Abstract
Due to the phaseout of polybrominated diphenyl ether (PBDE) flame retardants, new chemicals, such as 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) 2,3,4,5-tetrabromophthalate (TBPH), have been used as replacements in some commercial flame retardant mixtures. Both chemicals have been detected in indoor dust at concentrations approaching the concentrations of PBDEs; however, little is known about their fate, metabolism, or toxicity. The goal of this study was to investigate the potential metabolism of these two brominated flame retardants in human and rat tissues by conducting in vitro experiments with liver and intestinal subcellular fractions. In all the experiments, TBB was consistently metabolized to 2,3,4,5-tetrabromobenzoic acid (TBBA) via cleavage of the 2-ethylhexyl chain without requiring any added cofactors. TBBA was also formed in purified porcine carboxylesterase, but at a much faster rate of 6.29 ± 0.58 nmol min-1 mg protein-1. The estimated Km and Vmax values for TBB metabolism in human microsomes were 11.1 ± 3.9 μM and 0.644 ± 0.144 nmol min-1 mg protein-1, respectively. A similar Km of 9.3 ± 2.2 μM was calculated for porcine carboxylesterase, indicating similar enzyme specificity. While the rapid formation of TBBA may reduce the bioaccumulation potential of TBB in mammals and may be useful as a biomarker of TBB exposure, the toxicity of this brominated benzoic acid is unknown and may be a concern based on its structural similarity to other toxic pollutants. In contrast to TBB, no metabolites of TBPH were detected in human or rat subcellular fractions. However, a metabolic product of TBPH, mono(2-ethylhexyl) tetrabromophthalate (TBMEHP), was formed in purified porcine carboxylesterase at an approximate rate of 1.08 pmol min-1 mg protein-1. No Phase II metabolites of TBBA or TBMEHP were observed. More research is needed to understand the in vivo toxicokinetics and health effects of these compounds given their current ubiquitous presence in most US households and the resulting probability of chronic exposure, particularly to young children.
Keywords: TBB, TBPH, metabolism, tetrabromobenzoic acid, human, rat, carboxylesterase, biotransformation, flame retardants
Introduction
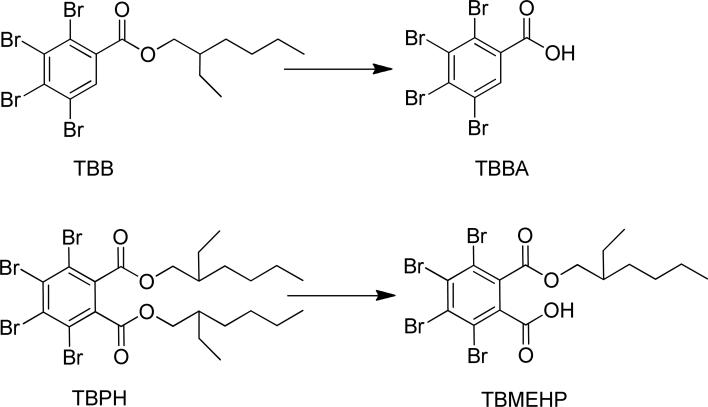
Brominated compounds are often used as additive flame retardants because of the halogen radicals’ ability to quench gas phase free radicals that propagate the fire cycle.1 The addition of halogens to aromatic and aliphatic backbones increases the flame retardant properties of these compounds, but also increases their potential for bioaccumulation and persistence in the environment. Several polybrominated diphenyl ether (PBDEs) flame retardant mixtures were used heavily until they were phased out in several regions2, 3 and were later listed as persistent organic pollutants (POPs) due to their persistence and bioaccumulation potential according to the Stockholm Convention.4 Currently, DecaBDE is the only commercial PBDE mixture still in use, but it is scheduled for phase out in the United States by 2013.5 Because flame retardants are used in consumer products, such as couches, electronics, and upholstery, they are present in the indoor environment. A study characterizing flame retardants in house dust identified 2-ethylhexyl tetrabromobenzoate (TBB) and 2-ethylhexyl tetrabromophthalate (TBPH) at concentrations often exceeding 1 μg g-1 (see structures in Figure 1).6 These compounds are components of several Firemaster® commercial flame retardant mixtures marketed by Chemtura (West Lafayette, IN, USA) for use as replacements for the PentaBDE commercial mixture. However, the physical properties of TBB and TBPH (estimated log KOW of 8.8 and 12, respectively) indicate a potential for biomagnification similar to other persistent organic pollutants.7 These compounds enter wastewater treatment plants, where they have been detected in sewage sludge and eventually enter the environment via effluent discharge or land-applied biosolids.8 TBB and TBPH were both detected in marine mammals from China, but no other published studies have analyzed sediment, lower trophic level organisms, or marine mammals from other regions.9 Therefore, little is known about the environmental fate of TBB and TBPH. A summary of the published studies investigating the levels of TBB and TBPH in the environment is included in Table 1.
Figure 1.
Chemical structures of TBB, TBPH, TBBA, and TBMEHP
Table 1.
