Abstract
The apolipoprotein E (APOE) ε4 allele increases the risk for late-onset Alzheimer's disease (AD) and age-related cognitive decline. We investigated whether ε4 carriers show reductions in gray matter volume compared to ε4 non-carriers decades prior to the potential onset of AD dementia or healthy cognitive aging. Fourteen cognitively normal ε4 carriers, ages 26 to 45, were compared with 10 age-matched, ε4 non-carriers using T1-weighted volumetric magnetic resonance imaging (MRI) scans. All had reported first or second-degree family histories of dementia. Group differences in gray matter were tested using voxel-based morphometry (VBM) and a multivariate model of regional covariance, the Scaled Subprofile Model (SSM). A combination of the first two SSM MRI gray matter patterns distinguished the APOE ε4 carriers from non-carriers. This combined pattern showed gray matter reductions in bilateral dorsolateral and medial frontal, anterior cingulate, parietal, and lateral temporal cortices with co-varying relative increases in cerebellum, occipital, fusiform, and hippocampal regions. With these gray matter differences occurring decades prior to the potential onset of dementia or cognitive aging, the results suggest longstanding, gene-associated differences in brain morphology that may lead to preferential vulnerability for the later effects of late onset AD or healthy brain aging.
Keywords: Apolipoprotein E, Late-Onset Alzheimer's Disease, Magnetic Resonance Imaging, Voxel-Based Morphometry, Multivariate Analysis, Gray Matter Volume
1. Introduction
The apolipoprotein E (APOE) ε4 allele, a common susceptibility gene for late-onset Alzheimer's disease (AD), increases the risk for dementia (Saunders et al., 1993) and has been associated with cognitive decline in healthy aging (Caselli et al., 2004; Caselli et al., 2009). In cognitively normal, young and late-middle aged adults, studies measuring cerebral metabolism with positron emission tomography (PET) have shown reductions in ε4 carriers compared to non-carriers (Reiman et al., 1996, 2004, 2005) in brain areas previously shown to be affected in AD, including in parietal, temporal, and frontal regions (Frackowiak et al., 1981; de Leon et al., 1983; Duara et al., 1986; Alexander et al., 2002). Recent studies have shown an association between APOE ε4 and increased deposition of β-amyloid with PET C11PIB in late middle age and older adults (Reiman et al., 2009; Morris et al., 2010), but no cortical binding of the PIB amyloid tracer was observed in younger individuals 45 to 49 years of age (Morris et al., 2010).
The question of whether and when observable differences in structural brain anatomy occur in relation to increased genetic risk for late onset AD is less clear. Patients with AD in the early stages of dementia show atrophy affecting the medial and lateral temporal lobes, but also extending into parietal and frontal cortices (Jack et al., 1992; Basso et al., 2006; Whitwell et al., 2007; Hua et al., 2009). In non-demented adults with average ages in late middle age or older, some studies have found greater reductions in medial temporal lobe structures in ε4 carriers than non-carriers (Plassman et al., 1997; den Heijer et al., 2002; Wishart et al., 2006; Burggren et al., 2008; Mueller et al., 2008), whereas others have observed no APOE group differences (Schmidt et al., 1996; Reiman et al., 1998; Trivedi et al., 2006). Studies of young adults using magnetic resonance imaging (MRI) univariate voxel-based morphometry (VBM) to evaluate regional gray matter throughout the brain found no differences between ε4 carriers and non-carriers (Mondadori et al., 2007; Filippini et al., 2009; Dennis et al., 2009), whereas one study of children and adolescents observed reductions of MRI cortical thickness in the entorhinal cortex in ε4 carriers compared to non-carriers (Shaw et al., 2007). The variability of findings across studies may be, in part, related to differences in the subject samples studied, as well as differences in MRI post-processing methods ranging from manual traced regions of interest for selective brain structures to the use of voxel-based whole brain approaches that have often relied on different inter-subject registration algorithms. In addition, these studies have all applied univariate analysis techniques that do not consider group differences in the patterns of covariance across brain regions. Multivariate covariance analysis methods may provide greater sensitivity in detecting regionally distributed effects of interest in neuroimaging data (Alexander and Moeller, 1994; Habeck et al., 2005).
We investigated whether genetic risk for late onset AD in adults with the APOE ε4 allele is associated with regional gray matter reductions occurring decades before the potential onset of dementia or healthy cognitive aging by using MRI VBM with the Scaled Subprofile Model (SSM; Moeller et al., 1987). The SSM is a multivariate analysis method that tests for regional covariance patterns in neuroimaging data and is sensitive to group differences without requiring conservative correction for multiple comparisons across voxel-based brain regions. This approach has been applied in numerous functional neuroimaging studies (Alexander and Moeller, 1994; Eidelberg et al., 1995; Alexander et al., 1999; Smith et al., 2006); and applications to structural MRI VBM in humans and non-human primates have shown this method to be sensitive in identifying robust regional patterns of gray matter associated with healthy aging (Alexander et al., 2006, 2008; Brickman et al., 2007, 2008; Bergfield et al., 2010), with frontal reductions as a common feature.
In this study, structural MRI scans from cognitively normal APOE ε4 carriers and non-carriers between the ages of 26 and 45 reporting first or second-degree family histories of dementia were investigated using Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, London, UK) VBM with DARTEL combined with multivariate SSM analysis. We hypothesized that APOE ε4 carriers would show a regionally-distributed pattern of gray matter reductions in brain regions known to be preferentially affected in late onset AD and healthy cognitive aging, including temporal, parietal, and frontal regions.
