Abstract
“Bang-sensitive” mutants of Drosophila display characteristic repertoires of distinct seizure-and-paralysis behaviorsupon mechanical shock (Ganetzky & Wu, 1982). We found that each of the bang-sensitive mutants described here (bas, bss, eas, and tko) also displayed similar behavioral repertoires upon exposure to either high or low temperature. These repertoires are composed of interspersed periods of seizure and paralysis, and appear to have interesting parallels with vertebrate epileptiform behavior. Analysis of gynandromorph mosaics of these bang-sensitive mutant flies indicated that anatomical foci required for these two types of behaviors do not totally overlap as they were separable among mosaic flies. Observations on mosaic and decapitated flies demonstrated an all-or-none expression of the seizure-and-paralysis behaviors, indicating global activity and long-range interactions in the nervous system. Therefore, the diverse collection of currently available Drosophila bang-sensitive mutants may serve as a rich source for mutational and cellular analysis to identify interacting molecular networks that are responsible for seizure phenotypes.
INTRODUCTION
“Bang-sensitive” mutants of Drosophila have attracted much attention since their initial descriptions in the early 1970’s because they are known for characteristic repertoires of striking seizure-and-paralysis behaviors (Benzer, 1971; Judd et al., 1972; Grigliatti et al., 1973; Ganetzky & Wu, 1982). The stress-evoked seizure repertoire is interspersed with periods of paralysis, representing an interesting parallel with vertebrate epileptic behavior (Ganetzky & Wu, 1982; Burg, 1987; Pavlidis & Tanouye, 1995; Lee & Wu, 2002; 2006). As in mammalian epilepsy, in which a large number of genes and neuronal mechanisms have been associated with seizure disorders (McNamara, 1994; Shoffner et al., 1990; Noebels, 2003; Klassen et al., 2011), several studies on Drosophila bang-sensitive mutants have documented a number of genes encoding proteins of different functional categories, including ion channels (Parker et al., 2011), mitochondrial proteins (Roydon et al., 1987; Fergestad et al., 2006) and lipid metabolism (Pavlidis & Tanouye, 1995; Pavlidis et al., 1994). The resultant cellular phenotypes induced in these mutants span from altered synaptic transmission and nerve excitability (Ganetzky & Wu, 1982; Jan & Jan, 1978; Engel & Wu, 1994; Marley & Baines, 2011), to spike frequency coding (Engel, 1995), as well as general seizure-like nerve and muscle spike discharge and neural pathway failure (Pavlidis & Tanouye, 1995; Kuebler & Tanouye, 2000; 2001; Lee & Wu, 2002; 2006). Therefore, the Drosophila bang-sensitive mutants may provide a rich source for mutational and cellular analysis to identify interacting molecular and cellular networks that are responsible for seizure phenotypes.
It is also known that many bang-sensitive mutants display extremely low threshold for the electrical stimulus-induced seizure response (Pavlidis & Tanouye, 1995; Kuebler & Tanouye, 2000; 2001; Lee & Wu, 2002; 2006; Fergestad et al., 2006; Marley & Baines, 2011). Here we describe a close association between temperature stress- and mechanical stress-induced behavioral repertoires of the seizure-and-paralysis response in bang-sensitive mutants. The seizure-and-paralysis response to temperature stressors represents a separate form of seizure behavior that has not been documented in detail for bang-sensitive mutants. To complement the molecular and cellular phenotypes determined at the biochemical and physiological levels, we adopted the genetic mosaic technique and surgical manipulations in this study to characterize behavioral consequences of the expression of bang-sensitive mutations in different body parts. We examined four representative “classical” bang-sensitive mutants, i.e. the earliest mutants reported in the literature: bang-sensitive (bas: Ganetzky & Wu, 1982), bang-senseless (bss: Jan & Jan, 1978; Ganetzky & Wu,1982), easily-shocked (eas: Ganetzky & Wu,1982), and technical knock-out (tko: Judd et al., 1972; Ganetzky & Wu,1982).
We found that the mechanical and temperature stress-induced behaviors are separable because their occurrence was not strictly coupled in individual mosaic flies, even though these two forms of seizures consisted of similar behavioral repertoires in each mutant genotype. In addition, observations on genetic mosaics and decapitated bang-sensitive flies indicated global activity and long-range interaction in the CNS in generating hyperactivity in the nervous system underlying the seizure-and-paralysis behavior.
This report is based on the work performed in our lab several decades ago, described in an unpublished thesis chapter (Burg, 1987). The findings have been subsequently confirmed (Wu, unpublished observations) and have become relevant to the active research in hyperexcitability and seizure phenotypes currently conducted in the Drosophila research community. We dedicate this paper to Dr. William L. Pak to commemorate his lifelong contributions to Drosophila neurogenetics.
