17%) | 6 (13%) | 6 (13%) | 0.87 |
| Nausea | 7 (15%) | 6 (7%) | 8 (17%) | 0.28 |
| Stomach pain | 12 (26%) | 13 (28%) | 14 (30%) | 0.97 |
| Diarrhea | 10 (22%) | 7 (15%) | 12 (26%) | 0.48 |
| Other symptoms | 25 (54%) | 25 (54%) | 23 (50%) | 0.93 |
Data are n (%).
3.4 Changes in fatty acids
Baseline levels of plasma fatty acids as well as changes over time for the three groups are summarized in Table 3. As expected, randomization produced groups that did not differ on EPA (p = 0.73), DHA (p = 0.38), or total n-3 (p = 0.41) at baseline. By the end of the study period plasma levels of EPA were approximately 3.5-fold higher in the 1.25 g/d n-3 group and 6-fold higher in the 2.5 g/d n-3 group (p<0.0001 for both), and plasma DHA levels were approximately 1.4-fold higher in the 1.25 g/d n-3 group and 1.5-fold higher in the 2.5 g/d n-3 group (p<0.0001 for both). The n-6:n-3 ratio was significantly decreased after supplementation for both low and high dose groups (p < 0.0001 for both).
Table 3.
Plasma fatty acids1
| Baseline (week 0) | Change Visits 0–5 | P for change2 | P for comparison of changes3 | |
|---|---|---|---|---|
| EPA | <.0001 | |||
| Placebo | 0.46 ± 0.10 | 0.17 ± 0.13 | 0.19 | |
| 1.25 g/d n-3 | 0.53 ± 0.10 | 1.4 ± 0.13 | <.0001 | |
| 2.5 g/d n-3 | 0.56 ± 0.10 | 2.9 ± 0.13 | <.0001 | |
| DHA | <.0001 | |||
| Placebo | 1.5 ± 0.09 | 0.07 ± 0.08 | 0.40 | |
| 1.25 g/d n-3 | 1.4 ± 0.09 | 0.60 ± 0.08 | <.0001 | |
| 2.5 g/d n-3 | 1.6 ± 0.09 | 0.73 ± 0.08 | <.0001 | |
| Total n-3 | <.0001 | |||
| Placebo | 3.7 ± 0.18 | 0.28 ± 0.24 | 0.25 | |
| 1.25 g/d n-3 | 4.0 ± 0.18 | 2.1 ± 0.24 | <.0001 | |
| 2.5 g/d n-3 | 4.0 ± 0.18 | 4.3 ± 0.24 | <.0001 | |
| Total n-6 | 0.01 | |||
| Placebo | 41 ± 0.82 | −0.90 ± 0.86 | 0.30 | |
| 1.25 g/d n-3 | 39 ± 0.82 | 0.41 ± 0.85 | 0.63 | |
| 2.5 g/d n-3 | 42 ± 0.82 | −3.2 ± 0.86 | 0.0004 | |
| n-6:n-3 Ratio | <.0001 | |||
| Placebo | 11 ± 0.35 | −0.81 ± 0.44 | 0.07 | |
| 1.25 g/d n-3 | 10 ± 0.35 | −3.4 ± 0.44 | <.0001 | |
| 2.5 g/d n-3 | 11 ± 0.35 | −6.1 ± 0.44 | <.0001 |
All values are means +/− SEMs from mixed effects models. Tests are for orthogonal contrasts in the models.
Within-group t-tests with degrees of freedom ranging from 132 to 136.
Between-group F-tests (visit by group interaction term) with degrees of freedom ranging from (2,133) to (2,136).
3.5 Primary Outcomes
Results for inflammatory outcomes and depression symptoms are summarized in Table 5. After adjusting for gender and sagittal abdominal diameter, there were significant supplementation effects on cytokines as evidenced by significant group by visit interactions for both TNF-α (p = 0.0002) and IL-6 (p = 0.0003). The estimated mean change in log-TNF-α from visit 1 to visit 5 was 0.11 units for the placebo group, corresponding to a 12% increase in the geometric mean of TNF-α. In comparison, the estimated mean change in log-TNF-α was 0.0002 units for the 1.25 g/d and −0.024 for the 2.5 g/d group, corresponding to changes of 0.2% and −2.3%, respectively. After Bonferroni-adjustment, these group differences were significant for the comparison of placebo to 1.25 g/d (p = 0.03) and placebo to 2.5 g/d (p = 0.004); no significant difference was noted between the two supplementation doses (p = 1.0).