2. Methods and Materials
2.1. Subjects
Twenty-four adults between the ages of 26 and 45 were selected from a cohort of 143 participants enrolled in a longitudinal study of genetic risk for late onset AD with ages ranging from 25 to 79 years. All subjects were medically screened to exclude any history of psychiatric or neurological illness or injury that could affect brain function and cognition. Details of the screening procedures have been previously described (Caselli et al., 2002) and included a neurological exam with a review of medical history and rating scales and tests of mental status, mood, psychiatric symptoms, and risk factors for cognitive impairment. Only subjects younger than 46 years of age with a reported first or second-degree family history of dementia and good quality MRI scans available were included in the current study. Participants were selected with either a first or second-degree family history as the indicator of self-reported familial risk, since many of the subjects had parents who were not yet in the age range to have a significant risk for dementia. Although the self-reported first-degree family history of dementia was numerically more frequent in the ε4 carrier group than ε4 non-carriers, the difference was not statistically significant (Table 1). The APOE ε4 carriers did not differ significantly from the comparison group of APOE ε4 non-carriers in age, gender, and years of education, but there was a trend for more years of education in the ε4 non-carriers (Table 1). Further, they did not differ significantly in the Mini-Mental Status Exam (Folstein et al., 1975). The ε4 carrier group consisted of 12 participants with ε3/4 and two with the ε4/4 genotype, whereas the non-carrier group included 10 participants with the ε3/3 genotype.
Table 1.
Subject demographic and clinical characteristics.
| APOE ε4 Carriers | APOE ε4 Non-Carriers | p-value | |
|---|---|---|---|
| N | 14 | 10 | |
| Age, mean (SD) | 38.5 (5.5) | 37.6 (4.8) | 0.63 |
| Gender, M/F | 3/11 | 4/6 | 0.39 |
| Education, mean (SD) | 15.6 (1.8) | 17.0 (1.7) | 0.10 |
| MMSE, mean (SD) | 29.8 (0.4) | 29.6 (0.7) | 0.67 |
| Family Hx, 1st/2nd degree | 5/9 | 1/9 | 0.34 |
APOE = apolipoprotein E; SD = standard deviation; MMSE = Mini-Mental State Exam; Family Hx, 1st/2nd degree = Family history of dementia, closest reported relative is first or second degree.
2.2. Neuropsychological testing
A battery of neuropsychological tests was administered to each participant to assess performance on measures of general cognitive ability and specific domains of verbal memory, attention, language, and visuospatial function. The tests included the Dementia Rating Scale (DRS; Mattis, 1976), a 12-item, 12-trial version of the Selective Reminding Test (SRT; Buschke, 1973), the Trail Making Test, parts A and B (Reitan, 1958), the Stroop Color-Word Interference Test (Dodrill, 1978), Boston Naming Test (Kaplan, 1983), a measure of verbal fluency (FAS; Benton and Hamsher, 1976), and the Extended Range Drawing Test (ERDT; Haxby et al., 1985).
2.3. APOE genotyping
APOE alleles were determined from genomic DNA obtained from blood samples with a polymerase chain reaction (PCR) method that has been described (Saunders et al., 1993). Briefly, genotyping was performed with DNA amplification by PCR using APOE primers and the HhaI restriction isotyping enzyme. Amplified DNA was digested by HhaI, resolved on a polyacrylamide gel, and autoradiographed for allele detection. Each autoradiograph was visualized by two independent observers who were blind to subject characteristics.
2.4. Image acquisition and processing
Volumetric T1-weighted spoiled gradient-recalled acquisition in a steady state (SPGR) MRI scans were acquired for each subject with a 1.5T GE Signa Horizon LX scanner (General Electric Medical Systems, Waukesha, WI, USA) at Banner Good Samaritan Medical Center with 124 contiguous axial 1.5 mm thick slices and 0.94 by 1.25 in plane resolution (repetition time = 33.3 msec, echo time = 5 msec, flip angle = 30 deg, matrix = 256 × 256, number of excitations = 1, field of view = 24 cm). The MRI scans were processed using Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, London, UK) with VBM (Ashburner and Friston, 2000; Good et al., 2001) and the DARTEL toolbox, which was used to perform diffeomorphic anatomical inter-subject registration and to produce the segmented gray matter maps (Ashburner, 2007; Ashburner and Friston, 2009). Briefly, we used the DARTEL routine in SPM8 to first segment gray and white matter, which were used subsequently for the simultaneous registration and customized template generation in the sample's average MRI space, performed iteratively with increasing accuracy. The non-linear spatial normalization information in this iterative procedure reflects flow fields, mapping the individual brain to a set of increasingly refined templates over all subjects in the sample. These segmented gray matter maps were subsequently brought into the standard Montreal Neurological Institute (MNI) template space for the SSM analysis and reporting of regional findings. Each gray matter map was processed to preserve volume information with spatial deformation and filtered using a Gaussian kernel with 8 mm full width at half maximum to produce smoothed maps of gray matter volume. An estimate of total intracranial volume (eTIV) was obtained by combining the gray, white, and cerebrospinal fluid segments in the native MRI brain scan for each subject.
2.5. Statistical analyses
SSM analysis was performed on the MRI VBM smoothed gray matter volume maps using MATLAB (Math Works, Natick, Massachusetts, USA). SSM assumptions and procedures have been described in detail (Moeller et al., 1987; Alexander and Moeller, 1994). Briefly, the means across regions and subjects were subtracted at each voxel, after the natural log transformation of the gray matter maps. A principal component analysis (PCA) was subsequently performed, producing a set of regional covariance patterns and corresponding subject scores reflecting the degree to which each subject expressed the identified network patterns. We performed SSM on VBM gray matter images to identify patterns of gray matter volume that distinguished the APOE ε4 carriers from non-carriers in this young to early middle-aged group of healthy adults. Multiple regression analysis was used to identify the best set of SSM component patterns distinguishing the APOE groups. We used the Akaike Information Criterion with adjustment for small sample sizes (AICc; Burnham and Anderson, 2002) as a model selection method that seeks to optimize a trade-off between bias and variance in selecting the best set of components in the regression model (Habeck et al., 2005). In this case, the first set of sequential components that together produced the lowest AICc value in the regression model to identify the APOE ε4 related network was selected as the best set of component predictors. Regression analyses were subsequently used to test the effect of gender, age, and education on the APOE ε4 related gray matter network pattern. The eTIV was used as a covariate to test for individual differences in total intracranial size. In addition, we performed follow up regression analyses to test the robustness of observed group differences in pattern expression after removing the subjects with two copies of the ε4 allele and those with a first degree family history of dementia from the group comparisons.
We applied a bootstrap re-sampling procedure (Efron and Tibshirani, 1994) with 500 iterations to the SSM analysis as previously described (Alexander et al., 2008; Bergfield et al., 2010) to provide reliability estimates at each voxel for the observed pattern weights associated with APOE group. In this case, the computed bootstrap distributions were used to provide confidence intervals for the voxel weights of the linearly combined network pattern associated with the subject score prediction that differed between APOE groups.
