Abstract
Acetaminophen (APAP) overdose leads to severe hepatotoxicity, increased oxidative stress and mitochondrial dysfunction. S-adenosyl-L-methionine (SAMe) protects against APAP toxicity at a mmol/kg equivalent dose to N-acetylcysteine (NAC). SAMe acts as a principle biological methyl donor and participates in polyamine synthesis which increase cell growth and has a role in mitochondrial protection. The purpose of the current study tested the hypothesis that SAMe protects against APAP toxicity by maintaining critical antioxidant enzymes and markers of oxidative stress. Male C57Bl/6 mice were treated with vehicle (Veh; water 15 ml/kg, ip), SAMe (1.25 mmol/kg, ip), APAP (250 mg/kg, ip), and SAMe + APAP (SAMe given 1 h following APAP). Liver was collected 2 and 4 h following APAP administration; mitochondrial swelling as well as hepatic catalase, glutathione peroxidase (GPx), glutathione reductase, and both Mn- and Cu/Zn-superoxide dismutase (SOD) enzyme activity were evaluated. Mitochondrial protein carbonyl, 3-nitrotyrosine cytochrome c leakage were analyzed by Western blot. SAMe significantly increased SOD, GPx, and glutathione reductase activity at 4 h following APAP overdose. SAMe greatly reduced markers of oxidative stress and cytochrome C leakage following APAP overdose. Our studies also demonstrate that a 1.25 mmol/kg dose of SAMe does not inhibit CYP 2E1 enzyme activity. The current study identifies a plausible mechanism for the decreased oxidative stress observed when SAMe is given following APAP.
Keywords: S-adenosyl-L-methionine, Acetaminophen, Hepatotoxicity, Oxidative stress, Antioxidant, Nitrotyrosine
1. Introduction
Acetaminophen (APAP) is the leading cause of drug induced liver injury. APAP overdose is a serious clinical problem resulting in over 26,000 hospitalizations per year in the United States (Nourjah et al., 2006). APAP toxicity results in severe hepatic centrilobular necrosis (Anundi et al., 1993). Currently, N-acetylcysteine (NAC) is the accepted treatment for APAP overdose in humans.
APAP toxicity is requires biotransformation by cytochrome P450 isozymes 2E1, 1A2, and 3A4 of APAP to N-acetyl-p-benzoquinoneimine (NAPQI), the ultimate hepatotoxic metabolite (Dahlin et al., 1984; Patten et al., 1993; Chen et al., 1998). Excess NAPQI rapidly depletes cellular stores of reduced glutathione (GSH) and subsequently adducts proteins in the liver precipitating cellular dysfunction (Hinson et al., 1995). The current treatment for APAP toxicity, NAC, works by replenishing cellular GSH to prevent the cell from being overwhelmed by NAPQI (Smilkstein et al., 1988). Because of the rapid conversion of APAP to NAPQI, prompt administration of NAC is critical to improving the clinical outcome; therefore, therapeutic interventions that work longer term to combat APAP toxicity are desirable.
APAP hepatotoxicity is characterized by greatly increased oxidative stress due to mitochondrial dysfunction and the generation of superoxide (Andersson et al., 1990). A common indicator of oxidative stress is lipid peroxidation, which has been found to be greatly increased in response to APAP toxicity (Wendel et al., 1979). In addition to the generation of reactive oxygen species (ROS), APAP also causes the production of reactive nitrogen species (RNS) leading to nitrotyrosine adduction of proteins (Hinson et al., 2000). Histology indicates that nitrotyrosine adduction is localized to the centrilobular region where APAP toxicity is most prevalent.
A key defense modulating the severity of APAP hepatotoxicity are the antioxidant enzymes glutathione reductase (GSSG reductase), glutathione peroxidase (GPx), catalase and superoxide dismutase (SOD). APAP toxicity has previously been demonstrated to reduce antioxidant enzyme activities and a few exogenous treatments have been tested to restore enzymatic function (Olaleye et al., 2008; Wu et al., 2010). NAC has also been demonstrated to protect the function of GPx and SOD when administered 2 h prior to APAP overdose (300 mg/kg body weight) (Wang et al., 2010). An endogenously produced molecule, such as S-adenosyl-L-methionine (SAMe), with the same protective benefits could prove a beneficial therapeutic compound.
Mitochondrial dysfunction is another hallmark of APAP toxicity and a contributing factor to the greatly increased oxidative stress observed with APAP overdose. Masubuchi and others determined that APAP injury induces mitochondrial permeability transition (MPT) in mice which is characterized by the loss of membrane potential and cellular ATP depletion (Masubuchi et al., 2005). MPT is also associated with the exodus of proteins from the mitochondrial inner membrane such as cytochrome c which has a role in initiating apoptosis (El-Hassan et al., 2003). ATP depletion occurs with APAP toxicity and acts as a precursor to the necrotic damage by inhibiting completion of apoptosis (Jaeschke and Bajt, 2006).
Prior research by our lab has demonstrated the ability of S-adenosyl-L-methionine (SAMe) to protect against APAP toxicity at a mmol/kg dose comparable to NAC (Terneus et al., 2007 and 2008). Humans produce 6-8 g per day of SAMe which is necessary for methylation of cellular proteins, DNA, and phospholipids. In addition to transmethylation reactions, SAMe also participates in the transsulfuration pathway to aid in the replenishment of GSH (Lu, 2000). SAMe is known to participate in polyamine synthesis which has a critical role in enhancing liver regeneration following partial hepatectomy (Fernandez et al., 2003; Brosnan and Brosnan, 2006). Polyamine synthesis and the ability to aid in liver regeneration are both unique properties that SAMe possesses over NAC as a potential therapeutic intervention. SAMe has already been recognized to protect mitochondria from alcohol-induced dysfunction (Bailey et al., 2006; Song et al., 2007). Additionally, our lab has previously demonstrated SAMe's ability to reduce oxidative stress markers such as the formation of protein carbonyls associated with APAP hepatic toxicity (Terneus et al., 2008).
Given SAMe's proven ability to protect against APAP toxicity, the current study sought to deepen the understanding of the mechanism of SAMe protection. First, subcellular protection by SAMe for APAP hepatic damage within the mitochondria and cytosol is not known. Second, information is lacking on the temporal protection of antioxidant enzyme activity by SAMe following exposure to APAP overdose. If protection of antioxidant enzyme activity by SAMe is minimal or only occurs after hepatic damage is manifested, then maintaining antioxidant enzyme activity is not a primary mechanism for SAMe protection of APAP hepatic toxicity. Additionally, protein carbonyl formation and nitrotyrosine adduction were analyzed on mitochondrial samples to determine levels of oxidative stress at a mitochondrial level with SAMe treatment after APAP overdose.
2. Methods and Materials
2.1 Reagents
SAMe toluene sulfonate salt was used for the experiments and was purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). All other chemicals were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA). The SOD Assay kit was purchased from Sigma Chemical Company (19160; St. Louis, MO). The OxyBlot™ Protein Oxidation Detection Kit purchased from Millipore (S7150; Temecula, CA). Alanine aminotransferase (ALT) was assayed using a method based on the discontinued Sigma 505-P kit as described by Patel and associates (2011).
