Abstract
Objective
Assess the relative effects of a variety of illicit and licit drugs on risk for adverse birth outcomes.
Methods
We used data from two large prospective investigations, and a novel analytic method, recursive partitioning class analysis to identify risk factors associated with preterm birth and delivering a small for gestational age infant.
Results
Compared to cocaine and opiate non-users, cocaine users were 3.53 times as likely (95% Cl: 1.65–7.56; p=0.001) and opiate users 2.86 times as likely (95% Cl: 1.11–7.36; p=0.03) to deliver preterm. The odds of delivering a small for gestational age infant for women who smoked more than two cigarettes daily was 3.74, (95% Cl: 2.47–5.65; p<0.0001) compared to women who smoked two or less cigarettes daily and had one previous child. Similarly, less educated, nulliparous women who smoked two or fewer cigarettes daily were 4.12 times as likely (95% Cl: 2.04–8.34; p<0.0001) to have a small for gestational age infant.
Conclusions
Among our covariates, prenatal cocaine and opiate use are the predominant risk factors for preterm birth; while tobacco use was the primary risk factor predicting small for gestational age at delivery. Multi-substance use did not substantially increase risk of adverse birth outcomes over these risk factors.
Keywords: Preterm birth, PTB, Small for Gestational Age, SGA, Cocaine, Opiate, Cigarette, Poly-substance use
Introduction
Children born to women who use hazardous substances in pregnancy are at increased risk of a variety of adverse birth outcomes. Tobacco exposure is strongly associated with reduced fetal growth [1–3]. Alcohol is a known teratogen linked to fetal alcohol syndrome [4] and low birth weight [5].
Data on illicit drug use are less consistent. Some [6,7] but not all [8,9] studies of marijuana use in pregnancy, report associations with low birth weight and shorter gestations. Cocaine [10,11] and opiate [12,13] use has been repeatedly linked with low birth weight, intrauterine growth restriction, and preterm birth, but not all studies agree [1,8]. Incremental risks resulting from multi-substance use have received little attention with varied findings; limiting the work is the fact that most results are derived from retrospective cohorts [12,14].
Of note, women who use hazardous substances in pregnancy frequently have other risk factors for poor birth outcomes, although these variables are infrequently included in studies of drug exposure. In particular, there has been a concern that the effects attributed to cocaine are instead due to poor health habits and use of nicotine cigarettes [15]. Zhang and Bracken analyzed a full spectrum of risk factors to determine the leading cause of small for gestational age and preterm birth, but data on substances only included cigarettes, alcohol and marijuana [16].
We hypothesized that in utero exposure to hazardous substances would constitute primary risks for many of the adverse birth outcomes in infants of substance using mothers. To test this we used data from two comprehensive, prospective cohort studies and recursive partitioning analysis to characterize the effects of in utero hazardous substance exposure on preterm birth (PTB) and small for gestational age (SGA) infant, in a pregnant population that included multi-substance users.
Methods
We used data from two comprehensive, multicenter, prospective studies, the Psychosocial Research to Improve Drug Treatment in Pregnancy study (PRIDE-P) and the Pink and Blue study, both of which are described elsewhere in detail [17,18]. Both studies took place at the same time and although recruitment for the Pink and Blue study was more extensive, all subjects screened for the PRIDE-P study were simultaneously considered for Pink and Blue. The PRIDE-P study is composed primarily of hazardous substance users and the Pink and Blue study had a substantial number of participants who used tobacco only or used no hazardous substances. Data collection instruments in both studies were similar. Human Subjects Boards approved and monitored safety of study procedures for all subjects.
Psychosocial Research to Improve Drug Treatment in Pregnancy (PRIDE-P) Study
Women were eligible to participate in the integrated obstetrical/substance abuse treatment program if they were at least 16 years of age, fluent in English or Spanish, were less than 29 completed weeks gestation at the time of screening, and were planning on delivering at either Yale-New Haven Hospital in New Haven, Connecticut or Bridgeport Hospital in Bridgeport, Connecticut. They must have self-reported use of alcohol or an illicit drug, other than opiates, within the 28 days prior to being screened for the study, or scored at least a 3 on the modified TWEAK. Women were ineligible if they were already engaged in substance use treatment, were using nicotine or an opiate as their only substance, had plans to relocate, were not competent to or were unwilling to provide consent, were an imminent danger to themselves or their fetus or required inpatient treatment.
Subjects were recruited from three local prenatal clinics affiliated with the Yale School of Medicine. Women who consented to be screened completed a health questionnaire that included demographic information, the Patient Health Questionnaire-2 (PHQ-2), the 4 P’s, the modified TWEAK, and a substance abuse calendar that documented use of cigarettes, alcohol, marijuana, cocaine, opiates and “other” within the previous 28 days. The TWEAK was designed to screen pregnant women for hazardous alcohol use and it was modified in this study to assess use of a range of hazardous substances in pregnancy [19]. For women who were potentially eligible, research staff explained the study, risks and benefits and obtained written, informed consent before enrollment.