MATERIALS AND METHODS
Mosaic generation
The ring-X chromosome, In(1)wvC, was used to produce gynandromorph mosaics (Hinton, 1955; Hotta & Benzer, 1972; Burg & Wu, 1986;1989; Lee & Wu, 2002). Heterozygous females were generated carrying the ring-X chromosome and a second X chromosome which bore one of the bang-sensitive mutant genes (tko, bas, eas, or bss). Upon spontaneous loss of the ring-X chromosome during development, patches of hemizygous male tissue were generated, surrounded by heterozygous female tissue. The non ring-X chromosome also carried recessive cuticular markers y (yellow) and cho (chocolate), which became visible to demarcate the hemizygous (mutant) patch tissue, representing clonal descendants derived from a cell that underwent a ring-X chromosome loss.
Behavioral testing
Flies 2–3 days of age were tested for bang-sensitive or temperature-sensitive seizure behavioral phenotypes. Intact flies were kept in clean glass vials (9.3 cm × 2.4 cm.) and given a 10 sec vibration using a vortex mixer (Model S8223, Sci. Prod., McGaw Park, IL) at the highest speed, 10 (Ganetzky & Wu, 1982; Burg, 1987) or immersed in a temperature controlled water bath with a magnifying viewer to observe fly behavior at high (39 °C) or low (8 °C) restrictive temperatures. Decapitated flies were kept on moistened filter paper in a petri dish and initially tested within 20 hours of decapitation(Burg, 1987). Mechanical vibration was delivered to decapitated flies by using a vortex mixer to vibrate decapitated flies being kept in small petri dishes. Cold- or heat-sensitivity of decapitated flies was determined using a peltier junction Hot-Cold plate (model 3832, Cole-Palmer, Chicago, IL), while intact flies were tested using a temperature controlled water bath. For temperature effects on the kinetics of recovery from mechanical shock-induced seizure and paralysis, ten flies were housed in glass vials and acclimated for at least two hours before testing in environmental chambers of temperature at 19 and 26 °C.
RESULTS
Bang sensitive seizure behavioral repertoire
Mechanical shock, such as tapping a culture vial, causes bang-sensitive mutants to lose coordination and leads to a characteristic sequence of behaviors, or “repertoire”, prior to full recovery (Fig. 1). The behavioral components in a “repertoire” are typically a combination of contorted posturing, uncoordinated movement and shaking of the legs, raising and buzzing of the wings, and spinning and uncontrolled flight, that are interspersed with periods of immobility or paralysis (Fig. 1). We have examined in detail the previously identified bang-sensitive mutants tko25t, eas2, bas, and bss1 (Judd et al., 1972; Grigliatti et al., 1973; Ganetzky & Wu, 1982). Each mutant has a distinct behavioral “repertoire”, composed of different sequences of the similar behavioral components (Burg, 1987).
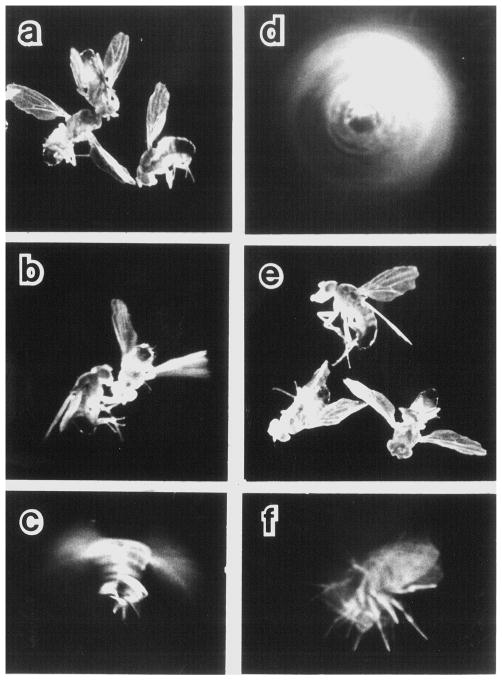
Figure 1.
Sequence of seizure-and-paralysis responses observed in a bang-sensitive mutant following mechanical shock (vortexing). The behavioral repertoire of bas eas double-mutant flies is shown here as an example. Initially, paralysis occurs with contorted posturing (a), followed by a period of uncoordinated leg movement (b). After a period of quiescence, wing buzzing may occur (c), sometimes even causing the fly to spin (d). The fly may resume the contorted posture (e). Such episodes (a–e) can be repeated one or more times. Eventually, the fly recovers to right itself (f) and walk away. Similar components of seizure-and-paralysis behaviors are seen in the response sequence during temperature-induced paralysis (see Fig. 2). Such components are also found in the behavioral repertoire of all bang-sensitive mutants studied here but the exact sequence differs between individual mutants (Burg, 1987). Photo exposure time = 5 sec.