Table 5.
Group effects1 on primary outcomes (natural log-transformed).
| Baseline (Visit 1) | Change, Visit 1 to 5 | Difference in Change versus Placebo | 95% CI | P-value2 | |
|---|---|---|---|---|---|
| log(TNF-α) | |||||
| Placebo | 0.66 (0.25) | 0.11 (0.030) | |||
| 1.25 g/d n-3 | 0.74 (0.25) | 0.002 (0.028) | −0.11 (0.04) | −0.028 to −0.19 | 0.03 |
| 2.5 g/d n-3 | 0.71 (0.25) | −0.024 (0.029) | −0.13 (0.04) | −0.052 to −0.22 | 0.004 |
| log(IL-6) | |||||
| Placebo | 0.91 (0.60) | 0.31 (0.077) | |||
| 1.25 g/d n-3 | 1.02 (0.60) | −0.106 (0.070) | −0.41 (0.10) | −0.21 to −0.62 | 0.0003 |
| 2.5 g/d n-3 | 0.85 (0.60) | −0.123 (0.074) | −0.43 (0.11) | −0.22 to −0.64 | 0.0002 |
| CES-D, log | |||||
| Placebo | 1.5 (0.17) | −0.056 (0.15) | |||
| 1.25 g/d n-3 | 1.5 (0.17) | −0.29 (0.15) | −0.23 (0.21) | −0.64 to 0.18 | 0.80 |
| 2.5 g/d n-3 | 1.6 (0.17) | −0.26 (0.15) | −0.20 (0.21) | −0.61 to 0.21 | 1.00 |
Least squares means (SE) adjusted for gender. Cytokine models additionally adjusted for sagittal abdominal diameter.
Comparison to placebo, Bonferroni-adjusted within outcome to account for multiple testing. Tests are t-tests with degrees of freedom ranging from 451 to 530. There were no significant differences between supplement doses.
A similar pattern was observed for IL-6. The estimated mean change in log-IL-6 from visit 1 to visit 5 was 0.31 units for the placebo group (36% increase), −0.106 for the 1.25 g/d group (10% decrease), and −0.123 for the 2.5 g/d group (12% decrease). Significant differences were observed between placebo and 1.25 g/d (p = 0.0003) and placebo and 2.5 g/d (p = 0.0002), but not between the two doses of fish oil (p = 1.0). To ensure that results were not driven by a small number of highly influential data points, residual plots were examined and one subject in the placebo group who appeared to be an outlier was removed and analyses were rerun. Resulting conclusions were the same and are not shown.
There did not appear to be group effects on depression (p = 0.86), adjusting for gender. There was a trend toward larger decreases in depression from visit 1 to visit 5 for the two fish oil groups than the placebo, but no differences were statistically significant.
4. Discussion
4.1 Intervention-related reductions in inflammation
Omega-3 supplementation significantly altered production of serum cytokines. IL-6 decreased by 10% and 12% in our low and high dose n-3 groups, respectively, compared to a 36% increase in the placebo group. Similarly, low and high dose n-3 groups showed modest 0.2% and −2.3% changes in TNF-α, compared to a 12% increase in the control group. This is the first well-powered trial to show significant changes in serum cytokines in healthy middle-aged and older adults.
4.2 Randomized PUFA trials
The largely negative serum cytokine data from prior n-3 PUFA trials have led to the suggestion that cytokine production is relatively insensitive to the n-3 PUFAs among healthy individuals, i.e., people who do not have chronic inflammatory diseases such as inflammatory bowel disease, rheumatoid arthritis, chronic obstructive pulmonary disease, or diabetes (Sijben and Calder, 2007; Wu, 2004). However, there are several notable differences between our study and prior RCTs. We carefully assessed variables known to influence inflammation including smoking, medication use, physical activity, and abdominal adiposity. We had minimal attrition; only 5 of our 138 subjects failed to complete the full trial. Our rigorous exclusion criteria produced a group of overweight sedentary adults who were more likely to have an inflammatory profile and who were otherwise healthy aside from their weight. In addition, our supplement’s 7:1 EPA: DHA ratio may be particularly beneficial based on evidence for EPA’s anti-inflammatory properties (Ariel and Serhan, 2007; Sijben and Calder, 2007).