Enrollees completed a comprehensive, computerized intake assessment that included past and present pregnancy events and complications, the Addictions Severity Index, the Inventory of Depressive Symptomatology, the MINI Neuropsychiatric Interview, and the modified TWEAK. Research staff assessed subjects during each of their subsequent prenatal visits after intake assessment. There was no limit on the number of encounters, but subjects were encouraged to attend at least 6 sessions. At each encounter a substance use calendar was completed. In addition urine toxicology and alcohol breath tests were obtained.
We screened 2684 women for study eligibility, of which 283 (10%) were eligible and 188 enrolled in the study. Of enrolled women, 1 miscarried, 5 had twins and 4 women were lost to follow up for a final sample of N = 178 participants with a singleton live birth.
Pink and Blue study
Women were eligible for the Pink and Blue study if they were at least 18 years of age, fluent in English or Spanish, were less than 17 weeks gestation at the time of screening and intended to deliver at a participating hospital. Requirements also included a positive screen for major depressive disorder (MDD), posttraumatic stress disorder (PTSD), or treatment for MDD within the prior five years. We randomly selected one of every three women who had neither a diagnosis of, nor treatment for MDD or PTSD in the last five years to participate as controls for comparison. Women were ineligible if they had a multi-fetal pregnancy, intended to terminate pregnancy, required insulin for diabetes, had plans to relocate, or were unwilling to provide consent.
Women were recruited from a total of 137 clinicians’ offices or hospital-based clinics in Connecticut and Western Massachusetts. Research staff approached pregnant women receiving care at the participating clinics as they waited for their prenatal visit to invite participation. Women who were willing were subsequently screened over the phone after informed consent was obtained.
Women who remained eligible completed a face-to-face interview prior to 17 weeks gestation, and a phone interview at 28 (±2) weeks gestation and at 8 (±4) weeks postpartum. During the interviews, subjects were administered the depression and anxiety modules of the World Mental Health Composite International Diagnostic Interview (WMH-CIDI), adjusted to assess illness in 28-day intervals across pregnancy. Information was collected on antidepressant and pharmacotherapy prior to pregnancy and monthly during pregnancy in addition to data about alcohol, drug use, race, ethnicity, socioeconomic status and medical complications.
We screened 9525 women, including 1905 (20%) who were eligible and had a history of MDD in the last five years, or were taking antidepressant medication. Another 4533 (48%) were eligible and did not have any of the stipulated exposures, of which 1612 (36%) were randomly selected to participate in the comparison group. Of the 3517 potentially eligible women, 2,793 were interviewed. Of enrolled women, 12 women terminated pregnancy, 75 miscarried, 7 had a stillborn infant, 8 had twins and 37 were lost to follow up. The 24 women who participated in both PRIDE-P and Pink and Blue were excluded from the Pink and Blue cohort, for a final sample of N = 2630 women with a singleton gestation.
Exposure and Outcome Assessment
Trained staff members who were blind to data collected in the corresponding studies reviewed hospital and outpatient medical records for maternal and neonatal birth outcomes. Information regarding prenatal care, labor and delivery, mental health, substance abuse and newborn infant data was collected.
Preterm birth (PTB) was defined as delivery of an infant before 37 completed weeks of gestation. Small for gestational age (SGA) was defined as birth weight less than 10th percentile for gestation accounting for race and gender. Gravidity was defined as the total number of times a women was pregnant and parity as the number of live births.
Statistical Analyses
Recursive partitioning class analysis is a technique whereby the sample is split into subgroups that maximally differ based on the specified outcome [16]. This nonparametric method finds the predictor, or for continuous variables the cut point(s) on that predictor, that maximizes differences between groups and uses it to split the sample into two to three subgroups called nodes. More concretely, the first split of the sample is based on the strongest predictor of the outcome. This process is repeated for each resulting node until the split results in subgroups that are either too small to further split, or do not differ significantly based on the outcome. We used a minimum node size of 25 or 1% of the sample [20]. The result is a set of terminal nodes or “tree”, effectively a division of the sample into relevant subgroups.
We constructed trees for two outcomes, PTB and SGA. We used the same potential risk factors for each outcome with the exception that the algorithm we used to calculate SGA included race whereas the definition for PTB did not. These factors included: marital status, race/ethnicity, age, education, gravidity, parity, body mass index (BMI), smoking in pregnancy, passive smoking, alcohol use, alcohol abuse, marijuana, cocaine, narcotics, and other drug use, number of daily cigarettes, number of drugs used in pregnancy, and an additional indicator for PRIDE-P/Pink and Blue cohort.