Temperature-induced seizures
We found that bang-sensitive mutants could also undergo seizures by exposure to either high (39 °C in bas and tko) or low (8 °C in eas and bss) temperatures, at which wild-type flies were unaffected for short periods of time (Fig. 2B, C). Interestingly, the behavioral repertoire characteristic of bang-sensitive mutants (Fig. 1) was also induced by restrictive temperature on the way to, and recovery from, paralysis. Notably, one additional bang-sensitive mutant, sda (Zhang & Tanouye, 2002) showed a similar seizure-and-paralysis repertoire in response to low-temperature exposure (below 10 °C, C.-F. Wu, unpublished observation). Therefore, these bang-sensitive mutants also represent a unique class of temperature-sensitive paralytic mutants. Many previously identified temperature-sensitive mutants, such as parats, napts, or shits are not sensitive to mechanical shocks and do not typically display seizure-like behavior when recovering from temperature-induced paralysis (Grigliatti et al, 1973; Wu et al., 1978).
Figure 2.
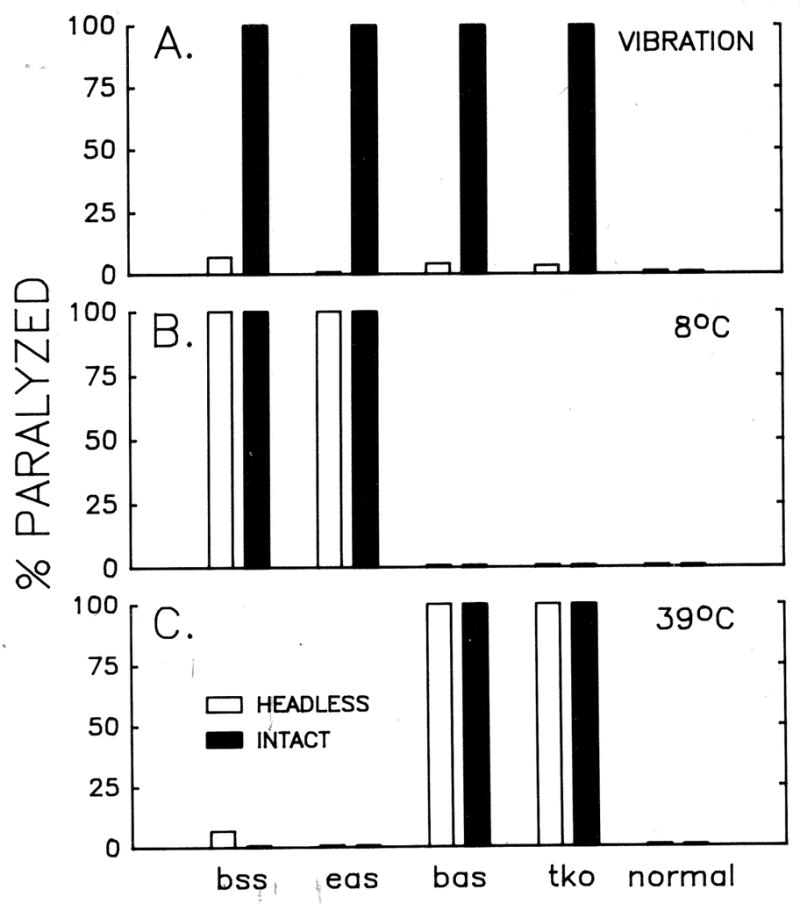
Mechanical and temperature stressor-induced seizure-and-paralysis behaviors in intact and decapitated bang-sensitive mutants. Results of behavioral tests are shown for intact (filled bars) or decapitated (empty bars) flies in response to (A) vibration (B) low temperature (8 °C), or high temperature (39 °C). The percentages of flies of different genotypes that became paralyzed are quantified. While intact mutant flies readily displayed the seizure-and-paralysis response at the vortex strength and temperature setting used in this experiment, decapitated flies required longer time to become paralyzed at the temperature setting (B and C). Similarly, these decapitated flies could become paralyzed at increased vortex strengths (see text). Note that wild-type (normal) flies (far-right column) were not paralyzed by any of the treatments given. N = 50 per treatment in (A), 20 for intact flies and 10 for decapitated flies in (B) and (C).
Furthermore, we investigated how temperature affects the mechanical shock-induced seizure and paralysis phenotypes in these bang-sensitive mutants. As can be seen in Table 1, the ambient temperature had a general effect on the time to recovery in each of these mutants. As temperature decreases, the recovery time was substantially prolonged in both male and female mutant flies. Interestingly, this held true for both high temperature-sensitive (bas and tko) and low temperature-sensitive (bss and eas) mutants within the temperature range tested (19–26 °C). Notably, the phenotype of tko, which was less extreme than others, became poorly expressed since a large portion of tko flies lost bang sensitivity at a higher temperature (Table 1). The results demonstrate a strong modulatory effect of temperature on the expression of the bang-sensitive phenotype.
Table 1.
