Abstract
OBJECTIVE
Diagnosis of insomnia disorder by Diagnostic and Statistical Manual (DSM)-IV, and as proposed by DSM-V, includes criteria for impairment in occupational- or social functioning due to sleep complaints. This study evaluated the clinical and polysomnographic correlates of impairment in daytime functioning in older adults with insomnia.
METHODS
In older adults with DSM-IV chronic insomnia (n=68), clinical and demographic information, and measures of health functioning, medical co-morbidity, and polysomnographic sleep were obtained. Four questions that evaluated difficulties or distress in occupational- or social functioning related to sleep complaints were used to code DSM threshold criteria for impairment in daytime functioning. Stepwise regression was used to identify predictors of impairment in daytime functioning.
RESULTS
Impairment in daytime functioning was significantly associated with younger age (p<0.05), and the amount of wake time after sleep onset as assessed by polysomnography (p<0.001), controlling for health functioning and minority racial status.
CONCLUSIONS
Amount of wake time after sleep onset uniquely contributes to criteria symptoms of impairment in daytime functioning among older adults with insomnia. Treatments that target sleep maintenance have the potential to improve social and occupational functioning in older adults with sleep complaints.
INTRODUCTION
Sleep disturbance constitutes one of the most common difficulties facing older adults, with nearly 60% of the community-dwelling elderly reporting sleep problems at least a few nights per week and over 15% of older adults fulfilling diagnostic criteria for insomnia [1]. In addition to complaints about sleep quantity or quality, and reported difficulties with sleep onset or sleep maintenance, the diagnosis of insomnia requires evidence of significant distress or impairment in daytime functioning, namely, symptoms of fatigue, sleepiness, attentional- or memory complaints, and/or mood disturbance, which impair occupational, social or interpersonal functioning [2, 3]. Whereas the clinical correlates of sleep complaints have been studied broadly across the life span including older adults [4, 5], less is known about the factors that contribute to impairment in daytime functioning as indexed by DSM criteria, which is a consequence of sleep complaints [3]. Moreover, no study to our knowledge has examined the relationship between polysomnographic sleep and DSM criteria of impairment in occupational and/or social functioning in older adults with chronic insomnia. In older adults, Gooneratne et al. reported that the combination of insomnia symptoms and sleep related breathing disorder, as indexed by polysomnography, was associated with daytime fatigue and sleepiness, although criteria symptoms of impaired functioning were not assessed[6]. This study examined the clinical and polysomonographic correlates of DSM criteria symptoms of functional impairment in older adults with insomnia.
METHODS
Subjects
In 2006, a randomized controlled trial (NCT00280020) was begun to evaluate the efficacy of behavioral treatments for DSM-IV insomnia in older adults. The present study is based on cross-sectional, baseline analyses of the first 68 participants who were enrolled in this trial. Male and female subjects older than 55 years were recruited via local print advertisements as approved by the Institutional Review Board. After telephone screening, participants were invited for interview, gave informed consent, and underwent a Structured Clinical Interview for DSM-IV (SCID)[7]. Diagnosis of insomnia was made in weekly consensus meetings in which criterion validity and reliability were monitored by the study psychiatrist (MRI). Regular viewing and scoring of videotaped interviews, along with the consensus meeting, maintained high inter-rater reliability (r=0.94). All eligible participants fulfilled criteria for the presence of primary insomnia as defined by the DSM-IV-TR[8], with exclusion of those had another current Axis I psychiatric disorder and/or medical comorbidities that were judged to impact sleep (e.g. unstable cardiovascular disease). We excluded subjects who were diagnosed with current major depressive disorder and those who used sedative hypnotic medications in the week prior to assessment of polysomnographic sleep.
Procedures
Demographic and clinical information was obtained by interview and questionnaire. To assess health functioning, the Short Form-36 (SF-36) was administered with use of its summary score for Physical Functioning (PCS)[9]. To evaluate the presence of medical co-morbidity, a physician (LK) obtained a medical history and completed a review of medical systems and physical examination; this information was used in the scoring of the Cumulative Illness Rating Scale-Geriatric Score (CIRS-G)[10]. Alcohol and tobacco use histories were obtained by interview.
DSM criteria symptoms of impairment in daytime functioning were coded using four “yes / no” format questions that asked whether sleep difficulties “interfered with working; decreased participation in recreational activities like sports; interfered with taking care of household responsibilities; or caused problems with friends or family.” These four questions parallel the criteria symptoms of impaired daytime functioning delineated in DSM-IV-TR and also capture the criteria symptoms of impaired occupation, academic, social, and interpersonal functioning as proposed by DSM-V. These responses were summarized by a 0–4 scale measure indicating the number of “yes” answers.
After completion of baseline evaluation, subjects were asked to refrain from alcohol-and/or sedative hypnotic use for one week prior to entry into the polysomnography sleep protocol that included a night of adaptation to the sleep laboratory followed by two nights in the UCLA General Clinical Research Center as previously described [11, 12]. No subject was allowed to use a hypnotic medication during the sleep protocol. During the night of adaptation, the presence of periodic limb movement and/or sleep apnea was evaluated, with exclusion of subjects who showed >15 limb movements per hour with arousal and/or > 15 hypopneic or apneic episodes (>4% desaturation) per hour with arousal, respectively. Data were averaged across the two nights of sleep after completion of the night of adaptation. All sleep records were visually scored in accordance with Rechtschaffen and Kales criteria [13].