We conducted logistic regression analyses to obtain unadjusted and adjusted odds ratio estimates, associated 95% confidence intervals (CIs) and p values for the resulting subgroups. For each outcome, we chose the subgroup with the greatest number of study participants to be the reference group. Adjusted analysis for PTB and SGA accounted for the same potential confounding variables determined a priori. However, to avoid over controlling, factors were removed from the analysis if they were already used to determine risk subgroup. For PTB, we included marital status, education, gravidity and BMI while for SGA we included age, marital status, race/ethnicity and BMI. History of PTB was not included in the analysis because it was unknown whether prior PTBs were the result of substance use; if so controlling for prior PTB would result in over adjusting for substance use. We were unable to analyze other causes of PTB such as sexually transmitted disease because data quality between cohorts was not comparable.
Fisher’s exact test for categorical variables and the Wilcoxon rank sum test for continuous variables were used to compare demographic variables across cohorts. SPSS AnswerTree 3.1 was used for the recursive partitioning class analysis and SAS 9.1.3 was used for other analyses.
Results
Patient characteristics, birth outcomes and exposures for each analytic cohort are shown in Table I. Women participating in the PRIDE-P study had worse birth outcomes (15% PTB and 15% SGA) compared to women participating in the Pink and Blue study (8% PTB, 8% SGA). On average, women in PRIDE-P were younger, less educated, and more overweight. Women in PRIDE-P were also more likely to be smokers (69%), alcohol abusers (22%), and marijuana (51%), cocaine (16%), opiate (10%) and other drug users (11%), as compared to participants in the Pink and Blue study. Among PRIDE-P participants 12% did not use drugs in pregnancy, while 60% used multiple drugs, including nicotine cigarettes and alcohol. In comparison, 76% of Pink and Blue participants did not use drugs, and 6% were poly-substance users.
Table I.
Characteristics, outcomes and exposures of the combined and individual cohorts.
| Combined Cohorts (N = 2808) | PRIDE-P (N = 178) | Pink and Blue (N = 2630) | ||
|---|---|---|---|---|
| Characteristic/Outcome/Exposure | N (%) | N (%) | N (%) | P value |
| Preterm birth | 249 (9) | 27 (15) | 222 (8) | 0.004 |
| Small for Gestational Age (SGA) | 242 (9) | 25 (15) | 217 (8) | 0.0063 |
| Marital status | <0.0001 | |||
| Married/cohabiting | 2363 (85) | 63 (35) | 2300 (87) | |
| Divorced/separated/widowed | 74 (3) | 10 (6) | 64 (2) | |
| Never married | 371 (13) | 105 (59) | 266 (10) | |
| Race/ethnicity | <0.0001 | |||
| White | 1990 (71) | 41 (23) | 1949 (74) | |
| Black | 280 (10) | 94 (53) | 186 (7) | |
| Hispanic | 415 (15) | 39 (22) | 376 (14) | |
| Other | 122 (4) | 3 (2) | 119 (5) | |
| Smoked in pregnancy | 493 (18) | 123 (69) | 370 (14) | <0.0001 |
| Passive smoking | 608 (22) | 117 (66) | 491 (19) | <0.0001 |
| Alcohol use in pregnancy | 1336 (48) | 96 (54) | 1240 (47) | 0.09 |
| Alcohol abuse in pregnancy | 334 (12) | 40 (22) | 294 (11) | <0.0001 |
| Marijuana use in pregnancy | 197 (7) | 90 (51) | 107 (4) | <0.0001 |
| Cocaine use in pregnancy | 38 (1) | 29 (16) | 9 (<1) | <0.0001 |
| Opiates use in pregnancy | 40 (1) | 18 (10) | 22 (1) | <0.0001 |
| Other drug use in pregnancy | 24 (1) | 19 (11) | 5 (<1) | <0.0001 |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 30.62 (5.91) | 25.03 (5.86) | 30.99 (5.72) | <0.0001 |
| Education (years) | 15.12 (2.85) | 11.86 (1.67) | 15.34 (2.78) | <0.0001 |
| Gravidity* | 2.58 (1.62) | 3.19 (2.66) | 2.54 (1.52) | 0.01 |
| Parity** | 0.87 (0.98) | 1.14 (1.40) | 0.85 (0.94) | 0.07 |
| BMI | 26.05 (6.41) | 28.15 (8.32) | 25.91 (6.23) | 0.001 |
| Number of daily cigarettes | 0.77 (2.56) | 3.10 (4.62) | 0.61 (2.27) | <0.0001 |
| Number of drugs used in pregnancy | 0.40 (0.76) | 1.79 (1.13) | 0.31 (0.62) | <0.0001 |
Gravidity: Total number of times a woman has been pregnant.
Parity: Number of live births.