The MNI coordinates for the local minima and maxima values for the bootstrapped SSM pattern weights were selected with Z scores ≥ +2.0 and ≤ -2.0 and were converted to coordinates of the Talairach and Tournoux (1988) brain atlas (http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.html). This Z score threshold was used to provide brain maps with color scales showing the full extent of regions robustly contributing to the SSM APOE group related pattern.
Group differences in demographic characteristics and neuropsychological test scores were evaluated using the Fisher's exact test and non-parametric Mann-Whitney U test where appropriate to address the non-normal distributions with small samples.
3. Results
The APOE ε4 carrier and non-carrier groups did not differ significantly on measures of neuropsychological function, but there was a trend for better performance on one long-term memory score of the Selective Reminding Test in the ε4 carriers than non-carriers (Table 2).
Table 2.
Subject neuropsychological performance.
| APOE ε4 Carriers | APOE ε4 Non-Carriers | p-value | |
|---|---|---|---|
| General Cognition | |||
| DRS Total | 142.5 (1.6) | 141.7 (1.6) | 0.26 |
| Memory | |||
| SRT Total | 131.8 (6.1) | 125.0 (10.6) | 0.29 |
| SRT LTR | 128.7 (8.4) | 118.3 (14.2) | 0.06 |
| SRT CLTR | 134.8 (7.0) | 124.0 (23.4) | 0.40 |
| SRT 30min Delay | 11.1 (0.7) | 10.0 (1.5) | 0.10 |
| Attention/Executive Function | |||
| Trails A | 23.4 (6.4) | 21.3 (4.6) | 0.51 |
| Trails B | 45.9 (17.2) | 49.8 (14.3) | 0.47 |
| Stroop I | 80.5 (15.8) | 78.4 (14.4) | 0.71 |
| Stroop II | 176.6 (37.9) | 173.5 (44.2) | 0.93 |
| Language | |||
| Boston Naming | 58.2 (1.7) | 57.8 (1.9) | 0.59 |
| FAS | 49.4 (12.7) | 45.0 (12.2) | 0.51 |
| Visuospatial | |||
| ERDT | 17.9 (2.0) | 18.1 (1.5) | 0.98 |
Values are means (standard deviations). APOE = apolipoprotein E; DRS = Dementia Rating Scale; SRT = Selective Reminding Test; LTR = long-term recall; CLTR = consistent long-term recall; FAS = measure of verbal fluency; ERDT = Extended Range Drawing Test.
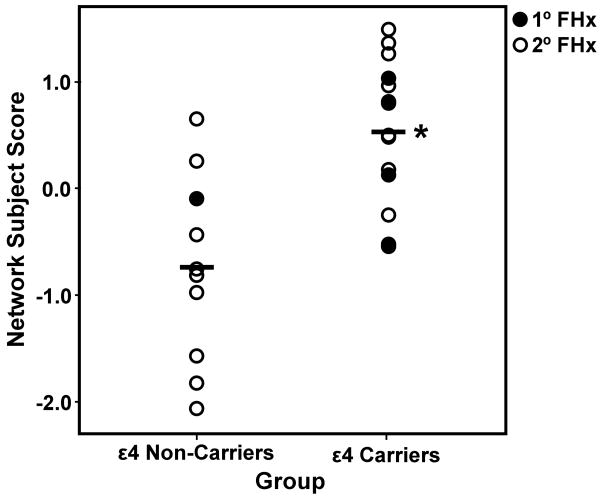
The group difference between the APOE ε4 carriers and non-carriers in the SSM analysis of the MRI VBM gray matter maps was investigated with a multiple regression model using the AICc method to select the best linear combination of components that differed between groups. This model included the first two components and accounted for 43.6% of the variance in distinguishing the APOE ε4 carrier from non-carrier groups (F(2,21) = 8.11, p ≤ 0.002) with the ε4 carriers showing higher expression of the network pattern than non-carriers (Figure 1). With eTIV added to the regression model, the APOE group effect remained significant (F(1,21) = 11.03, p ≤ 0.003) and eTIV was not a significant predictor (p = 0.97). To evaluate the potential effects of demographics on the APOE-related gray matter pattern, we subsequently tested the SSM pattern prediction by APOE group after including demographic covariates in the model. After entering age, gender, and years of education in separate multiple regression models combined with APOE group, APOE status remained a highly significant predictor of the APOE-related gray matter network pattern (F's(1,21) ≥ 11.71, p ≤ 0.003), with age (p = 0.27), gender (p = 0.56), and years of education (p = 0.25) not significantly contributing to the respective models.
Figure 1.
Gray matter network subject scores and apolipoprotein E (APOE) ε4 carrier status. Multiple regression of Scaled Subprofile Model (SSM) subject scores from the network analysis of magnetic resonance imaging (MRI) voxel-based morphometry in cognitively normal, young to middle-aged carriers (n= 14) and non-carriers (n=10) of the APOE ε4 allele. The scatterplot shows that the APOE ε4 carrier group has a higher expression of the MRI network pattern than does the ε4 non-carrier group (*R2 = 0.44, p ≤ 0.002, n = 24) and this difference remained significant after removing those subjects (filled circles) with a first-degree family history of dementia from the analysis (*R2 = 0.55, p ≤ 0.002, n = 19). The APOE ε4-related network subject scores were derived from the linear combination of the first two SSM component patterns. 1° FHx = subjects with a first-degree family history of dementia; 2° FHx = subjects with a second-degree family history of dementia. Bars = mean network subject score for the APOE groups.