2.2 Animals
Male C57BL/6 mice were obtained from Hilltop Lab Animals Inc. (Scottsdale, PA). Animals included in the study were between 4-8 weeks of age and weighed 16-24 g. Mice were maintained in an American Association for Accreditation of Laboratory Animal Care (AAALAC) accredited facility. Mice were maintained at controlled temperature (21-23°C), humidity (40-55%), and 12-hr light cycles (lights on 6:00 AM to 6:00PM). An acclimation period of 7 days was observed prior to the beginning of any experiment. The animals received Purina rodent chow and water ad libitum. The mice were fasted for 16 h prior to any experiment, but were provided free access to water.
2.3 SAMe Treatment Following APAP Overdose
Mice were randomly allocated into the following groups: Vehicle (Veh; 15 mL/kg water by intraperitoneal (ip) injection), SAMe (1.25 mmol/kg 5mL/kg ip injection), APAP (250 mg/kg 15mL/kg ip injection), and SAMe administered 1 h after APAP (SAMe + APAP; doses same as previously listed). SAMe was administered 1 h following APAP. Mice were anesthetized with carbon dioxide 2 and 4 h after APAP administration. Blood was collected by cardiac puncture in heparin-rinsed 1 mL syringes for determination of plasma ALT activity, which serves as an indicator of liver injury. Livers were then isolated and placed in ice cold Kreb's buffer (126 mM NaCl, 5 mMKCl, 3 mM MgSO4, 3 mM Na2HPO4, 1 mM CaCl2; pH 7.4), blotted, and weighed.
2.4 Mitochondrial Isolation
Mitochondria were isolated using a modification of a previously published protocol by Gogvadze and colleagues (2006). Briefly, the liver was isolated, blotted, weighed and placed in Mitochondrial Isolation Buffer A (225 mM sucrose, 3 mM KH2PO4, 5 mM MgCl2, 20 mM KCl, 20 mM triethanolamine, 2 mM EGTA; pH 7.4). The liver was minced and homogenized in a Dounce homogenizer on ice. Following homogenization, the liver was centrifuged at 600 × g for 10 minutes. The resultant pellet was discarded and the supernatant was centrifuged at 15,000 × g for 5 minutes. After the final centrifugation, the supernatant was retained for analysis of cytosolic SAMe levels. The pellet containing the mitochondria was resuspended in Mitochondrial Isolation Buffer B (Same as Buffer A except lacking EGTA) for a final concentration of 1 mg tissue weight/μL Buffer B. Samples were stored at -80°C until analysis.
2.5 Catalase Activity Assay
The protocol for determination of catalase activity was based on a paper by Zhang and coworkers (2004). Briefly, tissue was homogenized in Kreb's buffer (10 mL/g tissue) and centrifuged at 1000 × g for 15 minutes retaining the supernatant. For the assay, the disappearance of 15 mM H2O2 was measured at 240 nm and the change in absorbance was recorded over 1 minute. The level of catalase was calculated with the extinction coefficient (43.6 M-1 cm-1).
2.6 GPx Activity Assay
Lawrence and Burk (1976) developed a protocol which was employed to determine hepatic GPx activity. Liver tissue (100 mg) was homogenized in 1 mL of phosphate buffer (50 mM KH2PO4, 1 mM sodium azide, 1 mM EDTA; pH 7.2). The reaction mixture consisted of 0.5 mL phosphate buffer, 0.1 mL 2 mM NADPH, 0.1 mL glutathione reductase (1 Unit), 0.1 mL 10 mM reduced glutathione, and 0.1 mL sample. The reaction was initiated with the addition of H2O2 at a final concentration of 0.25 mM and disappearance of NADPH was measured for 1 minute at 340 nm. Activity was calculated using the extinction coefficient for NADPH (6.22 × 103 M-1 cm-1).
2.7 GSSG Reductase Activity Assay
GSSG reductase activity was determined based on a protocol developed by Mannervik with modifications (1999). Briefly, 100 mg of liver was homogenized in Kreb's buffer (10 mL/g tissue) and centrifuged 15 minutes at 1000 × g. 50 μL of sample was added to a test tube containing 2.7 mL phosphate buffer (120 mM KH2PO4; pH 7.2), 0.1 mL 15 mM EDTA, and 0.05 mL 65.3 mM glutathione dissulfide. The reaction was initiated by the addition of 0.05 mL 10 mM NADPH. Consumption of NADPH was monitored for 1 minute at 340 nm. A standard curve of known quantities of GSSG reductase was constructed for calculations.
2.8 SOD Activity Assay
SOD activity was determined using a Fluka designed spectrophotometric kit purchased from Sigma Chemical Company (19160; St. Louis, MO). Cu/Zn-SOD was inhibited by incubating the sample at room temperature sodium diethyldithiocarbamate (DDTC) at a final concentration of 25 mM. The assay was completed according to manufacturer's recommendations.
2.9 Mitochondrial Swelling Assay
Mitochondrial swelling was determined by a turbidometric technique (Gogvadze et al., 2006). Mitochondrial suspension containing 1 mg protein was set to constant spinning in 2 mL incubation buffer (150 mM KCl, 0.5 mM KH2PO4, 5 mM Tris Base, 100 mM succinic acid; pH 7.4). Subsequently, 2 μL 2.5 mM rotenone, 5.5 μL 10 mM CaCl2, and 20 μL 0.5 M KH2PO4 were added at one minute intervals to initiate the reaction which was monitored at 540 nm for 5 minutes recording absorbance every 15 seconds.
2.10 Western Blotting
Western blot analysis was conducted to examine expression of mitochondrial and cytosolic cytochrome c and mitochondrial 3-nitrotyrosine (3-NT) protein adduction. A 100 μg protein aliquot was denatured by boiling for 5 minutes. Samples were separated on a 12.5% polyacrylamide gel and transferred to a NC membrane (Whatman; Dassel, Germany). Transfer efficiency was verified using MemCode® Reversible Protein Stain Kit (Thermo Scientific; Rockford, IL). The membrane was then blocked using a 5% (w/v) milk/TBST solution (10 mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20; pH 8.0) for 1 hour. Membranes were next incubated overnight with constant shaking at 4°C in antibody for cytochrome c (sc-7159, Santa Cruz Biotechnology; Santa Cruz, CA) or 3-NT (ab61392, Abcam; Cambridge, MA) in 5% (w/v) milk/TBST. The membranes were washed four times with TBST. Appropriate secondary antibodies were incubated with the membranes for 1 hour. The membrane was again washed with TBST and developed using Amersham™ ECL™ Western Blotting Detection Reagents (GE Healthcare; Buckinghamnshire, UK). Densitometry was performed on each gel (n = 3-5 mice/group).
2.11 OxyBlot Analysis
Mitochondrial protein oxidation was assessed using an OxyBlot™ kit. The manufacturer's instructions were followed and 20 μg of protein was derivatized for each sample. Protein loaded was confirmed by MemCode® Reversible Protein Stain staining as in the Western blot protocol and results were quantified by densitometry (n = 3-5 mice/group).
2.12 Cyp 2E1Enzyme Activity and expression
In vitro hepatic biotransformation of aniline to p-aminophenol (PAP) was used as an indicator of CYP2E1 hepatic enzyme activity. These experiments were conducted in order to evaluate whether SAMe inhibited P450 mediated APAP metabolism. Microsomes were isolated from livers 2 and 4 h post APAP treatment using the methods of Schlenkman and Jansson (1999). Aniline para-hydroxylation to PAP was measured as described previously (Valentovic et al., 1988). Values were reported as nmol/mg protein/20 min incubation.
2.13 Statistical Analysis
Values represent Mean ± S.E.M. with n=3-5 animals/group. Differences in the groups were analyzed using a one-way ANOVA followed by a Tukey's post-hoc test (SigmaStat; SPSS Inc. Chicago, IL). All statistical analyses were conducted using a 95% confidence interval.