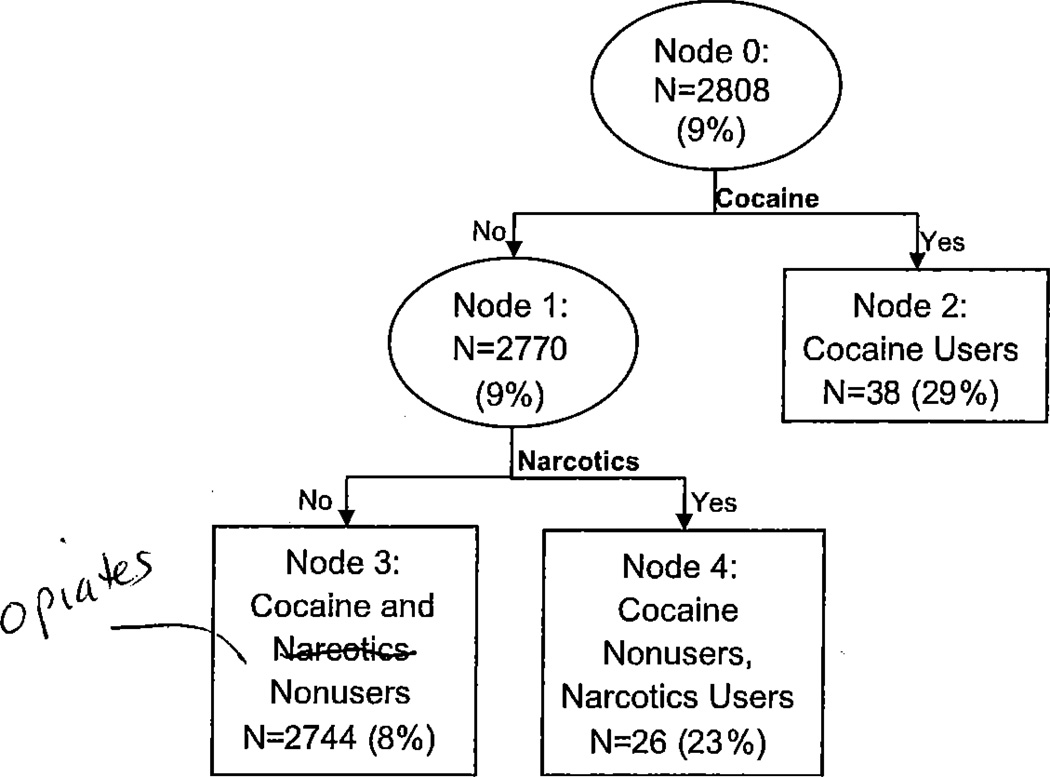
The tree analysis for risk factors associated with PTB is shown in Figure 1. Within each node, we show the number of women in the group and the proportion of those women who delivered preterm (in parentheses). The analysis resulted in 3 risk groups (nodes 2, 3, and 4). The primary split was based on cocaine use in pregnancy. The node for cocaine non-users was further split based on opiate use. The nodes for cocaine users (node 2) and cocaine nonusers, opiate users (node 4) were too small to split again. The node for nonusers of both cocaine and opiates (node 3) did not split again. It is interesting to note that none of the splits were based upon maternal risk factors associated with PTB or number of substances used. Cocaine users had the highest rate of PTB (node 2, 29%), followed by cocaine nonusers, opiate users (node 4,23%). Cocaine and opiate nonusers (8%) had the lowest PTB rates.
Figure 1.
Classification tree for assessing a women’s likelihood of preterm birth (PTB). Ovals indicate decision nodes, while rectangles indicate terminal nodes. The number of women and preterm birth rate (in parentheses) are displayed within each node.
Unadjusted and adjusted odds ratios for PTB risk subgroups are shown in Table II. Compared to non-users of cocaine and opiates, cocaine users were 3.53 times as likely to have a PTB (95% CI: 1.65–7.56; p=0.001), after adjusting for confounding factors. Opiate users who did not use cocaine had 2.86 times the odds (95% CI: 1.11–7.36; p= 0.03) of having PTB compared to non-users. Overall, 20% of preterm births occurred at 32 weeks or before defined as very preterm birth. Among the cocaine users subgroup, 6 of 11 (55%) were very preterm births.
Table II.
Adjusted odds ratios for preterm birth by risk group.