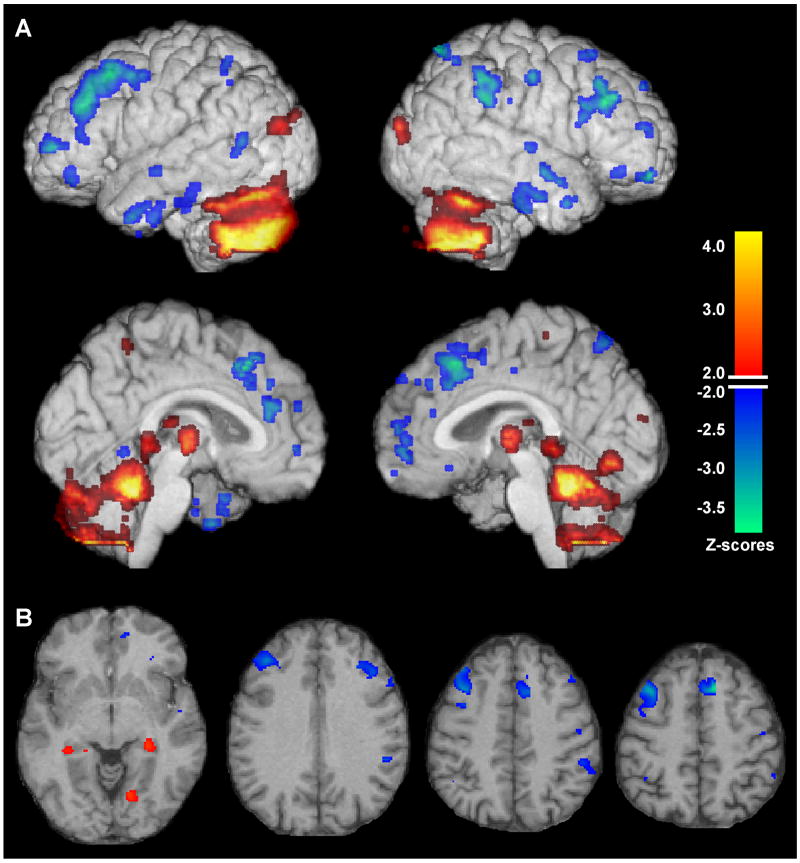
Bootstrap re-sampling of the linearly combined pattern of the first two SSM components was characterized by gray matter volume reductions indicated by voxels with maximal negative Z scores mainly occurring in the vicinities of bilateral inferior frontal, middle frontal, superior frontal, superior medial frontal, anterior cingulate, inferior and middle temporal, inferior parietal and precuneus, and right pre/post-central regions. Areas showing relative increases indicated by voxels with maximal positive Z scores were observed in vicinities of the middle occipital cortex, bilateral cerebellum, bilateral fusiform and right lingual gyri, bilateral thalamus, and small areas in bilateral hippocampal regions (Figure 2; Table 3).
Figure 2.
Regional gray matter network pattern related to apolipoprotein E (APOE) ε4 carrier status. Magnetic resonance imaging (MRI) gray matter pattern reflecting the linear combination of the first two Scaled Subprofile Model (SSM) components, whose mean subject scores differed between the APOE ε4 carriers and non-carriers in cognitively normal, young to early middle-aged adults. Voxels with Z scores ≥ +2.0 or ≤ -2.0 after bootstrap re-sampling with 500 iterations to provide robust regional pattern weights are superimposed on MRI projection maps (A) showing the right and left lateral and medial surfaces, as well as selected axial slices (B) spatially normalized with statistical parametric mapping (SPM8). The blue end of the color scale indicates brain regions showing lower gray matter volume in relation to APOE ε4 carrier status, whereas the orange end of the scale shows co-varying areas of relatively increased gray matter in relation to the presence of the ε4 allele. Regions showing greater gray matter volume reductions in the APOE ε4 carriers compared to non-carriers include areas in the vicinities of bilateral inferior frontal, middle frontal, superior frontal, superior medial frontal, anterior cingulate, inferior and middle temporal, inferior parietal, and right pre/post-central regions. Areas showing relative increases in the ε4 carriers compared to non-carriers are in the vicinities of the bilateral cerebellum, occipital cortex, thalamus, fusiform and right lingual gyri, and small bilateral areas in the vicinity of the hippocampus.
Table 3.
Minima and maxima values for network pattern of gray matter distinguishing APOE ε4 carriers and non-carriers.
| Talairach coord. | ||||||
|---|---|---|---|---|---|---|
| Region | H | BA | x | y | z | Z |
| Minima | ||||||
| Inf frontal | R | 47 | 29.7 | 38.4 | -8.7 | -2.56 |
| L | 46 | -35.6 | 36.0 | 20.3 | -2.90 | |
| Mid frontal | R | 9 | 31.7 | 34.4 | 27.8 | -2.66 |
| R | 8 | 45.5 | 29.2 | 39.1 | -2.29 | |
| L | 8 | -37.6 | 23.5 | 43.0 | -3.15 | |
| L | 11 | -27.7 | 44.1 | -12.3 | -2.25 | |
| Sup frontal | R | 10 | 23.8 | 55.0 | 12.0 | -2.20 |
| R | 8 | 23.8 | 22.1 | 54.2 | 2.03 | |
| L | 8 | -35.6 | 25.8 | 50.3 | -2.34 | |
| Sup med frontal | R | 8 | 9.9 | 23.7 | 46.7 | -3.13 |
| R | 10 | 4.0 | 52.3 | -2.6 | -2.10 | |
| L | 9 | -13.9 | 39.9 | 20.1 | -2.51 | |
| Anterior cingulate | R | 32 | 9.9 | 19.5 | 39.6 | -2.72 |
| R | 32 | 9.9 | 28.8 | 31.7 | -2.16 | |
| L | 32 | -9.9 | 37.7 | 16.5 | -2.17 | |
| Pre/Postcentral | R | 4 | 47.5 | -9.4 | 44.7 | -2.26 |
| Precuneus | R | 7 | 15.8 | -61.0 | 62.0 | -2.32 |
| Inf temporal | R | 20 | 49.5 | -18.3 | -15.9 | -2.33 |
| L | 20 | -37.6 | -5.4 | -30.0 | -2.48 | |
| Mid temporal | R | 21 | 51.5 | -2.4 | -10.0 | -2.21 |
| L | 39 | -45.5 | -53.9 | 10.1 | -2.56 | |
| L | 21 | -39.6 | 2.5 | -27.0 | -2.23 | |
| Inf parietal | R | 40 | 51.5 | -35.3 | 31.3 | -3.02 |
| L | 40 | -39.6 | -43.9 | 53.8 | -2.12 | |
| Maxima | ||||||
| Mid occipital | R | 18 | 31.7 | -86.5 | 19.1 | 2.39 |
| L | 19 | -37.6 | -74.7 | 22.2 | 2.67 | |
| Lingual | R | 18 | 13.9 | -68.2 | -3.3 | 2.67 |
| Fusiform | R | 37 | 41.6 | -53.3 | -17.5 | 3.04 |
| L | 37 | -39.6 | -51.4 | -17.6 | 2.11 | |
| Thalamus | R | 21.8 | -31.0 | 1.6 | 2.71 | |
| L | -15.8 | -32.8 | 5.3 | 2.81 | ||
| Hippocampus | R | 27.7 | -31.3 | -5.2 | 2.30 | |
| L | -31.7 | -33.3 | -5.1 | 2.09 | ||
| Cerebellum | R | 39.6 | -48.5 | -37.9 | 3.62 | |
| R | 17.8 | -49.3 | -14.4 | 3.29 | ||
| L | -39.6 | -52.4 | -37.8 | 4.23 | ||
| L | -23.8 | -51.4 | -17.6 | 3.85 | ||
APOE = apolipoprotein E; H = hemisphere; BA = Brodmann area; x, y, z = anatomical atlas coordinates from Talairach and Tournoux (58) in the x, y, and z planes; Z = z score; Inf = inferior; Mid = middle; Sup = superior; med = medial.