Summary of previous studies detecting TBB and TBPH in the environment.
| Study Type | Matrix | Results | Location | Author |
|---|---|---|---|---|
| Indoor Environment | House Dust | TBB: 6.6–15,030 ng g-1 TBPH: 1.5 to 10,630 ng g-1 |
Boston, MA | Stapleton, 20086 |
| House Dust | TBB: 450–75,000 ng g-1 TBPH: 300–47,110 ng g-1 |
Boston, MA | Stapleton, 200922 | |
| Dust from E-waste Processing Facility |
TBB: 18 ng g-1 mean TBPH: 270 ng g-1 mean |
Thailand | Ali, 201123 | |
| Polyurethane Foam From Baby Products |
TBB/TBPH: 5.85–42.5 mg g-1 | USA | Stapleton, 201124 | |
| Outdoor Environment | Finless Porpoise | TBB: 5.6 ± 17 ng g-1 lipid TBPH: 342 ± 883 ng g-1 lipid |
Hong Kong, China | Lam, 20099 |
| Sewage Sludge | TBB: 3,430–89,900 ng g-1 TOC TBPH: 64–33,500 ng g-1 TOC |
Mid-Atlantic Region, USA | La Guardia, 20108 | |
| Sewage Sludge | TBB: 128 and 2,490 ng g-1 dry TBPH: 220–1340 ng g-1 dry |
North Carolina and California, USA | Davis, 201225 | |
| Atmosphere | TBB/TBPH: 0.050–290 pg m-3 | Great Lakes Region, USA | Ma, 201126 | |
Because of their use in consumer products and prevalence in house dust, the probability of human exposure to TBB and TBPH is high, particularly for children. A previous study demonstrated the ability of a commercial mixture containing both TBB and TBPH to bioaccumulate in fathead minnows (Pimephales promelas) after dietary exposure.7 TBB was metabolized to form unidentified brominated metabolites, but TBPH was resistant to metabolic degradation.7 Because TBB and TBPH were only recently discovered in the environment, there is very little toxicological data on these compounds. The nonbrominated analogs of TBB and TBPH, 2-ethyhexyl benzoate (EHB) and bis(2-ethylhexyl) phthalate (DEHP), respectively, are commonly used industrial chemicals. Only slight toxicity has been observed with EHB, which is approved for use as a food additive,10 but the chronic toxicity of DEHP has been extensively studied.11 In general, the metabolites of DEHP, including the hydrolysis metabolite 2-monoethylhexyl phthalate (MEHP), mediate the toxicity of DEHP.11 Lipases and esterases in various organs mediate the bioactivation of DEHP via hydrolysis of one ethylhexyl group.12 For TBB, toxicity data is not available from the manufacturer, and only one previous study that attempted to assess the toxicity of the commercial BZ-54 mixture, which contains both TBB and TBPH, reported bioaccumulation of TBB and TBPH in fathead minnows with evidence of potential genotoxicity.7 Some acute toxicological data on TBPH are available from the EPA's high production volume chemical database,13 but the potential developmental toxicity and endocrine disrupting effects of TBPH are unknown.
The metabolism of TBB and TBPH in humans has not yet been evaluated, and no studies have investigated the levels of these flame retardants in human tissues. Based on the metabolism of DEHP, it is likely that TBB and TBPH undergo similar cleavage of the ethylhexyl groups during metabolism. Because metabolism may lead to bioactivation of these compounds, and because knowledge of the metabolic stability will allow for predictions of the half-life of these compounds in human tissues, it is important to understand the metabolic potential of TBB and TBPH in humans. Furthermore, because toxicology studies are usually performed in rats, it is also important to compare species-specific differences in metabolism, which may mediate differences in toxicity. The objectives of this study were to identify the metabolites of TBB and TBPH in human liver microsomes (HLM), calculate the enzyme kinetics, and compare the metabolic rates among liver and intestinal subcellular fractions from both humans and rats.
Experimental Procedures
Materials
HLM (50 donors pooled) and human liver cytosol (50 donors pooled) were purchased from Invitrogen (Austin, TX, USA). Human intestinal microsomes (20 donors pooled) were purchased from BD Biosciences (Franklin Lakes, NJ, USA). An additional preparation of HLM (50 donors pooled) was purchased from Celsis (Chicago, IL, USA). All the human subcellular fractions represented mixed-gender donors. Crude, lyophilized hepatic porcine carboxylesterase was purchased from Sigma (less than 5% buffer salts) (St. Louis, MO, USA). Rat microsomes and cytosol were prepared from the liver and small intestinal tissues from adult, female Wistar rats (n=4) using our previously published method.14 Protein concentrations were determined using the Bradford assay with a microplate reader.15 TBB (95% purity) and TBPH (99% purity) were purchased as neat solutions from AccuStandard, Inc. (New Haven, CT, USA), and stock solutions were prepared in DMSO at concentrations ranging from 0.0078–31.1 mM. The internal standard 4’-fluoro-2,3’,4,6-tetrabromodiphenylether (FBDE 69) was purchased from Chiron (Trondheim, Norway), while the internal standards 2,3,5-triiodobenzoic acid (TIBA) and monohexyl-2,3,4,5-tetrachlorophthalate (TCMHP) were purchased from Sigma. The metabolic product 2,3,4,5-tetrabromobenzoic acid (TBBA; estimated >98% purity by H1-NMR) was synthesized by the Duke Small Molecule Synthesis Facility (the detailed synthesis procedure is reported in the Supplemental Information). Mono(2-ethylhexyl) tetrabromophthalate (TBMEHP) was a gift from Dr. Kim Boekelhide's group at Brown University. All solvents and other materials were HPLC grade.