Dietary intakes of both the n-3 and omega-6 (n-6) PUFAs influence inflammation. Arachidonic acid (AA) is an n-6 polyunsaturated fatty acid derived from linoleic acid. The eicosanoids produced from enzymatic hydroxylation of AA increase proinflammatory cytokine production (Hibbeln et al., 2006). In contrast, the eicosanoids derived from n-3 PUFAs curb the production of AA-derived eicosanoids (Blasbalg et al., 2011; Pischon et al., 2003). Thus, both higher plasma levels of n-3 PUFAs as well as lower plasma n-6:n-3 ratios should restrain proinflammatory cytokine production (Ferrucci et al., 2006). In this context it is noteworthy that our participants’ average n-6:n-3 ratio at baseline was 10.82 (SD=2.48, range=3.3–18.8), considerably lower than the 15:1 to 17:1 ratios reported for the contemporary North American diet (Hibbeln et al., 1997; Simopoulos, 2002). Accordingly, our data probably underestimate the potential impact of the n-3 PUFAs.
The absolute magnitude of the change in both IL-6 and TNF-α levels in the two treatment groups was smaller than the alterations in the placebo group. The n-3 PUFA’s anti-inflammatory benefits may be both prophylactic (preventing an increase in inflammation) as well as therapeutic (decreasing elevated inflammatory cytokines), an important point for potential clinical utility.
Depressive symptoms were quite low at baseline and did not change significantly in response to supplementation; on entry into the trial, only 16 of our participants had a CES-D score of 16 or greater, the standard clinical cutoff used to define case status (Weissman et al., 1977). In a recent meta-analysis of randomized controlled trials; the authors concluded that n-3 PUFA supplementation benefited clinically depressed individuals, but not those with less severe depressed mood (Appleton et al., 2010).
In prior work from our laboratory, 68 medical students received either 2.5 g/d n-3 or a placebo for 12 weeks (Kiecolt-Glaser et al., 2011). Compared to controls, those students who received n-3 showed a 14% decrease in lipopolysaccharide (LPS) stimulated IL-6 production. Planned secondary analyses that used the plasma n-6:n-3 ratio in place of treatment group showed that decreasing n-6:n-3 ratios led to reductions in stimulated IL-6 and TNF-α production, as well as marginal differences in serum TNF-α. The absence of significant serum inflammatory changes was likely related to the very low baseline levels of serum cytokines in the healthy, young, and relatively thin population.
The majority of n-3 PUFA trials have used stimulated (ex vivo) production assays to assess changes in inflammation, rather than serum (in vivo) assays, and results of these trials have been mixed (Calder et al., 2009; Fritsche, 2006; Sijben and Calder, 2007). TNF-α and IL-6 are produced by a variety of types of cells, and thus serum cytokine levels may better reflect the overall inflammatory profile than stimulated production (Pischon et al., 2003). The absence of both normal values and clinical correlates for ex vivo assays limits inferences about n-3 PUFA’s clinical therapeutic potential. Indeed, the health and aging literatures have focused on serum proinflammatory cytokine levels (Farzaneh-Far et al., 2009; Penninx et al., 2003; Taaffe et al., 2000). Thus, our finding that omega-3 supplementation can substantially change serum cytokines is important.
In accord with our findings, a recent study showed that consumption of 1.8 g/d of EPA + DHA changed the expression of 1040 genes, including decreased expression of genes involved in inflammatory and atherogenic pathways as well as NF-kB signaling (Bouwens et al., 2009). In contrast, consumption of the high-oleic sunflower oil control only changed the expression of 298 genes (Bouwens et al., 2009).