Time to recovery from mechanical stress-induced seizure-and-paralysis behavior
| Genotype | Male
|
Female
|
||||
|---|---|---|---|---|---|---|
| 19 °C | 22 °C | 26 °C | 19 °C | 22 °C | 26 °C | |
| bss | 140 ± 58 (12/12) | 94 ± 28 (13/13) | 68 ± 32 (12/12) | 131 ± 46(13/13) | 113 ± 22 (13/13) | 77 ± 16 (13/13) |
| eas | 96 ± 28 (11/11) | 60 ± 24 (15/15) | 32 ± 5.1 (16/16) | 109 ± 29 (16/16) | 78 ± 23 (16/16) | 42 ± 8.1 (15/16) |
| bas | 107 ± 37 (11/11) | 99 ± 30 (11/11) | 73 ± 52 (10/11) | 105 ± 27 (12/12) | 95 ± 28 (13/13) | 61 ± 43(12/12) |
| tko | 46 ± 23 (14/16) | 58 ± 13 (14/16) | 7.5 ± 2.9 (4/16) | 43 ± 24 (13/14) | 56 ± 11 (14/14) | 9.0 ± 3.5 (7/16) |
Mean ± S.D. in seconds are shown. Numbers in parentheses indicate (# of flies paralyzed/# of flies tested). The flies which did not pass out are excluded from recovery time statistics. Note that majority of tko mutant flies did not paralyze at 26 °C.
Decreased sensitivity to mechanical and temperature stressors in decapitated bang-sensitive mutant flies
Normal flies, following decapitation, are still able to stand, retain responses to tactile stimuli, and show a righting response when pushed over (Vandervorst & Ghysen, 1980; Burg & Wu, 1986; 1989). When decapitated, bang-sensitive flies behaved similarly to decapitated wild type flies, but, surprisingly, were more resistant to mechanical jolt (Figs. 2A and 3). Upon mechanical shocks, these flies seldom underwent a seizure at a stimulus level (vortex speed 7, duration 10 s) sufficient to induce seizure and paralysis in the corresponding intact bang-sensitive mutant flies. Even though they were knocked over, they struggled and were able to stand up (Fig. 3). In contrast, intact bang-sensitive flies were unable to stand after mechanical vibration, even with assistance by a brush (Fig. 3), until the behavioral repertoire had reached completion (Fig 1f). It should be noted that upon further increase in the stimulus level (speed 10, duration 10 s), a portion of these decapitated bang-sensitive flies did display seizure-and-paralysis behavior.
Figure 3.

Suppression of the mechanical stress-induced seizure in decapitated bang-sensitive mutants. Shown are bas eas double-mutant flies (with visible markers for recombination, g bas1 sd eas2 f). While intact flies were paralyzed by mechanical vibration (upper 3 flies, note bodies in contorted positions, cf. Fig. 1a, e), decapitated flies were unaffected, being able to stand after mechanical vibration (lower 3 flies, note accompanying heads). The prolonged seizure phenotype in the double mutant allowed for easy photographic recording. Exposure time = 5 sec.
In addition, decapitation appeared to suppress the behavioral repertoire at seizure-inducing temperatures. Minutes after intact bang-sensitive flies became paralyzed (Fig. 2B, C), decapitated mutants eventually collapsed at the temperature that caused intact ones to undergo seizure and paralysis. We found that the association with temperature sensitivity and suppression by decapitation are common among bang-sensitive mutants studied here.
Separation of vibration- and temperature-induced seizures in gynandormorph mosaics
The suppression of seizure behavior in decapitated bang-sensitive flies suggests that a full blown, all-or-none, global activity of interacting foci in the nervous system is needed to generate seizure (e.g., motor patterns reflecting discharges of certain thoracic ganglion circuits can be modulated by input from the head). We further investigated this idea by using genetic mosaics, in which patches of mutant tissue coexist within phenotypically normal tissue (Hotta & Benzer, 1972; Burg & Wu, 1986; 1989). The mutants examined here were recessive except for bss, which showed a semi-dominant effect (Ganetzky & Wu, 1982). Mosaics were generated for each mutant (Burg, 1987) in an attempt to determine the anatomical foci for seizure (Fig. 4). The behavior of individual mosaic flies was observed after mechanical vibrations. The most significant result was the all-or-none nature of the seizure behavior. We never observed mosaic flies that displayed seizure-like behavior involving only a portion of the animal in a large number of samples examined (tko, 247; bas, 601; eas, 406; bss, 205).