Metabolic Incubations
All incubations were performed in 100 mM potassium phosphate incubation buffer (pH 7.4) in glass culture tubes in a shaking water bath at 37°C. The enzyme kinetics were evaluated using a microsomal protein concentration of 40 μg mL-1 for 10 min incubations. The incubation conditions were chosen to minimize substrate depletion, especially in incubations with low concentrations, while producing sufficient product to measure using our LC/MS/MS assay. In addition, range finding experiments indicated that metabolism was linear between 40-200 μg protein mL-1. The incubations for kinetics analyses were performed with 9 TBB concentrations ranging from 0.0078–31.1 μM. The reactions were stopped by the addition of an equal volume of 1 M HCl. The incubations to assess the Phase II metabolism of TBBA and TBMEHP were conducted in the presence of the following cofactors: 50 μM PAPS (phosphoadenosine phosphosulfate) and 8 mM MgCl2 for sulfation; 2 mM UDPGA (uridine diphosphate glucuronic acid), 25 μg mL-1 alamethicin, and 8 mM MgCl2 for glucuronidation; and 10 μM reduced glutathione for glutathione conjugation. The Phase II incubations were performed for 90 min.
Sample Preparation
After stopping the reactions with HCl, 25 ng each of FBDE 69, TIBA, and TCMHP were added as internal standards for TBB and TBPH, TBBA, and TBMEHP, respectively. The reaction mixtures were extracted using Agilent SampliQ OPT cartridges (3 mL, 60 mg; Agilent Technologies, Inc., Santa Clara, CA, US) using a method similar to our previously published extraction procedure for thyroid hormones.16 After conditioning the SPE cartridges with 3 mL of methanol and 3 mL of water, the entire incubation mixture was added. The cartridges were washed with 3 mL of water, and the analytes were eluted with 2 mL of methanol and 2 mL of dichloromethane. The eluent fractions were combined, evaporated under nitrogen, and reconstituted in methanol.
GC/MS Identification of Brominated Metabolites
To identify potential metabolites using GC/MS, the extracts were reconstituted in hexane, derivatized using diazomethane (a methylating agent), and analyzed using full scan GC/ECNI (electron capture negative ionization)-MS and GC/EI-MS using our lab's previously published GC conditions.6 The mass spectra of peaks not present in control incubations (without microsomes) were analyzed to determine the structural characteristics of the potential metabolites based on fragmentation patterns and bromine isotope signatures.
LC/MS/MS Quantification
The TBB and TBPH dosing concentrations were evaluated using LC/MS/MS with negative APCI (atmospheric pressure chemical ionization) using previously published MRMs and operating parameters.17 Isochratic LC separation was performed using a Synergi XB-C18 column (100 × 2.10 mm, 2.6 μm; Phenomenex, Torrance, CA, US) with 98% methanol and 2% water as the mobile phase for 9 min. The method detection limits for TBB and TBPH were 5.05 ng mL-1 and 2.41 ng mL-1, respectively.
An LC/MS/MS method using negative ESI (electrospray ionization) was developed to quantify TBBA without derivatization and to confirm the GC/MS results. Separation of a 15-μL injection was performed on a Synergi Polar-RP column (50 × 2.0 mm, 2.5 μm; Phenomenex) with water and acetonitrile mobile phases with 5 mM acetic acid. A gradient method at a flow rate of 0.4 mL min-1 was used to separate TBBA, TBMEHP, and their surrogate standards, TIBA and TCMHP, starting with 30% acetonitrile, increasing to 98% acetonitrile over 5 min, and returning to 30% acetonitrile over 3 min. TBBA and TIBA ionized and fragmented similarly, forming [M-H]- precursor ions and [M-CO2]- and [M-X]- product ions (X= Br or I; Table 2). TBMEHP formed an [M-CO2]- parent ion (m/z=549.0) using ESI and fragmented to form a [Br]- product ion (80.9). Multiple reaction monitoring (MRM) was used with the transitions and conditions shown in Table 2. The mean recoveries of 10 ng of the analytes and 50 ng of the internal standards spiked into buffer containing 50 μg of bovine albumin were calculated and are shown in Table 2. The calibration curves for TBBA and TBMEHP were linear (R2>0.99) over a calibration range of 1.00–1,000 ng mL-1. Phase II metabolites (i.e., glucuronide- and sulfate-conjugated metabolites) were screened using a combination of techniques including neutral loss scans (176 Da for glucuronide cleavage) and predicted MRM analyses as described in a previous study.18
Table 2.
LC/MS/MS conditions, analyte recoveries, and method detection limits for the two metabolites, TBBA and TBMEHP, and the internal standards used for their quantification, 2,3,5-triiodobenzoic acid (TIBA) and monohexyl-2,3,4,5-tetrachlorophthalate (TCMHP), respectively.
| Compound | Fragmentor Voltage | Collision Energy | Gas Temp (°C) | MRM Transition | Mean Recoveryb | Method Detection Limit |
|---|---|---|---|---|---|---|
| TBBA | 75 | 5 | 250 | 436.6 → 392.6 | 63 ± 5% | 1.25 ng mL-1 |
| TIBA | 75 | 5 | 250 | 498.7 → 454.8 | 63 ± 6% | |
| TBMEHP | 120 | 30 | 250 | 548.8a → 80.9 | 110 ± 3% | 1.20 ng mL-1 |
| TCMHP | 80 | 5 | 250 | 343.0a → 215.0 | 108 ± 1% |
The parent ion in the MRM transition represents [M-CO2]-
Recovery of 10 ng of TBBA and TBMEHP (n=6) and 50 ng of TIBA and TCMHP (n=3)
Quality Assurance
The results in this study are reported as the means ± SE of at least 2 independent experiments including 2–4 samples in each experiment along with controls containing 50 μg of bovine albumin as lab blanks. The mean concentrations of TBBA, TBMEHP, TBB, TBPH in the lab blanks were 0.474 ± 0.092, 0.288 ± 0.125, 2.77 ± 0.29, and 0.938 ± 0.186 ng mL-1, respectively. Method detection limits were defined as the mean of the blank measurements plus 3 times the SD of the blank measurements. Statistical analyses and enzyme kinetics were calculated using JMP 9 (Cary, NC, USA). A Michaelis-Menten model was used to calculate enzyme kinetics as follows:
| Eq. 1 |
where ν is the initial velocity at a given substrate concentration (S). The goodness of fit of the Michaelis-Menten models is reported using the coefficient of determination R2. ANOVA was used to determine whether significant interactions existed between the formation rates and the tissue preparations. Tukey's post-hoc test was used to compare TBBA formation rates among all the tissues with a significance level of p<0.05.