4.3 Dosage and risks
Neither our IL-6 nor our TNF-α data showed significant differences between our 1.25 and 2.5 g/d n-3 dose, although both clearly differed from the placebo. One review concluded that while the effects were inconsistent, it appeared that significant changes in cytokine production by lymphocytes only occurred with ≥ 2.0 g/d of EPA + DHA (Sijben and Calder, 2007). In addition, variables such as typical dietary intake influence responses (Yee et al., 2010), and our sample had a higher than expected average n-6:n-3 ratio at baseline, as described earlier. The FDA has concluded that intakes of up to 3 g/d of marine n-3 PUFAs are “Generally Recognized As Safe” (Kris-Etherton et al., 2002); our higher dose, 2.5 g/d, fell within that range and would appear to be a good choice for future studies.
Side effects were infrequent and did not differ between groups. These data are in accord with the low incidence reported in large n-3 PUFA studies (Leaf et al., 1994, 1999).
4.4 Health implications
Several large studies have linked higher n-3 PUFA levels with lower all-cause mortality (Lee et al., 2009; Pottala et al., 2010), including a large 3.5 year trial (Marchioli et al., 2002). The n-3 PUFA’s anti-inflammatory properties provide one obvious pathway for these reductions in mortality. Inflammation is a robust and reliable predictor of all-cause mortality in older adults (Pedersen and Febbraio, 2008). Chronic inflammation has been linked to a spectrum of health problems including depression, cardiovascular disease, osteoporosis, cancer, and arthritis (Pedersen and Febbraio, 2008). In fact, more globally, chronic inflammation has been suggested as one key biological mechanism that may fuel declines in physical function leading to frailty, disability, and, ultimately, death. Our data suggest that n-3 PUFAs can reduce inflammation in overweight, sedentary middle-aged and older adults, and thus could have broad health benefits. Although the n-3 PUFAs cannot take the place of good health behaviors like exercise, individuals who are at risk because of established inflammatory diseases or conditions may profit from their use. These data provide a window into the ways in which the n-3 PUFAs may impact disease initiation, progression, and resolution.
Highlights.
The data suggest that n-3 PUFAs can reduce inflammation in overweight, sedentary middle-aged and older adults.
Acknowledgments
The study was supported in part by NIH grants AG029562, UL1RR025755, and CA16058. OmegaBrite (Waltham, MA) supplied the omega-3 supplement and placebo without charge and without restrictions; OmegaBrite did not influence the design, funding, implementation, interpretation, or publication of the data.
Footnotes
Conflict of interest statement: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Intakes of 19 individual fatty acids: results from 1994–96, continuing survey of food intakes by individuals. U. S. Department of Agriculture, Agricultural Research Service; http://www.barc.usda.gov/bhnrc/foodsurvey/home.htm. [Google Scholar]
- Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91:757–770. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends in Immunology. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Basco MR, Krebaum SR, Rush AJ. Outcome measures of depression. In: Strupp HH, Horowitz LM, Lambert MJ, editors. Measuring patient changes in mood, anxiety, and personality disorders. American Psychological Association; Washington D. C: 1997. pp. 207–245. [Google Scholar]
- Belury MA, Kempa-Steczko A. Conjugated linoleic acid modulates hepatic lipid composition in mice. Lipids. 1997;32:199–204. doi: 10.1007/s11745-997-0025-0. [DOI] [PubMed] [Google Scholar]
- Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh WJ, Dyer EG. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemical Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blok WL, Deslypere JP, Demacker PN, van der Ven-Jongekrijg J, Hectors MP, van der Meer JW, Katan MB. Pro- and anti-inflammatory cytokines in healthy volunteers fed various doses of fish oil for 1 year. Eur J Clin Invest. 1997;27:1003–1008. doi: 10.1046/j.1365-2362.1997.2240775.x. [DOI] [PubMed] [Google Scholar]