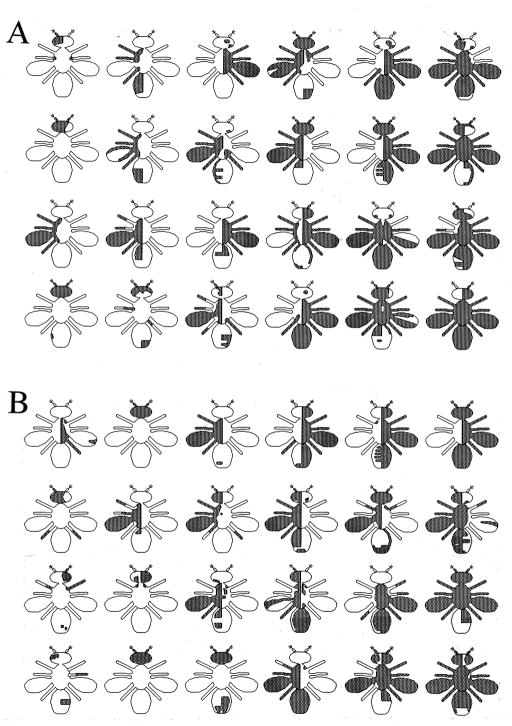
Figure 4.
Separation of temperature- and vibration-induced seizure in bas mosaic flies. Shaded areas indicate hemizygous mutant tissue, expressing recessive y and cho visible markers, in otherwise phenotypically normal mosaic flies. (A) A sample of mosaics that displayed seizure induced by high temperature (39°C for 3 minutes) but not by vibration. (B) A sample of mosaics that displayed seizures induced by vibration but not by high temperature. The phenotype was all-or-none. In no case did any mosaic exhibit seizure-like behavior involving only a portion of the animal. Total number of mosaics examined = 601.
To investigate the possibility that the foci for seizure triggered mechanically and by temperature are separable, we examined individual bas mosaics for sensitivity to temperature conditions compared to mechanical shocks. In a fraction of bas mosaics, some (53/601) were paralyzed by temperature but not by mechanical shock (Fig. 4A). Conversely, some other mosaic flies (99/601) were paralyzed only by mechanical vibration but not by temperature (Fig. 4B), while a larger number of mosaics (237/601) were sensitive to both mechanical vibration and temperature. This result indicates that the anatomical foci responsible for the initiation of seizure by temperature and mechanical vibration are different.
The above observations suggest that there are distributed foci rather than distinct, exclusive foci for vibration or temperature stress induction of seizures. Especially, the mosaicism involving the entire head being mutant does not guarantee expression of both vibration- and temperature-induced seizures (Fig. 4). Although a descending central control clearly exerts influence over the peripheral expression of hyperexcitability, the circuit for seizure expression is likely localized in the thorax, which could be triggered locally. This is consistent with the suppression effect of decapitation on seizure expression (Fig. 3), albeit such headless flies could still express the same seizure repertoire at elevated stress levels. In electrophysiological experiments on both intact and decapitated preparations, electroconvulsive-induced seizures recorded in flight muscles could be induced with stimulation applied to the head across the brain or directly applied to the thorax (Lee and Wu, 2002).
DISCUSSION
We have shown that the classical bang-sensitive mutants in our study also exhibit a temperature-induced seizure, caused either by a low or high restrictive temperature. In gynandormorph mosaics of these mutants, we have been able to separate the temperature and vibration-induced behaviors, suggesting that different anatomical foci are required for seizure induction by either temperature or mechanical stressors. Analysis of mosaic or decapitated flies also indicate that bang-sensitive behavior is an all-or-none, full-blown expression involving interactive anatomical foci within the nervous system since seizure-like behavior involving only a portion of the animal was not observed. This study also showed that the seizure-and-paralysis behavioral repertoire could be evoked in decapitated bang-sensitive flies, suggesting the release of a sequence of motor programs generated in thoracic ganglion. This is consistent with a previous report of electroconvulsive discharge triggered by direct stimuli on the thorax in headless flies (Lee & Wu, 2002). However, the seizure expression in decapitated mutant flies required more extreme mechanical or temperature stress, reflecting a strong modulatory influence from the head, likely the input from brain, on the seizure motor activity generated in thoracic ganglion.
The molecular basis of the physiological and behavioral defects of bang-sensitive mutations has been investigated for some time. In mammals, a large number of genes have been associated with epileptiform disorders (Noebels, 2003; Klassen et al., 2011). These identified genes are relevant to a wide variety of cellular functions, including membrane excitability and synaptic transmission, as well as neural circuit development and maintenance. To date, molecular genetic analysis in Drosophila indicates that the tko locus encodes a mitochondrial ribosomal protein (Roydon et al., 1987), which may be important for the high energy demand of the nervous system, eas encodes an ethanolamine kinase (Pavlidis et al, 1994), an important enzyme for membrane lipid metabolism, and bss1 is a gain of function mutation in the sodium channel gene, para (Parker et al, 2011), which is critical in action potential generation. Among a number of homologous genes that when mutated lead to seizure-related disorders in both humans and Drosophila, a few well-documented cases include mutations of both potassium channel (Kcna 1 in humans: Glasscock et al., 2007; Shaker (Sh) in Drosophila: Wu & Ganetzky, 1992; Fox et al., 2005) and sodium channel (Nacna in humans: Clases et al., 2001; Escayg et al., 2000; para in Drosophila: Parker et al, 2011) genes.