Results and Discussion
In Vitro Metabolism of TBB
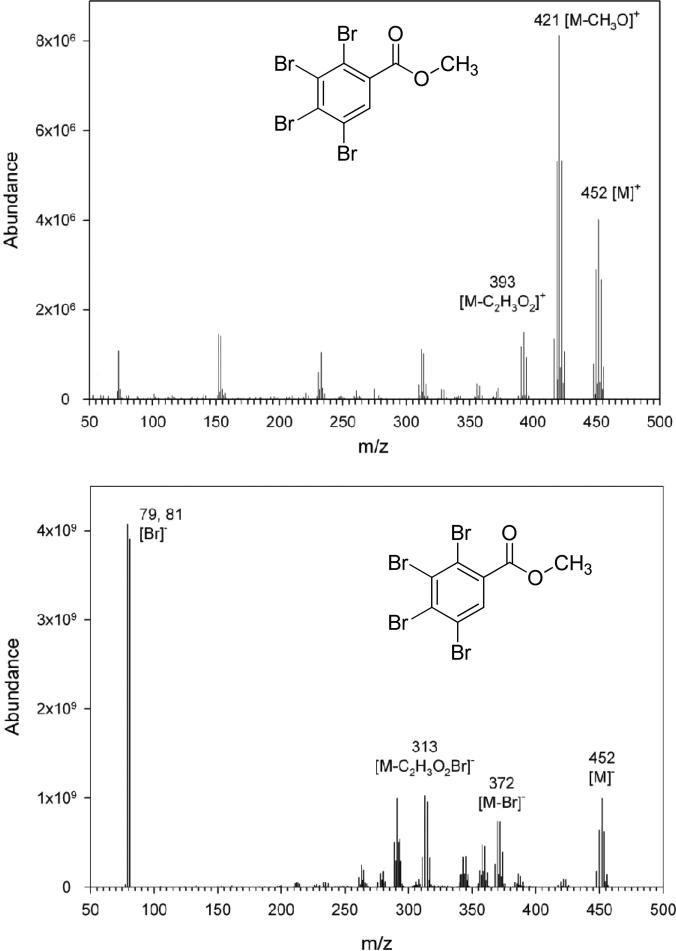
The results from our experiments indicated that TBB was rapidly metabolized in both HLM and rat microsomes. To initially identify the potential metabolites of TBB, 60-min incubations were performed with HLM. The sample extracts were analyzed before and after methyl-derivatization with diazomethane. Using full-scan GC/MS with ECNI and EI ionization, no potential debrominated or other metabolites were detected in the nonderivatized samples for TBB (i.e. no peaks were observed), but a 79.9 ± 4.6% reduction in the concentration of TBB was observed. In samples treated with diazomethane, TBBA was identified as the major metabolite of TBB based on both ECNI and EI mass spectra of the methyl-derivative of TBBA, as shown in Figure 2, and no other potential metabolites were detected (Supplemental Figure 3). A peak with an m/z of 452.0 was present in both ECNI and EI mass spectra that corresponded to the molecular ion of the TBBA-methyl derivative along with other fragment ions (Figure 2). To further confirm the identification, an analytical standard for TBBA was synthesized, and a more robust LC/MS/MS method using negative ESI was developed using 2,3,5-triiodobenzoic acid (TIBA) as an internal standard. Comparison of the metabolite with the synthesized TBBA confirmed that TBBA was the primary metabolite of TBB.
Figure 2.
Mass spectra of the TBBA methyl-derivative obtained using GC/MS operated in EI (top) and ECNI (bottom) ionization modes with labeled fragments.