Since two subjects in the APOE ε4 carrier group were ε4 homozygotes (each with a first-degree family history of dementia), we performed a regression analysis to evaluate whether the group difference in SSM pattern expression could be related to the inclusion of these two participants with two copies of the ε4 allele. Analysis of the group difference for only the ε4 heterozygotes (n = 12) and ε4 non-carriers (n = 10), showed that the group effect was unchanged, accounting for 47.5% of the variance (F(2,19) = 8.59, p ≤ 0.002). The overall APOE group difference in the regional pattern of gray matter was also present after removing subjects 40 years of age and older, with the ε4 carriers (n = 8) showing a higher pattern expression than non-carriers (n = 7; F(2,12) = 8.95; p ≤ 0.004).
To test whether the observed difference between APOE groups in the gray matter pattern expression could be explained by the relatively higher occurrence of first-degree relatives with dementia in the ε4 carrier group, we subsequently performed a regression analysis evaluating the ability of the linear combination of the first two component patterns to distinguish the groups, including only subjects reporting a second-degree family history of dementia in the ε4 carriers (n = 9) and non-carriers (n = 9). The difference between these two APOE subgroups in SSM subject scores remained significant, accounting for 56.5% of the variance (F(2,15) = 9.75, p ≤ 0.002) with ε4 carriers showing a higher expression of the combined SSM pattern (Figure 1).
4. Discussion
Our study investigating the effects of APOE genotype in cognitively normal young to early middle-aged adults identified a regional network pattern of MRI gray matter reductions that showed greater expression in ε4 carriers than non-carriers. This APOE-related pattern was characterized by reductions in dorsolateral and medial prefrontal, lateral temporal, and parietal cortices. The brain regions showing gray matter differences in the young to early middle-aged ε4 carriers are among those previously found in studies of healthy aging and late onset AD (Alexander et al., 2006; Whitwell et al., 2007; Fjell et al., 2009; Hua et al., 2009; Bergfield et al., 2010). Our findings demonstrate brain morphological differences occurring in young to early middle age adulthood in relation to APOE genotype, with structural effects observed decades prior to the potential onset of the dementia of late onset AD or the effects of cognitive aging.
Functional neuroimaging studies with PET have shown cerebral hypometabolism in relation to APOE genotype in cognitively normal late middle-aged adults, with ε4 carriers showing reductions in brain regions affected early in late onset AD (Reiman et al., 1996; 2005), including parietotemporal, posterior cingulate, and to a lesser extent frontal brain regions. These PET findings were further demonstrated in cognitively normal young adults, ages 20 to 39 (Reiman et al., 2004). Some studies of brain activity in similarly young adults with fMRI performed during cognitive tasks and in a “default mode” resting state have suggested alterations in the efficiency of functional networks in APOE ε4 carriers compared to non-carriers (Filippini et al., 2009; Dennis et al., 2009). Differences in activation in these fMRI studies, with increased activity in medial temporal lobes, as well as increased functional connectivity, suggests that some regions supporting frontal and temporal mediated cognitive functions may be preferentially affected in early adulthood in ε4 carriers.
Previous studies investigating structural brain differences in relation to APOE genotype have typically relied on univariate analyses for structures, such as the hippocampus (Reiman et al., 1998; Mueller et al., 2008), or throughout the brain using voxel-based methods such as VBM (Filippini et al., 2009; Dennis et al., 2009). We used a multivariate network analysis method with VBM and bootstrap re-sampling that is sensitive for identifying robust regionally-distributed patterns of gray matter throughout the brain without the need for conservative correction for multiple comparisons often required with univariate analyses (Habeck et al., 2005; Bergfield et al., 2010). Further, we performed image post-processing with SPM8 VBM and DARTEL, a method that has been suggested to provide greater accuracy for MRI registration (Klein et al., 2009) than the voxel-based methods used in previous APOE group studies in young healthy adults. The use of multivariate analysis methods, like SSM, combined with improved MRI registration algorithms may afford greater sensitivity in detecting regionally distributed patterns of gray matter differences in those carrying an increased risk for brain aging and dementia in later life.
A limitation of this study was the small sample of young and early middle-age, APOE ε4 carriers and non-carriers in our cohort who reported family histories of dementia and had MRI scans available for analysis. Despite this limitation, we observed highly significant differences using our multivariate network analysis with bootstrap re-sampling methods. The robustness of our findings was further supported by the APOE group effect remaining significant after we controlled for age, gender, educational level, and eTIV. The APOE group difference in the regional pattern of gray matter was also present after removing two ε4 homozygotes from the analysis, and after removing subjects 40 years of age and older. Further research is needed with larger cohorts of young and older cognitively normal adults to evaluate the consistency and individual differences in expression of this APOE-related network gray matter pattern across multiple samples and age groups.