All bang-sensitive mutants reported here have been shown to display high-frequency spike discharges in the flight muscles following high-frequency electroconvulsive stimulation to the brain (Pavlidis & Tanouye, 1995; Engel, 1995; Kuebler & Tanouye, 2000; 2001; Lee & Wu, 2002; 2006; Fergestad et al., 2006; Parker et al., 2011). In addition, a synaptic defect has been found in the bss mutation, with altered long-term facilitation at the larval neuromuscular junction (Jan & Jan, 1978), associated with multiple firing in the motor neuron (Ganetzky & Wu, 1982). Moreover, the bas, bss, eas, and tko mutations have been demonstrated to alter spike coding in mechanosensory cells (Engel & Wu, 1994). Significantly, the bang-sensitive behavior in the above mutants is suppressed by the napts mutation (Wu et al., 1978; Ganetzky & Wu, 1982), which acts to reduce nerve excitability by a decrease of sodium channel density (Kernan et al., 1991). The napts mutation also suppresses the physiological defects in bss, including enhanced synaptic transmission and nerve hyperexcitability at larval neuromuscular junctions (Ganetzky & Wu, 1982) and abnormal seizure spike discharge in response to electroconvulsive stimuli in adult flies (Lee & Wu, 2006).
The seizure-and-paralysis behavioral repertoire characteristic of the mutants described here may be applicable to some other bang-sensitive flies, including slam-dance (sda; Zhang & Tanouye, 2002), which is also cold sensitive (see Results). However, this typical behavioral repertoire is not shared by all recently described “bang-sensitive” mutants. For example, mechanical shock induces only transient immobilization or uncoordinated movements in focal adhesion kinase (Ueda et al., 2002) and ATPalpha (Palladino et al., 2003; Fergestad et al., 2008), without seizure or clear display of refractory period (see below). Further investigations may reveal different neural substrates responsible for these two types of “bang sensitivity.”
Studies of bang-sensitive mutants in Drosophila may open a door to reveal basic mechanisms underlying seizure generation. Notably, these Drosophila mutants present interesting parallels with some well-known characteristics of vertebrate epileptic behavior. 1) Synchronized spike discharges can be recorded along different body axes in bang-sensitive mutants (Lee & Wu, 2002), in contrast to spike discharge activities in different brain foci associated with epileptic episodes (Bannister, 1985). 2) A refractory period is found in these bang-mutants (Ganetzky & Wu, 1982). It is known in clinical cases and animal models for epilepsy, post-ictal hypo-excitability or quiescence occurs (Heinemann & Gutnick, 1979). 3) The present study demonstrates the global, all-or-none expression of the bang-sensitive behavioral repertoire. It is known that in many cases of epilepsy, symptoms can be relieved by surgical isolation of interacting foci in the brain (Gordon et al., 1971; Ojemann, 1987). 4) We have shown separate anatomic foci for seizure behavior triggered by different stressors. Similar examples in mammals include photogenic and audiogenic forms of epilepsy, the triggering of which is mediated by different neuro-anatomical pathways (Bannister, 1985).
Taken together, across the phyla, seizure behaviors could reflect some general properties intrinsic to neuronal interactions and interconnections of functional loci in the nervous systems. Many of the basic molecular and cellular mechanisms underlying this complex phenomenon may well be conserved, since numerous examples exist where important molecules in the nervous system share high homology between Drosophila and man (Buchner, 1991; McQuilton et al., 2011). Therefore, identification of additional bang-sensitive mutants and the corresponding gene products in Drosophila may allow a better understanding of the interacting molecular and cellular networks responsible for seizure behavior. This specific class of Drosophila mutants will allow an incisive analysis of what specific properties of neurons and features of the neural circuits, when altered, result in highly stereotypic physiological and behavioral abnormalities (Shoffner et al., 1990; McNamara, 1994).
Acknowledgments
We thank Dr. Atsushi Ueda for participation in replicating the observations of decapitated bang-sensitive flies described in this paper and his assistance in the construction of Table 1. We also thank Drs. M.A. Howard III and M.-m. Poo for comments on an earlier version of this manuscript. This work was in part supported by NIH grants NS18500 and GM 088804 and an NIH pre-doctoral training grant (GM07091, MGB).