Carboxylesterase-Mediated Metabolism
To determine whether cytochrome P450 metabolizing enzymes were involved in the metabolism of TBB, incubations were performed in the presence and absence of the necessary cofactor, NADPH. The addition of NADPH (0, 0.3, 3.0 and 30 mM) did not significantly affect the formation rate of TBBA (data not shown). Based on the observed mechanism of ester hydrolysis in the absence of cofactors, we hypothesized that carboxylesterases were responsible for the metabolism of TBB. Esterases represent an extensive class of cytosolic and microsomal enzymes with broad substrate specificity and widespread expression in mammalian tissues.19 Furthermore, the nonhalogenated analog of TBB, 2-ethylhexyl benzoate, is a well-characterized substrates of carboxylesterases.20 Therefore, the metabolism of TBB was also examined in a purified preparation of hepatic porcine carboxylesterase (PCE). Although the use of PCE in this study makes it difficult to determine whether or not similar metabolism of TBB and TBPH may be expected from human carboxylesterases, previous studies have shown that human and porcine carboxylesterases catalyze similar metabolic reactions with differences only in the observed reaction rates.21 The PCE enzyme preparation rapidly metabolized TBB to TBBA, and no other metabolites were detected. Because various types of esterases are widely expressed in vertebrates and bacteria, TBB hydrolysis may occur in many different organisms, and bacterial degradation may occur in the environment or during wastewater treatment.19
Enzyme Kinetics
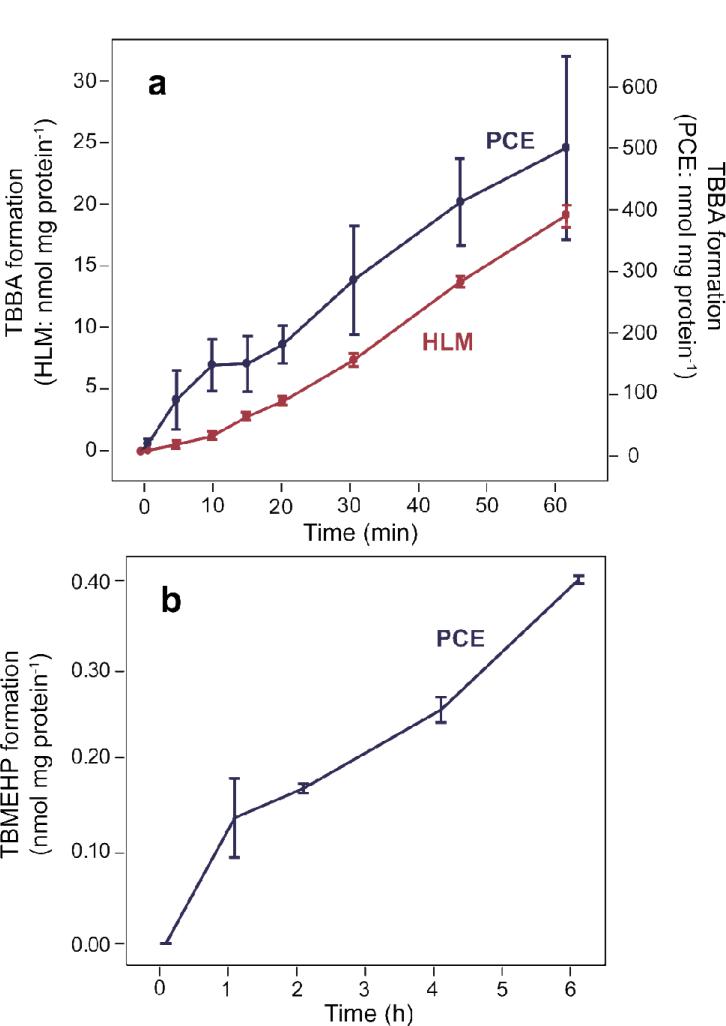
The hydrolysis of TBB in the liver to form TBBA may influence the distribution and potential toxicity of TBB in vivo. To determine the maximum rate of metabolism and the enzyme specificity for TBB in HLM and PCE, we calculated the Michaelis-Menten kinetic parameters. To optimize the incubation conditions for the kinetics experiments, the time courses of both HLM- and PCE-mediated TBB hydrolysis were evaluated over 60 min with either 40 μg protein mL-1 of HLM or 2 μg protein mL-1 of PCE at a TBB concentration of 27.1 ± 1.3 μM. TBBA formation continued at an approximately linear rate for the entire 60-min incubation for both preparations, as shown in Figure 3a.
Figure 3.
Formation of TBBA over time in (a) human liver microsomes (40 μg protein mL-1) and (b) porcine hepatic carboxylesterase (2 μg protein mL-1) at a TBB concentration of 27.1 ± 1.3 μM showing approximately linear formation for 60 min. The values represent the mean of 4 experiments.
Attempts were made to characterize the rate of TBBA formation relative to the metabolic loss of TBB (i.e., a mass balance). TBBA increased over time during the incubation, producing approximately 1 nmol during the 60-min incubation. TBB, on the other hand, decreased by approximately 11% (e.g., 2.9 nmol) during the 60-min incubation; therefore, the formation of TBBA appeared to account for approximately 30% of the substrate loss of TBB over the 60-min incubation with HLM. However, given the standard error in the measurement of TBB, this loss was not statistically significant.
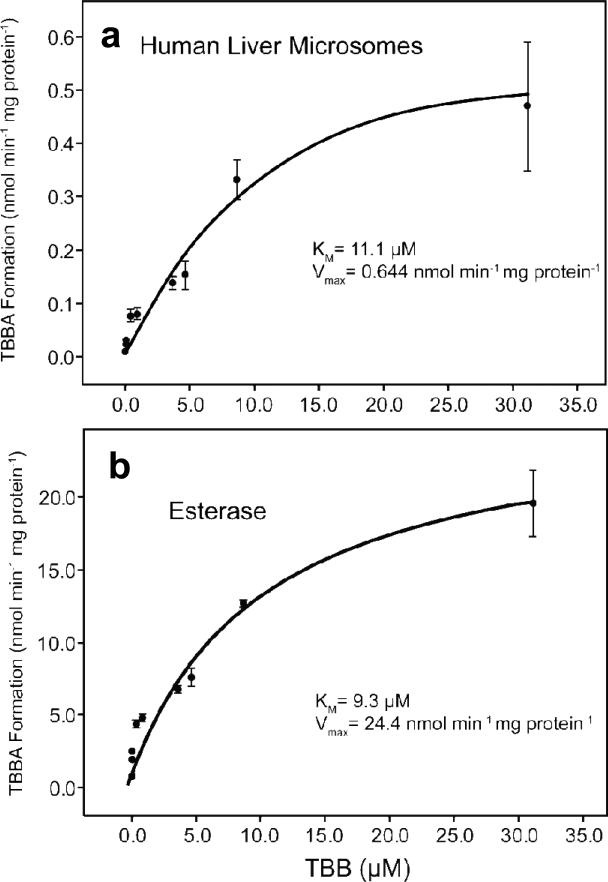
The kinetics of TBBA formation in HLM and PCE were evaluated by performing incubations with a range of TBB concentrations. The results of the kinetics experiments are shown in Fig. 4. Using a Michaelis-Menten model, the Km and Vmax for TBBA formation in HLM were determined to be 11.1 ± 3.9 μM and 0.644 ± 0.144 nmol min-1 mg protein-1, and the Km and Vmax in PCE were 9.3 ± 2.2 μM and 24.4 ± 2.6 nmol min-1 mg protein-1, respectively. The Km was approximately 10 μM in both HLM and PCE, which indicates similar enzyme specificity among the enzymes in both preparations. Due to the limited solubility of TBB in the incubation buffer, approximately 30 μM was the highest concentration that could be used to evaluate the kinetics without introducing unacceptably high variability in the substrate concentrations and, thus, the reaction velocities.