There is strong support for an association between APOE ε4 and increased deposition of Alzheimer's disease pathology, assessed by PET and cerebrospinal fluid markers in late middle age and older adults (Reiman et al., 2009; Morris et al., 2010). In a study using the PET C11PIB amyloid tracer, however, no cortical binding was observed in those 45 to 49 years of age with or without the ε4 allele (Morris et al., 2010). In addition, a recent study of neuropathology for subjects with ages ranging from 0 to 97 years found no differences between APOE groups for senile plaques from 0 to 49 years, but increases were observed for ε4 carriers compared to non-carriers in those ages 50 and older (Kok et al., 2009).
Together, these results suggest that the morphological brain changes observed in our sample likely pre-date the potential for significant residual deposition of amyloid associated with the ε4 allele. In combination with previous neuroimaging findings, our results suggest a time course for the impact of APOE ε4 on brain aging that includes young adult to early middle age differences in regional morphology, metabolism, and network connectivity followed by increased accumulation of amyloid with aging that begins to show appreciable effects in the brain during the sixth decade and beyond. It is now well established that patients with late onset AD have high levels of fibrillar amyloid compared to healthy controls, but 20-50% of healthy non-demented elderly show positive PIB binding consistent with amyloid deposition that may impact age-related brain function and connectivity (Sperling et al., 2009).
APOE ε4 may exert effects on brain structure in early adulthood, promoting regional vulnerability in brain networks as we age and affecting the clinical expression of late onset AD dementia or the cognitive effects of healthy aging in later life. It is possible that altered efficiency in functional networks may develop early as a form of neural compensation to maintain high levels of cognitive performance in the context of reduced regional gray matter. The ability of the brain to compensate may represent an important component of a “cognitive reserve” that can help to delay or diminish the cognitive expression of developing neurodegenerative disease or the effects of cognitive aging (Stern et al., 1995, 2005; Alexander et al., 1997; Grady, 2008; Stern, 2009). With reduced gray matter related to APOE ε4, compensatory connectivity in the development of functional networks supporting frontal and temporal mediated cognitive functions may occur by early adulthood, but at a potential cost that may ultimately lead to greater vulnerability to the effects of cognitive aging and age-related deposition of late onset AD pathology.
It is also possible that the observed APOE group differences in structural neuroimaging in young and middle-age adults represent one of multiple distinct effects of APOE genotype on the brain over the lifespan, some having direct effects early in life on the organization of brain anatomy, function, and connectivity, while others having potentially independent effects on the accumulation of late onset AD pathology in later life. Multiple mechanisms underlying APOE ε4-related risk for brain aging and dementia have been suggested, including effects on amyloid metabolism and accumulation, effects on lipid metabolism and synaptic plasticity, regulatory effects on brain immunoreactivity, and direct neurotoxic effects (Keene et al., 2011; Verghese et al., 2011). Such multiple APOE effects may interact with other genetic, environmental, or disease risk factors that together contribute to individual differences in neural and functional compensation reflected in neuroimaging studies and the trajectory toward cognitive aging or the development of dementia.
Further research is needed to evaluate the time course and underlying mechanisms of structural and functional brain effects of the APOE ε4 allele over the full age spectrum and to help determine why some individuals go on to develop dementia or age-related cognitive decline and others demonstrate “successful cognitive aging” without overt declines in cognitive abilities.
Recent studies have reported that having a first-degree family history of dementia may affect brain function, suggesting that additional, as yet unidentified, genetic factors may interact with APOE genotype to influence functional brain changes associated with an increased risk for dementia (Johnson et al., 2006). In our study, greater expression of the APOE-related gray matter pattern in the ε4 carriers than non-carriers was observed with and without those with a first-degree family history of dementia. We cannot, however, exclude the possibility that our young adults with only a current second-degree family history of dementia will subsequently develop a first-degree familial risk.
In the APOE-related gray matter network pattern we observed areas of co-varying relative increases of gray matter in the ε4 carriers compared to ε4 non-carriers, including in bilateral cerebellar, occipital, bilateral thalamus, bilateral fusiform and right lingual gyri regions. The implications of these areas of relative increases in gray matter are not clear, but may be part of a regionally distributed network of brain regions that help to support and maintain normal cognition in the context of reduced gray matter in frontal, lateral temporal, and parietal brain regions among younger adult ε4 carriers.
We also observed small areas of relative gray matter increases in bilateral hippocampal regions. Lower fMRI cognitive activation in the medial temporal regions has been observed in some studies of older adult APOE ε4 carriers compared to ε4 non-carriers (Trivedi et al., 2006), but greater activation has been reported in the medial temporal lobes in both young and old adult ε4 carriers than non-carriers (Filippini et al., 2009; Dennis et al., 2009; Bookheimer et al., 2000). Areas of greater cortical thickness have also been reported in older adult APOE ε4 carriers than non-carriers including in frontal, occipitotemporal, and parahippocampal regions (Espeseth et al., 2008). The relative increases with our network analysis of gray matter suggest that those APOE ε4 carriers with greater regionally-distributed reductions in young to early middle age adulthood were also those with the greater relative co-varying increases in these regions of the medial temporal lobes known to be vulnerable to developing late onset AD pathology in later life.
With confirmation in larger samples, such relative increases in hippocampal gray matter in the context of reductions in prefrontal, lateral temporal, and parietal cortices could help explain preferential vulnerability to cognitive aging and dementia in some ε4 carriers. In this case, it is possible that ε4 carriers rely on an intact hippocampus to a greater degree in performing the normal functions of the brain network engaged in frontal and temporal lobe mediated cognitive abilities. Some indirect support for this possibility is indicated by fMRI studies reporting both greater hippocampal activation in young adult ε4 carriers than non-carriers while performing memory encoding tasks, as well as increased resting state connectivity between hippocampal and other brain regions (Filippini et al., 2009; Dennis et al., 2009). When late onset AD pathology begins to develop in key medial temporal brain regions to a greater extent in ε4 carriers in later life, the longstanding APOE ε4-associated gray matter reductions in other brain areas may produce a neural memory system with a diminished capacity for functional compensation and greater vulnerability to the developing effects of cognitive aging and dementia. Thus, it is possible that for ε4 carriers the inherent compensatory ability of this network is altered by early adulthood and becomes more reliant on the hippocampus to maintain normal cognitive performance, which in turn makes the network more vulnerable when this brain structure becomes a target for AD pathology in later life.