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| Subgroup | OR | 95% CI | P value | OR | 95% CI | P value |
| Node 2: Cocaine Users | 3.53 | (1.65–7.56, 9.01) | <0.0001 | 5.55 | (2.32, 13.29) | 0.0001 |
| Node 3: Cocaine and Narcotics Nonusers | REF | REF | ||||
| Node 4: Cocaine Nonusers, Narcotics Users | 3.25 | (1.29, 8.17) | 0.01 | 2.86 | (1.11, 7.36) | 0.03 |
Adjusted for age, marital status, race/ethnicity, education, gravidity and BMI
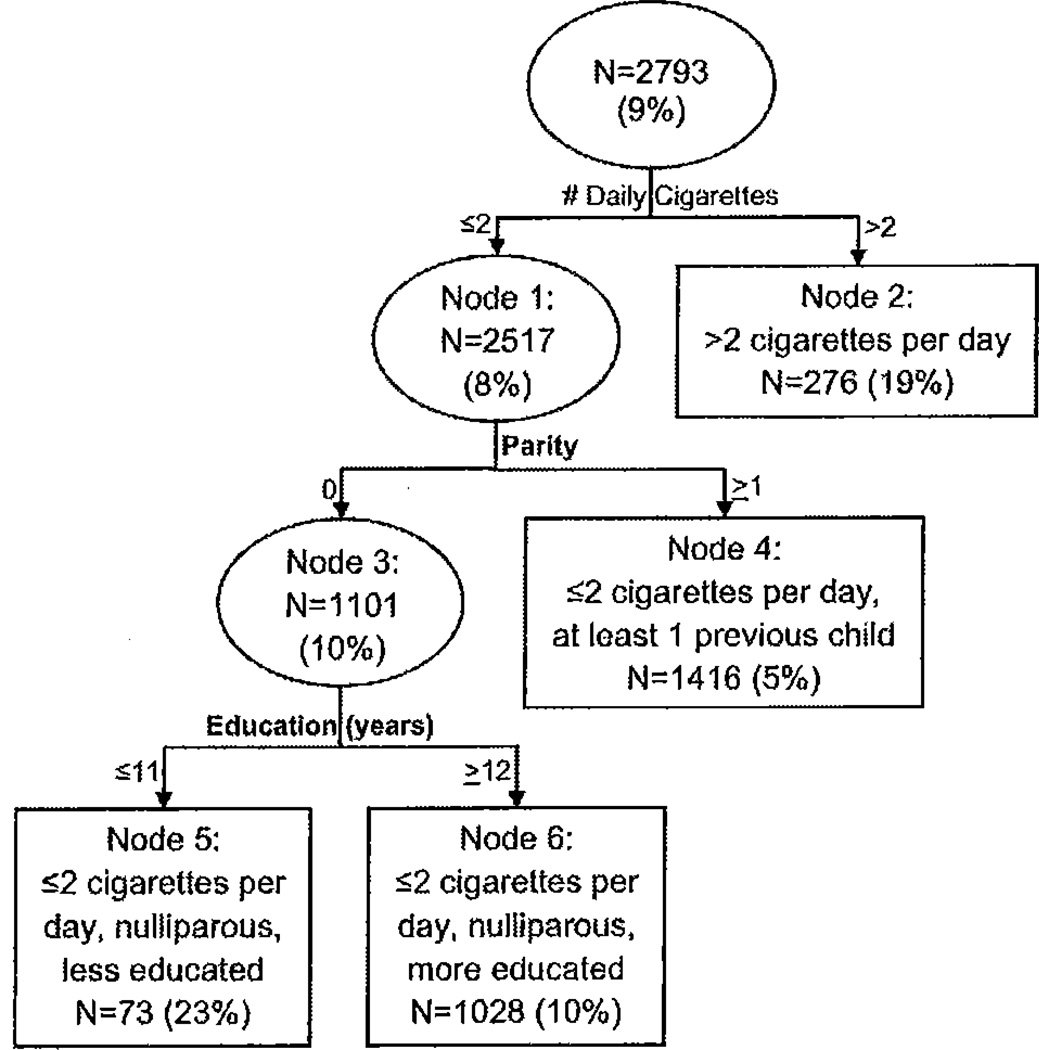
The tree analysis for SGA, a four-subgroup (nodes 2, 4, 5, and 6) classification, is shown in Figure 2. The first split was based on number of cigarettes smoked, where a cut point of 2 cigarettes per day maximized differences in SGA. The node for nonsmokers or women who smoked 2 or fewer cigarettes per day further split on parity, and the node representing nulliparous women split again based on education. Again we should note that the subgroup of women who smoked more than 2 cigarettes per day (node 2) did not split further, and none of the splits were based upon other substances used or number of substances used. Women who smoked more than 2 cigarettes per day, (node 2, 19%) and less educated, nulliparous women who smoked 2 or fewer cigarettes per day (node 5, 23%) had the highest SGA rates.
Figure 2.
Classification tree for assessing a woman’s likelihood for small for gestation age (SGA). Ovals indicate decision nodes, while rectangles indicate terminal nodes. The number of women and SGA rate (in parentheses) are displayed within each node.
Table III shows unadjusted and adjusted odds ratios for SGA births across tree-based risk groups. Women who smoked more than 2 cigarettes per day had 3.74 times the odds (95% CI: 2.47–5.65; p < 0.0001) of having an SGA infant compared to women with at least 1 child who smoked 2 or fewer cigarettes per day. Similarly, among women who smoked 2 or fewer cigarettes per day, less educated, nulliparous women were 4.12 times as likely (95% CI: 2.04–8.34; p < 0.0001) to have an SGA infant compared to women with at least one child.