Figure 4.
Initial velocity of TBBA formation at various substrate concentrations fit to a Michaelis-Menten model using 10-min incubations with human liver microsomes and porcine hepatic carboxylesterase with R2 values of 0.782 and 0.862, respectively. The values represent the mean of 4 experiments.
Multiple Species and Tissue Comparison
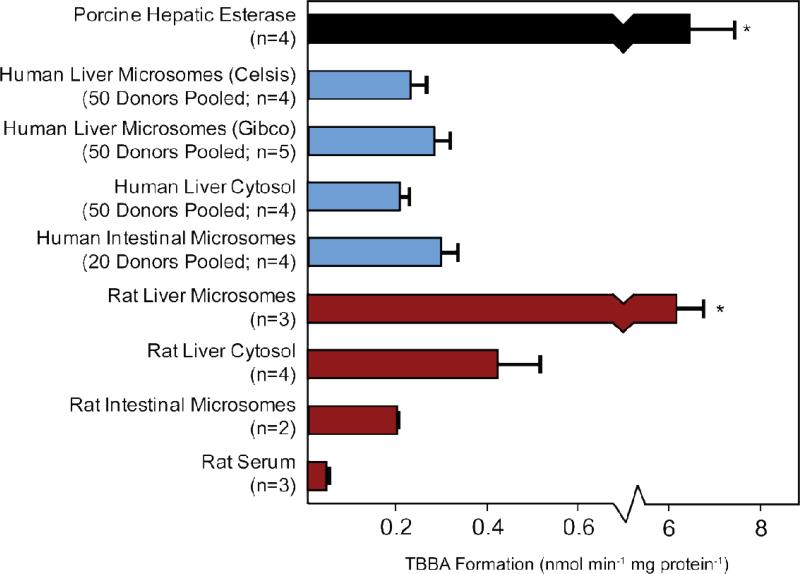
TBB metabolism was compared in commercial samples purchased from two different suppliers and between sub-cellular fractions prepared from different human tissues (human liver cytosol and human intestinal microsome fractions). TBB metabolism was evaluated in simultaneous 10-min incubations with 40 μg protein mL-1 of each subcellular fraction at a nominal TBB concentration of 20 μM (the actual mean concentration was measured to be 5.25 ± 0.31 μM, which was likely due to the limited solubility in the DMSO dosing solvent). The results of these experiments are shown in Figure 5. TBBA formation rates observed in the two commercial preparations of HLM were not significantly different. TBB metabolism was slightly lower in the cytosol (0.207 ± 0.020 nmol min-1 mg protein-1) than the intestinal and liver microsomes (0.297 ± 0.037 and 0.259 ± 0.033 nmol min-1 mg protein-1, respectively), but these differences were also not statistically significant.
Figure 5.
TBBA formation rates for incubations performed with human and rat tissues normalized to protein content. In pooled samples, n equals the number of individual experiments performed with the pooled sample. In the rat tissues, n equals the number of animals from which individual microsomal or serum samples were prepared. The asterisk indicates significant difference from samples without an asterisk using Tukey's post-hoc test (p<0.01) after ANOVA indicated a main effect of tissue on formation rate (p<0.001).
Potential TBB metabolism was compared among rat tissues to evaluate species-specific differences between rats and humans. TBB metabolism was investigated in rat liver microsomes and cytosol, intestinal microsomes, and serum. TBB was metabolized to form TBBA in all the rat tissues at formation rates ranging from 0.203 ± 0.004 and 0.422 ± 0.093 nmol min-1 mg protein-1 in intestinal microsomes and liver cytosol, respectively, to 6.25 ± 0.58 nmol min-1 mg protein-1 in liver microsomes, as shown in Figure 5. TBB metabolism also occurred in rat serum but at a much slower rate than in the other tissues (0.0418 ± 0.0090 nmol min-1 mg protein-1). The formation of TBBA in rat liver microsomes occurred at a significantly faster formation rate than all the other rat and human tissues, but was similar to the rate of TBBA formation in PCE (6.29 ± 0.92 nmol min-1 mg protein-1). Unlike human tissues, TBB metabolism was significantly slower in rat intestinal microsomes than in rat liver microsomes. While the metabolism of TBB was similar among human liver and intestinal microsomes and human cytosol, TBB metabolism in rat liver microsomes occurred much faster. This species-specific difference in TBB metabolism should be considered in toxicity studies performed using rats due to the more rapid elimination of TBB in rat livers. However, the rat data presented here are representative of a small number of female rats (n=4), and further work is necessary to confirm whether these values are representative of male rats and other rat strains.