References
- Bannister R. Brains’s Clinical Neurology. 6. London: Oxford Univ. Press; 1985. [Google Scholar]
- Benzer S. From the gene to behavior. J Amer Med Assoc. 1971;218:1015–1022. [PubMed] [Google Scholar]
- Buchner E. Genes expressed in the adult brain of Drosophila and effects of their mutations on behavior: A survey of transmitter- and second messenger-related genes. J Neurogenet. 1991;7(4):153–192. doi: 10.3109/01677069109167432. [DOI] [PubMed] [Google Scholar]
- Burg MG. PhD Thesis. Univ. of Iowa; Iowa City, Iowa, USA: 1987. Genetic and mosaic analysis of mutations which alter never and muscle excitability in Drosophila melanogaster: Effects on development and behavior. [Google Scholar]
- Burg MG, Wu CF. Differentiation and central projections of peripheral sensory cells with action-potential block in Drosophila mosaics. J Neurosci. 1986;6:2968–2976. doi: 10.1523/JNEUROSCI.06-10-02968.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MG, Wu CF. Central projections of peripheral mechanosensory cells with increased excitability in Drosophila mosaics. Developmental Biology. 1989;131:505–514. doi: 10.1016/s0012-1606(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Clases L, Ceulemans B, Audenaert D, Smets K, Lofgren A, Del-Favero J, Ala-Mello S, Basel-Vanagaite L, Plecko B, Raskin S, Thiry P, Wolf N, Van Broeckhoven C, DeJonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–1332. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JE. PhD thesis. University of Iowa; Iowa City, Iowa, USA: 1995. Effects of second messenger and excitability mutations upon identified neural circuits underlying activity-dependent plasticity of behavior in Drosophila. [Google Scholar]
- Engel J, Wu CF. Altered mechanoreceptor response in Drosophila bang-sensitive mutants. J Comp Physiol [A] 1994;175:267–278. doi: 10.1007/BF00192986. [DOI] [PubMed] [Google Scholar]
- Escayg A, MacDonald B, Meisler M, Baulac S, Huberfeld G, An-Gourfinkel I, Brice A, Leguern E, Moulard B, Chaigne D, Bruesi C, Malafosse A. Mutations of SCN1A encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–345. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- Fergestad T, Bostwick B, Ganetzky B. Metabolic disruption in Drosophila bang-sensitive mutants. Genetics. 2006;173:1357–1364. doi: 10.1534/genetics.106.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergestad T, Olson L, Patel KP, Miller R, Palladino M, Ganetzky B. Neuropathology in Drosophila mutants with increased seizure susceptibility. Genetics. 2008;178:947–956. doi: 10.1534/genetics.107.082115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox L, Ueda A, Berke B, Peng I-F, Wu C-F. Movement Disorders in Drosophila Mutants of Potassium Channels and Biogenic Amine Pathways. In: LeDoux MS, editor. Animal Models of Movement Disorders. San Diego: Academic Press/Elsevier; 2005. pp. 487–504. [Google Scholar]
- Ganetzky B, Wu CF. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics. 1982;100:597–614. doi: 10.1093/genetics/100.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasscock E, Qian J, Yoo JW, Noebels JL. Masking epilepsy by combining two epilepsy genes. Nat Neurosci. 2007;10:1554–1558. doi: 10.1038/nn1999. [DOI] [PubMed] [Google Scholar]
- Gordon HW, Bogen JE, Sperry RW. Absence of deconnexion syndrome in two patients with partial section of the neocommissures. Brain. 1971;94:327–336. doi: 10.1093/brain/94.2.327. [DOI] [PubMed] [Google Scholar]
- Grigliatti T, Hall L, Rosenbluth R, Suzuki DT. Temperature-sensitive mutations in Drosophila melanogaster. Molec Gen Genet. 1973;120:107–14. doi: 10.1007/BF00267238. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Gutnick MJ. Relation between extracellular potassium concentration and neuronal activities in cat thalamus (VPL) during projection of cortical epileptiform discharge. Electroencephalogr Clin Neurophysiol. 1979;47:345–347. doi: 10.1016/0013-4694(79)90285-2. [DOI] [PubMed] [Google Scholar]
- Hinton CW. The behavior of an unstable ring chromosome of Drosophila melanogaster. Genetics. 1955;40:951–961. doi: 10.1093/genetics/40.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y, Benzer S. Mapping of behaviour in Drosophila Mosaics. Nature. 1972;240:527–535. doi: 10.1038/240527a0. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Genetic dissection of short-term and long-term facilitation in Drosophila. Proc Nat Acad Sci U S A. 1978;75:515–519. doi: 10.1073/pnas.75.1.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd BH, Shen MW, Kaufman TC. The anatomy and function of a segment of the X chromosome of Drosophila melanogaster. Genetics. 1972;71:139–156. doi: 10.1093/genetics/71.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan MJ, Kuroda MI, Kreber R, Baker BS, Ganetzky B. napts, a mutation affecting sodium channel activity in Drosophila, is an allele of mle a regulator of X chromosome transcription. Cell. 1991;66:949–959. doi: 10.1016/0092-8674(91)90440-a. [DOI] [PubMed] [Google Scholar]