- Bouwens M, van de Rest O, Dellschaft N, Bromhaar MG, de Groot LC, Geleijnse JM, Muller M, Afman LA. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr. 2009;90:415–424. doi: 10.3945/ajcn.2009.27680. [DOI] [PubMed] [Google Scholar]
- Browning LM. n-3 Polyunsaturated fatty acids, inflammation and obesity-related disease. Proc Nutr Soc. 2003;62:447–453. doi: 10.1079/pns2003252. [DOI] [PubMed] [Google Scholar]
- Buckley MS, Goff AD, Knapp WE. Fish oil interaction with warfarin. Ann Pharmacother. 2004;38:50–52. doi: 10.1345/aph.1D007. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Calder PC, Albers R, Antoine JM, Blum S, Bourdet-Sicard R, Ferns GA, Folkerts G, Friedmann PS, Frost GS, Guarner F, Lovik M, Macfarlane S, Meyer PD, M’Rabet L, Serafini M, van Eden W, van Loo J, Vas Dias W, Vidry S, Winklhofer-Roob BM, Zhao J. Inflammatory disease processes and interactions with nutrition. Br J Nutr. 2009;101(Suppl 1):S1–45. doi: 10.1017/S0007114509377867. [DOI] [PubMed] [Google Scholar]
- Clasey JL, Bouchard C, Teates CD, Riblett JE, Thorner MO, Hartman ML, Weltman A. The use of anthropometric and dual-energy X-ray absorptiometry (DXA) measures to estimate total abdominal and abdominal visceral fat in men and women. Obes Res. 1999;7:256–264. doi: 10.1002/j.1550-8528.1999.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Diggle P, Heagerty P, Liang KY, Zeger SL. Analysis of longitudinal data. Claredon Press; USA, Oxford: 2002. [Google Scholar]
- Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2009;205:538–543. doi: 10.1016/j.atherosclerosis.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Real JM, Broch M, Vendrell J, Ricart W. Insulin resistance, inflammation, and serum fatty acid composition. Diabetes Care. 2003;26:1362–1368. doi: 10.2337/diacare.26.5.1362. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610. [DOI] [PubMed] [Google Scholar]
- Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129:210–223. doi: 10.1016/j.pain.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Hallahan B, Garland MR. Essential fatty acids and mental health. Br J Psychiatry. 2005;186:275–277. doi: 10.1192/bjp.186.4.275. [DOI] [PubMed] [Google Scholar]
- Harada ND, Chiu V, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33:962–970. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WEM. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83:1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Umhau JC, George DT, Salem NJ. Do plasma polyunsaturates predict hostility and depression? World Rev Nutr Diet. 1997;82:175–186. doi: 10.1159/000059633. [DOI] [PubMed] [Google Scholar]
- James KE, Bloch DA, Lee KK, Kraemer HC, Fuller RK. An index for assessing blindness in a multi-centre clinical trial: disulfiram for alcohol cessation--a VA cooperative study. Stat Med. 1996;15:1421–1434. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1421::AID-SIM266>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Jenkins CD, Krueger BE, Rose RM, Hurst MW. Use of a monthly health review to ascertain illness and injuries. Am J Public Health. 1980;70:82–84. doi: 10.2105/ajph.70.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EJ, Schaefer EJ. Potential role of dietary n-3 fatty acids in the prevention of dementia and macular degeneration. Am J Clin Nutr. 2006;83S:1494s–1498s. doi: 10.1093/ajcn/83.6.1494S. [DOI] [PubMed] [Google Scholar]
- Kalogeropoulos N, Panagiotakos DB, Pitsavos C, Chrysohoou C, Rousinou G, Toutouza M, Stefanadis C. Unsaturated fatty acids are inversely associated and n-6/n-3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clin Chim Acta. 2010;411:584–591. doi: 10.1016/j.cca.2010.01.023. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain Behav Immun. 2011;25:1725–1734. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Porter K, Beversdorf D, Lemeshow S, Glaser R. Depressive symptoms, omega-6:omega-3 fatty acids, and inflammation in older adults. Psychosom Med. 2007;69:217–224. doi: 10.1097/PSY.0b013e3180313a45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- Leaf A, Jorgensen MB, Jacobs AK, Cote G, Schoenfeld DA, Scheer J, Weiner BH, Slack JD, Kellett MA, Raizner AE. Do fish oils prevent restenosis after coronary angioplasty? Circulation. 1994;90:2248–2257. doi: 10.1161/01.cir.90.5.2248. [DOI] [PubMed] [Google Scholar]