Statistical analyses
Data obtained were converted in SPSS [version16.0; SPSS Inc, Chicago, IL] for analysis. As this was an analysis of the correlates of impairment in daytime functioning, the goal was to maximize the number of potential correlates given a modest sample size. Three domains of variables (demographic- and clinical; health functioning and medical co-morbidity; and polysomnography) were categorized: the demographic and clinical (i.e., age, sex, ethnicity, minority racial status, marital status, employment status, body mass index); health functioning and medical co-moribidity (i.e., SF-36-PCS, CIRS-G); and polysomnography (i.e., total sleep time, sleep onset latency, wake time after sleep onset (WASO), and percentages of NREM stage 2, slow wave, and rapid eye movement sleep). (Note: some additional measures, e.g., sleep efficiency, percentage of NREM stage 1 sleep, were excluded due to mathematical co-linearity with included variables).
Stepwise multiple regression analyses were then utilized to add variables to the prediction model in the following order: clinical and demographic variables, health functioning and medical co-morbidity, and polysomnographic measures. Only variables showing some trend (p<.10) were retained when the next set in the order was tested; these variables, however, were always retained even if, in subsequent models, they no longer achieved the set criterion (p<.10). The final model thus included all variables that had at one step met the specified threshold. The dependent variable in these analyses was a composite measure of impaired occupation, interpersonal, and/or social function as defined by DSM criteria symptoms, on a 0–4 scale.
RESULTS
Subjects (N=68) fulfilled criteria for chronic primary insomnia as determined by SCID-IV and had a mean age of 66.2 years [SD=7.7]; education level of 15.5 years [SD=1.5]; and body mass index [BMI] of 25.9 [SD=3.7]. Of the sample, 73.5% were female, 11.8% were non-white race, 8.8% were Hispanic ethnicity, 33.8% were married or cohabitating, 47.1% were employed.
In this older adult sample, measures of health status indicated that persons were generally in good medical health; subjects had PCS as determined by the SF-36 of 47.9 [SD=8.4] and CIRS-G scores of 2.1 [SD=2.1]. None of the subjects were smokers, and alcohol use was infrequent; only 53% reported drinking in the last 90 days, with an with an average of 0.3 [SD=0.6] drinks per day. All subjects reported refraining from use of alcohol in the week prior to assessment of polysomnographic sleep.
Measures of polysomnographic sleep showed prominent disturbances of sleep continuity in this insomnia sample as shown in Table 1. The composite measure of DSM impairment in daytime function ranged from 0 to 4 with a mean of 1.87 [SD=1.43]. The distribution of scores was not skewed (skew=.20, SE skew = .29), and was appropriate for use in multiple regression without the need for any transformations.
Table 1.
Polysomnographic Sleep Measures in Older Adults with Insomnia
| Variable | mean | SD |
|---|---|---|
| Total sleep time (min) | 350.0 | 56.7 |
| Sleep onset latency (min) | 28.2 | 23.2 |
| Sleep efficiency (%) | 72.9 | 11.9 |
| WASO (min) | 92.6 | 43.8 |
| Stage 1 (%) | 11.3 | 17.0 |
| Stage 2 (%) | 64.0 | 11.6 |
| Stage 3–4 (%) | 1.1 | 3.4 |
| REM sleep (%) | 23.6 | 6.3 |
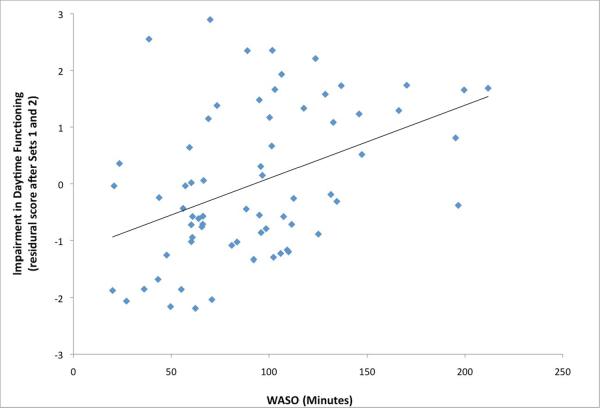
To determine the clinical and polysomnographic correlates associated with DSM impairment in daytime function, we used stepwise multivariate regression analyses with variables across three domains including demographic characteristics, self-reported health status, and polysomnographic sleep. In the first set, age [t=1.89; p=0.06] and minority status [t=2.16, p<0.05] entered the model. Of the second set, only PCS was significant [t=2.00, p=0.05], when age and minority status were included in the model. Finally in the third regression examining the polysomnographic measures (and including age, minority status and PCS), only WASO was retained [t=1.66, p=0.10]. The final regression model showed that severity of impairment in daytime function was associated with younger age (= −0.26, p< 0.05), and only marginally to minority status and PCS (see Table 2). On the other hand, severity of impairment in daytime function was highly related to one measure of polysomnographic sleep, amount of awake time after sleep onset (= 0.40, p < 0.001).(Figure 1)
Table 2.