3. Results
3.1 SAMe Attenuation of Hepatic APAP toxicity
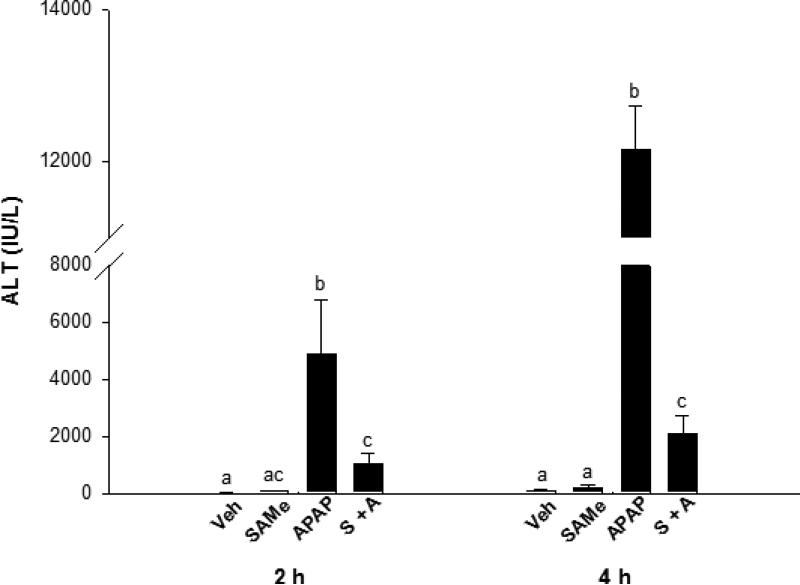
Body weights were similar between all treatment groups (Table 1). Liver weight/10 g body weight were increased 2 and 4 h after APAP treatment relative to the Veh mice. Plasma ALT levels were significantly increased 2 and 4 h (p<0.05) in the APAP group confirming APAP overdose (Figure 1). SAMe partially protected the liver from APAP toxicity as indicated by a less marked increase in ALT levels compared to the APAP group. ALT values were 80% lower in the SAMe + APAP group compared to the APAP mice. SAMe afforded partial correction as ALT values were still higher than Veh ALT at either the 2 or 4 h time period. The ALT measurement indicate that SAMe administered 1 h after APAP reduced hepatic toxicity.
Table 1.
Comparison of liver and body weight following APAP administration in C57BI/6 mice
| Group | Body Wt (g) | Liver wt/10 g body wt | |
|---|---|---|---|
| Veh | 25.20 ± 1.02a | 0.424 ± 0.011a | |
| 2 h | SAMe | 24.80 ± 0.80a | 0.416 ± 0.013a |
| APAP | 24.40 ± 0.75a | 0.505 ± 0.042b | |
| S + A | 23.60 ± 0.75a | 0.515 ± 0.029b | |
| 4 h | Veh | 23.60 ± 0.40a | 0.421 ± 0.007a |
| SAMe | 23.60 ± 0.40a | 0.407 ± 0.025ab | |
| APAP | 23.20 ± 0.49a | 0.543 ± 0.021c | |
| S + A | 22.40 ± 0.98a | 0.493 ± 0.026b |
Value are mean ± S.E.M. with n=5 animals per group.
Superscripts denote statistical differences between groups (p<0.05).
Superscripts denote statistical differences between groups (p<0.05).
Superscripts denote statistical differences between groups (p<0.05).
Superscripts denote statistical differences between groups (p<0.05).
Fig. 1.
ALT levels in blood plasma 2 and 4 h following APAP administration to C57Bl/6 mice. Mice were randomly allocated into Veh (water), SAMe (1.25 mmol/kg), APAP (250 mg/kg), and SAMe administered 1 h following APAP (S + A). Values represent mean ± S.E.M. with n=5 mice per group and superscripts denoting statistically significant differences (p<0.05).
3.2 Antioxidant Enzyme Activity protection by SAMe
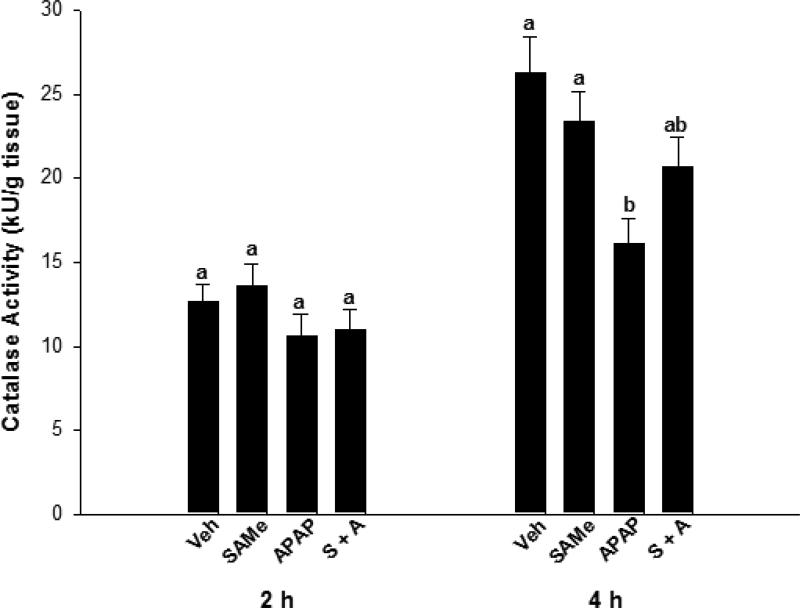
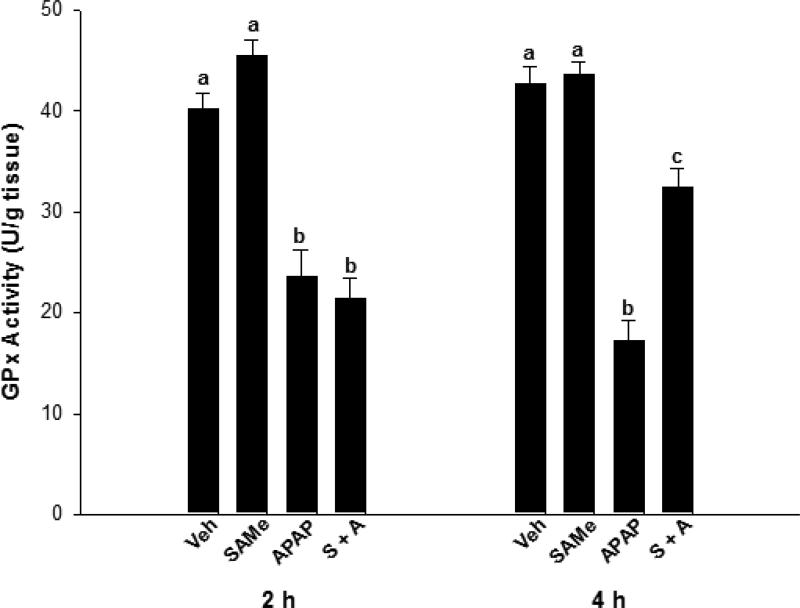
Relative to the Veh group, SAMe did not alter catalase or GPx enzyme activity (Figures 2 & 3). Catalase activity was similar for all groups at the 2 h time period but at 4 h, APAP diminished catalase enzyme activity over 30% when compared to Veh and SAMe groups (p<0.05). The SAMe + APAP group did not show significant elevations in catalase activity compared with the APAP group, but there was a trend toward improvement. APAP depressed GPx activity at both the 2 and 4 h (p<0.05) time periods when compared to the Veh and SAMe groups (Figure 3). GPx was diminished at 2 h in the SAMe + APAP group compared to the Veh and SAMe groups but activity was partially restored at 4 h in the SAMe + APAP group. SAMe did not totally reverse the effects of APAP as GPx activity in the SAMe + APAP group were higher than the APAP group but still lower than the Veh and SAMe groups 4 h (p<0.05).