- Lee JH, O’Keefe JH, Lavie CJ, Harris WS. Omega-3 fatty acids: cardiovascular benefits, sources and sustainability. Nature Reviews Cardiology. 2009;6:753–758. doi: 10.1038/nrcardio.2009.188. [DOI] [PubMed] [Google Scholar]
- Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F, Investigators GIP. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction - Time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- Orts K, Sheridan JF, Robinson-Whelen S, Glaser R, Malarkey WB, Kiecolt-Glaser JK. The reliability and validity of a structured interview for the assessment of infectious illness. J Behav Med. 1995;18:517–530. doi: 10.1007/BF01857893. [DOI] [PubMed] [Google Scholar]
- Patterson RE, Kristal AR, Carter RA, Fels-Tinker LF, Bolton MP, Argurs-Collins T. Measurement characteristics of the Women’s Health Initiative Food Frequency Questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Penninx BWJH, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: Results from the Health, Aging, and Body Composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- Pottala JV, Garg S, Cohen BE, Whooley MA, Harris WS. Blood eicosapentaenoic and docosahexaenoic acids predict all-cause mortality in patients with stable coronary heart disease: The Heart and Soul Study. Circ-Cardiovasc Qual Outcomes. 2010;3:406–412. doi: 10.1161/CIRCOUTCOMES.109.896159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Resnicow K, McCarty F, Blissett D, Wang T, Heitzler C, Lee RE. Validity of a modified CHAMPS physical activity questionnaire among African-Americans. Med Sci Sports Exerc. 2003;35:1537–1545. doi: 10.1249/01.MSS.0000084419.64044.2B. [DOI] [PubMed] [Google Scholar]
- Shantha NC, Decker EA, Hennig B. Comparison of methylation methods for the quantitation of conjugated linoleic acid isomers. J AOAC Int. 1993;76:644–649. [Google Scholar]
- Shelton RC, Miller AH. Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Prog Neurobiol. 2010;91:275–299. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijben JWC, Calder PC. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc. 2007;66:237–259. doi: 10.1017/S0029665107005472. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med Sci Sports Exerc. 2001a;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Verboncoeur CJ, McLellan BY, Gillis DE, Rush S, Mills K, King AC, Ritter P, Brown B, Bortz WM. Physical activity outcomes of CHAMPS II: A physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001b;56A:m465–m470. doi: 10.1093/gerona/56.8.m465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll AL, Damico KE, Daly BP, Severus WE, Marangell LB. Methodological considerations in clinical studies of omega 3 fatty acids in major depression and bipolar disorder. World Rev Nutr Diet. 2001;88:58–67. doi: 10.1159/000059745. [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- Valagussa F, Franzosi MG, Geraci E, Mininni N, Nicolosi GL, Santini M, Tavazzi L, Vecchio C, Marchioli R, Bomba E, Chieffo C, Maggioni AP, Schweiger C, Tognoni G, Barzi F, Flamminio AV, Marfisi RM, Olivieri M, Pera C, Polidoro A, Santoro E, Zama R, Pagliaro L, Correale E, Del Favero A, Loi U, Marubini E, Campolo L, Casari A, Di Minno G, Donati MB, Galli M, Gattone M, Garattini S, Mancini M, Marino P, Santoro GM, Scardulla C, Specchia G, Cericola A, Di Gregorio D, Di Mascio R, Levantesi G, Mantini L, Mastrogiuseppe G, Tucci C, Mocarelli P, Baldinelli R, Ceriotti F, Colonna A, Cortese C, Fortunato G, Franzini C, Gonano F, Graziani MS, Investigators GIP. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML, Licinio J, Gold PW, Hermida RC, Mastorakos G, Chrousos GP. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- Wu DY. Modulation of immune and inflammatory responses by dietary lipids. Curr Opin Lipidol. 2004;15:43–47. doi: 10.1097/00041433-200402000-00009. [DOI] [PubMed] [Google Scholar]
- Yee LD, Lester JL, Cole RM, Richardson JR, Hsu JC, Li Y, Lehman A, Belury MA, Clinton SK. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am J Clin Nutr. 2010;91:1185–1194. doi: 10.3945/ajcn.2009.29036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusof HM, Miles EA, Calder P. Influence of very long-chain n-3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot Essent Fatty Acids. 2008;78:219–228. doi: 10.1016/j.plefa.2008.02.002. [DOI] [PubMed] [Google Scholar]