Table III.
Adjusted odds ratios for SGA by risk group.
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
| Subgroup | OR | 95% CI | P value | OR | 95% CI | P value |
| Node 2: Smoking >2 cigarettes/day | 4.31 | (2.95, 6.30) | <0.0001 | 3.74 | (2.47, 5.65) | <0.0001 |
| Node 4: Nonsmokers or smoking ≤2 cigarettes/day, at least 1 previous child | REF | REF | ||||
| Node 5: Nonsmokers or smoking ≤2 cigarettes/day, nulliparous, less educated | 5.51 | (3.05, 9.94) | <0.0001 | 4.12 | (2.04, 8.34) | <0.0001 |
| Node 6: Nonsmoker or smoking ≤2 cigarettes per day, nulliparous, more educated | 1.91 | (1.40, 2.61) | <0.0001 | 1.84 | (1.33, 2.54) | 0.0002 |
Adjusted for age, marital status, race/ethnicity, and BMI.
Discussion
We found cocaine use and opiate use were most predictive of PTB. The well-established risk factor of tobacco use eclipsed other licit and illicit drugs as a predictor of an SGA infant. Uneducated, nulliparous women were also at high risk to deliver an SGA infant. Although these positive associations are not novel, we have systematically eliminated other drugs and characteristics as leading predictors of PTB and SGA. Number of drugs used, passive smoking, alcohol use and abuse, marijuana use, narcotics use, other drug use, gravidity, BMI, and marital status were less potent factors for both outcomes.
Despite a relatively small number of cocaine and opiate users in our sample, we found cocaine and opiate use to be the primary risk factors for PTB, which speaks to the strength of these associations. Our findings are consistent with a recent study by Almario et al. that included opiate addicted women who used other drugs and found that both opiate and cocaine use were predictive of PTB [12], Other [10–12, 21] but not all previous work [8] has shown prenatal cocaine and opiate use to be associated with PTB. We extend prior findings by including other potential risk factors for PTB in our model. It is alarming that women in our cohort who used cocaine and opiates had a rate of PTB (28.9%, 23% respectively) that is more than twice the national average in 2006 (12.8%) [22].
We did not find tobacco to be linked with PTB, but others uncommonly find this [2,22]. Opiates have been connected with PTB in prior work [12,23]. Notably, in a report of opiate addicted women who used other drugs, cocaine was the only additional substance that predicted PTB [12]. Furthermore, the rate of PTB among Pink and Blue participants (8%) is not a population estimate for the rate of PTB in Connecticut. The lower rate of PTB among this group may be attributable to participation bias given that patients entered the study voluntarily.
We found that women who smoked more than two cigarettes per day have the highest rates of delivering an SGA infant. Such results are consistent with the literature [1–3], however we show that even in the setting of illicit drug and alcohol use cigarette smoking is the predominant risk factor for SGA. Previous studies have found a dose effect relationship between smoking and SGA [3], and it may be more accurate to model cigarette consumption as a continuous variable. However a tree-type approach required a 2–3 level categorization to recursively assess risk. The CHAID (Chi-square Automatic Interaction Detector) algorithm did not choose a third level, although this may be intuitive, to discourage model over fitting.
The effect of cocaine use on fetal growth is reported more consistently than for other illicit substances and alcohol [1]; however, most studies do not account for the effects of smoking [10,25]. We did not show an association between cocaine use and SGA, which may be because we accounted for the effects of cigarette smoking among cocaine users.
Level of education, which was used as a proxy for socioeconomic status, and parity were also identified as risk factors for SGA. We found less educated, nulliparous women to be of high risk. Similarly Jansen and collegues [26], found that less educated women were at increased risk of delivering an SGA infant. These findings highlight the importance of controlling for associated risk factors in analyses of drug use in pregnancy.
Other studies have identified race as a predictor of PTB and SGA [26, 27]. Zhang and Bracken found race to be the primary risk factor for both PTB and SGA; however, their potential risk factors did not include cocaine nor opiate use. After accounting for these factors, we did not find race to be a significant risk factor for PTB. For SGA our results were more consistent, as they found smoking to be the second-leading risk factor after race [16].
A unique component of our study was the ability to prospectively explore associations between multi-substance use in pregnancy and birth outcomes. Such investigations are scarce and inconclusive [12,14]. We did not find that the number of drugs used was associated with PTB or SGA, particularly when compared to individual drugs. Our analysis on the various combinations of drugs was admittedly limited in power by the low frequency of each unique combination.
This study has several advantages. First, data were derived from two large, comprehensive, prospective cohorts that provided a range of exposures. Second, trained staff, blind to drug exposure, extracted birth outcomes from medical records. Third, we used a statistical technique that allowed us to evaluate the independent effects of specific substances, possible interactions in multiple-substance users and a comprehensive set of potentially confounding variables on PTB and SGA.