In summary, our findings suggest that genetic risk for late onset AD with APOE ε4 is associated with a regional pattern of gray matter reductions involving prefrontal, parietal, and selective temporal brain regions that occurred decades prior to the potential onset of dementia or age-related cognitive decline in our sample. The use of multivariate network analyses with SSM MRI VBM can help identify regionally distributed patterns of brain morphology associated with measures of genetic risk to help elucidate mechanisms underlying individual differences associated with healthy and pathological aging.
Acknowledgments
We thank Tricia Merkeley and Melaney Aschenbrenner for their technical assistance and help with data collection. This work was supported by the Alzheimer's Association (IIRG-02-3784), the National Institute of Mental Health (MH57899), the National Institute on Aging (AG025526, AG19610, and AG031581), the state of Arizona and Arizona Department of Health Services, Arizona Advanced Research Institute for Biomedical Imaging, and the Evelyn F. McKnight Brain Institute.
Footnotes
Disclosure Statement: The authors have no conflicts of interest relevant to the subject of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, Chen K, Merkley TL, Reiman EM, Caselli RJ, Aschenbrenner M, Lewis DJ, Pietrini P, Teipel SJ, Hampel H, Rapoport SI, Moeller JR. Regional network of magnetic resonance imaging gray matter volume in healthy aging. NeuroReport. 2006;17:951–56. doi: 10.1097/01.wnr.0000220135.16844.b6. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer's disease treatment studies. Am J Psychiatry. 2002;159:738–55. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Chen K, Aschenbrenner M, Merkley TL, Santerre-Lemmon LE, Shamy JL, Skaggs WE, Buonocore MH, Rapp PR, Barnes CA. Age-related regional network of magnetic resonance imaging gray matter in the rhesus macaque. J Neurosci. 2008;28:2710–18. doi: 10.1523/JNEUROSCI.1852-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, Schapiro MB. Association of premorbid intellectual function with cerebral metabolism in Alzheimer's disease: implications for the cognitive reserve hypothesis. Am J Psychiatry. 1997;154:165–172. doi: 10.1176/ajp.154.2.165. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Mentis MJ, Van Horn JD, Grady CL, Berman KF, Furey ML, Pietrini P, Schapiro MB, Rapoport SI, Moeller JR. Individual differences in PET activation of object perception and attention systems predict face matching accuracy. NeuroReport. 1999;10:1965–71. doi: 10.1097/00001756-199906230-00032. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Moeller JR. Application of the Scaled Subprofile Model to functional imaging in neuropsychiatric disorders: a principal component approach to modeling regional patterns of brain function in disease. Hum Brain Mapp. 1994;2:79–94. [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Computing average shaped tissue probability templates. NeuroImage. 2009;45:333–41. doi: 10.1016/j.neuroimage.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, de S. Multilingual Aphasia Examination. University of Iowa; Iowa City, IA: 1976. [Google Scholar]
- Bergfield KL, Hanson KD, Chen K, Teipel SJ, Hampel H, Rapoport SI, Moeller JR, Alexander GE. Age-related networks of regional covariance in MRI gray matter: reproducible multivariate patterns in healthy aging. NeuroImage. 2010;49:1750–59. doi: 10.1016/j.neuroimage.2009.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Habeck C, Ramos MA, Scarmeas N, Stern Y. A forward application of age associated gray and white matter networks. Hum Brain Mapp. 2008;29:1139–46. doi: 10.1002/hbm.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Habeck C, Zarahn E, Flynn J, Stern Y. Structural MRI covariance patterns associated with normal aging and neuropsychological functioning. Neurobiol Aging. 2007;28:284–95. doi: 10.1016/j.neurobiolaging.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, Bookheimer SY. NeuroImage. 2008;41:1177–83. doi: 10.1016/j.neuroimage.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson D. Model Selection and Multimodel Inference. Springer Verlag; New York, NY: 2002. [Google Scholar]
- Buschke H. Selective reminding for analysis of memory and learning. J Verbal Learning and Verbal Behavior. 1973;12:543–50. [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, Locke DE, Snyder CH, Alexander GE, Rademakers R, Reiman EM. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361:255–63. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Hentz JG, Osborne D, Graff-Radford NR, Barbieri CJ, Alexander GE, Hall GR, Reiman EM, Hardy J, Saunders AM. Apolipoprotein E and intellectual achievement. J Am Geriatr Soc. 2002;50:49–54. doi: 10.1046/j.1532-5415.2002.50007.x. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GE. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE ε4 allele. Neurology. 2004;62:1990–95. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Ferris SH, George AE, Reisberg B, Christman DR, Kricheff II, Wolf AP. Computed tomography and positron emission transaxial evaluations of normal aging and Alzheimer's disease. J Cereb Blood Flow Metab. 1983;3:391–94. doi: 10.1038/jcbfm.1983.57. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, Breteler MMB. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–48. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh-Bohmer KA, Cabeza R. Temporal lobe functional activity and connectivity in young adult APOE ε4 carriers. Alzheimer's & Dementia. 2010;6:303–11. doi: 10.1016/j.jalz.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodrill CB. A neuropsychological battery for epilepsy. Epilepsia. 1978;19:611–623. doi: 10.1111/j.1528-1157.1978.tb05041.x. [DOI] [PubMed] [Google Scholar]
- Duara R, Grady CL, Haxby JV, Sundaram M, Cutler NR, Heston L, Moore AM, Schlageter NL, Larson S, Rapoport SI. Positron emission tomography in Alzheimer's disease. Neurology. 1986;36:879–87. doi: 10.1212/wnl.36.7.879. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. CRC Press; New York, NY: 1994. [Google Scholar]
- Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Chaly T, Belakhlef A, Mandel F, Przedborski S, Fahn S. Early differential diagnosis of Parkinson's disease with 18F-fluorodeoxyglucose and positron emission tomography. Neurology. 1995;45:1995–2004. doi: 10.1212/wnl.45.11.1995. [DOI] [PubMed] [Google Scholar]