Fig. 2.
Catalase enzymatic activity in the liver 2 and 4 h following APAP overdose in C57Bl/6 mice was assessed with mice randomly divided into Veh (water), SAMe (1.25 mmol/kg), APAP (250 mg/kg), and SAMe administered 1 h post-APAP (S + A). Values represent mean ± S.E.M. with n=5 mice per group and superscripts denoting statistical differences (p<0.05).
Fig. 3.
Liver GPx activity alterations when SAMe was administered 1 h after APAP overdose. C57Bl/6 mice were randomly divided into Veh (water), SAMe (1.25 mmol/kg), APAP (250 mg/kg), and SAMe administered 1 h following APAP (S + A). Livers were collected 2 and 4 h following APAP overdose. All values represent mean ± S.E.M. Each group represents 5 mice with superscripts denoting statistically significant differences (p<0.05).
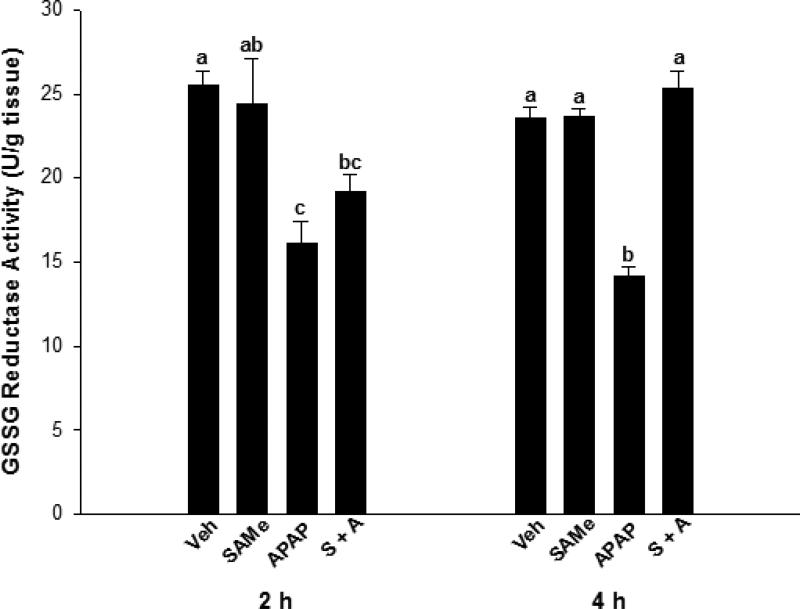
SAMe treatment did not alter GSSG reductase enzyme activity at either the 2 or 4 h time periods when compared to Veh (Figure 4). APAP treatment depressed GSSG reductase activity (p<0.05) by 36% and 42% of Veh levels, at the 2 and 4 h time periods, respectively. GSSG reductase activity at 2 h in the SAMe + APAP treatment group, was similar to the APAP and SAMe levels. However, at 4 h SAMe + APAP had returned GSSG reductase activity to levels comparable to Veh and SAMe activity.
Fig. 4.
GSSG reductase activity in the liver following APAP overdose in C57Bl/6 mice was determined by enzymatic assay. Mice were randomly allocated into Veh (water), SAMe (1.25 mmol/kg), APAP (250 mg/kg), and SAMe administered 1 h following APAP (S + A) and livers collected 2 and 4 h following APAP overdose. Each group represents 5 experiments with different mice and superscripts denote statistically significant differences (p<0.05).
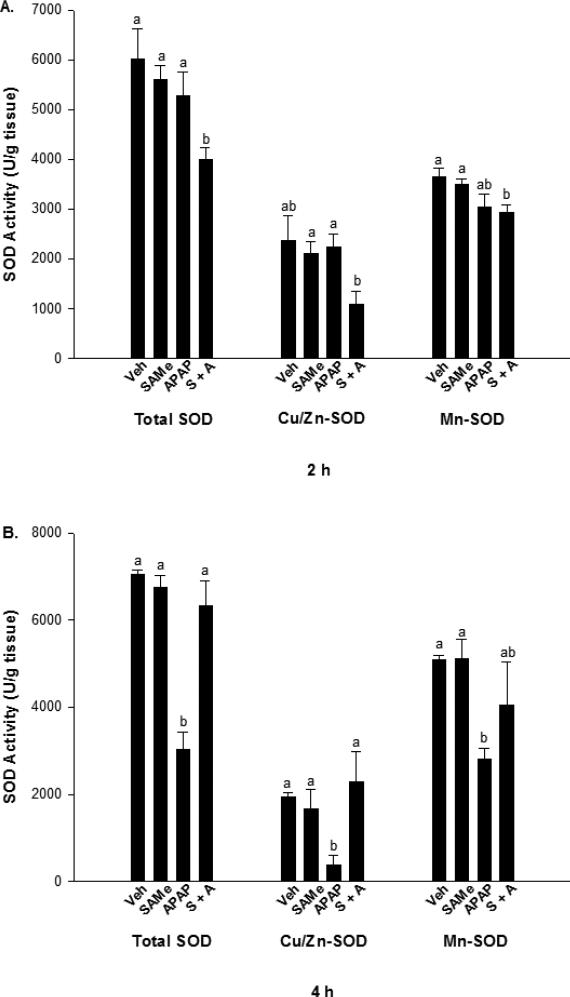
SOD activity was measured for 2 and 4 h treatment groups and expressed as total, cytosolic (Cu/Zn-SOD), and mitochondrial (Mn-SOD). SAMe treatment did not alter total, Cu/Zn, and Mn-SOD at any time period examined (Figure 5). In the 2 h treatment group, total SOD and Mn-SOD activity were lower (p<0.05) in the SAMe + APAP group compared to the Veh and SAMe mice. The Veh, SAMe, and APAP groups showed no significant difference (Figure 5, Panel A) for any form of SOD at 2 h. The Veh group did not differ significantly from the SAMe + APAP group for Cu/Zn-SOD. Mn-SOD was significantly decreased (p<0.05) in the SAMe + APAP group when compared to the Veh and SAMe groups at 2 h, and no difference was observed when compared to the APAP treatment group.
Fig. 5.
SOD activity following APAP overdose in C57Bl/6 mice. Cu/Zn-SOD was inhibited with DDTC for determination of Mn-SOD. Panel A represents SOD levels 2 h following APAP overdose, while panel B represents SOD levels 4 h following APAP overdose. Superscripts denote statistically significant differences (p<0.05) with n=5 mice per group.
In the 4 h treatment groups, total, Cu/Zn, and Mn-SOD activities were decreased (p<0.05) in the APAP group when compared with the Veh and SAMe groups (Figure 5, Panel B). It appeared that a longer time period of 4 h was needed to produce a decline in SOD activity. Treatment with SAMe (SAMe + APAP) increased total and Cu/Zn-SOD activity to levels similar to Veh and SAMe. The 4 h Mn-SOD activity in the SAMe + APAP group was not significantly different from the APAP group.