Predictors of DSM Criteria Symptoms of Impairment in Daytime Function
| Variable | B | Beta | t-value | p-value |
|---|---|---|---|---|
| Constant | 5.503 | |||
| Age1 | −.048 | −.258 | 2.44 | .018 |
| Minority status1 | −.700 | −.158 | 1.48 | .145 |
| PCS2 | −.033 | −.195 | 1.83 | .073 |
| WASO3 | .013 | .401 | 3.78 | <.001 |
Superscript represents which set the variable entered in the final model.
Figure 1.
Association between amount of wake after sleep onset in minutes, as indexed by polysomnography, impairment in daytime function in older adults with insomnia in the final model. (= 0.40, p < 0.001)
DISCUSSION
Among older adults with insomnia, criteria symptom of daytime impairment in functioning was most consistently associated with younger age in this older adult sample and amount of wake time after sleep onset. Relative youth may indicate potential for insomnia's greater impact on a comparatively higher level of functionality. It is also possible that the older subjects in the sample had greater chronicity to their insomnia and had perhaps learned to function with the adverse daytime consequences experienced. Alternatively, it is possible that younger people were more likely to be working and needed to perform at a higher level, although employment status was not related to daytime functioning.
Nocturnal awakenings disrupt the sleep of about one-third of the general population[2]. In this study, increased wake time after sleep onset was found to be a significant correlate of impairment in daytime functioning. Whereas recent work has found that self-reported difficulty resuming sleep was associated with subjective shorter sleep duration, poorer sleep quality, greater daytime impairment, greater consultations for sleep disturbances and greater likelihood of receiving a sleep medication [2], we believe this was the first study to incorporate polysomnographic-derived wake time after sleep onset data as a correlate of impaired occupational and/or social functioning in older adults with insomnia. Together, these findings suggest that interventions targeting objective wake time after sleep onset could potentially reduce some of the daytime consequences of sleep complaints. Such approaches might include the use benzodiazepine receptor agonists [14], or behavioral approaches such as stimulus control instructions that improves sleep maintenance beyond the initial short-term treatment time [15].
There were several limitations to this study, including the relative outnumbering of males by females in the sample. Second, impairment in daytime functioning relied on reported difficulties in occupational and social function without objective assessment of dysfunction, although this method is consistent with DSM diagnostic criteria. Third, subjects in this sample were relatively healthy and may not have been representative of the older adult population. Nevertheless, these findings demonstrate that amount of wake time after sleep onset as indexed by polysomnography uniquely contributes to criteria symptoms of impairment in daytime function in older adults with insomnia.
ACKNOWLEDGMENTS
This work was supported in part by grants HL 079955, AG 026364, CA 10014152, CA116778, RR00827, P30-AG028748, General Clinical Research Centers Program, the UCLA Cousins Center at the Semel Institute for Neurosciences, and the UCLA Older Americans Independence Center Inflammatory Biology Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial gain related to the outcome of this research, and there are no potential conflicts of interest.
REFERENCES
- 1.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM, Krystal A, Roehrs TA, Roth T, Vitiello MV. Using difficulty resuming sleep to define nocturnal awakenings. Sleep Med. 2010;11:236–41. doi: 10.1016/j.sleep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohayon MM, Riemann D, Morin C, Reynolds CF., 3rd Hierarchy of insomnia criteria based on daytime consequences. Sleep Med. 2012;13:52–7. doi: 10.1016/j.sleep.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohayon MM. Prevalence and correlates of nonrestorative sleep complaints. Arch Intern Med. 2005;165:35–41. doi: 10.1001/archinte.165.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Phillips B, Mannino D. Correlates of sleep complaints in adults: the ARIC study. Journal of clinical sleep medicine : J Clin Sleep Med. 2005;1:277–83. [PubMed] [Google Scholar]
- 6.Gooneratne NS, Gehrman PR, Nkwuo JE, Bellamy SL, Schutte-Rodin S, Dinges DF, Pack AI. Consequences of comorbid insomnia symptoms and sleep-related breathing disorder in elderly subjects. Arch Intern Med. 2006;166:1732–8. doi: 10.1001/archinte.166.16.1732. [DOI] [PubMed] [Google Scholar]
- 7.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. Patient Edition, Version 2.0 New York State Psychiatric Institute; New York, New York: 1996. [Google Scholar]
- 8.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Text Revision - 4th ed Author; Washington, DC: 2000. [Google Scholar]
- 9.McHorney CA, Ware JE, Jr., Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995;43:130–7. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 11.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24:54–7. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 13.Rechtschaffen A, Kales A. A Manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. NINDS; Bethesda: 1968. [DOI] [PubMed] [Google Scholar]
- 14.Nowell PD, Mazumdar S, Buysse DJ, Dew MA, Reynolds CF, 3rd, Kupfer DJ. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA. 1997;278:2170–7. [PubMed] [Google Scholar]
- 15.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]