Our study also has limitations. A major limitation was the use of self-report data given that woman under report the use of hazardous substances during pregnancy [28], While the PRIDE-P study collected urine analyses on drug use, we employed self-report in our analyses to render data from the two cohorts more similar. Previous analyses with the PRIDE-P cohort found that self-report was comparable to findings documented through urine analyses (Yonkers et al., in Press). Also, the use of two cohorts and the fact that women who used drugs and alcohol were predominantly in the PRIDE-P cohort is a limitation. Because we targeted specific exposures (drug users in PRIDE-P and depressed women in the Pink and Blue study) and considered a relatively small number of women screened for participation (10% in PRIDE and 68% in PAB), these cohorts do not represent the general population. Furthermore, women in the PRIDE-P cohort were part of a treatment program; however, preliminary analysis shows that the program did not have substantial efficacy. Finally, unlike the self-poisoning model [29], which is able to determine the effect of high doses of hazardous substances we evaluated participants who enrolled voluntarily and may not have included women with more severe drug use conditions.
In summary, among the broad range of substances studied, we found that cocaine and opiate use in pregnancy are the predominant risk factors for PTB. Likewise, the major risk factor for SGA in this group was tobacco use. Finally, multi-substance use did not substantially increase risk of adverse birth outcomes over these risk factors. These strong findings should provide further impetus for mothers to seek treatment and clinicians to actively encourage substance use treatment in pregnancy given the increased risk of infant morbidity and mortality associated with such adverse outcomes [30].
Acknowledgements
This work was supported by a grant from the Doris Duke Charitable Foundation and the Yale Child Study Center. The PRIDE-P study was supported by Grant R01 DA 019135 from the National Institute on Drug Abuse to Drs. Yonkers, and Rounsaville and Grant R01HD045735 from the National Institute of Child Health and Human Development to Dr. Yonkers.
Footnotes
Declaration of Interest: Dr Yonkers is recipient of honoraria from Up To Date. Grants given to Yale but used by Dr. Yonker’s lab include an investigator initiated grant to treat postpartum depression that is funded by Lilly. In addition, study medications have been received from Pfizer to support a multicenter study on the treatment of premenstrual dysphoric disorder. We have no other disclosures among the remaining authors.
References
- 1.Bada HS, Das A, Bauer CR, Shankaran S, Lester BM, Gard CC, Wright LL, et al. Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J Perinatal. 2005;25:631–637. doi: 10.1038/sj.jp.7211378. [DOI] [PubMed] [Google Scholar]
- 2.Hammoud AO, Bujold E, Sorokin Y, Schild C, Krapp M, Baumann P. Smoking in pregnancy revisited: findings from a large population-based study. Am J Obstet Gynecol. 2005;192:1856–1862. doi: 10.1016/j.ajog.2004.12.057. discussion 1862. [DOI] [PubMed] [Google Scholar]
- 3.McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol. 2009;23:779–793. doi: 10.1016/j.bpobgyn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Keegan J, Parva M, Finnegan M, Gerson A, Belden M. Addiction in pregnancy. J Addict Dis. 2010;29:175–191. doi: 10.1080/10550881003684723. [DOI] [PubMed] [Google Scholar]
- 5.Jaddoe VW, Bakker R, Hofman A, Mackenbach JP, Moll HA, Steegers EA, Witteman JC. Moderate alcohol consumption during pregnancy and the risk of low birth weight and preterm birth. The generation R study. Ann Epidemiol. 2007;17:834–840. doi: 10.1016/j.annepidem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Fergusson DM, Horwood LJ, Northstone K ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Maternal use of cannabis and pregnancy outcome. BJOG. 2002;109:21–27. doi: 10.1111/j.1471-0528.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- 7.El-Mohandes A, Herman AA, Nabil El-Khorazaty M, Katta PS, White D, Grylack L. Prenatal care reduces the impact of illicit drug use on perinatal outcomes. J Perinatal. 2003;23:354–360. doi: 10.1038/sj.jp.7210933. [DOI] [PubMed] [Google Scholar]