3.3 SAMe attenuates mitochondrial APAP induced Protein Carbonyl and 3-Nitrotyrosine adduction
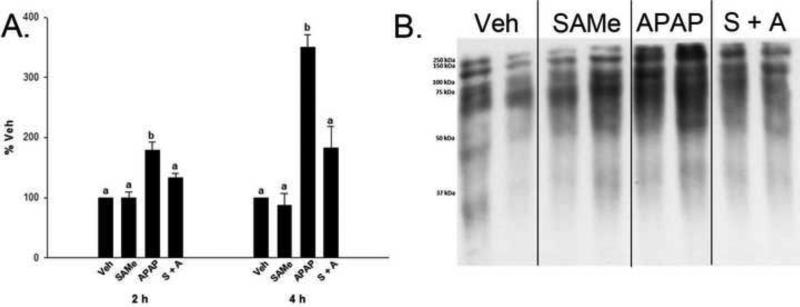
As a global measure of the effect of SAMe's ability to reduce mitochondrial oxidative stress, OxyBlots were performed on mitochondrial samples to detect protein carbonyl formation. In both 2 and 4 h samples, densitometry was significantly increased (p<0.05) in the APAP treatment groups when compared to the Veh and SAMe groups (Figure 6). When SAMe was administered following APAP, the density returned to be comparable to the Veh and SAMe treatment groups indicating that SAMe treatment lessens the formation of protein carbonyls in the mitochondria of APAP treated C57Bl/6 mice. These results are consistent with the protection observed by SAMe + APAP for the antioxidant enzyme activities (Figures 2-5).
Fig. 6.
Protein carbonyl formation in C57Bl/6 mouse mitochondria 2 and 4 h following APAP overdose. Protein carbonyls were assessed using an OxyBlot™ kit. Panel A contains densitometry from 2 and 4 h OxyBlots™ following APAP overdose. Densitometry was normalized to total protein staining (not shown) and is expressed as % Veh. All values represent mean ± S.E.M. with n=4 mice per group. Superscripts denote statistical difference (p<0.05). Panel B is a representative 4 h blot with lanes 1-2 corresponding to Veh, 3-4 SAMe, 5-6 APAP, and 7-8 SAMe administered 1 h following APAP (S + A).
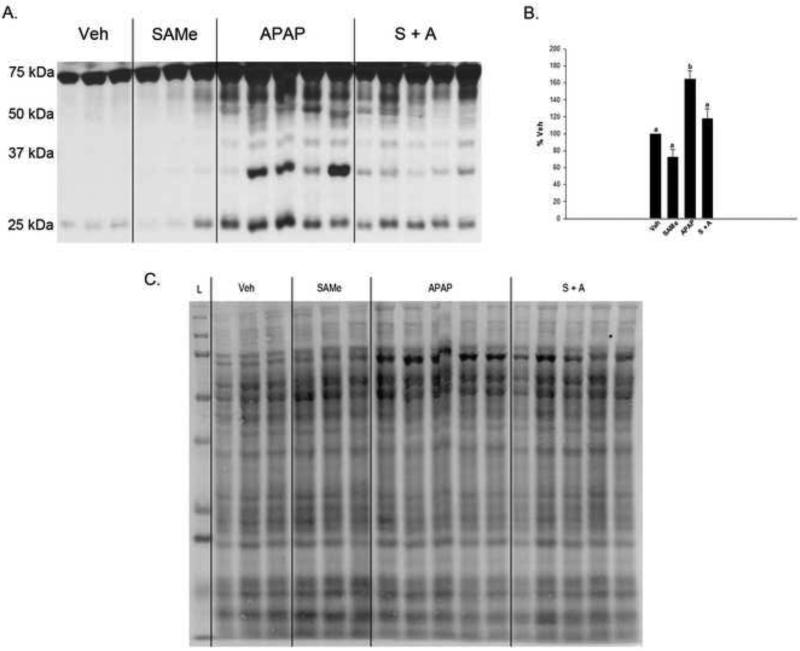
APAP significantly increased mitochondrial 3-NT protein adduction 4 h following overdose when compared to Veh and SAMe treatment groups (Figure 7). SAMe decreased the amount of mitochondrial 3-NT adduction when administered following APAP. Even though there was a similar increase in mitochondrial 3-NT adduction in the 2 h group, SAMe was less effective at decreasing the amount of adduction observed at this time period (data not shown).
Fig 7.
Mitochondrial 3-NT protein adduct formation 4 h following APAP overdose. Panel A is a representative 4 h blot containing mitochondrial samples from the indicated groups. Panel B is the densitometry from panel A for whole lane 3-NT adduction normalized to total lane protein staining. Superscripts represent statistical significance, and values are mean ± S.E.M. with n=3-5 mice per group.
3.4 Cytochrome c release
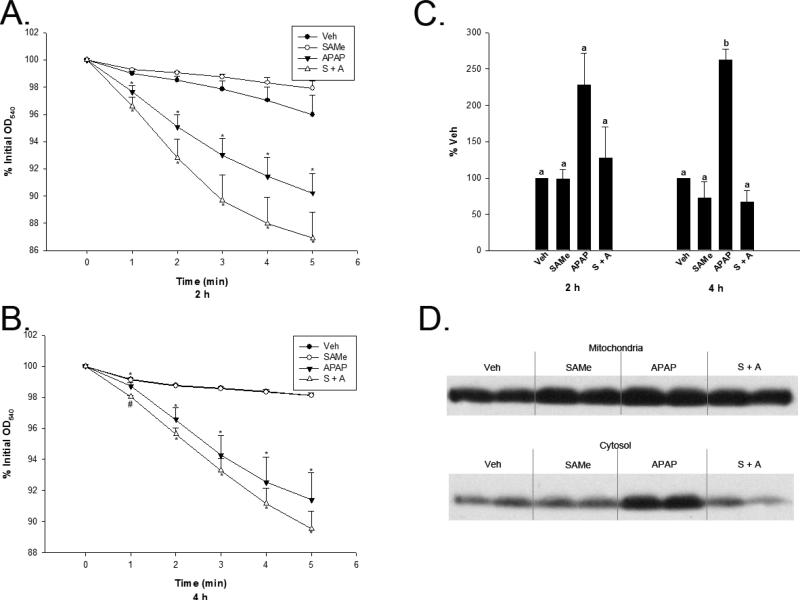
Mitochondrial function was also analyzed by examining mitochondrial swelling following APAP overdose. There was a significant increase in mitochondrial swelling at both 2 and 4 h following APAP overdose (Figure 8, Panels A and B). SAMe was not able to prevent the increased swelling observed during these time periods, and there was not a significant difference observed between the APAP and SAMe + APAP groups except in the 4 h treatment group 1 min after swelling was induced when the SAMe + APAP group was significantly lower than the APAP group.
Fig 8.
Mitochondrial swelling and cytochrome c leakage were used to assess mitochondrial function following APAP overdose. Mice were randomly allocated into Veh (water), SAMe (1.25 mmol/kg), APAP (250 mg/kg), and SAMe administered 1 h following APAP. Livers were collected 2 and 4 h following APAP administration and analyzed for mitochondrial swelling (Panel A and B). Panel C represents densitometry conducted on cytosolic cytochrome c levels following APAP overdose when analyzed at 2 and 4 h. Panel D is a representative 4 h cytosol cytochrome c blot depicting Veh (Lanes 1 and 2), SAMe (Lanes 3 and 4), APAP (Lanes 5 and 6), and S + A (Lanes 7 and 8). All values represent mean ± S.E.M. with at least 4 mice represented in each group. Super and subscripts denote statistical differences (p<0.05).