TBPH Metabolism
Unlike TBB, in experiments with HLM, a significant loss of TBPH was not observed, and no metabolites were detected by GC/MS analysis of the sample extracts. An LC/MS/MS method was developed to monitor tetrabromo-MEHP (TBMEHP), a potential hydrolysis metabolite of TBPH (Figure 1). After a 6-h incubation in HLM, TBMEHP was not detected as a metabolite of TBPH and no significant loss of TBPH was observed. However, TBPH was slowly metabolized to form TBMEHP in the presence of 0.1 mg mL-1 of PCE. This reaction was monitored at multiple time points up to 6 h and maintained linearity at an approximate rate of 1.08 pmol min-1 mg esterase-1 as shown in Fig. 3b. In a previous study with PCE, DEHP (50 μM) was metabolized to form MEHP at a rate of 127 pmol min-1 mg protein-1.12 This rate was approximately 100 times faster than the hydrolysis of TBPH observed in this study (1.08 pmol min-1 mg protein-1). The prominent difference between the metabolic hydrolysis of DEHP and TBPH may be a result of steric hindrance by the fully brominated phenyl ring of TBPH.
Phase II Metabolism
The potential Phase II metabolism of TBBA and TBMEHP was evaluated in HLM and cytosol. Potential sulfation and glutathione conjugation were evaluated in cytosol, and glucuronidation was evaluated in microsomes. Using a combination of LC/MS/MS techniques, no Phase II metabolites were detected for either compound. Furthermore, there were no significant losses of the parent compounds, TBBA or TBMEHP, during the incubations.
TBBA may prove to be a useful biomarker of human exposure to TBB. Although this study shows that TBBA is likely formed in vivo via metabolism, the half-life of TBBA in human tissues is unknown. No Phase II metabolites of TBBA were detected in this study after performing incubations with cofactors specific for sulfation, glucuronidation, and glutathione conjugation. If TBBA is excreted in the urine, it may be an important indicator of human TBB exposure; therefore, future studies should evaluate the distribution and excretion of both TBB and TBBA.
The results for TBPH provide some insight into the fate and potential toxicity of TBPH. Because TBPH is apparently more recalcitrant to metabolism than TBB, TBPH may have a longer half-life after absorption in vivo. A study analyzing marine mammals from the Pearl River Delta in China reported mean concentrations of 5.6 ± 17 and 342 ± 883 ng g lipid-1 for TBB and TBPH, respectively,9 even though the approximate ratio of TBB to TBPH in the Firemaster 550 commercial mixture is 4:1.6 Metabolism of TBB may be an important factor in these observed differences in TBB and TBPH concentrations and in the ratios of TBB and TBPH in other environmental matrices.
The metabolism of TBB to form TBBA has several toxicological implications. While metabolism apparently reduces the potential for bioaccumulation of TBB, it introduces a metabolite, TBBA, with unknown toxicity and fate. This metabolite may be used as a biomarker of TBB exposure for public health and exposure studies once its excretion pathway has been characterized. The metabolism of TBPH to form TBMEHP may have implications on the toxicity of TBPH, but may not be rapid enough to affect the bioaccumulation of TBPH. However, its persistence in tissues and the ubiquity of carboxylesterases in other organs and tissues may facilitate TBPH metabolism in mammalian tissues. In vivo toxicological studies should assess the in vivo accumulation and metabolism of both TBB and TBPH in mammals and evaluate the toxicity of both TBBA and TBMEHP.
Supplementary Material
Acknowledgements
We thank Dave Gooden and Ramesh Gopalaswamy in the Duke Small Molecule Synthesis Facility for synthesizing TBBA.
Funding Sources
This study was funded by a grant from the National Institute of Environmental Health Sciences, R01ESO16099.
Abbreviations
- PBDE
polybrominated diphenyl ether
- TBB
2-ethylhexyl-2,3,4,5-tetrabromobenzoate
- TBPH
bis(2-ethylhexyl) 2,3,4,5 tetrabromophthalate
- HLM
human liver microsomes
- PCE
porcine hepatic carboxylesterase
- TBBA
2,3,4,5-tetrabromobenzoic acid
- TBMEHP
mono(2-ethylhexyl) 2,3,4,5-tetrabromophthalate
- TIBA
2,3,5-triiodobenzoic acid
- TCMHP
monohexyl-2,3,4,5-tetrachlorophthalate
- EHB
2-ethylhexyl benzoate
- DEHP
bis(2-ethylhexyl) phthalate
- MEHP
mono(2-ethylhexyl) phthalate
- MS/MS
tandem mass spectrometry
- ESI
electrospray ionization
- ECNI
electron capture negative ionization
- APCI
atmospheric pressure chemical ionization
References
- 1.Rahman F, Langford KH, Scrimshaw MD, Lester JN. Polybrominated diphenyl ether (PBDE) flame retardants. Sci. Total Environ. 2001;275:1–17. doi: 10.1016/s0048-9697(01)00852-x. [DOI] [PubMed] [Google Scholar]
- 2.Tullo A. Great Lakes to phase out flame retardants. Chemical & Engineering News. 2003;81:13–13. [Google Scholar]
- 3.EU Directive 2003/11/EC of the European Parliament and of the Council of 6 February 2003. Official Journal of the European Union, Directive 76/769/EEC. :45–46. [Google Scholar]
- 4.EU Stockholm Convention on Persistent Organic Pollutants. Adoption of Amendments to Annexes A, B, and C. Decisions SC-4/13, 14, 18. 2009 Available at chm.pops.int.