- Espeseth T, Westlye LT, Fjell AM, Walhovd KB, Rootwelt H, Reinvang I. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E ε4. Neurobiol Aging. 2008;29:329–40. doi: 10.1016/j.neurobiolaging.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Filippini N, Macintosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc Natl Acad Sci USA. 2009;106:7209–14. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19:2001–12. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frackowiak RSJ, Pozzill IC, Legg NJ, DuBoulay GH, Marshall J, Lenzi GL, Jones T. Regional cerebral oxygen supply and utilization in dementia: a clinical and physiological study with oxygen-15 and positron emission tomography. Brain. 1981;104:753–78. doi: 10.1093/brain/104.4.753. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnstrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Ann NY Acad Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Habeck C, Krakauer JW, Ghez C, Sackeim HA, Eidelberg D, Stern Y, Moeller JR. A new approach to spatial covariance modeling of functional brain imaging data: ordinal trend analysis. Neural Computation. 2005;17:1602–45. doi: 10.1162/0899766053723023. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Duara R, Grady CL, Cutler NR, Rapoport SI. Relations between neuropsychological and cerebral metabolic asymmetries in early Alzheimer's disease. J Cereb Blood Flow Metab. 1985;5:193–200. doi: 10.1038/jcbfm.1985.25. [DOI] [PubMed] [Google Scholar]
- Hua X, Lee S, Yanovsky I, Leow AD, Chou YY, Ho AJ, Gutman B, Toga AW, Jack CR, Jr, Bernstein MA, Reiman EM, Harvey DJ, Kornak J, Schuff N, Alexander GE, Weiner MW, Thompson PM Alzheimer's Disease Neuroimaging Initiative. Optimizing power to track brain degeneration in Alzheimer's disease and mild cognitive impairment with tensor-based morphometry: an ADNI study of 515 subjects. NeuroImage. 2009;48:668–81. doi: 10.1016/j.neuroimage.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, O'Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–88. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, Asthana S, Hermann BP, Sager MA. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J Neurosci. 2006;26:6069–76. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test, Experimental Version. Boston Veterans Administration Center; Boston, MA: 1983. [Google Scholar]
- Keene CD, Cudaback E, Li X, Montine KS, Montine TJ. Apolipoprotein E isoforms and regulation of the innate immune response in brain of patients with Alzheimer's disease. Curr Opin Neurobiol. 2011;21:1–9. doi: 10.1016/j.conb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok E, Haikonen S, Luoto T, Hubtala H, Goebeler S, Haapasalo H, Karhunen PJ. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesion begins in middle age. Ann Neurol. 2009;65:650–57. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellack L, Karasu TB, editors. Geriatric Psychiatry. Grune & Stratton; New York, NY: 1976. pp. 77–121. [Google Scholar]
- Moeller JR, Strother SC, Sidtis JJ, Rottenberg DA. Scaled subprofile model: a statistical approach to the analysis of functional patterns in positron emission tomographic data. J Cereb Blood Flow Metab. 1987;7:649–58. doi: 10.1038/jcbfm.1987.118. [DOI] [PubMed] [Google Scholar]
- Mondadori CRA, de Quervain DJF, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, Boesiger P, Hock C, Nitsch RM, Papassotiropoulos A, Henke K. Better memory and neural efficiency in young apolipoprotein E ε4 carriers. Cereb Cortex. 2007;17:1934–47. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau pathology in cognitively normal aging. Ann Neurol. 2010;67:122–31. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Raptentsetsang S, Elman J, Weiner MW. Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer's disease using high resolution MRI at 4T. NeuroImage. 2008;42:42–8. doi: 10.1016/j.neuroimage.2008.04.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Welsh-Bohmer KA, Bigler ED, Johnson SC, Anderson CV, Helms MJ, Saunders AM, Breitner JC. Apolipoprotein E epsilon 4 allele and hippocampal volume in twins with normal cognition. Neurology. 1997;48:985–89. doi: 10.1212/wnl.48.4.985. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein. E N Engl J Med. 1996;334:752–58. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Dandy D, Osborne D, Saunders AM, Hardy J. Correlations between apolipoprotein E ε4 gene dose and lower brain-imaging measurements of regional glucose metabolism. Proc Natl Acad Sci USA. 2005;102:8299–302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci USA. 2004;101:284–89. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JBS, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ. Fibrillar amyloid-β burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci USA. 2009;106:6820–25. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Uecker A, Caselli RJ, Lewis S, Bandy D, de Leon MJ, De Santi S, Convit A, Osborne D, Weaver A, Thibodeau SN. Hippocampal volume in cognitively normal persons at genetic risk for Alzheimer's disease. Ann Neurol. 1998;44:288–91. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Motor Skills. 1958;8:271–76. [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–72. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Schmidt R, Fazekas F, Semmler J, Kapeller P, Reinhart B, Kostner GM. ApoE 4 allele in the normal elderly: neuropsychologic and brain MRI correlates. Clin Genet. 1996;50:293–99. doi: 10.1111/j.1399-0004.1996.tb02377.x. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- Smith JF, Chen K, Johnson SC, Morrone-Strupinsky J, Reiman EM, Nelson A, Moeller JR, Alexander GE. Network analysis of single-subject fMRI during a finger opposition task. NeuroImage. 2006;32:325–32. doi: 10.1016/j.neuroimage.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamak IM, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–88. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Stricks L, Link B, Lennon MC, Mayeux R. Relationship between lifetime occupation and parietal flow: implications for a reserve against Alzheimer's disease pathology. Neurology. 1995;45:55–60. doi: 10.1212/wnl.45.1.55. [DOI] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, Flynn J, Sackeim H, van Heertum R. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex. 2005;15:394–402. doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System - an Approach to Cerebral Imaging. Thieme Medical Publishers; New York, NY: 1988. [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Torgerson BM, Sager MA, Hermann BP, Asthana S, Johnson SC. Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer's disease: a cross-sectional study. BMC Med. 2006;4:1. doi: 10.1186/1741-7015-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–52. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer's disease. Brain. 2007;130:1777–86. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, McAllister TW, Rabin LA, McDonald BC, Flashman LA, Roth RM, Mamourian AC, Tsongalis GJ, Rhodes CH. Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology. 2006;67:1221–24. doi: 10.1212/01.wnl.0000238079.00472.3a. [DOI] [PubMed] [Google Scholar]