Cytochrome c leakage was used to asses mitochondrial function associated with APAP toxicity. Cytochrome c leakage into the cytosol was 20% greater in the APAP group when compared with the Veh group 2 and 4 h following APAP overdose, although the results were not significant at the 2 h time period (Figure 8, Panel C). SAMe was able to prevent the release of cytochrome c into the cytosol at 4 h when compared to the APAP group. The increased cytosolic cytochrome c is also evident in a representative blot included in Figure 8, Panel D.
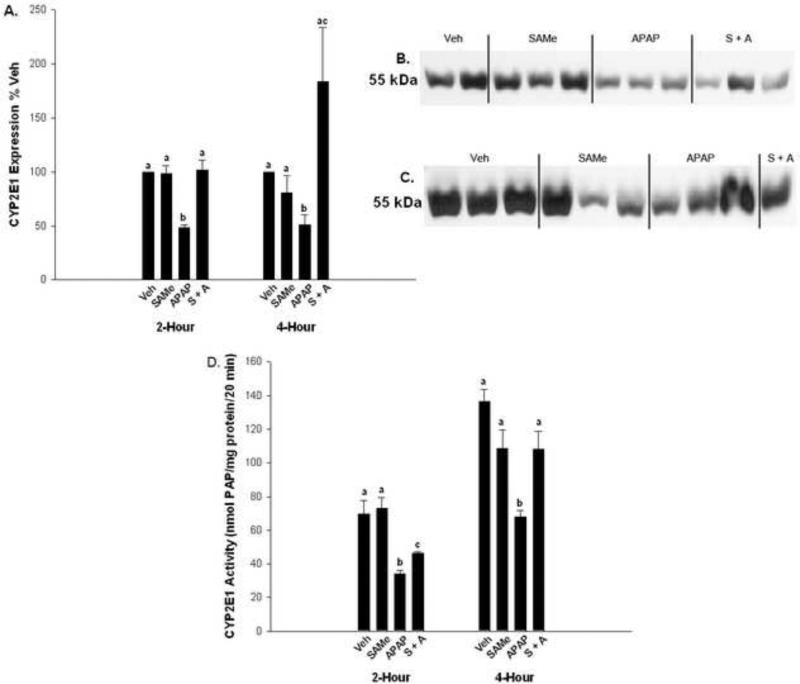
3.5 CYP 2E1 enzyme activity and expression
Aniline para-hydroxylation to PAP was used as an indicator of CYP2E1 enzyme activity. SAMe treatment, at the dose used in this study, did not alter aniline hydroxylation. APAP diminished CYP2E1 enzyme activity at 2 and 4 h relative to the Veh and SAMe groups (Figure 9). The SAMe + APAP group had higher enzyme activity than the APAP group at 2 h but PAP formation was slower than the Veh and SAMe groups at 2 h. Enzyme activity was similar to Veh and SAMe at 4 h. These results indicate that the mechanism for protection by SAMe is not due to inhibition of P450 metabolism at the dose used in our studies. Western analysis of protein expression also showed that SAMe does not alter CYP2E1 enzyme expression at 2 or 4 h. APAP diminished CYPE 2E1 expression at 2 and h compared to all other groups.
Fig. 9.
CYP2E1 enzyme activity and expression in APAP treated mice. Panel A represent densitometry for CYP2E1 enzyme expression measured at 2 h (Panel B) and 4 h (Panel C) using a Western blot. APAP inhibited CYP2E1 expression while SAMe did not inhibit CYP2E1 expression. Panel D represent para-hydroxylation of aniline to p-aminophenol (PAP) measured 2 and 4 h after APAP treatment in groups as described in Fig. 1. Enzyme activity was expressed as nmol PAP/mg protein/20 min. All values represent mean ± S.E.M. with at least 4 mice represented in each group. Superscripts denote statistical differences between groups as differences between groups are denoted by different letters. (p<0.05).
Discussion
Our lab has previously reported that SAMe reduced APAP toxicity in C57Bl/6 mice when administered 0 or 1 h after APAP (Terneus et al., 2007 & 2008). SAMe can reduce protein carbonylation and lipid peroxidation when administered after a toxic dose of APAP. Previous work established the importance of ROS and RNS generation in APAP toxicity (Cover et al., 2005). Damage caused by ROS and RNS is thought to be preceded by NAPQI induced GSH depletion, which we have previously demonstrated the ability of SAMe to prevent in APAP treated mice. However, the current study shows that SAMe also has the potential to protect the function of critical antioxidant enzymes normally altered by APAP toxicity.
SOD has been extensively studied with APAP toxicity. Decreased levels of Mn-SOD have been shown to significantly increase APAP toxicity, which is consistent with the generation of superoxide occurring primarily in the mitochondria with APAP toxicity (Yoshikawa et al., 2009; Ramachandran et al., 2011). Furthermore, Mn-SOD activity has recently been demonstrated to be decreased with nitration secondary to APAP toxicity (Agarwal et al., 2011). At 4 h, SAMe given 1 h after APAP (SAMe + APAP group) decreased 3-NT adducts globally in mitochondrial protein which is consistent with the observed correction in Mn-SOD activity observed for the SAMe + APAP group compared to APAP. The exact mechanism for SAMe decreasing APAP mediated nitration of tyrosine residues in proteins is not known. However, these results suggest SAMe interferes with APAP induced mitochondrial dysfunction and oxidative stress.
In contrast to decreased expression of Mn-SOD, Cu/Zn-SOD knockout mice show almost complete protection against APAP overdose (Zhu et al., 2006). SAMe given as an antidote to APAP toxicity clearly protects total SOD activity 4 h following APAP with significant increases in Cu/Zn-SOD activity and improvement. We clearly observed protection when SAMe was given following APAP indicating that, in our model, the modest increase in Mn-SOD may be of greater importance than the increase in Cu/Zn-SOD. In addition, full function of all antioxidant enzymes is important in reducing oxidative stress as hydrogen peroxide formed from MnSOD and Cu/Zn SOD is also toxic and is detoxified by catalase and glutathione peroxidase.
Evidence has existed for some time that the GPx-GSSG reductase system was protective in cases of APAP toxicity (Adamson et al., 1989). However, it is also apparent that over expression of intracellular GPx can increase APAP toxicity most likely as a result of enhanced GSH depletion (Mirochnitchenko et al., 1999). Our results clearly demonstrated a depression of GPx activity 2 and 4 h following APAP overdose, which was reversed at 4 h by SAMe administration. The fact that GPx activity was not returned to Veh level may be beneficial in light of the study on GPx over expression increasing APAP toxicity. Additionally, the increased activity of GSSG reductase at 4 h in the SAMe + APAP group would serve to increase cellular levels of GSH and decrease glutathione disulfide levels, which is consistent with prior results from our lab (Terneus et al., 2007 and 2008).
Catalase represents an important link in the detoxification of ROS generated as a result of APAP toxicity because it does not require GSH to function. Clear evidence exists that APAP can depress catalase activity at 6 and 24 h following APAP administration Yan et al., 2009; Chandrasekaran et al., 2011). Additionally, antioxidant administration prevents the observed decrease in catalase activity. SAMe does not have antioxidant properties and was still able to improve catalase function, although not significantly, 4 h following APAP administration.