- 5.USEPA DecaBDE phase-out initiative. 2010 Available at http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/deccadbe.html.
- 6.Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. Alternate and new brominated flame retardants detected in U.S. house dust. Environ. Sci. Technol. 2008;42:6910–6916. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- 7.Bearr JS, Stapleton HM, Mitchelmore CL. Accumulation and DNA damage in fathead minnows (Pimephales promelas) exposed to 2 brominated flame-retardant mixtures, Firemaster® 550 and Firemaster® BZ-54. Environ. Toxicol. Chem. 2010;29:722–729. doi: 10.1002/etc.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Guardia MJ, Hale RC, Harvey E, Chen D. Flame-retardants and other organohalogens detected in sewage sludge by electron capture negative ion mass spectrometry. Environ. Sci. Technol. 2010;44:4658–4664. doi: 10.1021/es9039264. [DOI] [PubMed] [Google Scholar]
- 9.Lam JCW, Lau RKF, Murphy MB, Lam PKS. Temporal trends of hexabromocyclododecanes (HBCDs) and polybrominated diphenyl ethers (PBDEs) and detection of two novel flame retardants in marine mammals from Hong Kong, South China. Environ. Sci. Technol. 2009;43:6944–6949. doi: 10.1021/es901408t. [DOI] [PubMed] [Google Scholar]
- 10.WHO Safety evaluation of certain food additives and contaminants. WHO Food Additives Series:64. 2011 [Google Scholar]
- 11.Tickner JA, Schettler T, Guidotti T, McCally M, Rossi M. Health risks posed by use of di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: A critical review. Am. J. Ind. Med. 2001;39:100–111. doi: 10.1002/1097-0274(200101)39:1<100::aid-ajim10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Niino T, Ishibashi T, Ishiwata H, Takeda K, Onodera S. Characterization of human salivary esterase in enzymatic hydrolysis of phthalate esters. J. Health Sci. 2003;49:76–81. [Google Scholar]
- 13.Horizons H. a. E., editor. Test plan for phthalic acid tetrabromo bis 2-ethylhexyl ester (CAS# 26040-51-7) Brominated Phthalate Ester Panel; Annapolis, MD: 2002. [Google Scholar]
- 14.Roberts SC, Noyes PD, Gallagher EP, Stapleton HM. Species-specific differences and structure-activity relationships in the debromination of PBDE congeners in three fish species. Environ. Sci. Technol. 2011;45:1999–2005. doi: 10.1021/es103934x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradford MM. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Wang DL, Stapleton HM. Analysis of thyroid hormones in serum by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2010;397:1831–1839. doi: 10.1007/s00216-010-3705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou S, Reiner E, Marvin C, Helm P, Riddell N, Dorman F, Misselwitz M, Shen L, Crozier P, MacPherson K, Brindle I. Development of liquid chromatography atmospheric pressure chemical ionization tandem mass spectrometry for analysis of halogenated flame retardants in wastewater. Anal. Bioanal. Chem. 2010;396:1311–1320. doi: 10.1007/s00216-009-3279-6. [DOI] [PubMed] [Google Scholar]
- 18.Clarke NJ, Rindgen D, Korfmacher WA, Cox KA. Peer reviewed: Systematic LC/MS metabolite identification in drug discovery. Analytical Chemistry. 2001;73:430 A–439 A. doi: 10.1021/ac012480y. [DOI] [PubMed] [Google Scholar]
- 19.Sogorb MA, Vilanova E. Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicol. Lett. 2002;128:215–228. doi: 10.1016/s0378-4274(01)00543-4. [DOI] [PubMed] [Google Scholar]
- 20.Albro PW, Corbett BJ, Hass JR. The mechanism for nonspecific lipase from rat pancreas. Biochim. Biophys. Acta. 1976;431:493–506. doi: 10.1016/0005-2760(76)90215-0. [DOI] [PubMed] [Google Scholar]
- 21.Huang TL, Shiotsuki T, Uematsu T, Borhan B, Li QX, Hammock BD. Structure-activity relationships for substrates and inhibitors of mammalian liver microsomal carboxylesterases. Pharmaceutical Research. 1996;13:1495–1500. doi: 10.1023/a:1016071311190. [DOI] [PubMed] [Google Scholar]
- 22.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ. Sci. Technol. 2009;43:7490–7495. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali N, Harrad S, Muenhor D, Neels H, Covaci A. Analytical characteristics and determination of major novel brominated flame retardants (NBFRs) in indoor dust. Anal. Bioanal. Chem. 2011;400:3073–3083. doi: 10.1007/s00216-011-4966-7. [DOI] [PubMed] [Google Scholar]
- 24.Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. Identification of flame retardants in polyurethane foam collected from baby products. Environ. Sci. Technol. 2011;45:5323–5331. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis EF, Klosterhaus SL, Stapleton HM. Measurement of flame retardants and triclosan in municipal sewage sludge and biosolids. Environ. Int. 2012;40:1–7. doi: 10.1016/j.envint.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Venier M, Hites RA. 2-Ethylhexyl tetrabromobenzoate and bis(2-ethylhexyl) tetrabromophthalate flame retardants in the Great Lakes atmosphere. Environ. Sci. Technol. 2011;46:204–208. doi: 10.1021/es203251f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.