- 8.Shiono PH, Klebanoff MA, Nugent RP, Cotch MF, Wilkins DG, Rollins DE, Carey JC, Behrman RE. The impact of cocaine and marijuana use on low birth weight and preterm birth: a multicenter study. Am J Obstet Gynecol. 1995;172:19–27. doi: 10.1016/0002-9378(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 9.van Gelder MM, Reefhuis J, Caton AR, Werler MM, Druschel CM, Roeleveld N National Birth Defects Prevention Study. Characteristics of pregnant illicit drug users and associations between cannabis use and perinatal outcome in a population-based study. Drug Alcohol Depend. 2010;109:243–247. doi: 10.1016/j.drugalcdep.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Bateman DA, Ng SK, Hansen CA, Heagarty MC. The effects of intrauterine cocaine exposure in newborns. Am J Public Health. 1993;83:190–193. doi: 10.2105/ajph.83.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kliegman RM, Madura D, Kiwi R, Eisenberg I, Yamashita T. Relation of maternal cocaine use to the risks of prematurity and low birth weight. J Pediatr. 1994;124:751–756. doi: 10.1016/s0022-3476(05)81370-8. [DOI] [PubMed] [Google Scholar]
- 12.Almario CV, Seligman NS, Dysart KC, Berghella V, Baxter JK. Risk factors for preterm birth among opiate-addicted gravid women in a methadone treatment program. Am J Obstet Gynecol. 2009;201:326.el–326.e6. doi: 10.1016/j.ajog.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 13.Bada HS, Das A, Bauer CR, Shankaran S, Lester B, Wright LL, Verter J, et al. Gestational cocaine exposure and intrauterine growth: maternal lifestyle study. Obstet Gynecol. 2002;100:916–924. doi: 10.1016/s0029-7844(02)02199-3. [DOI] [PubMed] [Google Scholar]
- 14.Pinto SM, Dodd S, Walkinshaw SA, Siney C, Kakkar P, Mousa HA. Substance abuse during pregnancy: effect on pregnancy outcomes. Eur J Obstet Gynecol Reprod Biol. 2010;150:137–141. doi: 10.1016/j.ejogrb.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Schempf AH, Strobino DM. Illicit drug use and adverse birth outcomes: is it drugs or context? J Urban Health. 2008;85:858–873. doi: 10.1007/s11524-008-9315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Bracken MB. Tree-based risk factor analysis of preterm delivery and small-for-gestational-age birth. Am J Epidemiol. 1995;141:70–78. doi: 10.1093/oxfordjournals.aje.a117347. [DOI] [PubMed] [Google Scholar]
- 17.Yonkers KA, Howell HB, Allen AE, Ball SA, Pantalon MV, Rounsaville BJ. A treatment for substance abusing pregnant women. Arch Womens Ment Health. 2009;12:221–227. doi: 10.1007/s00737-009-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yonkers KA, Smith MV, Gotman N, Belanger K. Typical somatic symptoms of pregnancy and their impact on a diagnosis of major depressive disorder. Gen Hosp Psychiatry. 2009;31:327–333. doi: 10.1016/j.genhosppsych.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonkers KA, Gotman N, Kershaw T, Forray A, Howell HB, Rounsaville BJ. Screening for prenatal substance use: development of the Substance Use Risk Profile-Pregnancy scale. Obstet Gynecol. 2010;116:827–833. doi: 10.1097/AOG.0b013e3181ed8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26:172–181. doi: 10.1207/S15324796ABM2603_02. [DOI] [PubMed] [Google Scholar]
- 21.Arlettaz R, Kashiwagi M, Das-Kundu S, Fauchere JC, Lang A, Bucher HU. Methadone maintenance program in pregnancy in a swiss perinatal center (II): Neonatal outcome and social resources. Acta Obstet Gynecol Scand. 2005;84(2):145–150. doi: 10.1111/j.0001-6349.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 22.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kimeyer S, Matthews TJ. National vital statistics report. Natl Vital Stat Rep. 2009;57:1–102. [Google Scholar]
- 23.Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol. 2000;182(2):465–472. doi: 10.1016/s0002-9378(00)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visscher WA, Feder M, Burns AM, Brady TM, Bray RM. The impact of smoking and other substance use by urban women on the birthweight of their infants. Subst Use Misuse. 2003;38:1063–1093. doi: 10.1081/ja-120017651. [DOI] [PubMed] [Google Scholar]
- 25.Eyler FD, Behnke M, Conlon M, Woods NS, Frentzen B. Prenatal cocaine use: a comparison of neonates matched on maternal risk factors. Neurotoxicol Teratol. 1994;16:81–87. doi: 10.1016/0892-0362(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 26.Jansen PW, Tiemeier H, Looman CW, Jaddoe VW, Hofman A, Moll HA, Steegers EA, et al. Explaining educational inequalities in birthweight: the Generation R Study. Paediatr Perinat Epidemiol. 2009;23:216–228. doi: 10.1111/j.1365-3016.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- 27.Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatal. 2006;23:325–328. doi: 10.1055/s-2006-947164. [DOI] [PubMed] [Google Scholar]
- 28.Gonczi L, Czeizel AE. Integrating smoking cessation into periconceptional care. Tob Control. 1996;5:160. doi: 10.1136/tc.5.2.160a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czeizel AE, Gidai J, Petik D, Timmermann G, Puhó EH. Self-poisoning during pregnancy as a model for teratogenic risk estimation of drugs. Toxicol Ind Health. 2008;24:11–28. doi: 10.1177/0748233708089020. [DOI] [PubMed] [Google Scholar]
- 30.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]