Formation of the reactive metabolite NAPQI has long been known to be a prerequisite for mitochondrial dysfunction associated with APAP toxicity (Weis et al., 1992). More recent work suggests that the observed changes in mitochondrial function are caused by JNK activation following both GSH depletion and resultant oxidative stress (Saito et al., 2010). Additionally, peroxynitrite formation has been demonstrated to increase fragmentation of mitochondrial DNA, which could be a cause of some of the observed mitochondrial dysfunction (Cover et al., 2005). We demonstrated a clear reduction in markers of oxidative stress when SAMe was given after APAP in the 4 h treatment group. Additionally, the reduction in mitochondrial 3-NT adduct formation at 4 h with SAMe administration could be beneficial to function following APAP overdose.
Cytochrome c has previously been used as a marker for APAP toxicity (Song et al., 2004). However, it is clear that APAP toxicity is a necrotic event and caspase activation associated with apoptosis is abrogated by ATP depletion associated with toxicity (Kon et al., 2004). SAMe prevents release of cytochrome c into cell cytosol at both 2 and 4 h following APAP toxicity indicating mitochondrial protection. The dose of APAP used in our studies induced necrosis as the percent release of cytochrome c into cytosol was less than 30% of total cytochrome c.
One finding of interest was that SAMe did not prevent mitochondrial swelling when administered following APAP overdose. This finding contradicts the work of Song and associates (2004) which clearly demonstrated SAMe decreased mitochondrial swelling when given as an antidote to APAP toxicity. Three factors may account for the difference in findings between the current and previous study. First, our lab used a much higher concentration of calcium to initiate swelling in combination with additional phosphate and rotenone, which were not used in the Song research. Waldmeier and others demonstrated that even a high concentration of cyclosporine A cannot indefinitely inhibit permeability transition when isolated mitochondria are exposed to high concentrations of both calcium and phosphate (Waldmeier et al, 2009). Second, the concentration of SAMe used by Song, et al. (2004) was double that used by our lab in the current experiment. Perhaps SAMe needs to be present at a higher level than was used in the current experiment to provide protection against APAP induced mitochondrial swelling. Additionally, APAP (200 mg/kg, ip) has been demonstrated to undergo rapid conversion to NAPQI depleting cellular glutathione within 20 min of administration (Bajt et al, 2011). The current protocol administered SAMe 1 h after APAP, allowing more than enough time for NAPQI to form and cause some damage that could weaken the mitochondria, rendering them more susceptible to swelling. However, given the bulk of the data presented in this paper, SAMe is providing clear protection following APAP overdose.
Biotransformation of APAP by cytochrome P450 is critical for formation of NAPQI and development of hepatotoxicity (Patten et al., 1993; Chen et al., 1998). Caro and Cederbaum (2005) reported that SAMe inhibited in vitro CYP2E1 metabolism in rat microsomes and in HepG2 cells. The IC50 was reported as 1.5 mM for CYP2E1. In our studies, inhibition of CYP2E1 by SAMe is not the mechanism for reduced hepatic APAP toxicity in vivo when we administered SAMe 1 h after APAP. In our study we administer a dose of 1.25 mmol/kg SAMe which corresponds to approximately 30 ummoles distributed within the entire animal. The low amount administered to the animal is not sufficient to inhibit P450 enzyme activity. However, we also measured in vitro CYP2E1 metabolism and expression (Figure 9) which confirms P450 metabolism is not inhibited by SAMe.
In conclusion, we present here a clear line of evidence that the observed decrease in oxidative stress mediated by SAMe is in part due to the maintenance of antioxidant enzyme function following APAP overdose. Antioxidant enzyme function is greatly improved 4 h following APAP overdose when SAMe is given as an antidote. Also, cytochrome c release is inhibited by SAMe 4 h following APAP, indicating a protection of mitochondrial function. Mitochondrial dysfunction due to oxidative stress and resultant ATP depletion are critical factors in APAP toxicity. By protecting against mitochondrial oxidative stress, SAMe provides a potentially useful therapeutic intervention following APAP toxicity.
We examined SAMe protection of oxidative stress enzyme activity from Acetaminophen.
Mitochondrial 3-nitrotyrosine adduction by acetaminophen was prevented by SAMe.
WE showed that SAMe protection is not by inhibition of CYP 2E1.
SAMe reduced mitochondrial protein carbonylation induced by acetaminophen
Acknowledgements
The current project was supported by NIH Grants 5P20RR016477 to the West Virginia IDeA Network for Biomedical Research Excellence, NIH grant 2P20RR016477-09S4 and the West Virginia NASA Space Grant Consortium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson GM, Harman AW. A role for the glutathione peroxidase/reductase enzyme system in the protection from paracetamol toxicity in isolated mouse hepatocytes. Biochem Pharmacol. 1989;38:3323–3330. doi: 10.1016/0006-2952(89)90630-8. [DOI] [PubMed] [Google Scholar]
- Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, Hinson JA. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther. 2011;337:110–116. doi: 10.1124/jpet.110.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson BS, Rundgren M, Nelson SD, Harder S. N-acetyl-p-benzoquinone imine-induced changes in the energy metabolism in hepatocytes. Chem Biol Interact. 1990;75:201–211. doi: 10.1016/0009-2797(90)90118-7. [DOI] [PubMed] [Google Scholar]
- Anundi I, Lahteenmaki T, Rundgren M, Moldeus P, Lindros KO. Zonation of acetaminophen metabolism and cytochrome P450 2E1-mediated toxicity studied in isolated periportal and perivenous hepatocytes. Biochem Pharmacol. 1993;45:1251–1259. doi: 10.1016/0006-2952(93)90277-4. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Robinson G, Pinner A, Chamlee L, Ulasova E, Pompilius M, Page GP, Chhieng D, Jhala N, Landar A, Kharbanda KK, Ballinger S, Darley-Usmar V. S-adenosylmethionine prevents chronic alcohol-induced mitochondrial dysfunction in the rat liver. Am J Physiol Gastrointest Liver Physiol. 2006;291:G857–867. doi: 10.1152/ajpgi.00044.2006. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. Apoptosis-Inducing Factor Modulates Mitochondrial Oxidant Stress in Acetaminophen Hepatotoxicity. Toxicol Sci. 2011;122:598–605. doi: 10.1093/toxsci/kfr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan JT, Brosnan ME. The sulfur-containing amino acids: an overview. J Nutr. 2006;136:1636S–1640S. doi: 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran VR, Periasamy S, Liu LL, Liu MY. 17beta-Estradiol protects against acetaminophen-overdose-induced acute oxidative hepatic damage and increases the survival rate in mice. Steroids. 2011;76:118–124. doi: 10.1016/j.steroids.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Inhibition of CYP2E1 catalytic activity in vitro by S-adenosyl-L-methionine. Biochem Pharmacol. 2005;69:1081–1093. doi: 10.1016/j.bcp.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Chen W, Koenigs LL, Thompson SJ, Peter RM, Rettie AE, Trager WF, Nelson SD. Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chem Res Toxicol. 1998;11:295–301. doi: 10.1021/tx9701687. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci U S A. 1984;8:327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PC, Hinton RH, Price SC, Kass GE. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: roles of cytochrome c, Bax, Bid, and caspases. Toxicol Appl Pharmacol. 2003;191:118–129. doi: 10.1016/s0041-008x(03)00240-0. [DOI] [PubMed] [Google Scholar]