- Klassen T, Davis C, Goldman A, Burgess D, Chen T, Wheeler D, McPherson J, Bourquin T, Lewis L, Villasana D, Morgan M, Muzny D, Gibbs R, Noebels J. Exome Sequencing of Ion Channel Genes Reveals Complex Profiles Confounding Personal Risk Assessment in Epilepsy. Cell. 2011;145:1036–1048. doi: 10.1016/j.cell.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler D, Tanouye M. Modifications of seizure susceptibility in Drosophila. J Neurophysiol. 2000;83:998–1009. doi: 10.1152/jn.2000.83.2.998. [DOI] [PubMed] [Google Scholar]
- Kuebler D, Tanouye M. Genetic suppression of seizure susceptibility in Drosophila. J Neurophysiol. 2001;86:1211–1225. doi: 10.1152/jn.2001.86.3.1211. [DOI] [PubMed] [Google Scholar]
- Lee J, Wu CF. Electroconvulsive seizure behavior in Drosophila: Analysis of the physiological repertoire underlying a stereotyped action pattern in bang-sensitive mutants. J Neurosci. 2002;22:11065–11079. doi: 10.1523/JNEUROSCI.22-24-11065.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Wu CF. Genetic modifications of seizure susceptibility and expression by altered excitability in Drosophila Na+ and K+ channel mutants. J Neurophysiol. 2006;96:2465–2478. doi: 10.1152/jn.00499.2006. [DOI] [PubMed] [Google Scholar]
- Marley R, Baines RA. Increased persistent Na+ current contributes to seizure in the slamdance bang-sensitive Drosophila mutant. J Neurophysiol. 2011;106:18–29. doi: 10.1152/jn.00808.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JO. Cellular and molecular basis of epilepsy. J Neurosci. 1994;14:3413–3425. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuilton P, St Pierre SE, Thurmond J the FlyBase Consortium. FlyBase 101 – the basics of navigating FlyBase. Nucleic Acids Research. 2011;40:21. doi: 10.1093/nar/gkr1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noebels J. The Biology of epilepsy genes. Ann Rev Neurosci. 2003;26:599–625. doi: 10.1146/annurev.neuro.26.010302.081210. [DOI] [PubMed] [Google Scholar]
- Ojemann GA. Surgical therapy for medically intractable epilepsy. J Neurosurg. 1987;66:489–499. doi: 10.3171/jns.1987.66.4.0489. [DOI] [PubMed] [Google Scholar]
- Palladino MJ, Bower JE, Kreber R, Ganetzky B. Neural dysfunction and neurodegeneration in Drosophila Na+/K+ ATPase alpha subunit mutants. J Neurosci. 2003;23:1276–1286. doi: 10.1523/JNEUROSCI.23-04-01276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L, Padilla M, Du Y, Dong K, Tanouye MA. Drosophila as a model for epilepsy: bss is a gain-of-function mutation in the para sodium channel gene that leads to seizures. Genetics. 2011;187:523–534. doi: 10.1534/genetics.110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P, Tanouye MA. Seizures and failures in the giant fiber pathway of Drosophila bang-sensitive paralytic mutants. J Neurosci. 1995;15(8):5810–5819. doi: 10.1523/JNEUROSCI.15-08-05810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P, Ramaswami M, Tanouye MA. The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell. 1994;79:23–33. doi: 10.1016/0092-8674(94)90397-2. [DOI] [PubMed] [Google Scholar]
- Roydon CS, Pirrotta V, Jan LY. The tko locus, site of a behavioral mutation in Drosophila melanogaster, codes for a protein homologous to prokaryotic ribosomal protein S12. Cell. 1987;51:165–173. doi: 10.1016/0092-8674(87)90144-9. [DOI] [PubMed] [Google Scholar]
- Shoffner JM, Lott MT, Lezza AMS, Seibel P, Ballinger SW, Wallace DC. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNALys mutation. Cell. 1990;61:931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Ueda A, Wu CF. Mutation of Drosophila focal adhesion kinase induces bang-sensitive behavior and disrupts glial function, axonal conduction and synaptic transmission. European J Neurosci. 2008;27:2860–2870. doi: 10.1111/j.1460-9568.2008.06252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervorst P, Ghysen A. Genetic control of sensory connections in Drosophila. Nature. 1980;286:65–67. doi: 10.1038/286065a0. [DOI] [PubMed] [Google Scholar]
- Wu C-F, Ganetzky B. Neurogenetic studies of ion channels in Drosophila. In: Narahashi T, editor. Ion Channels. Vol. 3. New York: Plenum Publishing Corp; 1992. pp. 261–314. [DOI] [PubMed] [Google Scholar]
- Wu CF, Ganetzky B, Jan LY, Jan YN, Benzer S. A Drosophila mutant with a temperature-sensitive block in nerve conduction. Proc Natl Acad Sci U S A. 1978;75:4047–4051. doi: 10.1073/pnas.75.8.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Tanouye M. The Drosophila slamdance gene: a mutation in an aminopeptidase can cause seizure, paralysis and neuronal failure. Genetics. 2002;162:1283–1299. doi: 10.1093/genetics/162.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]