- Fernandez L, Perez V, Munoz M, Corpa JM, Abad M, Carbajo MT. Effects of S-adenosylmethionine on hepatic regeneration after partial hepatectomy in the rat. J Physiol Biochem. 2003;59:63–64. doi: 10.1007/BF03179869. [DOI] [PubMed] [Google Scholar]
- Gogvadze V, Orrenius S, Zhivotovsky B. Analysis of Mitochondrial Dysfunciton during cell death. In: Maines MD, Costa LG, Reed DJ, Sassa S, Sipes IG, editors. Current Protocols in Toxicology. Vol. 1. John Wiley & Sons, Inc.; Hoboken, NJ: 2006. pp. 2.10.11–12.10.27. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Michael SL, Ault SG, Pumford NR. Western blot analysis for nitrotyrosine protein adducts in livers of saline-treated and acetaminophen-treated mice. Toxicol Sci. 2000;53:467–473. doi: 10.1093/toxsci/53.2.467. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Pumford NR, Roberts DW. Mechanisms of acetaminophen toxicity: immunochemical detection of drug-protein adducts. Drug Metab Rev. 1995;27:73–92. doi: 10.3109/03602539509029816. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Goh CW, Ong MM, Boelsterli UA. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 2007;45:412–421. doi: 10.1002/hep.21475. [DOI] [PubMed] [Google Scholar]
- Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Lu SC. S-Adenosylmethionine. Int J Biochem Cell Biol. 2000;32:391–395. doi: 10.1016/s1357-2725(99)00139-9. [DOI] [PubMed] [Google Scholar]
- Mannervik B. Measurement of Glutathione Reductase. In: Maines MD, Costa LG, Hodgson E, Reed DJ, Sipes IG, editors. Current Protocols in Toxicology. Vol. 2. John Wiley & Sons, Inc.; Hoboken, NJ: 1999. pp. 7.2.1–7.2.4. [Google Scholar]
- Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42:110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Mirochnitchenko O, Weisbrot-Lefkowitz M, Reuhl K, Chen L, Yang C, Inouye M. Acetaminophen toxicity. Opposite effects of two forms of glutathione peroxidase. J Biol Chem. 1999;274:10349–10355. doi: 10.1074/jbc.274.15.10349. [DOI] [PubMed] [Google Scholar]
- Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen (Paracetomal)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15:398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- Olaleye MT, Rocha BT. Acetaminophen-induced liver damage in mice: effects of some medicinal plants on the oxidative defense system. Exp Toxicol Pathol. 2008;59:319–327. doi: 10.1016/j.etp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Patel NN, Crincoli CM, Frederick DM, Tchao R, Harvison PJ. Effect of structural modifications on 3-(3,5-dichlorophenyl)-2,4-thiazolidinedione-induced hepatotoxicity in Fischer 344 rats. J Appl Toxicol. 2012;32:108–1710. doi: 10.1002/jat.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten CJ, Thomas PE, Guy RL, Lee M, Gonzalez FJ, Guengerich FP, Yang CS. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol. 1993;6:511–518. doi: 10.1021/tx00034a019. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2011;251:226–233. doi: 10.1016/j.taap.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman JB, Jansson I. Measurement of Cytochorme P450. In: Maines MD, Costa LG, Reed DJ, Sassa S, Sipes IG, editors. Current Protocols in Toxicology. Vol. 1. John Wiley & Sons, Inc.; Hoboken, NJ: 1999. pp. 4.1.1–4.1.14. [Google Scholar]
- Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- Song Z, McClain CJ, Chen T. S-Adenosylmethionine protects against acetaminophen-induced hepatotoxicity in mice. Pharmacology. 2004;71:199–208. doi: 10.1159/000078086. [DOI] [PubMed] [Google Scholar]
- Song Z, Zhou Z, Song M, Uriarte S, Chen T, Deaciuc I, McClain CJ. Alcohol-induced S-adenosylhomocysteine accumulation in the liver sensitizes to TNF hepatotoxicity: possible involvement of mitochondrial S-adenosylmethionine transport. Biochem Pharmacol. 2007;74:521–531. doi: 10.1016/j.bcp.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terneus MV, Brown JM, Carpenter AB, Valentovic MA. Comparison of S-adenosyl-L-methionine (SAMe) and N-acetylcysteine (NAC) protective effects on hepatic damage when administered after acetaminophen overdose. Toxicology. 2008;244:25–34. doi: 10.1016/j.tox.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terneus MV, Kiningham KK, Carpenter AB, Sullivan SB, Valentovic MA. Comparison of S-Adenosyl-L-methionine and N-acetylcysteine protective effects on acetaminophen hepatic toxicity. J Pharmacol Exp Ther. 2007;320:99–107. doi: 10.1124/jpet.106.111872. [DOI] [PubMed] [Google Scholar]
- Valentovic M, Elliott C, Teets VJ, Brown PI, Yang D, Rankin GO. Enzyme induction produced by N-(3,5-dichlorophenyl)succinimide (NDPS) in rats. Biochem Pharmacol. 1988;37:768–770. doi: 10.1016/0006-2952(88)90156-6. [DOI] [PubMed] [Google Scholar]
- Waldmeier PC, Feldtrauer JJ, Qian T, Lemasters JJ. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol Pharmacol. 2009;62:22–29. doi: 10.1124/mol.62.1.22. [DOI] [PubMed] [Google Scholar]
- Wang AY, Lian LH, Jiang YZ, Wu YL, Nan JX. Gentiana manshurica Kitagawa prevents acetaminophen-induced acute hepatic injury in mice via inhibiting JNK/ERK MAPK pathway. World J Gastroenterol. 2010;16:384–391. doi: 10.3748/wjg.v16.i3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis M, Kass GE, Orrenius S, Moldeus P. N-acetyl-p-benzoquinone imine induces Ca2+ release from mitochondria by stimulating pyridine nucleotide hydrolysis. J Biol Chem. 1992;267:804–809. [PubMed] [Google Scholar]
- Wendel A, Feuerstein S, Konz KH. Acute paracetamol intoxication of starved mice leads to lipid peroxidation in vivo. Biochem Pharmacol. 1979;28:2051–2055. doi: 10.1016/0006-2952(79)90223-5. [DOI] [PubMed] [Google Scholar]
- Wu YL, Jiang YZ, Jin XJ, Lian LH, Piao JY, Wan Y, Jin HR, Joon Lee J, Nan JX. Acanthoic acid, a diterpene in Acanthopanax koreanum, protects acetaminophen-induced hepatic toxicity in mice. Phytomedicine. 2010;17:475–479. doi: 10.1016/j.phymed.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Yan SL, Wu ST, Yin MC, Chen HT, Chen HC. Protective effects from carnosine and histidine on acetaminophen-induced liver injury. J Food Sci. 2009;74:H259–265. doi: 10.1111/j.1750-3841.2009.01330.x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Morita M, Hosomi H, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Knockdown of superoxide dismutase 2 enhances acetaminophen-induced hepatotoxicity in rat. Toxicology. 2009;264:89–95. doi: 10.1016/j.tox.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Zhang YT, Zheng QS, Pan J, Zheng RL. Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic Clin Pharmacol Toxicol. 2004;95:53–58. doi: 10.1111/j.1742-7843.2004.950202.x. [DOI] [PubMed] [Google Scholar]
- Zhu JH, Zhang X, McClung JP, Lei XG. Impact of Cu, Zn-superoxide dismutase and Se-dependent glutathione peroxidase-1 knockouts on acetaminophen-induced cell death and related signaling in murine liver. Exp Biol Med (Maywood) 2006;231:1726–1732. doi: 10.1177/153537020623101109. [DOI] [PubMed] [Google Scholar]